Size Matters? A Comprehensive In Vitro Study of the Impact of Particle Size on the Toxicity of ZnO
Abstract
1. Introduction
2. Materials and Methods
2.1. ZnO Particles and Reagents
2.2. Characterization of ZnO Particles
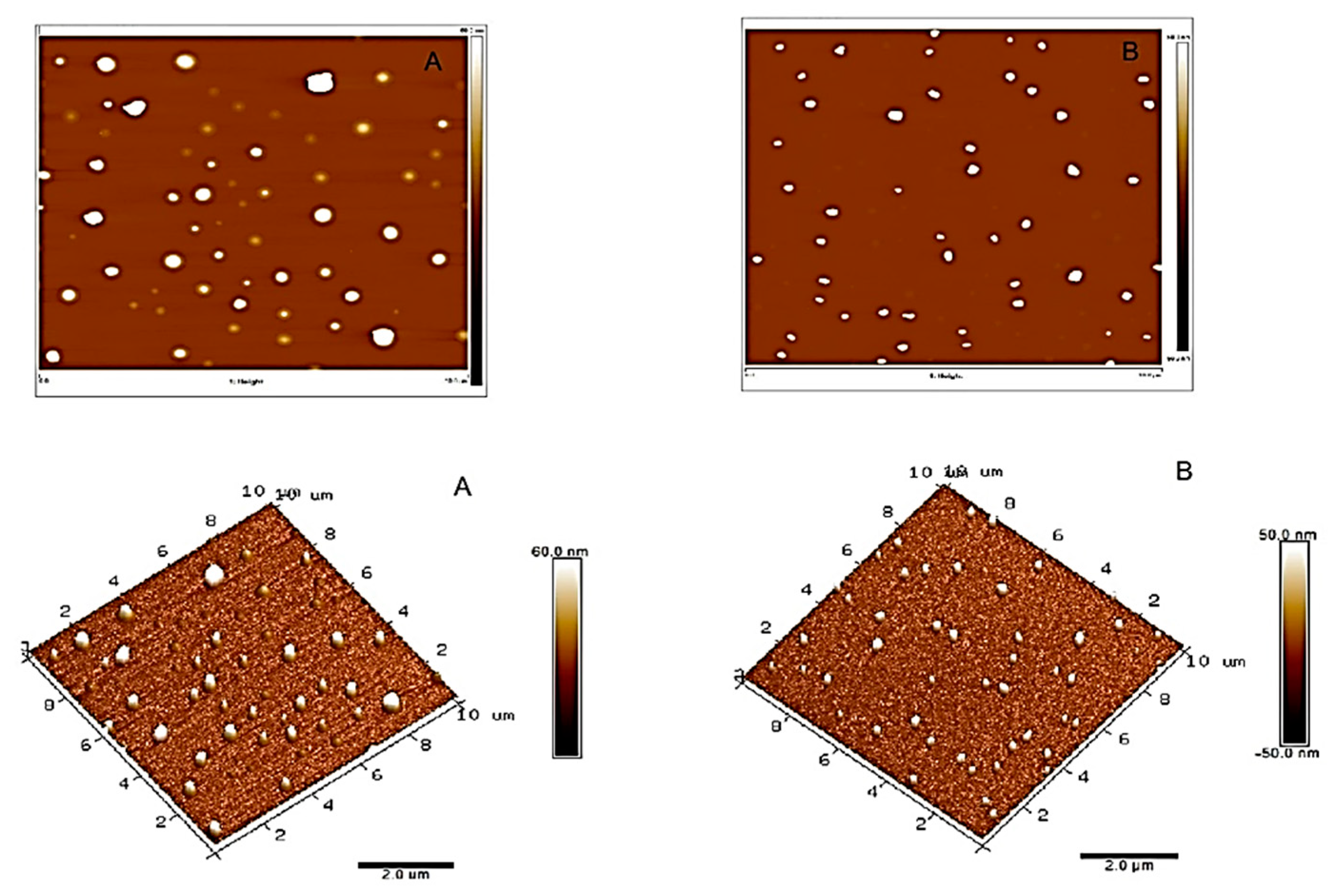
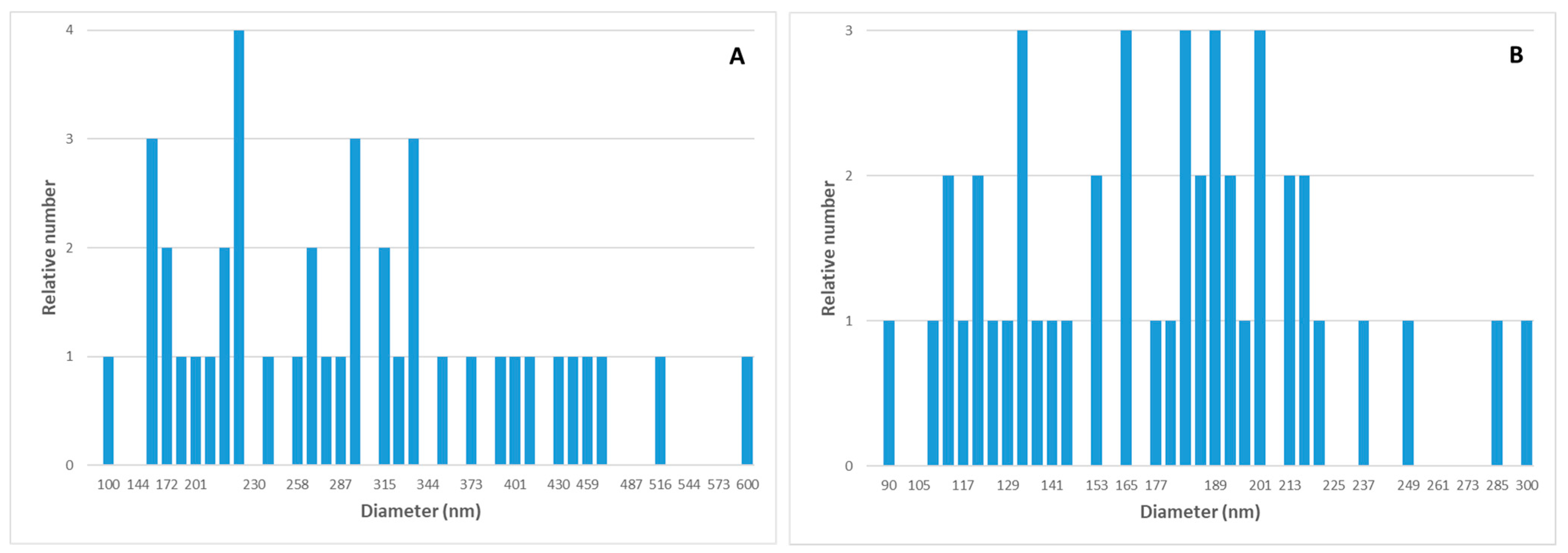
2.2.1. Atomic Force Microscopy (AFM)
2.2.2. Transmission Electron Microscopy (TEM)
2.2.3. Dynamic Light Scattering
2.3. Study of ZnO Albumin Interactions by Spectrometric Analysis
2.4. Hemocompatibility Studies
2.4.1. Blood Collection and Preparation of Erythrocyte Suspension
2.4.2. Potential Media Interaction on Hemoglobin Adsorption
pH 5.7, B = 15.5 mL
pH 7.4, B = 54.0 mL
2.4.3. Hemolytic Activity: Effect of Temperature and Albumin
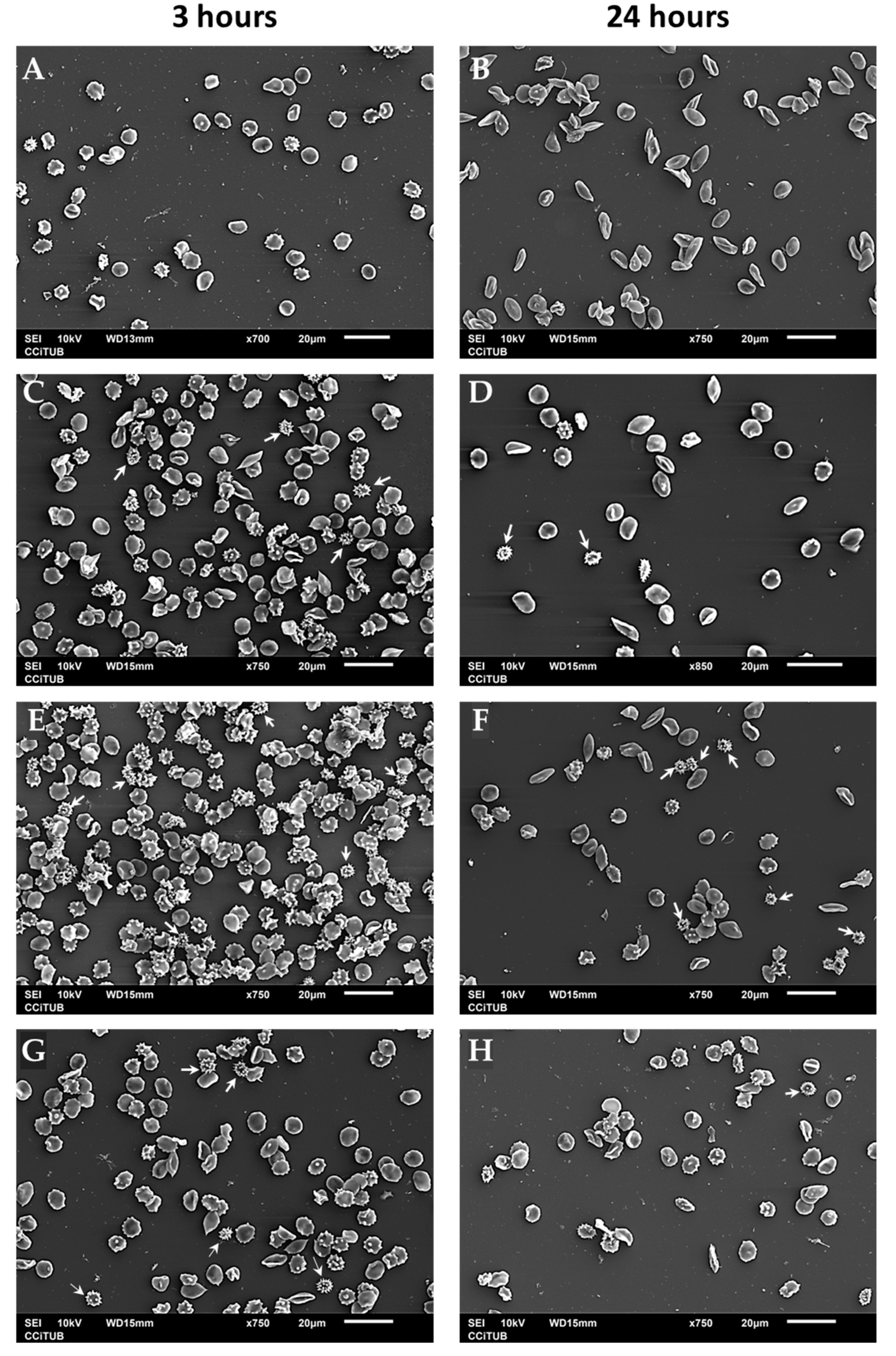
2.4.4. Effects of ZnO on Erythrocyte Morphology
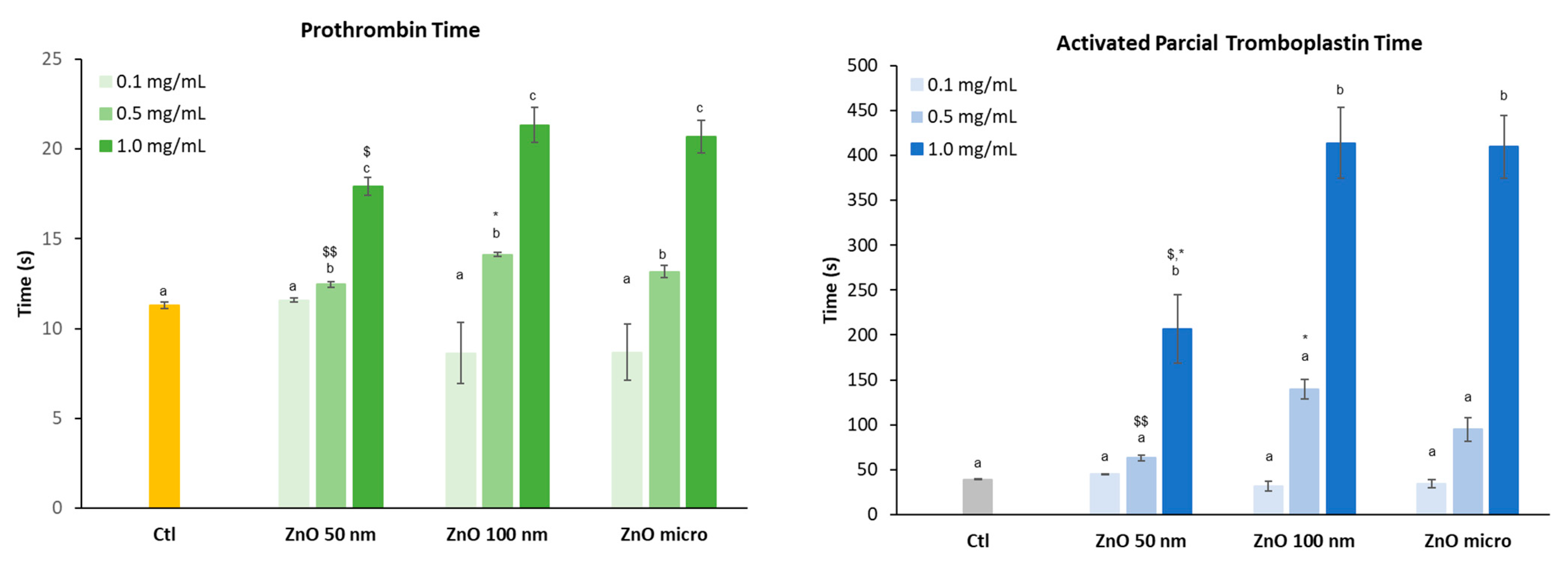
2.4.5. Coagulation Assays
