Carbon Nanotubes for Confinement-Induced Energetic Nanomaterials
Abstract
1. Introduction
2. Experimental Section
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Comet, M.; Martin, C.; Schnell, F.; Spitzer, D. Nanothermites: A short Review. Factsheet for Experimenters, Present and Future Challenges. Propellants Explos. Pyrotech. 2019, 44, 18–36. [Google Scholar] [CrossRef]
- Armstrong, R.W.; Baschung, B.; Booth, D.W.; Samirant, M. Enhanced Propellant Combustion with Nanoparticles. Nano Lett. 2003, 3, 253–255. [Google Scholar] [CrossRef]
- Bellott, B.J.; Noh, W.; Nuzzo, R.G.; Girolami, G.S. Nanoenergetic materials: Boron nanoparticles from the pyrolysis of decaborane and their functionalisation. Chem. Commun. 2009, 22, 3214–3215. [Google Scholar] [CrossRef] [PubMed]
- Slocik, J.M.; Crouse, C.A.; Spowart, J.E.; Naik, R.R. Biologically Tunable Reactivity of Energetic Nanomaterials Using Protein Cages. Nano Lett. 2013, 13, 2535–2540. [Google Scholar] [CrossRef] [PubMed]
- Zarko, V.E.; Gromov, A.A. (Eds.) Energetic Nanomaterials: Synthesis, Characterization, and Application; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780128027107. [Google Scholar]
- Zhao, F.; Yi, J.; Hong, W.; An, T.; Yang, Y. Chapter Ten—Preparation, Characterization, and Catalytic Activity of Carbon Nanotubes-Supported Metal or Metal Oxide. In Energetic Nanomaterials: Synthesis, Characterization, and Application; Elsevier: Amsterdam, The Netherlands, 2016; pp. 231–284. [Google Scholar] [CrossRef]
- Kim, J.H.; Ahn, J.Y.; Park, H.S.; Kim, S.H. Optical ignition of nanoenergetic materials: The role of single-walled carbon nanotubes as potential optical igniters. Combust. Flame 2013, 160, 830–834. [Google Scholar] [CrossRef]
- Manaa, M.R.; Mitchell, A.R.; Garza, R.G.; Pagoria, P.F.; Watkins, B.E. Flash Ignition and Initiation of Explosives−Nanotubes Mixture. J. Am. Chem. Soc. 2005, 127, 13786–13787. [Google Scholar] [CrossRef]
