Effect of α-Al2O3 Additive on the Surface Micro-Arc Oxidation Coating of Ti6Al4V Alloy
Abstract
1. Introduction
2. Experimental Materials and Methods
2.1. Micro-Arc Oxidation Coating Preparation
2.2. Organizational Structure Analysis and Performance Testing
3. Results and Discussion
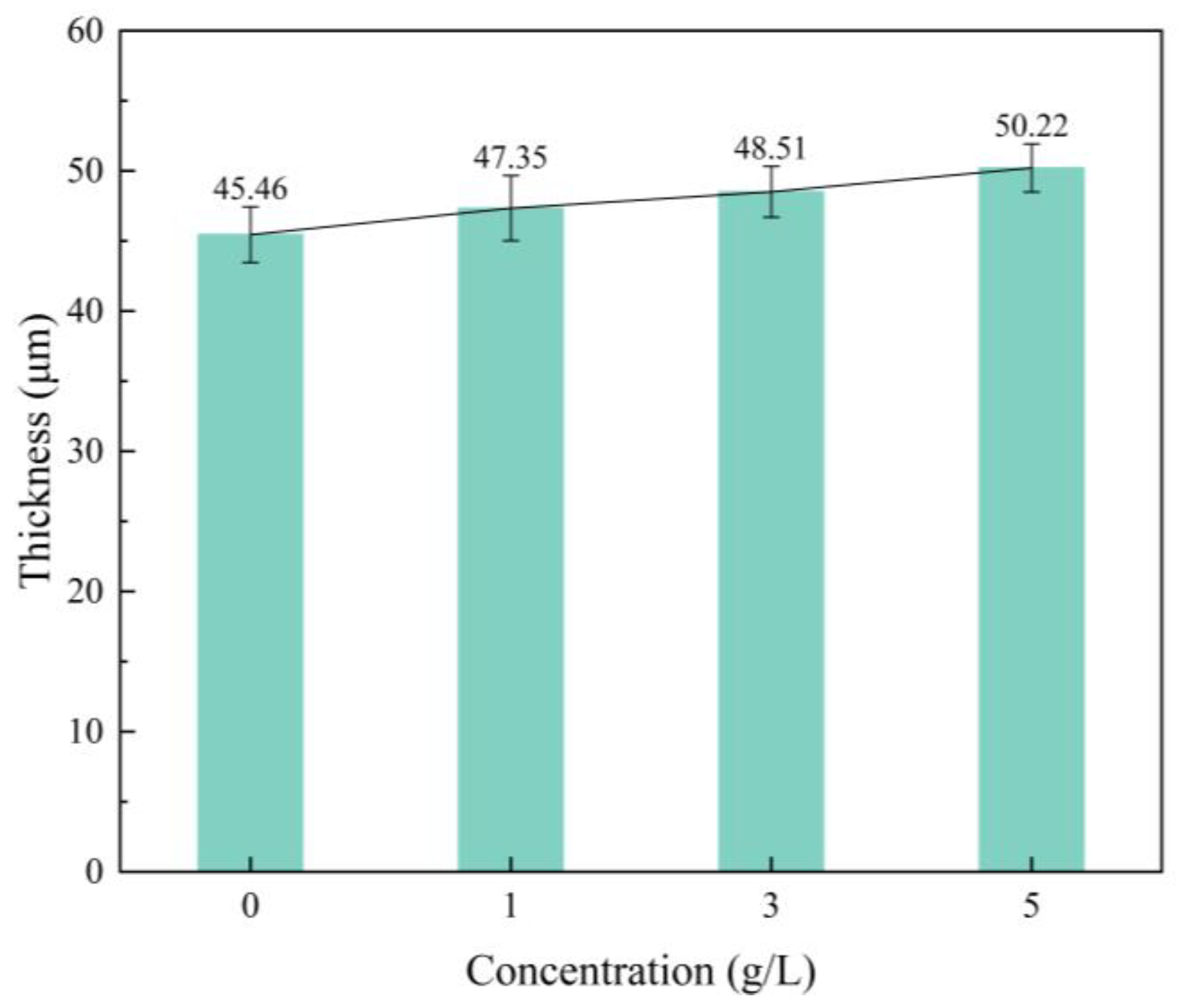