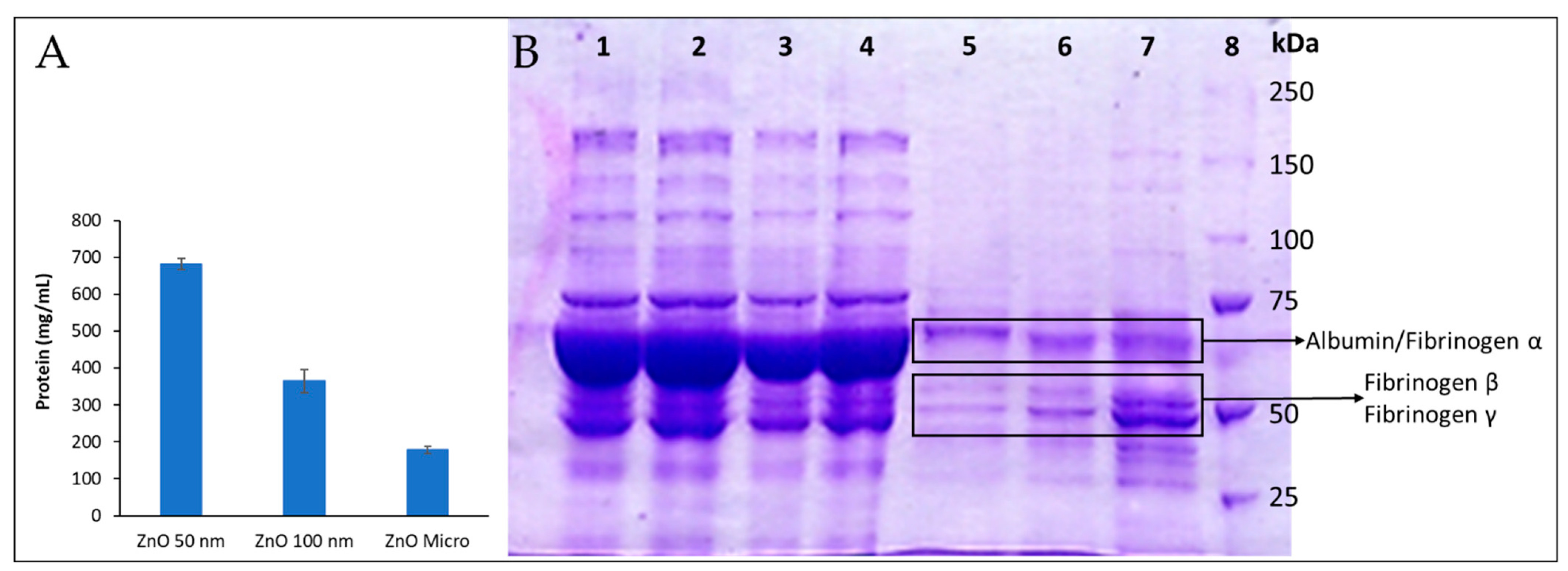
2.4.6. Two-Dimensional Gel Electrophoresis of Human Plasma Protein: SDS-PAGE
2.5. Cell Culture and Cytotoxicity Studies
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of ZnO Particles
3.1.1. Atomic Force Microscopy (AFM)
3.1.2. Transmission Electron Microscopy (TEM)
3.1.3. Dynamic Light Scattering (DLS)
3.1.4. Influence of Proteins on Hydrodynamic Diameter
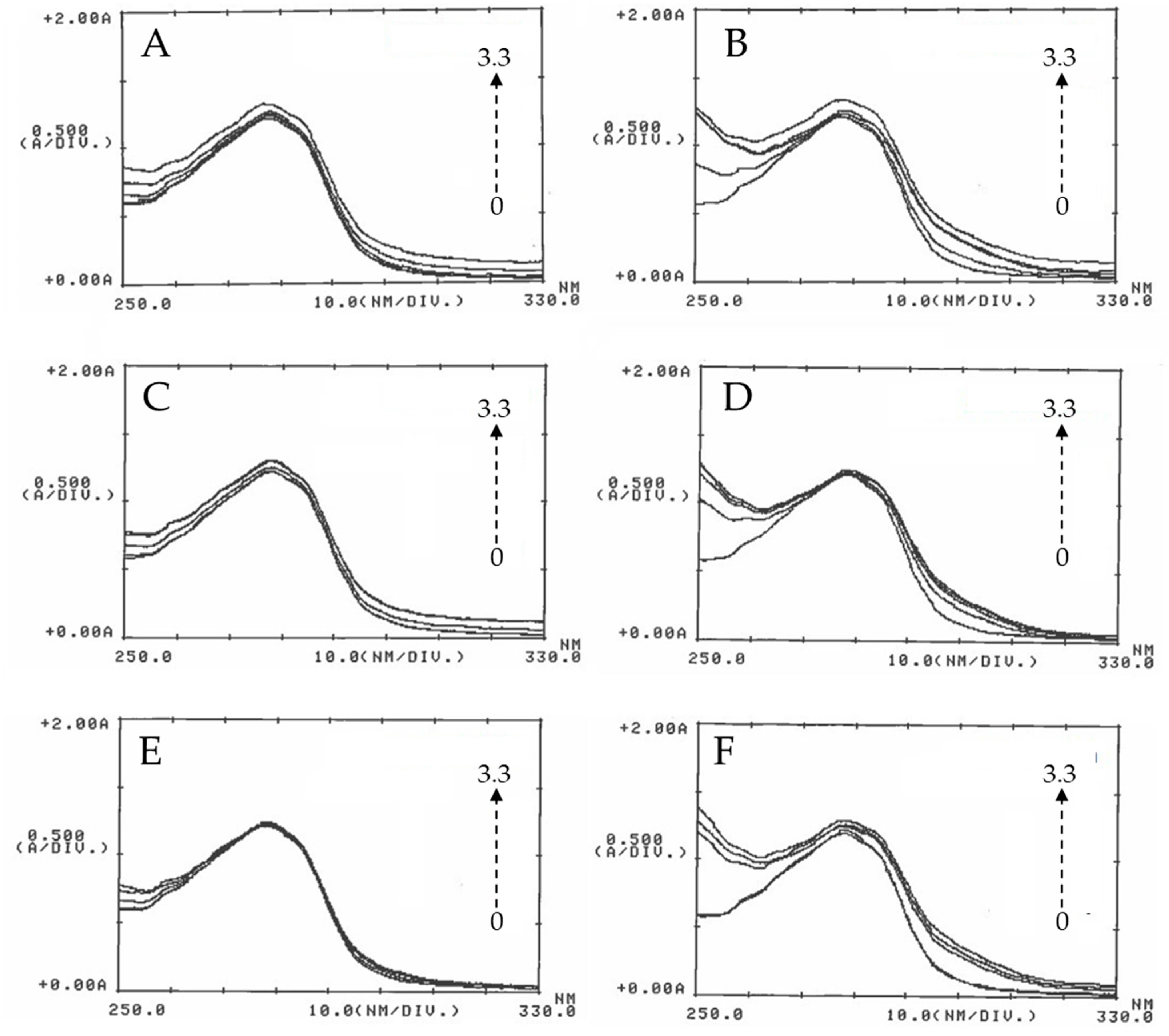
3.2. ZnO Albumin Interactions
3.2.1. UV-Visible Spectrophotometry
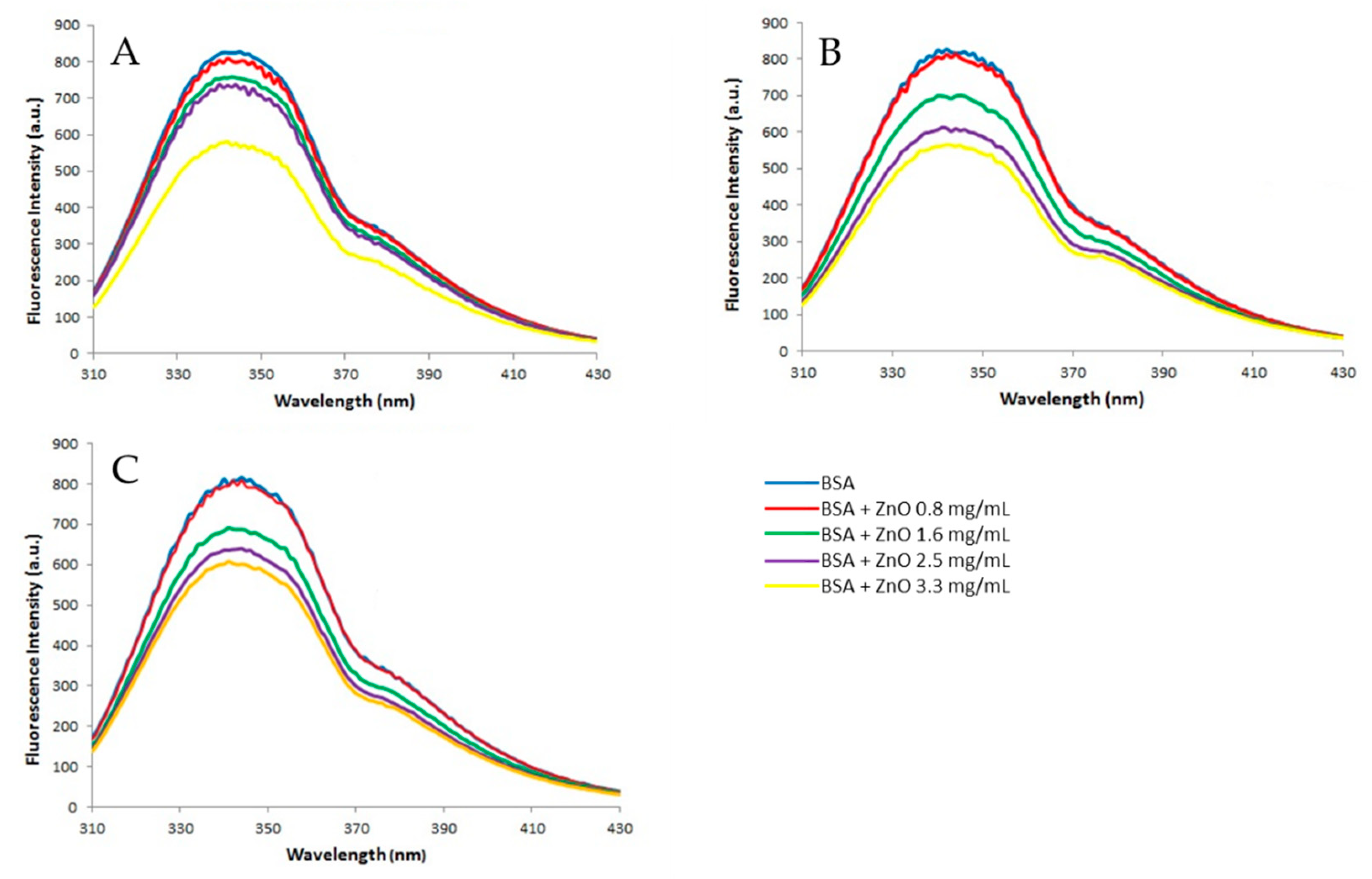
3.2.2. Fluorescence Measurements
3.3. Hemolytic Activity of Zn
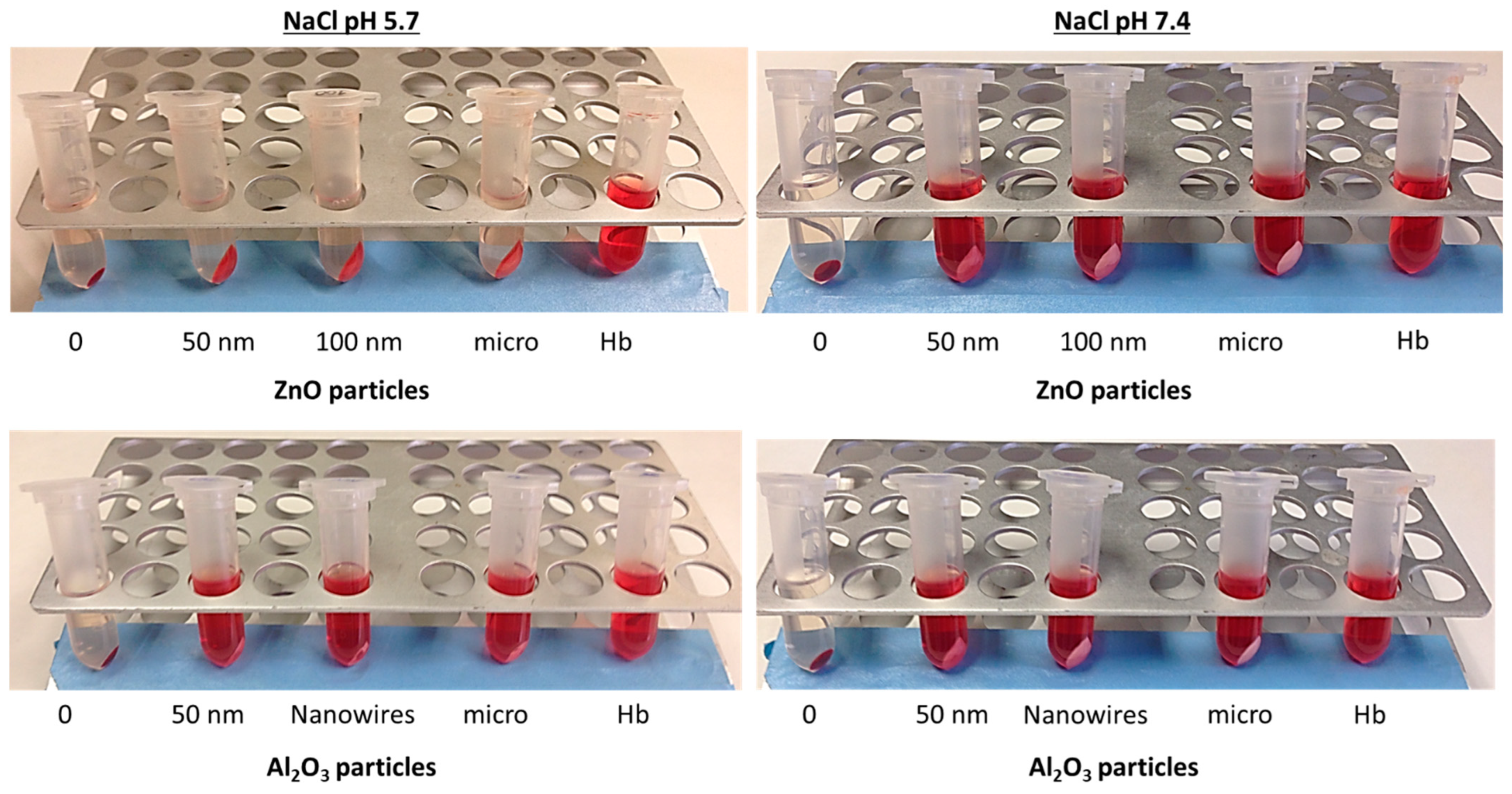
3.3.1. Media Interaction on Hemoglobin Adsorption
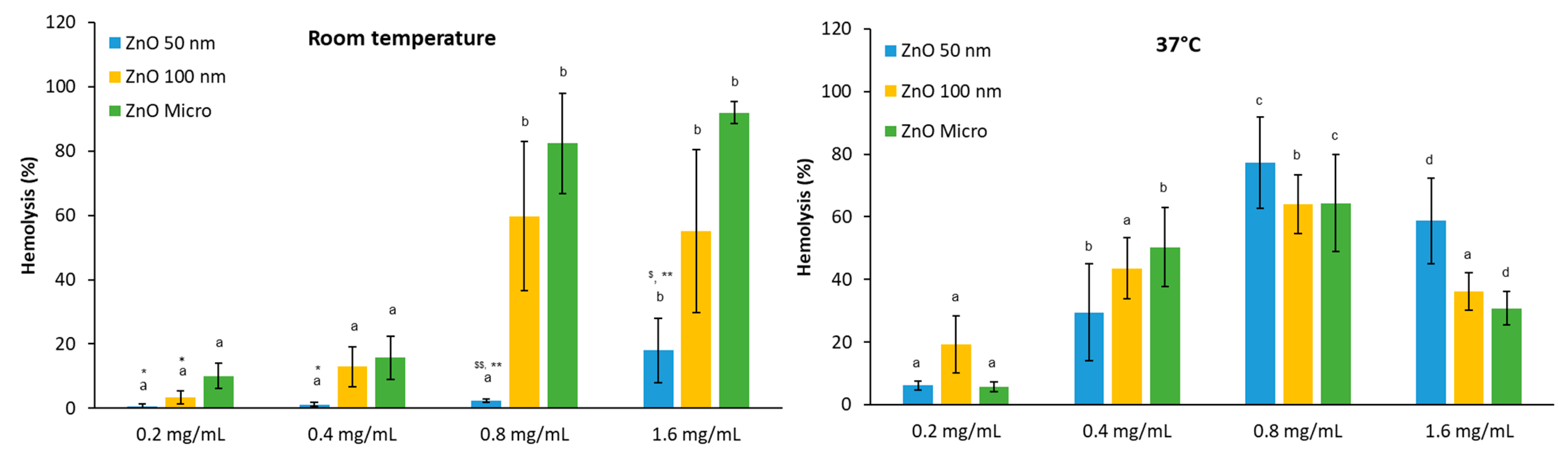
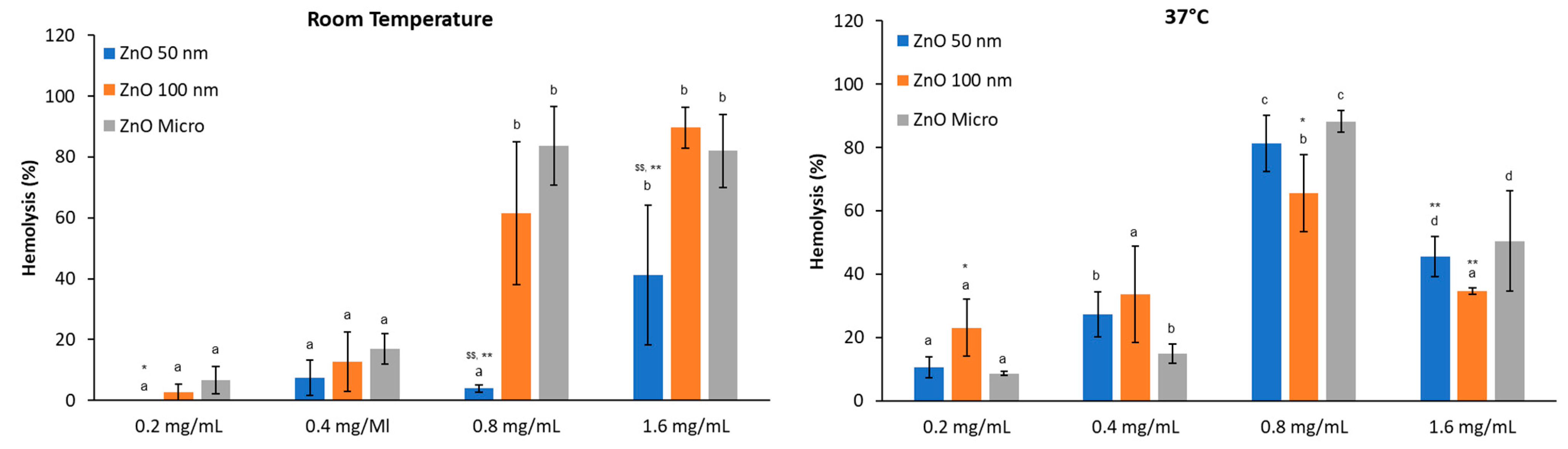
3.3.2. Hemolytic Activity in PBS Media, pH 7.4
- Effect of temperature
- Effect of albumin
3.3.3. Effects of ZnO on Erythrocyte Morphology