- Kim, J.H.; Cho, M.H.; Kim, K.J.; Kim, S.H. Laser ignition and controlled explosion of nanoenergetic materials: The role of multi-walled carbon nanotubes. Carbon 2017, 118, 268–277. [Google Scholar] [CrossRef]
- Visconti, P.; Primiceri, P.; De Fazio, R.; Strafella, L.; Ficarella, A.; Carlucci, A.P. Light-Induced ignition of Carbon Nanotubes and energetic nano-materials: A review on methods and advanced technical solutions for nanoparticles-enriched fuels combustion. Rev. Adv. Mater. Sci. 2020, 59, 26–46. [Google Scholar] [CrossRef]
- Choi, W.; Hong, S.; Abrahamson, J.T.; Han, J.-H.; Song, C.; Nair, N.; Baik, S.; Strano, M.S. Chemically driven carbon-nanotube-guided thermopower waves. Nat. Mater. 2010, 9, 423–429. [Google Scholar] [CrossRef]
- Hwang, H.; Yeo, T.; Um, J.-E.; Lee, K.; Kim, H.-S.; Han, J.-H.; Kim, W.-J.; Choi, W. Investigation of the effect of the structure of large-area carbon nanotube/fuel composites on energy generation from thermopower waves. Nanoscale Res. Lett. 2014, 9, 536. [Google Scholar] [CrossRef]
- Elghany, M.A.; Elbeih, A.; Hassanein, S. Thermal Behavior and Decomposition Kinetics of RDX and RDX/HTPB Composition Using Various Techniques and Methods. Cent. Eur. J. Energetic Mater. 2016, 13, 714–735. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.P.; Domínguez, J.G.; Hernández, F.G.; Vélez, L.F.; Domínguez, O. The Thermal Decomposition of Ammonium Perchlorate-Aluminum Propellants in Presence of Metallic Zinc Particles. Mater. Sci. Appl. 2017, 8, 436–447. [Google Scholar] [CrossRef]
- Pivkina, A.N.; Muravyev, N.V.; Monogarov, K.A.; Fomenkov, I.V.; Schoonman, J. Catalysis of HMX Decomposition and Combustion: Defect Chemistry Approach. In Energetic Nanomaterials: Synthesis, Characterization, and Application; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780128027107. [Google Scholar]
- Burnham, A.K.; Stanford, V.L.; Vyazovkin, S.; Kahl, E.M. Effect of pressure on TATB and LX-17 thermal decomposition. Therm. Acta 2021, 699, 178908. [Google Scholar] [CrossRef]