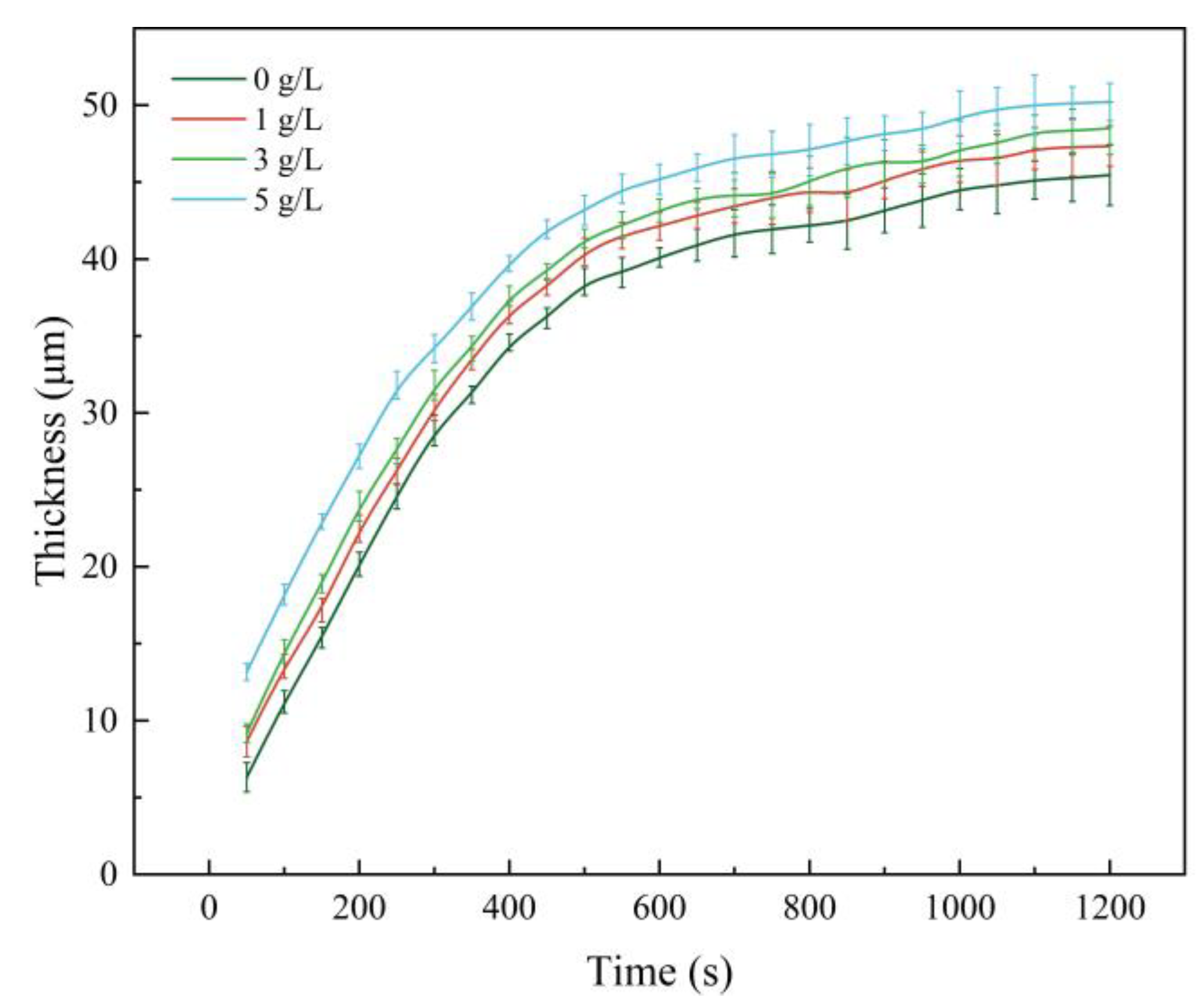
3.1. Effect of α-Al2O3 Concentration on the Thickness of Micro-Arc Oxidation Coatings
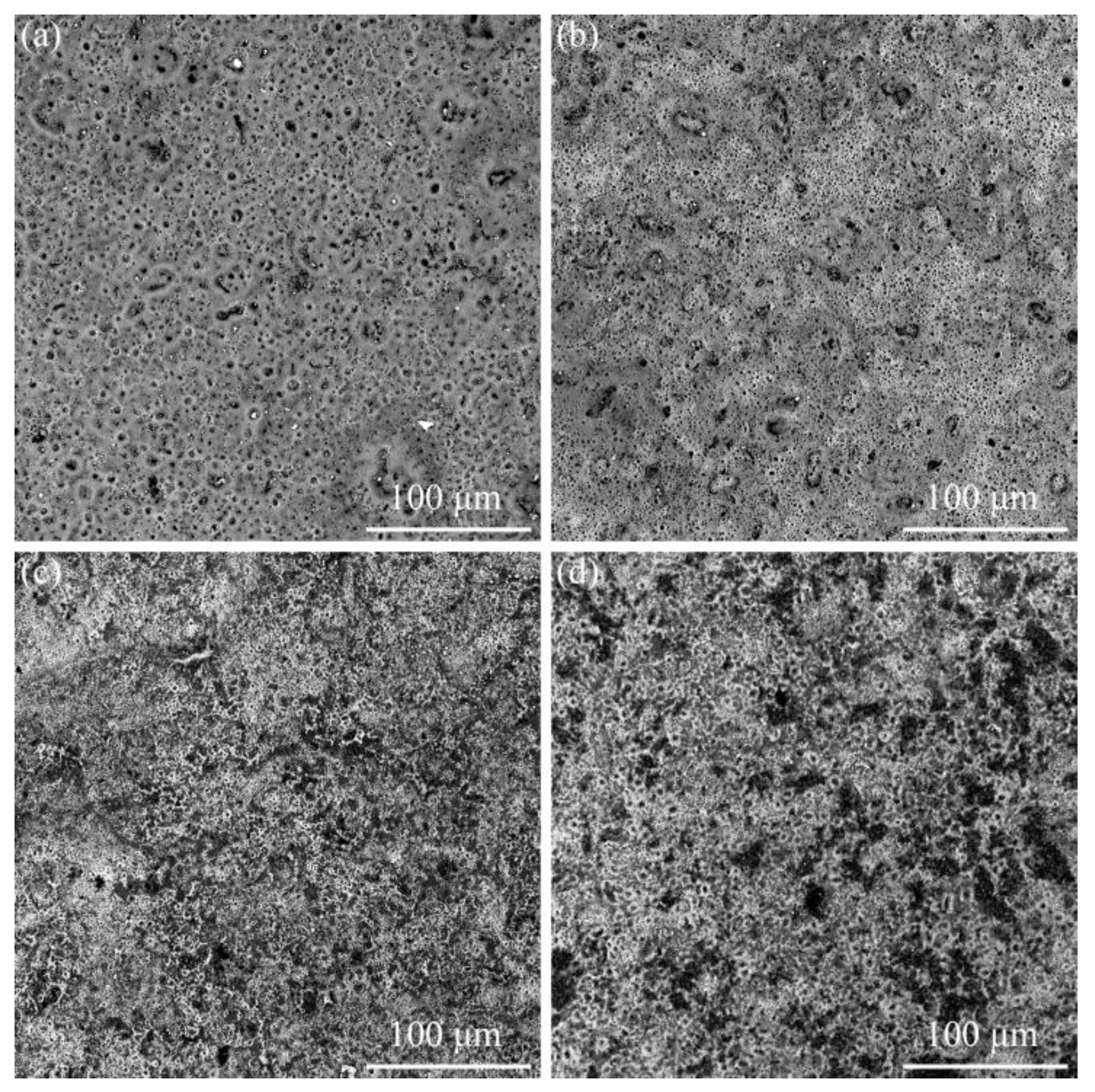
3.2. Effect of α-Al2O3 on the Microscopic Morphology of Micro-Arc Oxidation Coating
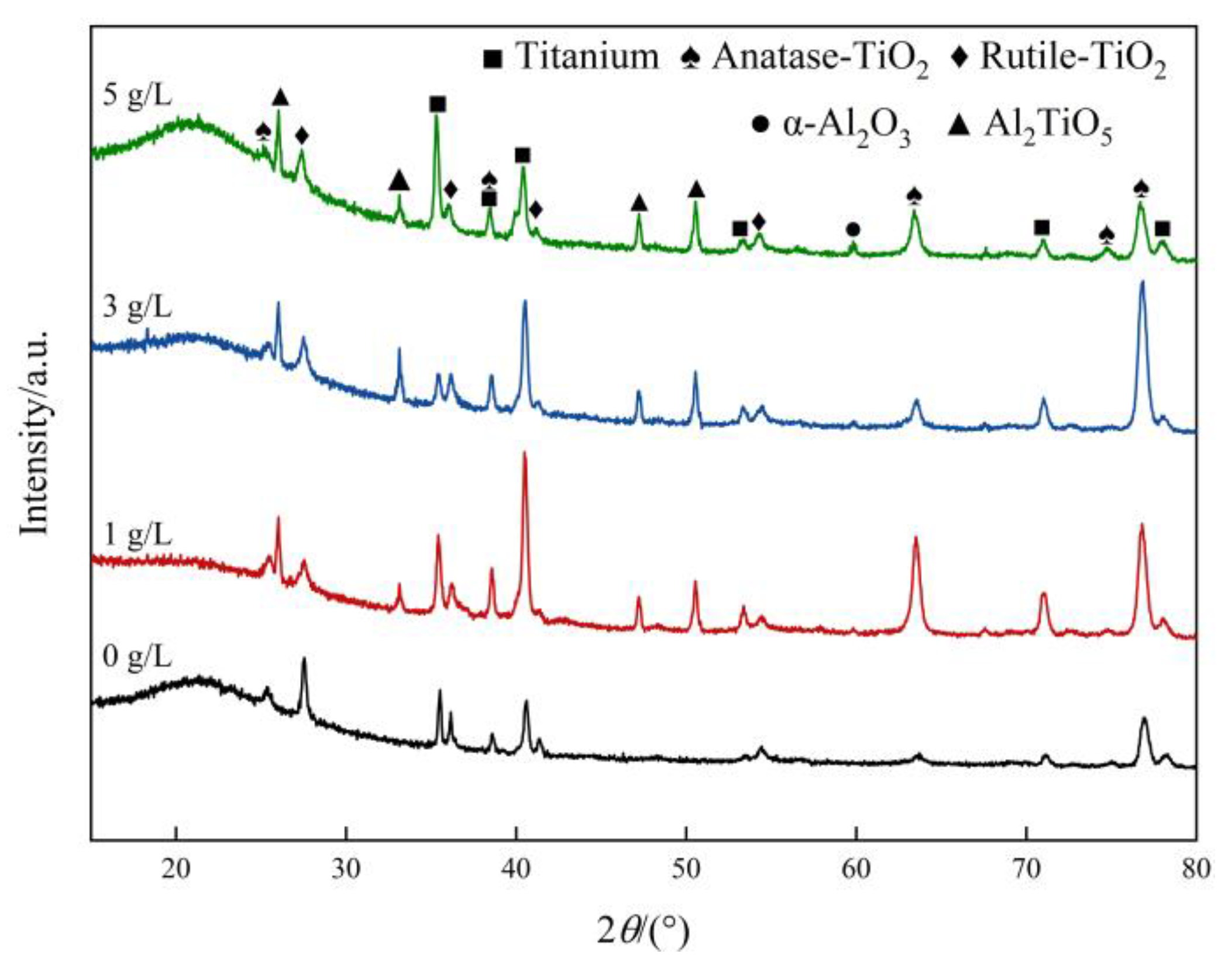