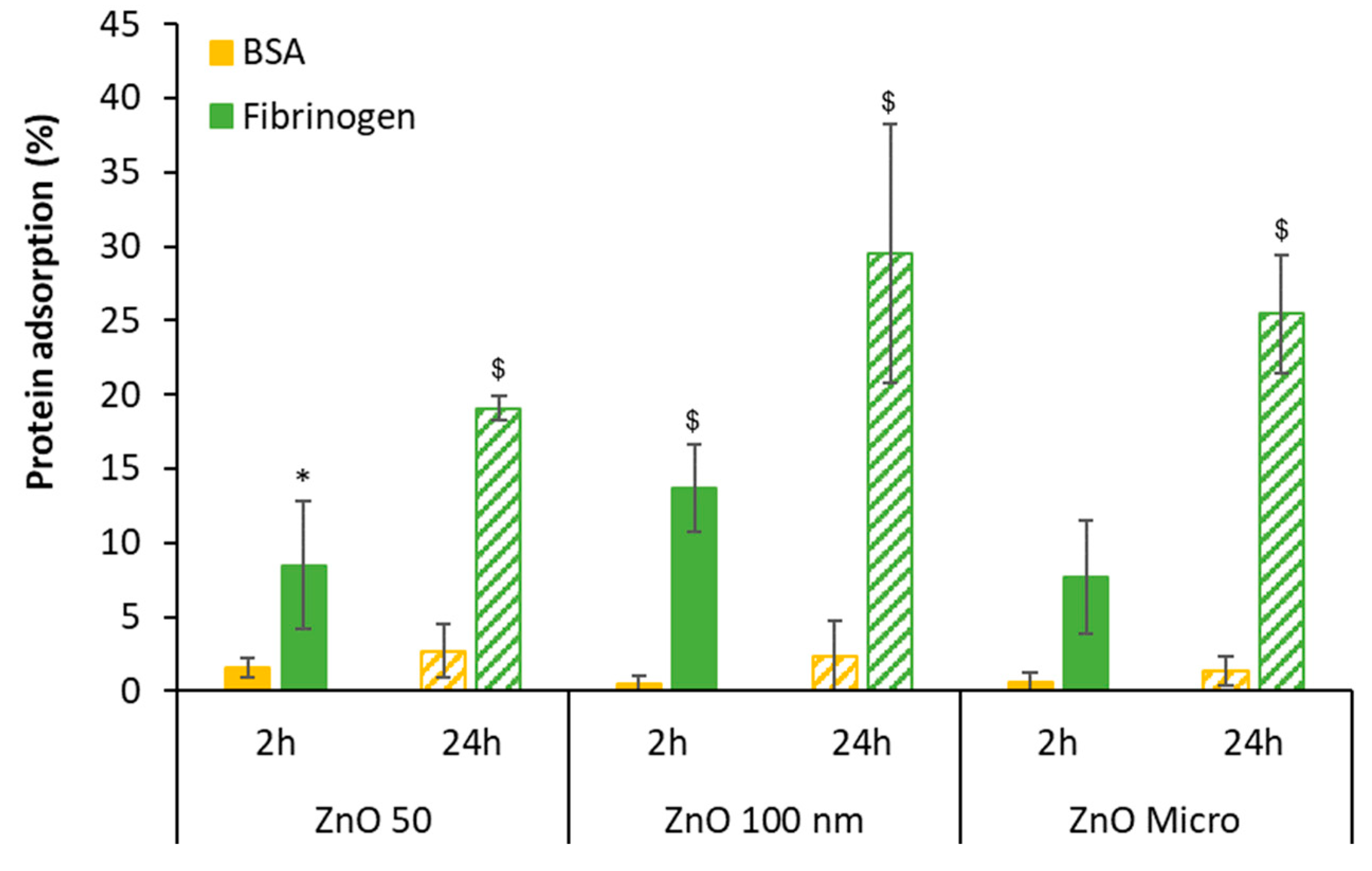
3.4. Coagulation Studies and Plasma Protein Interactions
3.4.1. Coagulation: Prothrombin Time and Activated Partial Thromboplastin Time
3.4.2. Two-Dimensional Gel Electrophoresis of Human Plasma Protein: SDS-PAGE
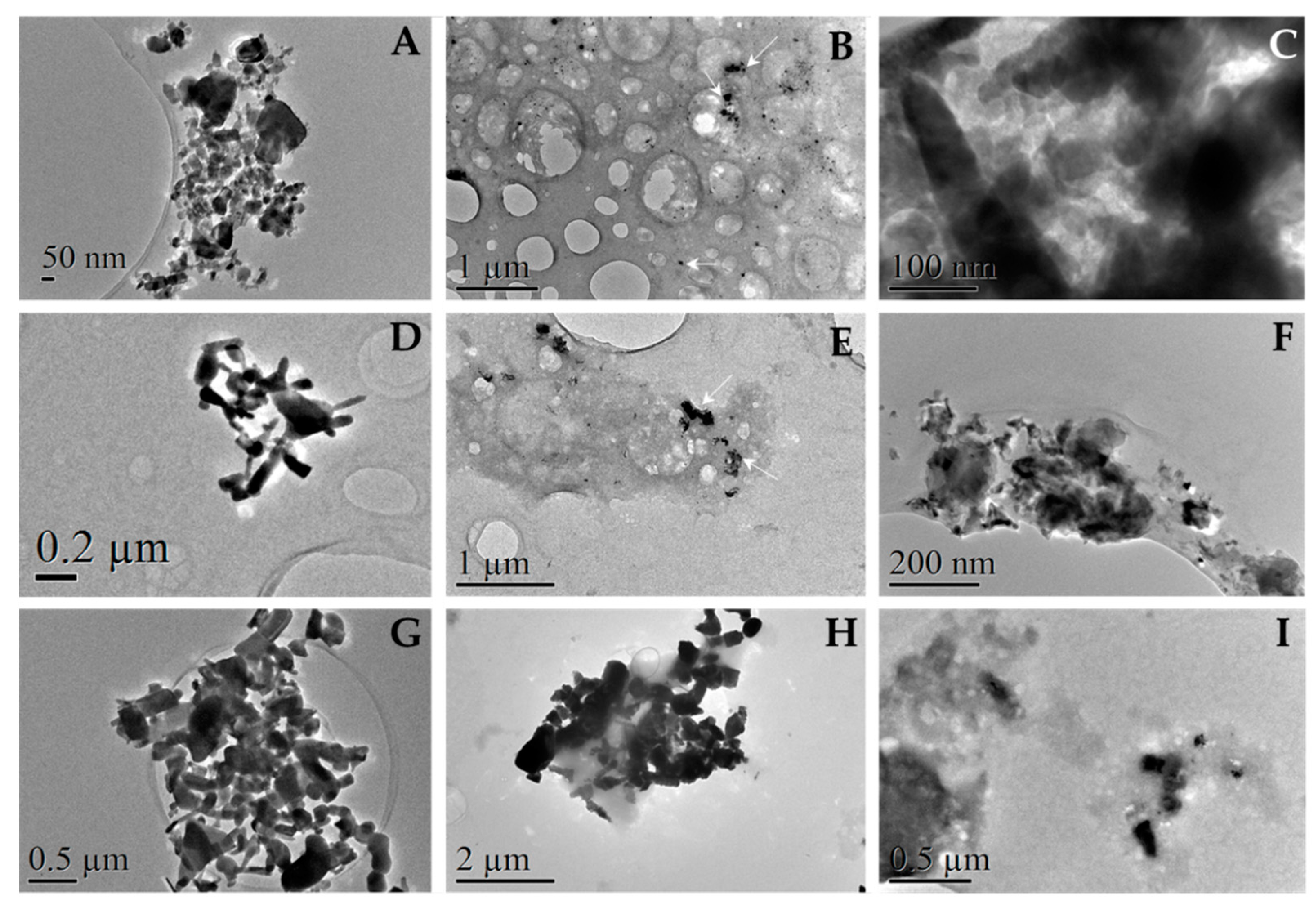
3.4.3. Observation of Protein Corona by TEM
3.5. Cytotoxicity of ZnO Particles in HaCaT and A549
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef] [PubMed]
- Allouni, Z.E.; Gjerdet, N.R.; Cimpan, M.R.; Høl, P.J. The effect of blood protein adsorption on cellular uptake of anatase TiO2 nanoparticles. Int. J. Nanomed. 2015, 10, 687–695. [Google Scholar]
- Fritea, L.; Banica, F.; Costea, T.O.; Moldovan, L.; Dobjanschi, L.; Muresan, M.; Cavalu, S. Metal Nanoparticles and Carbon-Based Nanomaterials for Improved Performances of Electrochemical (Bio)Sensors with Biomedical Applications. Materials 2021, 14, 6319. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, V.D.; Prasad, S.V.; Banerjee, A.; Gopinath, M.; Murugesan, R.; Marotta, F.; Sun, X.F.; Pathak, S. Health hazards of nanoparticles: Understanding the toxicity mechanism of nanosized ZnO in cosmetic products. Drug Chem. Toxicol. 2019, 42, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, R.; Kumar, A.; Tripathi, R.; Kumar, A. Advancing of Zinc Oxide Nanoparticles for Cosmetic Applications. In Handbook of Consumer Nanoproducts; Springer: Singapore, 2022; pp. 1057–1072. [Google Scholar]
- Sha, R.; Basak, A.; Maity, P.C.; Badhulika, S. ZnO nano-structured based devices for chemical and optical sensing applications. Sens. Actuators Rep. 2022, 4, 100098. [Google Scholar] [CrossRef]
- Weng, Z.; Xu, Y.; Gao, J.; Wang, X. Research progress of stimuli-responsive ZnO-based nanomaterials in biomedical applications. Biomater. Sci. 2022, 11, 76–95. [Google Scholar] [CrossRef]
- Alavi, N.; Maghami, P.; Fani Pakdel, A.; Rezaei, M.; Avan, A. The advance anticancer role of polymeric core-shell ZnO nanoparticles containing oxaliplatin in colorectal cancer. J. Biochem. Mol. Toxicol. 2023, 37, e23325. [Google Scholar] [CrossRef]
- Costa, B.A.; Abuçafy, M.P.; Barbosa, T.W.L.; da Silva, B.L.; Fulindi, R.B.; Isquibola, G.; da Costa, P.I.; Chiavacci, L.A. ZnO@ZIF-8 Nanoparticles as Nanocarrier of Ciprofloxacin for Antimicrobial Activity. Pharmaceutics 2023, 15, 259. [Google Scholar] [CrossRef]
- Urban, P.; Liptrott, N.J.; Bremer, S. Overview of the blood compatibility of nanomedicines: A trend analysis of in vitro and in vivo studies. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1546. [Google Scholar] [CrossRef]
- Kopac, C. Protein corona, understanding the nanoparticle interactions and future prespectives: A critical review. Int. J. Biol. Macromol. 2021, 169, 290–301. [Google Scholar] [CrossRef]
- Satzer, P.; Svec, F.; Sekot, G.; Jungbauer, A. Protein adsorption onto nanoparticles induces conformational changes: Particle size dependency, kinetics, and mechanisms. Eng. Life Sci. 2016, 16, 238–246. [Google Scholar] [CrossRef]
- Yu, J.; Kim, H.J.; Go, M.R.; Bae, S.H.; Choi, S.J. ZnO Interactions with Biomatrices: Effect of Particle Size on ZnO-Protein Corona. Nanomaterials 2017, 7, 377. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Seog, J.H.; Graham, L.M.; Lee, S.B. Experimental considerations on the cytotoxicity of nanoparticles. Nanomedicine 2011, 6, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Jedrzejczak-Silicka, M.; Mijowska, E. General Cytotoxicity and Its Application in Nanomaterial Analysis. In Cytotoxicity; IntechOpen: London, UK, 2018; Available online: http://dx.doi.org/10.5772/intechopen.72578 (accessed on 2 November 2021).