- Pelletier, V.; Bhattacharyya, S.; Knoke, I.; Forohar, F.; Bichay, M.; Gogotsi, Y. Copper Azide Confined Inside Templated Carbon Nanotubes. Adv. Funct. Mater. 2010, 20, 3168–3174. [Google Scholar] [CrossRef]
- Zhu, Z.P.; Su, D.S.; Lu, Y.; Schlögl, R.; Weinberg, G.; Liu, Z.Y. Molecular “Glass” Blowing: From Carbon Nanotubes to Carbon Nanobulbs. Adv. Mater. 2004, 16, 443–447. Available online: https://www.researchgate.net/publication/268293621_Blowing_Carbon_Nanotubes_to_Carbon_Nanobulbs (accessed on 10 October 2022). [CrossRef]
- Guo, R.; Hu, Y.; Shen, R.; Ye, Y.; Wu, L. A micro initiator realized by integrating KNO3@CNTs nanoenergetic materials with a Cu microbridge. Chem. Eng. J. 2012, 211–212, 31–36. [Google Scholar] [CrossRef]
- Guo, R.; Hu, Y.; Shen, R.; Ye, Y. Electro-explosion performance of KNO3 -filled carbon nanotubes initiator. J. Appl. Phys. 2014, 115, 174901. [Google Scholar] [CrossRef]
- Bortolamiol, T.; Lukanov, P.; Galibert, A.-M.; Soula, B.; Lonchambon, P.; Datas, L.; Flahaut, E. Double-walled carbon nanotubes: Quantitative purification assessment, balance between purification and degradation and solution filling as an evidence of opening. Carbon 2014, 78, 79–90. [Google Scholar] [CrossRef]
- Tsang, S.C.; Chen, Y.K.; Harris, P.J.F.; Green, M.L.H. A simple chemical method of opening and filling carbon nanotubes. Nature 1994, 372, 159–162. [Google Scholar] [CrossRef]
- Pradere, C.; Suhard, S.; Vendier, L.; Jacob, G.; Chaudret, B.; Sabo-Etienne, S. Heterometallic Werner complexes as energetic materials. Dalton Trans. 2008, 20, 2725–2731. [Google Scholar] [CrossRef] [PubMed]
- Suhard, S.; Fau, P.; Chaudret, B.; Sabo-Etienne, S.; Mauzac, M.; Mingotaud, A.-F.; Ardila-Rodriguez, G.; Rossi, C.; Guimon, M.-F. When Energetic Materials, PDMS-Based Elastomers, and Microelectronic Processes Work Together: Fabrication of a Disposable Microactuator. Chem. Mater. 2009, 21, 1069–1076. [Google Scholar] [CrossRef]