3.3. Effect of α-Al2O3 Concentration on the Physical Phase Composition of Micro-Arc Oxidation Coatings
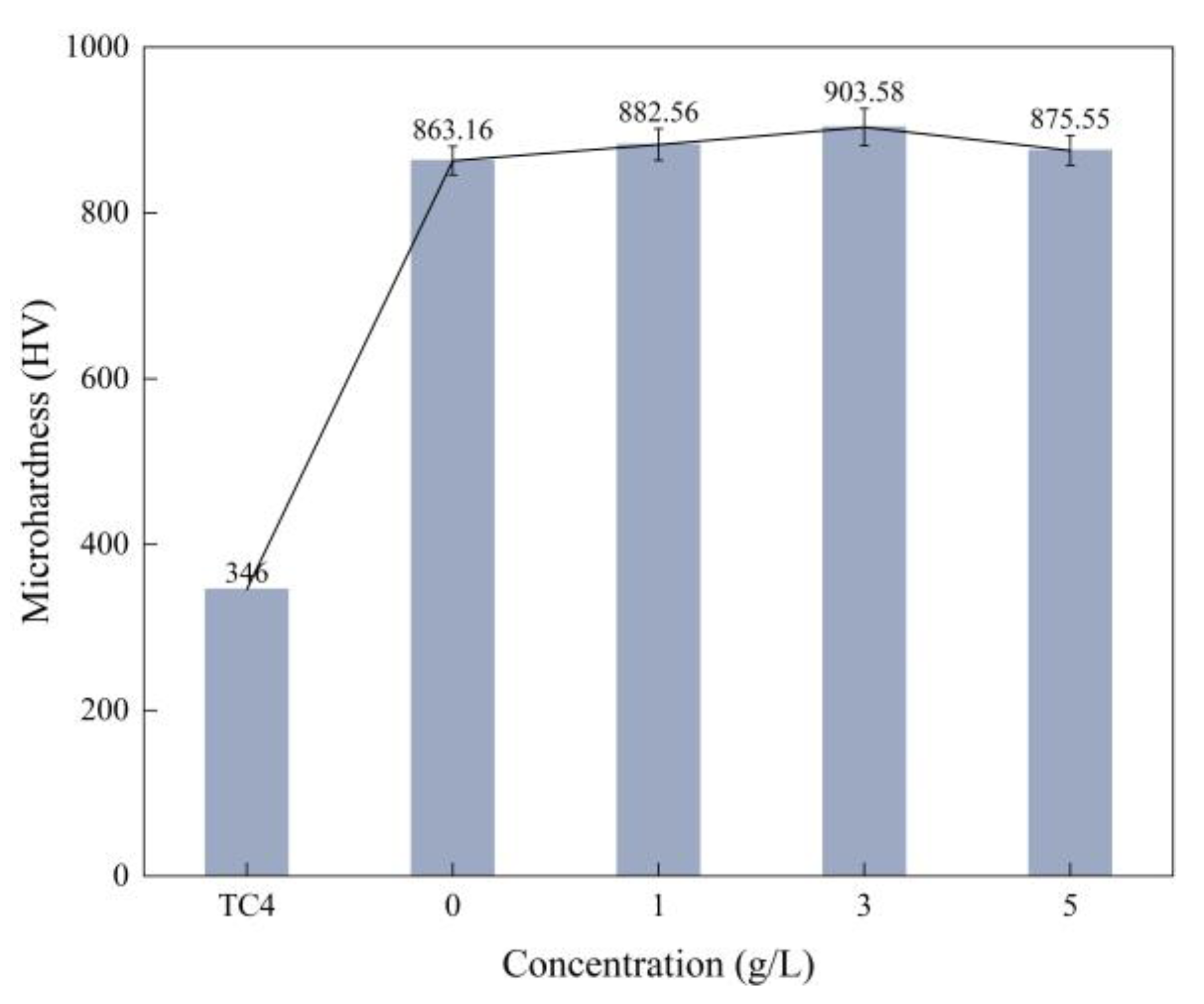
3.4. Effect of α-Al2O3 Concentration on the Microhardness of Micro-Arc Oxidation Coatings
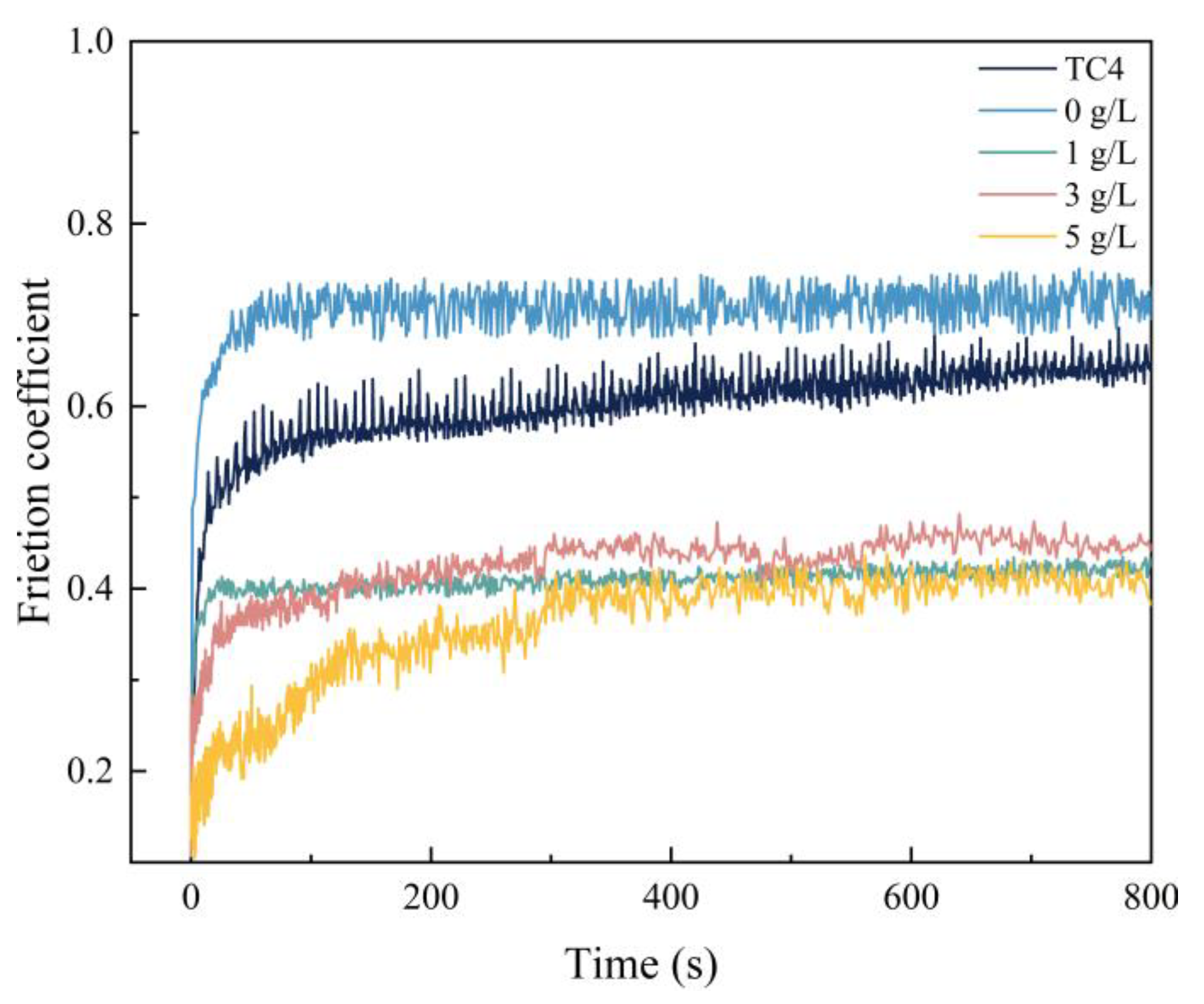