- Kruger, N.J. The Bradford method for protein quantitation. Methods Mol. Biol. 1994, 32, 9–15. [Google Scholar]
- Mariam, J.; Dongre, P.M.; Kothari, D.C. Study of Interaction of Silver Nanoparticles with Bovine Serum Albumin Using Fluorescence Spectroscopy. J. Fluoresc. 2011, 21, 2193–2199. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.; Duffin, R.; Bradley, M.; Megson, I.L.; Macnee, W.; Lee, J.K.; Jeong, J.; Donaldson, K. Predictive Value of in Vitro Assays Depends on the Mechanism of Toxicity of Metal Oxide Nanoparticles. Part. Fibre Toxicol. 2013, 10, 55. [Google Scholar] [CrossRef]
- Nogueira, D.R.; Mitjans, M.; Infante, M.R.; Vinardell, M.P. The Role of Counterions in the Membrane-Disruptive Properties of pH-Sensitive Lysine-Based Surfactants. Acta Biomater. 2011, 7, 2846–2856. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lan, J.; Xu, Z.; Chen, T. Toxicity and Biodistribution of Aqueous Synthesized ZnS and ZnO Quantum Dots in Mice. Nanotoxicology 2014, 8, 107–116. [Google Scholar] [CrossRef]
- Treuel, L.; Brandholt, S.; Maffre, P.; Wiegele, S.; Shang, L.; Nienhaus, G.U. Impact of protein modification on the protein corona on nanoparticles and nanoparticle-cell interactions. ACS Nano 2014, 8, 503–513. [Google Scholar] [CrossRef]
- Vinardell, M.P.; Sordé, A.; Díaz, J.; Baccarin, T.; Mitjans, M. Comparative Effects of Macro-Sized Aluminum Oxide and Aluminum Oxide Nanoparticles on Erythrocyte Hemolysis: Influence of Cell Source, Temperature, and Size. J. Nanopart. Res. 2015, 17, 80. [Google Scholar] [CrossRef]
- Potter, T.M.; Rodriguez, J.C.; Neun, B.W.; Ilinskaya, A.N.; Cedrone, E.; Dobrovolskaia, M.A. In Vitro Assessment of Nanoparticle Effects on Blood Coagulation. Methods Mol. Biol. 2018, 1682, 103–124. [Google Scholar] [PubMed]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fet, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Kielkopf, C.L.; Bauer, W.; Urbatsch, I.L. Bradford Assay for Determining Protein Concentration. Cold Spring Harb. Protoc. 2020, 2020, 102269. [Google Scholar] [CrossRef]
- Laemmli, U.H. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Vinardell, M.P.; Llanas, H.; Marics, L.; Mitjans, M. In Vitro Comparative Skin Irritation Induced by Nano and Non-Nano Zinc Oxide. Nanomaterials 2017, 7, 56. [Google Scholar] [CrossRef]
- Guadagnini, R.; Halamoda, B.; Walker, L.; Pojana, G.; Magdolenova, Z.; Bilanicova, D.; Saunders, L.; Juillerat-Jeanneret, L.; Marcomini, A.; Huk, A.; et al. Toxicity screenings of nanomaterials: Challenges due to interference with assay processes and components of classic in vitro tests. Nanotoxicology 2015, 9, 13–24. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Zanette, C.; Pelin, M.; Crosera, M.; Adami, G.; Bovenzi, M.; Larese, F.F.; Florio, C. Silver nanoparticles exert a long-lasting antiproliferative effect on human keratinocyte HaCaT cell line. Toxicol. In Vitro 2011, 25, 1053–1060. [Google Scholar] [CrossRef]
- Babich, H.; Borenfreund, E. Applications of the Neutral Red Cytotoxicity Assay to In Vitro Toxicology. Alt. Lab. Animals. 1990, 18, 129–144. [Google Scholar] [CrossRef]
- Dhawan, A.; Sharma, V. Toxicity assessment of nanomaterials: Methods and challenges. Anal. Bioanal. Chem. 2010, 398, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Yin, L.H.; Tang, M.; Pu, Y.P. ZnO, TiO2, SiO2 and Al2O3 Nanoparticles-Induced Toxic Effects on Human Fetal Lung Fibroblasts. Biomed. Environ. Sci. 2011, 24, 661–669. [Google Scholar]
- Šimundić, M.; Drašler, B.; Šuštar, V.; Zupanc, J.; Štukelj, R.; Makovec, D.; Erdogmus, D.; Hägerstrand, H.; Drobne, D.; Kralj-Iglič, V. Effect of engineered TiO2 and ZnO nanoparticles on erythrocytes, platelet-rich plasma and giant unilamelar phospholipid vesicles. BMC Vet. Res. 2013, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.B.; Yu, J.; Choi, S.J. Interaction between ZnO Nanoparticles and Albumin and Its Effect on Cytotoxicity, Cellular Uptake, Intestinal Transport, Toxicokinetics, and Acute Oral Toxicity. Nanomaterials 2021, 11, 2922. [Google Scholar] [CrossRef] [PubMed]
- Fornaguera, C.; Calderó, G.; Mitjans, M.; Vinardell, M.P.; Solans, C.; Vauthier, C. Interactions of PLGA nanoparticles with blood components: Protein adsorption, coagulation, activation of the complement system and hemolysis studies. Nanoscale 2015, 7, 6045–6058. [Google Scholar] [CrossRef] [PubMed]
- Bihari, P.; Vippola, M.; Schultes, S.; Praetner, M.; Khandoga, A.G.; Reichel, C.A.; Coester, C.; Tuomi, T.; Rehberg, M.; Krombach, F. Optimized dispersion of nanoparticles for biological in vitro and in vivo studies. Part. Fibre Toxicol. 2008, 5, 14. [Google Scholar] [CrossRef]
- Bhogale, A.; Patel, N.; Sarpotdar, P.; Mariam, J.; Dongre, P.M.; Miotello, A.; Kothari, D.C. Systematic Investigation on the Interaction of Bovine Serum Albumin with ZnO Nanoparticles Using Fluorescence Spectroscopy. Colloids Surf. B Biointerfaces 2013, 102, 257–264. [Google Scholar] [CrossRef]
- Sasidharan, N. Interaction of Colloidal Zinc Oxide Nanoparticles with Bovine Serum Albumin and its Adsorption Isotherms and Kinetics. Colloids Surf. B Biointerfaces 2013, 102, 195–201. [Google Scholar] [CrossRef]
- Simón-Vázquez, R.; Lozano-Fernández, T.; Peleteiro-Olmedo, M.; González-Fernández, Á. Conformational Changes in Human Plasma Proteins Induced by Metal Oxide Nanoparticles. Colloids Surf. B Biointerfaces 2014, 113, 198–206. [Google Scholar] [CrossRef]
- Lu, S.; Duffin, R.; Poland, C.; Daly, P.; Murphy, F.; Drost, E.; Macnee, W.; Stone, V.; Donaldson, K. Efficacy of simple short-term in vitro assays for predicting the potential of metal oxide nanoparticles to cause pulmonary inflammation. Environ. Health Perspect. 2009, 10, 241–247. [Google Scholar] [CrossRef]
- Nemmar, A.; Beegam, S.; Yuvaraju, P. Interaction of Amorphous Silica Nanoparticles with Erythrocytes in Vitro: Role of Oxidative Stress. Cell. Physiol. Biochem. 2014, 34, 255–265. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Abdelmonem, A.M.; Behzadi, S.; Clement, J.H.; Dutz, S.; Ejtehadi, M.R.; Hartmann, R.; Kantner, K.; Linne, U.; Maffre, P.; et al. Temperature: The “ignored” factor at the NanoBio interface. ACS Nano 2013, 7, 6555–6562. [Google Scholar] [CrossRef] [PubMed]
- Aisaka, Y.; Kawaguchi, R.; Watanabe, S. Hemolysis Caused by Titanium Dioxide Particles. Inhal. Toxicol. 2008, 20, 891–893. [Google Scholar] [CrossRef] [PubMed]
- Geekiyanage, N.M.; Balanant, M.A.; Sauret, E.; Saha, S.; Flower, R.; Lim, C.T.; Gu, Y. A coarsegrained red blood cell membrane model to study stomatocyte-discocyte-echinocyte morphologies. PLoS ONE 2019, 14, e0215447. [Google Scholar] [CrossRef]
- Neun, B.W.; Rodriguez, J.; Ilinskaya, A.; Dobrovolskaia, M. NCL Method ITA-12 Coagulation Assays; NIH: Bethesda, MD, USA, 2015; pp. 1–8. Available online: https://ncihub.cancer.gov/publications/147/?v=1 (accessed on 3 May 2023).