- Deblitz, R.; Hrib, C.G.; Blaurock, S.; Jones, P.G.; Plenikowski, G.; Edelmann, F.T. Explosive Werner-type Cobalt(iii) complexes. Inorg. Chem. Front. 2014, 1, 621–640. [Google Scholar] [CrossRef]
- Flahaut, E.; Peigney, A.; Bacsa, W.S.; Bacsa, R.R.; Laurent, C. CCVD synthesis of carbon nanotubes from (Mg,Co,Mo)O catalysts: Influence of the proportions of cobalt and molybdenum. J. Mater. Chem. 2004, 14, 646–653. [Google Scholar] [CrossRef]
- Flahaut, E.; Bacsa, R.; Peigney, A.; Laurent, C. Gram-scale CCVD synthesis of double-walled carbon nanotubes. Chem. Commun. 2003, 12, 1442–1443. [Google Scholar] [CrossRef]
- Bjerrum, J.; McReynolds, J.P.; Oppegard, A.L.; Parry, R.W. Hexamminecobalt(III) Salts. In Inorganic Syntheses; McGraw Hill: New York, NY, USA; London, UK, 1946; pp. 216–221. [Google Scholar] [CrossRef]
- Sloan, J.; Novotny, M.C.; Bailey, S.R.; Brown, G.; Xu, C.; Williams, V.C.; Friedrichs, S.; Flahaut, E.; Callender, R.L.; York, A.P.E.; et al. Two layer 4:4 co-ordinated KI crystals grown within single walled carbon nanotubes. Chem. Phys. Lett. 2000, 329, 61–65. [Google Scholar] [CrossRef]
- Flahaut, E.; Sloan, J.; Friedrichs, S.; Kirkland, A.I.; Coleman, K.S.; Williams, V.C.; Hanson, N.; Hutchison, J.L.; Green, M.L.H. Crystallization of 2H and 4H PbI2 in Carbon Nanotubes of Varying Diameters and Morphologies. Chem. Mater. 2006, 18, 2059–2069. [Google Scholar] [CrossRef]
- Sloan, J.; Matthewman, G.; Dyer-Smith, C.; Sung, A.-Y.; Liu, Z.; Suenaga, K.; Kirkland, A.I.; Flahaut, E. Direct Imaging of the Structure, Relaxation, and Sterically Constrained Motion of Encapsulated Tungsten Polyoxometalate Lindqvist Ions within Carbon Nanotubes. ACS Nano 2008, 2, 966–976. [Google Scholar] [CrossRef]
- Rinkenbach, W.H. The heats of combustion and formation of aromatic nitro compounds. J. Am. Chem. Soc. 1930, 52, 115–120. [Google Scholar] [CrossRef]