3.5. Effect of α-Al2O3 Concentration on the Frictional Wear Properties of Micro-Arc Oxidation Coatings
3.6. Effect of α-Al2O3 Concentration on the Corrosion Resistance of Micro-Arc Oxidation Coatings
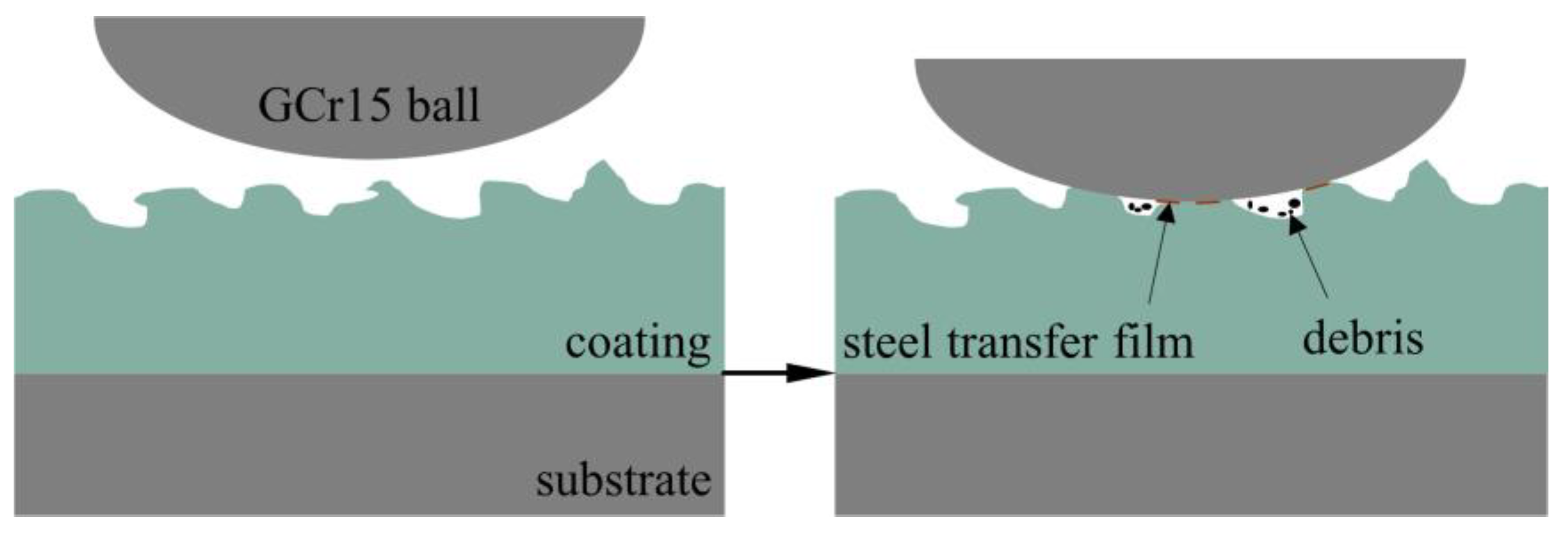
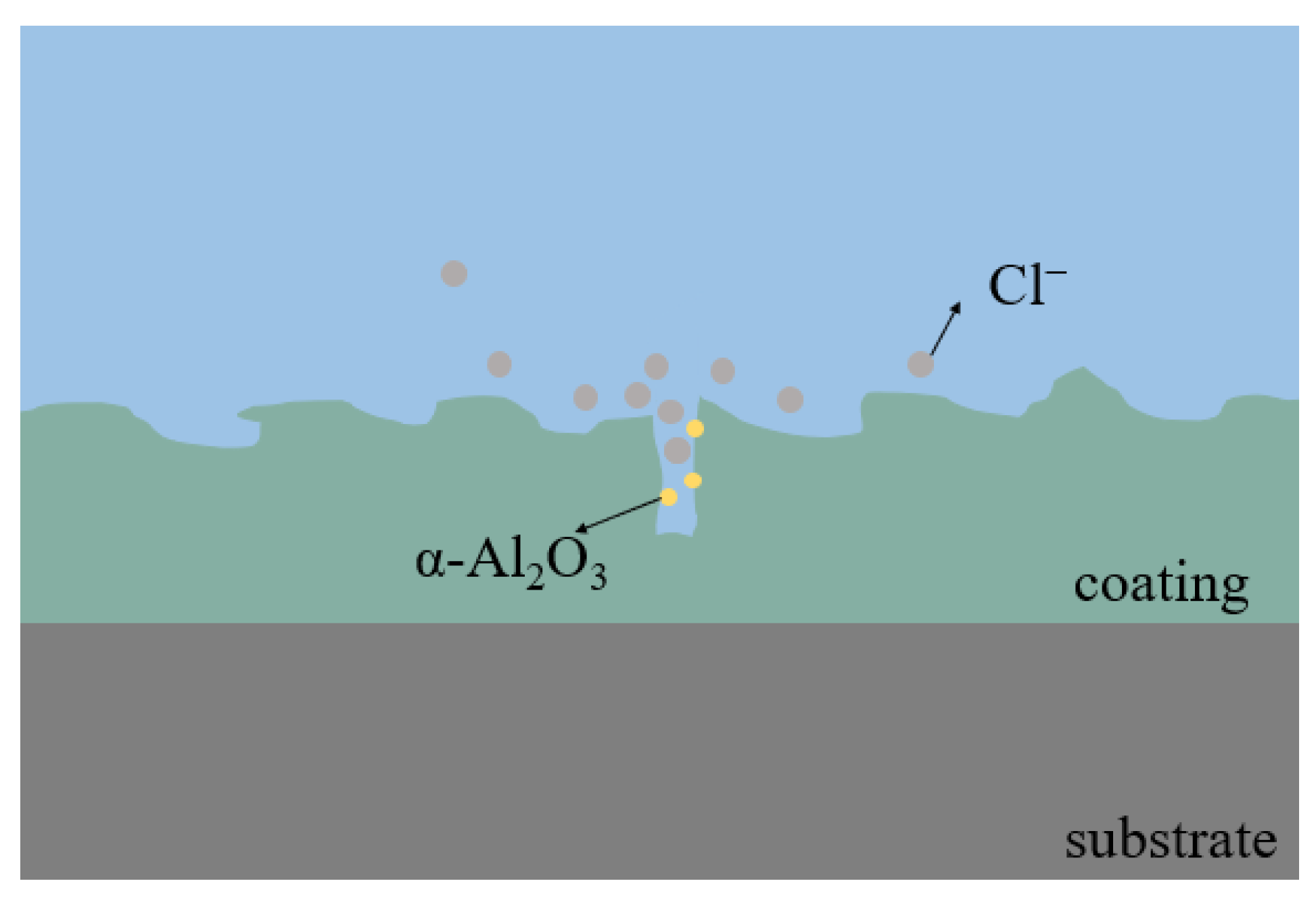
- The α-Al2O3 nanoparticles contained in the coating play a certain role in repairing the discharge channels (see Figure 10) and reducing the number of penetration holes. This makes the coating more dense, and effectively isolates the external corrosive environment from corrosive factors such as Cl− ions, which plays an important role in improving the corrosion resistance.