- Gunawan, R.; Zhang, D. Thermal stability and kinetics of decomposition of ammonium nitrate in the presence of pyrite. J. Hazard. Mater. 2009, 165, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Oommen, C. Ammonium nitrate: A promising rocket propellant oxidizer. J. Hazard. Mater. 1999, 67, 253–281. [Google Scholar] [CrossRef] [PubMed]
- Botoshansky, M.; Herbstein, F.H.; Kapon, M. Crystallography of Metal Picrates. II.* Crystal Structure of Yellow Thallium(I) Picrate; Relation Among Various Metal(I) Picrate Phase. Acta Cryst. 1994, B50, 589–596. [Google Scholar] [CrossRef]
- Lee, T.; Chen, J.W.; Lee, H.L.; Lin, T.Y.; Tsai, Y.C.; Cheng, S.L.; Lee, S.W.; Hu, J.C.; Chen, L.T. Stabilization and spheroidization of ammonium nitrate: Co-crystallization with crown ethers and spherical crystallization by solvent screening. Chem. Eng. J. 2013, 225, 809–817. [Google Scholar] [CrossRef]
- Kruger, G.J.; Reynhardt, E.C. Hexaamminecobalt(III) Chloride. Acta Cryst. 1978, B34, 915–917. [Google Scholar] [CrossRef]
- Brill, T.B.; James, K.J. Kinetics and mechanisms of thermal decomposition of nitroaromatic explosives. Chem. Rev. 1993, 93, 2667–2692. [Google Scholar] [CrossRef]
- Babrauskas, V.; Leggett, D. Thermal decomposition of ammonium nitrate. Fire Mater. 2020, 44, 250–268. [Google Scholar] [CrossRef]
- Miyake, A.; Kobayashi, H.; Echigoya, H.; Ogawa, T. Combustion and ignition properties of ammonium nitrate and activated carbon mixtures. Int. J. Energetic Mater. Chem. Propuls. 2009, 8, 411–419. [Google Scholar] [CrossRef]
- Dobrynin, O.S.; Zharkov, M.N.; Kuchurov, I.V.; Fomenkov, I.V.; Zlotin, S.G.; Monogarov, K.A.; Meerov, D.B.; Pivkina, A.N.; Muravyev, N.V. Supercritical antisolvent processing of nitrocellulose: Downscaling to nanosized, reducing friction sensitivity and introducing burning rate catalyst. Nanomaterials 2019, 9, 1386. [Google Scholar] [CrossRef]