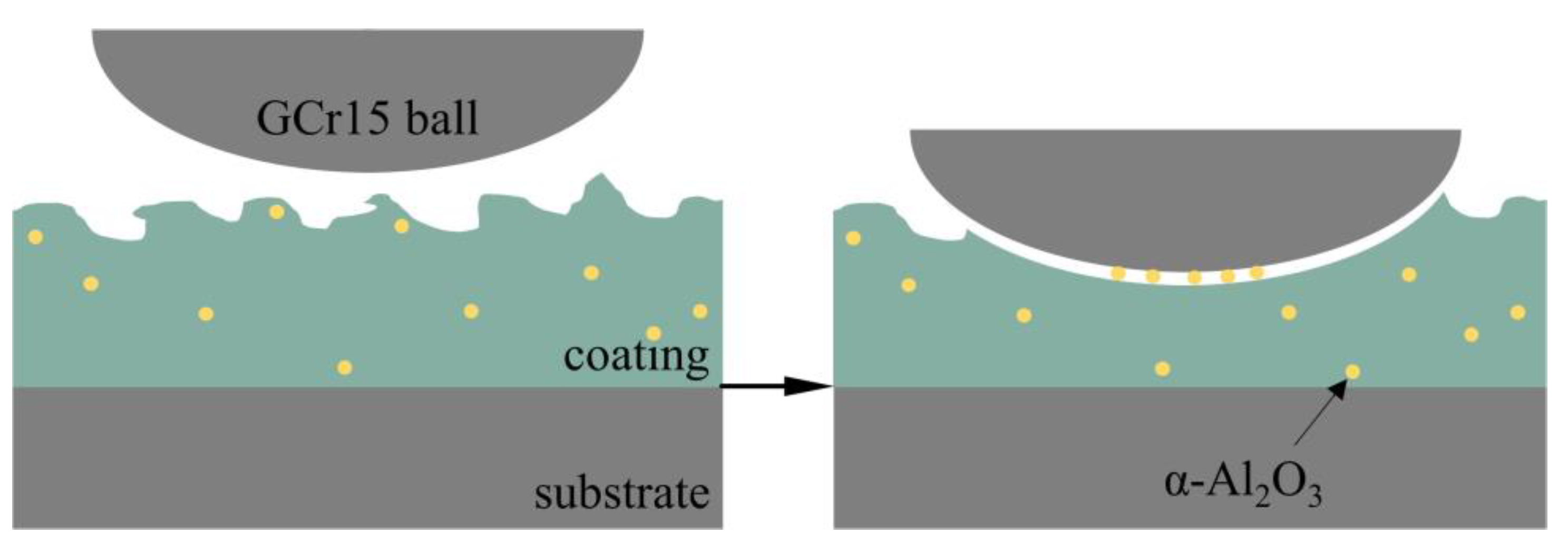
- After the addition of α-Al2O3 nanoparticles, the coating contains new phases of α-Al2O3 and Al2TiO5 in addition to Anatase-TiO2 and Rutile-TiO2. The α-Al2O3 nanoparticles are diffusely distributed in the coating, while Al2TiO5 has a high hardness and strong corrosion resistance. Its isolation reduces the electrochemical corrosion tendency of the specimen and further improves the corrosion resistance.
- The thickness of micro-arc oxidation coatings prepared by adding an appropriate amount of α-Al2O3 to the electrolyte increases due to the increased electrolyte conductivity. It helps to slow the intrusion of corrosive media to the substrate to a certain extent.
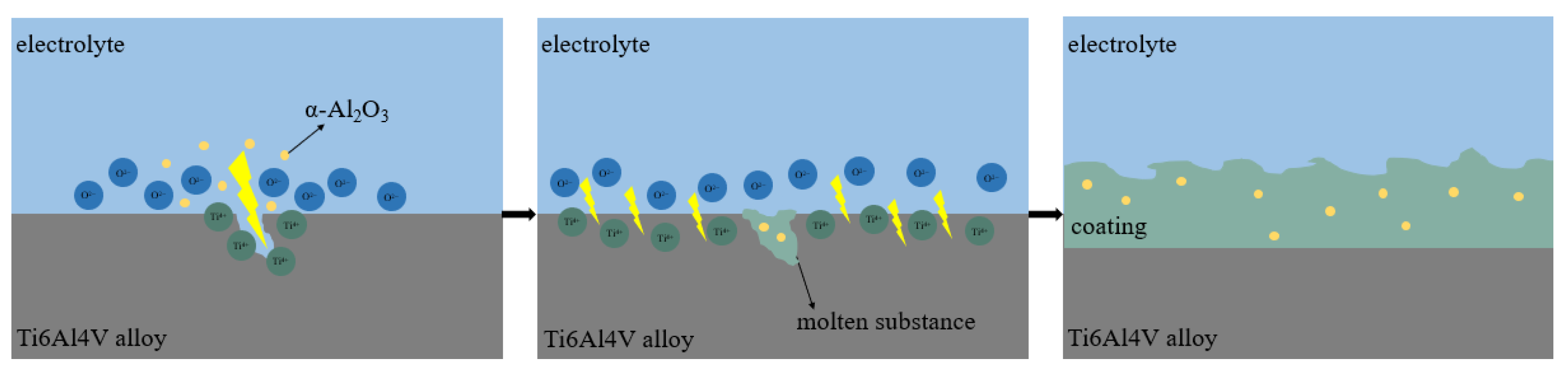
- The growth process of the coating is mainly by the breakdown melting of the substrate and the transient cooling of the molten material in the electrolyte. Based on the good toughness of α-Al2O3 nanoparticles, embedding them in the coating is beneficial to reduce internal stress, which is helpful to improve the corrosion resistance.
3.7. Growth Mechanism of the Ti6Al4V Alloy Micro-Arc Oxide Coatings
4. Conclusions
- The surface density of the coating is significantly improved by embedding α-Al2O3 nanoparticles, and the number of micropores and defects, such as grooves and pits, on the surface are reduced. The coating contains Ti, Anatase-TiO2 and Rutile-TiO2 phases. The diffraction peak intensity of the Rutile-TiO2 phase is improved by the addition of α-Al2O3 nanoparticles. In addition, some α-Al2O3 nanoparticles are embedded in the coating, and a new Al2TiO5 phase is generated.
- With the increase of α-Al2O3 nanoparticle concentration in the electrolyte, the thickness of the coating increased slightly, and reached a maximum value of 50.22 μm when the particle concentration was 5 g/L. The surface roughness of the coating decreased and then increased, and the roughness, Ra, reached a minimum value of 3.82 μm when the particle concentration was 3 g/L; the surface was the flattest at this time. The microhardness of the coating increased, then decreased, and reached a maximum value of 903.58 HV when the concentration was 3 g/L.