- Chang, C.W.; Tseng, J.M.; Horng, J.J.; Shu, C.M. Thermal decomposition of carbon nanotube/Al2O3 powders by DSC testing. Compos. Sci. Technol. 2008, 68, 2954–2959. [Google Scholar] [CrossRef]
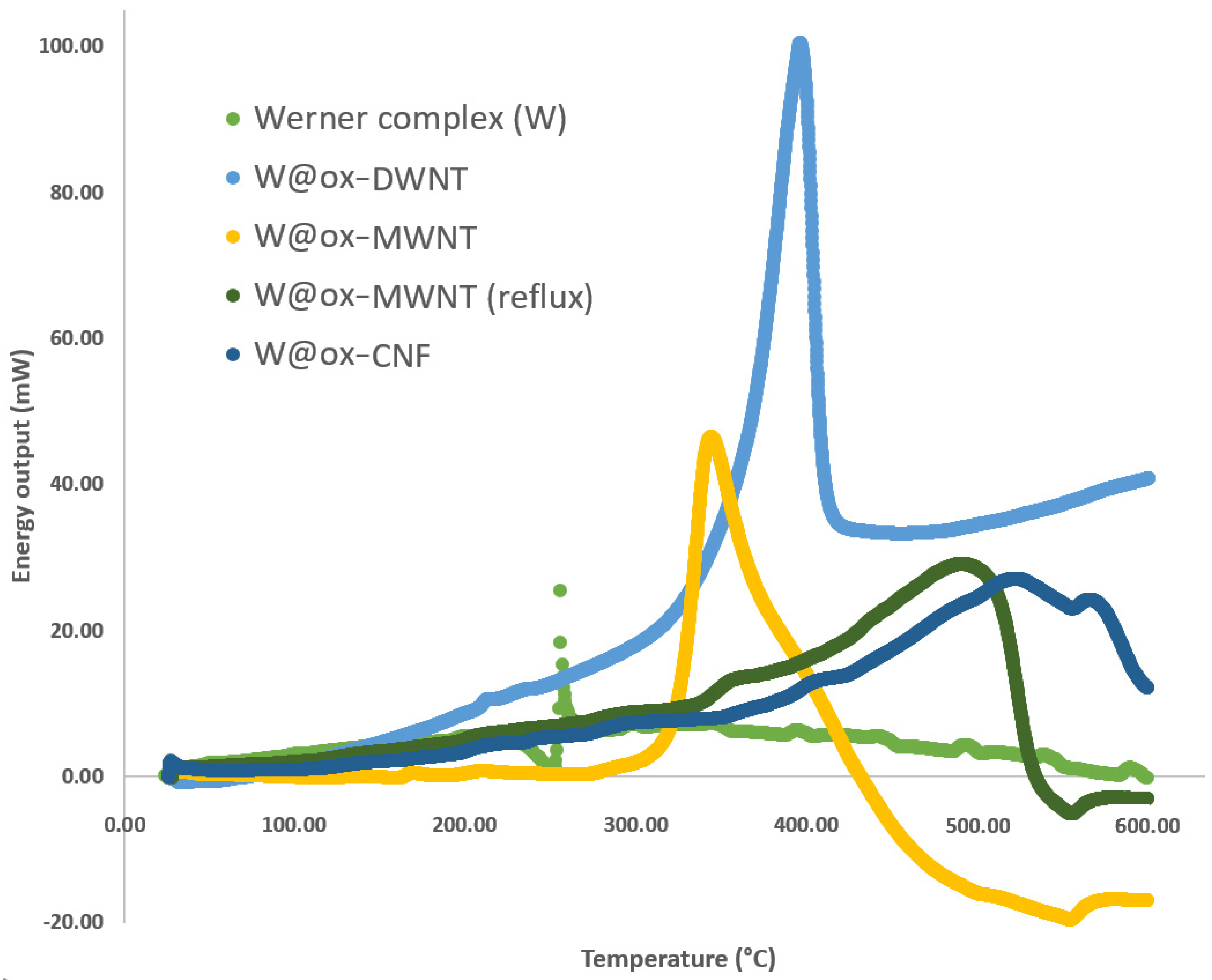
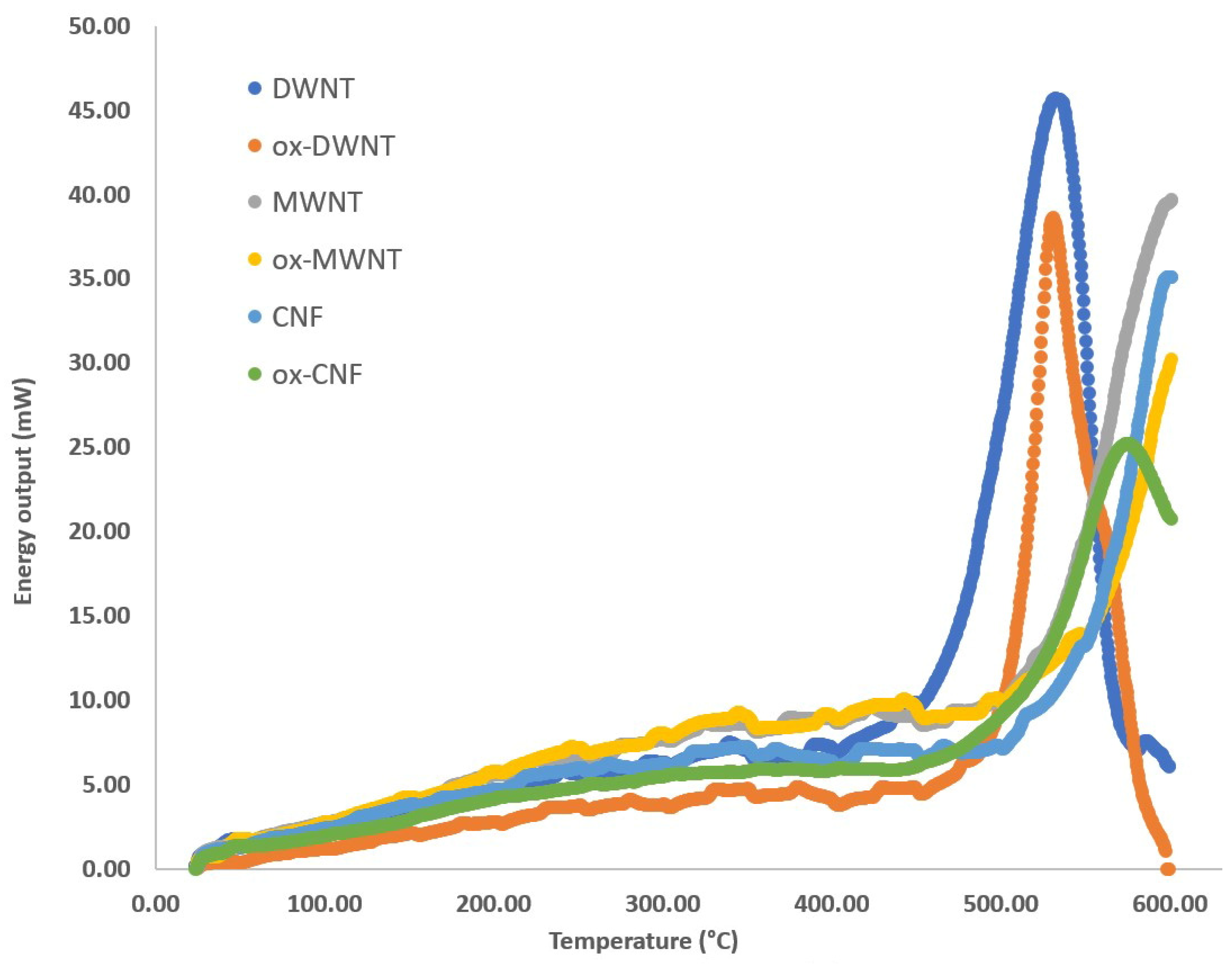
| Compound | Normalized Energy Output (kJ/g) | ΔE = E − Ew (kJ/g) | E/EW | Max. Peak T (°C) | Δ T = T − Tw (°C) | FWHM (°C) |
|---|---|---|---|---|---|---|
| Werner complex (W) | 0.5 | 0 | 1.0 | 253.2 | 0.0 | 3.2 |
| W@ox-DWNT | 14.6 | 14.1 | 29.0 | 396.3 | 143.1 | 52.0 |
| W@ox-MWNT | 23.3 | 22.8 | 47.0 | 342.2 | 89.0 | 59.5 |
| W@ox-MWNT (reflux) | 28.1 | 27.6 | 56.2 | 504.8 | 251.6 | 113.3 |
| W@ox-CNF | 12.1 | 11.6 | 24.0 | 515.3 | 262.1 | 128.0 |
| Compound | Normalized Energy Output (kJ/g) | Maximal Peak Temperature (°C) | FWHM (°C) | |
|---|---|---|---|---|
| W | 0.5 | 253.2 | 3.2 | This work |
| PA | 11.2 | 300 | [32] | |
| AN | 2.2 | 293.0 | [33,34] | |
| ox-DWNT | 6.7 | 530.7 (should. 546) | 31.0 | This work |
| W@ox-DWNT | 14.6 (7.2) | 396.3 | 52.0 | This work |
| PA@ox-DWNT | 15.0 (17.9) | 566.0 | 30.0 | This work |
| AN@ox-DWNT | 14.7 (8.9) | 585.6 | 38.0 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acevedo, R.; Soula, B.; Galibert, A.M.; Flahaut, E. Carbon Nanotubes for Confinement-Induced Energetic Nanomaterials. Nanomaterials 2023, 13, 1845. https://doi.org/10.3390/nano13121845
Acevedo R, Soula B, Galibert AM, Flahaut E. Carbon Nanotubes for Confinement-Induced Energetic Nanomaterials. Nanomaterials. 2023; 13(12):1845. https://doi.org/10.3390/nano13121845
Chicago/Turabian StyleAcevedo, Ruben, Brigitte Soula, Anne Marie Galibert, and Emmanuel Flahaut. 2023. "Carbon Nanotubes for Confinement-Induced Energetic Nanomaterials" Nanomaterials 13, no. 12: 1845. https://doi.org/10.3390/nano13121845
APA StyleAcevedo, R., Soula, B., Galibert, A. M., & Flahaut, E. (2023). Carbon Nanotubes for Confinement-Induced Energetic Nanomaterials. Nanomaterials, 13(12), 1845. https://doi.org/10.3390/nano13121845