- Compared with the Ti6Al4V alloy, the micro-arc oxidation coating has better wear resistance. The coating prepared with 3 g/L of α-Al2O3 nanoparticles has a stable friction factor of 0.41 and the best frictional wear performance, mainly because it has the lowest roughness and the highest surface hardness. The α-Al2O3 nanoparticles have a friction-reducing effect, and the surface micropores provide space for storage of generated abrasive chips.
- When the concentration of α-Al2O3 nanoparticles is 3 g/L, the good density and the isolation of Rutile-TiO2, α-Al2O3 and Al2TiO5 phases in the coating protect the substrate from corrosive solutions to a certain extent. The linear polarization resistance, Rp, is increased to 5.85 × 104 Ω/cm2, which is improved by one order of magnitude, and the corrosion potential of the specimen is 197 mV, an increase of 312 mV, and the corrosion current density, icorr, is 7.99 × 10−7 A/cm2, a decrease of two orders of magnitude.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Niazi, H.; Golestani-Fard, F.; Wang, W.; Shahmiri, M.; Zargar, H.R.; Alfantazi, A.; Bayati, R. Structure-property correlation in EEMAO fabricated TiO2-Al2O3 nanocomposite coatings. ACS Appl. Mater. Interfaces 2014, 6, 5538–5547. [Google Scholar] [CrossRef]
- Cui, C.; Hu, B.; Zhao, L.; Liu, S. Titanium alloy production technology, market prospects and industry development. Mater. Des. 2010, 32, 1684–1691. [Google Scholar] [CrossRef]
- Khanna, R.; Kokubo, T.; Matsushita, T.; Nomura, Y.; Nose, N.; Oomori, Y.; Yoshida, T.; Wakita, K.; Takadama, H. Novel artificial hip joint: A layer of alumina on Ti-6Al-4V alloy formed by micro-arc oxidation. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 55, 393–400. [Google Scholar] [CrossRef]
- Rodriguez, L.; Vieu, A.; Balsarin, M.; Combes, P.; Alexis, J.; Esvan, J.; Lesko, S.; Denape, J.; Paris, J.; Delbé, K. Physico-chemical characterisation and tribological behaviour of ground micro-arc oxidation coating on aluminium alloy—Comparison with hard anodised oxidation. Wear 2023, 516–517, 204591. [Google Scholar] [CrossRef]
- Rasool, G.; Stack, M. Tribo-oxidation maps for Ti against steel. Tribol. Int. 2015, 91, 258–266. [Google Scholar] [CrossRef]
- Ming, M.; Xinjian, Z.; Qian, X.; Jun, L.; Xiaodi, H. Preparation and tribological properties of self-lubricating TiO2/graphite composite coating on Ti6Al4V alloy. Appl. Surf. Sci. 2012, 258, 8570–8576. [Google Scholar]
- Sun, L.; Ma, Y.; Fan, B.; Wang, S.; Wang, Z. Investigation of anti-corrosion property of hybrid coatings fabricated by combining PEC with MAO on pure magnesium. J. Magnes. Alloys 2022, 10, 2875–2888. [Google Scholar] [CrossRef]
- Kaseem, M.; Choel, C. The effect of in-situ reactive incorporation of MoOx on the corrosion behavior of Ti-6Al-4V alloy coated via micro-arc oxidation coating. Corros. Sci. 2021, 192, 109764. [Google Scholar] [CrossRef]
- Somsanith, N.; Narayanan, T.S.; Kim, Y.K.; Park, I.S.; Bae, T.S.; Lee, M.H. Surface medication of Ti-15Mo alloy by thermal oxidation: Evaluation of surface characteristics and corrosion resistance in Ringer's solution. Appl. Surf. Sci. 2015, 356, 1117–1126. [Google Scholar] [CrossRef]
- Obadele, B.A.; Olubambi, P.A.; Andrews, A.; Pityana, S.; Mathew, M.T. Electrochemical behaviour of laser-clad Ti6Al4V with CP Ti in 0.1 M oxalic acid solution. J. Alloys Compd. 2015, 646, 753–759. [Google Scholar] [CrossRef]
- Hongxi, L.; Qian, X.; Xiaowei, Z.; Chuanqi, W.; Baoyin, T. Wear and corrosion behaviors of Ti6Al4V alloy biomedical materials by silver plasma immersion ion implantation process. Thin Solid Films 2012, 521, 89–93. [Google Scholar] [CrossRef]
- Diamanti, M.; Sebastiani, M.; Mangione, V.; Del Curto, B.; Pedeferri, M.; Bemporad, E.; Cigada, A.; Carassiti, F. Multi-step anodizing on Ti6Al4V components to improve tribomechanical performances. Surf. Coat. Technol. 2013, 227, 19–27. [Google Scholar] [CrossRef]
- Li, Y.; Yao, B.; Long, B.; Tian, H.; Wang, B. Preparation, characterization and mechanical properties of microarc oxidation coating formed on titanium in Al(OH)3 colloidal solution. Appl. Surf. Sci. 2012, 258, 5238–5243. [Google Scholar] [CrossRef]
- Roknian, M.; Fattah-Alhosseini, A.; Gashti, S.O.; Keshavarz, M.K. Study of the effect of ZnO nanoparticles addition to PEO coatings on pure titanium substrate: Microstructural analysis, antibacterial effect and corrosion behavior of coatings in Ringer's physiological solution. J. Alloys Compd. 2018, 740, 330–345. [Google Scholar] [CrossRef]
- Yasui, T.; Hayashi, K.; Fukumoto, M. Behaviors of Micro-Arcs, Bubbles, and Coating Growth during Plasma Electrolytic Oxidation of β-Titanium Alloy. Materials 2022, 16, 360. [Google Scholar] [CrossRef]
- Xiang, N.; Song, R.-G.; Zhao, J.; Li, H.; Wang, C.; Wang, Z.-X. Microstructure and mechanical properties of ceramic coatings formed on 6063 aluminium alloy by micro-arc oxidation. Trans. Nonferrous Met. Soc. China 2015, 25, 3323–3328. [Google Scholar] [CrossRef]
- Fazel, M.; Salimijazi, H.; Golozar, M.; Jazi, M.G. A comparison of corrosion, tribocorrosion and electrochemical impedance properties of pure Ti and Ti6Al4V alloy treated by micro-arc oxidation process. Appl. Surf. Sci. 2014, 324, 751–756. [Google Scholar] [CrossRef]
- Qin, D.; Xu, G.; Yang, Y.; Chen, S. Multiphase Ceramic Coatings with High Hardness and Wear Resistance on 5052 Aluminum Alloy by a Microarc Oxidation Method. ACS Sustain. Chem. Eng. 2018, 6, 2431–2437. [Google Scholar] [CrossRef]
- Zeng, R.C.; Cui, L.Y.; Jiang, K.; Liu, R.; Zhao, B.D.; Zheng, Y.F. In Vitro Corrosion and Cytocompatibility of a Microarc Oxidation Coating and Poly(L-lactic acid) Composite Coating on Mg-1Li-1Ca Alloy for Orthopedic Implants. ACS Appl. Mater. Interfaces 2016, 8, 10014–10028. [Google Scholar] [CrossRef]
- Pavarini, M.; Moscatelli, M.; Candiani, G.; Tarsini, P.; Cochis, A.; Rimondini, L.; Chiesa, R. Influence of frequency and duty cycle on the properties of antibacterial borate-based PEO coatings on titanium for bone-contact applications. Appl. Surf. Sci. 2021, 567, 150811. [Google Scholar] [CrossRef]
- Tavares, M.D.M.; Vitoriano, J.D.O.; da Silva, R.C.; Franco, A.R.; de Souza, G.B.; da Costa, J.A.P.; Alves-Junior, C. Effect of duty cycle and treatment time on electrolytic plasma oxidation of commercially pure Al samples. J. Mater. Res. Technol. 2019, 8, 2141–2147. [Google Scholar] [CrossRef]
- Wei, X.; Huang, H.; Sun, M.; Liu, W.; Qiu, J. Effects of honeycomb pretreatment on MAO coating fabricated on aluminum. Surf. Coat. Technol. 2019, 363, 265–272. [Google Scholar] [CrossRef]
- Seyfoori, A.; Mirdamadi, S.; Khavandi, A.; Raufi, Z.S. Biodegradation behavior of micro-arc oxidized AZ31 magnesium alloys formed in two different electrolytes. Appl. Surf. Sci. 2012, 261, 92–100. [Google Scholar] [CrossRef]
- Arunnellaiappan, T.; Babu, N.K.; Krishna, L.R.; Rameshbabu, N. Influence of frequency and duty cycle on microstructure of plasma electrolytic oxidized AA7075 and the correlation to its corrosion behavior. Surf. Coat. Technol. 2015, 280, 136–147. [Google Scholar] [CrossRef]
- Jiang, J.Y.; Xu, J.L.; Liu, Z.H.; Deng, L.; Sun, B.; Liu, S.D.; Wang, L.; Liu, H.Y. Preparation, corrosion resistance and hemocompatibility of the superhydrophobic TiO2 coatings on biomedical Ti-6Al-4V alloys. Appl. Surf. Sci. 2015, 347, 591–595. [Google Scholar] [CrossRef]
- Mu, M.; Liang, J.; Zhou, X.; Xiao, Q. One-step preparation of TiO2/MoS2 composite coating on Ti6Al4V alloy by plasma electrolytic oxidation and its tribological properties. Surf. Coat. Technol. 2013, 214, 124–130. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Rouhaghdam, A.S. Fabrication of TiC/WC ultra hard nanocomposite layers by plasma electrolysis and study of its characteristics. Surf. Coat. Technol. 2010, 205, S51–S56. [Google Scholar] [CrossRef]
- Liu, W.; Blawert, C.; Zheludkevich, M.L.; Lin, Y.; Talha, M.; Shi, Y.; Chen, L. Effects of graphene nanosheets on the ceramic coatings formed on Ti6Al4V alloy drill pipe by plasma electrolytic oxidation. J. Alloys Compd. 2019, 789, 996–1007. [Google Scholar] [CrossRef]
- Yang, W.; Wu, S.; Xu, D.; Gao, W.; Yao, Y.; Guo, Q.; Chen, J. Preparation and performance of alumina ceramic coating doped with aluminum nitride by micro arc oxidation. Ceram. Int. 2020, 46, 17112–17116. [Google Scholar] [CrossRef]
- Shokouhfar, M.; Allahkaram, S. Effect of incorporation of nanoparticles with different composition on wear and corrosion behavior of ceramic coatings developed on pure titanium by micro arc oxidation. Surf. Coat. Technol. 2017, 309, 767–778. [Google Scholar] [CrossRef]


| Concentration/(g/L) | Ecorr/V | icorr/(A/cm2) | βa/(V·dec−1) | βc/(V·dec−1) | Rp/(Ω/cm2) |
|---|---|---|---|---|---|
| Ti6Al4V | −0.509 | 2.25 × 10−5 | 0.20 | 0.20 | 1.93 × 103 |
| 0 | −0.283 | 6.63 × 10−6 | 0.19 | 0.31 | 7.71 × 103 |
| 1 | −0.217 | 1.05 × 10−6 | 0.14 | 0.19 | 3.30 × 104 |
| 3 | −0.197 | 7.99 × 10−7 | 0.19 | 0.24 | 5.85 × 104 |
| 5 | −0.228 | 2.70 × 10−6 | 0.20 | 0.23 | 1.70 × 104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Yuan, M. Effect of α-Al2O3 Additive on the Surface Micro-Arc Oxidation Coating of Ti6Al4V Alloy. Nanomaterials 2023, 13, 1802. https://doi.org/10.3390/nano13111802
Chen Y, Yuan M. Effect of α-Al2O3 Additive on the Surface Micro-Arc Oxidation Coating of Ti6Al4V Alloy. Nanomaterials. 2023; 13(11):1802. https://doi.org/10.3390/nano13111802
Chicago/Turabian StyleChen, Yuke, and Meini Yuan. 2023. "Effect of α-Al2O3 Additive on the Surface Micro-Arc Oxidation Coating of Ti6Al4V Alloy" Nanomaterials 13, no. 11: 1802. https://doi.org/10.3390/nano13111802
APA StyleChen, Y., & Yuan, M. (2023). Effect of α-Al2O3 Additive on the Surface Micro-Arc Oxidation Coating of Ti6Al4V Alloy. Nanomaterials, 13(11), 1802. https://doi.org/10.3390/nano13111802