- Kushida, T.; Saha, K.; Subramani, C.; Nandwana, V.; Rotello, V.M. Effect of nano-scale curvature on the intrinsic blood coagulation system. Nanoscale 2014, 6, 14484–14487. [Google Scholar] [CrossRef]
- Song, L.; Zhu, D.; Liu, L.; Dong, X.; Zhang, H.; Leng, X. Evaluation of the coagulation properties of arginine-chitosan/DNA nanoparticles. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 95, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Duran, N.; Silveira, C.P.; Duran, M.; Martinez, D.S.T. Silver nanoparticle protein corona and toxicity: A mini-review. J. Nanobiotechnol. 2015, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Tennent, G.A.; Brennan, S.O.; Stangou, A.J.; O’Grady, J.; Hawkins, P.N.; Pepys, M.B. Human plasma fibrinogen is synthesized in the liver. Blood 2007, 109, 1971–1974. [Google Scholar] [CrossRef] [PubMed]
- Verstraelen, S.; Remy, S.; Casals, E.; De Boever, P.; Witters, H.; Gatti, A.; Puntes, V.; Nelissen, I. Gene expression profiles reveal distinct immunological responses of cobalt and cerium dioxide nanoparticles in two in vitro lung epithelial cell models. Toxicol. Lett. 2014, 228, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, D.R.; Morán, M.C.; Mitjans, M.; Martínez, V.; Pérez, L.; Vinardell, M.P. New cationic nanovesicular systems containing lysine-based surfactants for topical administration: Toxicity assessment using representative skin cell lines. Eur. J. Pharm. Biopharm. 2013, 83, 33–43. [Google Scholar] [CrossRef]
- Farcal, L.; Torres Andón, F.; Di Cristo, L.; Rotoli, B.M.; Bussolati, O.; Bergamaschi, E.; Mech, A.; Hartmann, N.B.; Rasmussen, K.; Riego-Sintes, J.; et al. Comprehensive In Vitro Toxicity Testing of a Panel of Representative Oxide Nanomaterials: First Steps towards an Intelligent Testing Strategy. PLoS ONE 2015, 10, e0127174. [Google Scholar] [CrossRef]
- Liang, H.; Jin, C.; Tang, Y.; Wang, F.; Ma, C.; Yang, Y. Cytotoxicity of silica nanoparticles on HaCaT cells. J. Appl. Toxicol. 2014, 34, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Jeng, H.A.; Swanson, J. Toxicity of metal oxide nanoparticles in mammalian cells. J. Environ. Sci. Health A 2006, 41, 2699–2711. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Fujita, D.; Kajiwara, S.; Minowa, T.; Li, X.; Takemura, T.; Iwai, H.; Hanagata, N. Contribution of physicochemical characteristics of nano-oxides to cytotoxicity. Biomaterials 2010, 31, 8022–8031. [Google Scholar] [CrossRef] [PubMed]

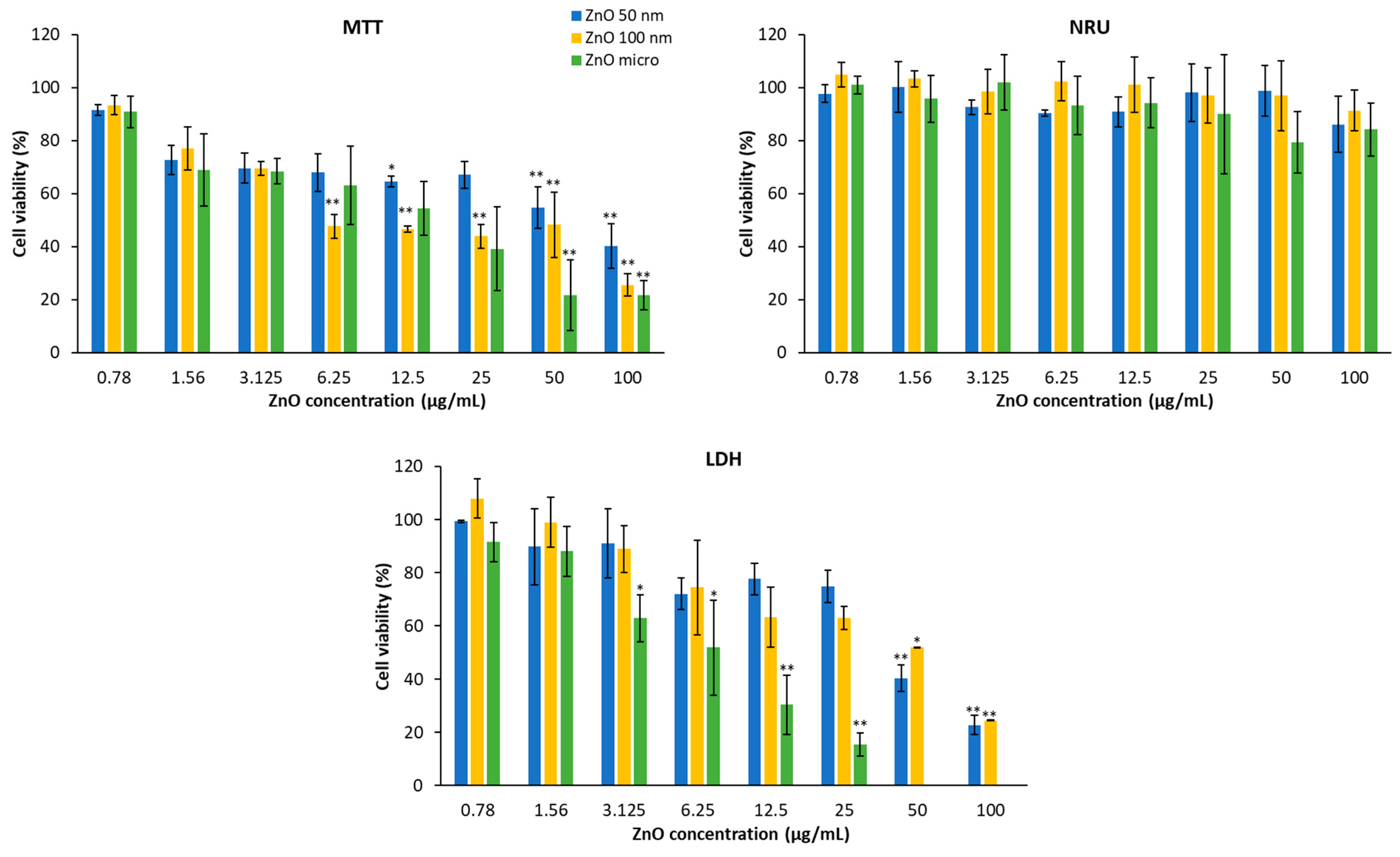
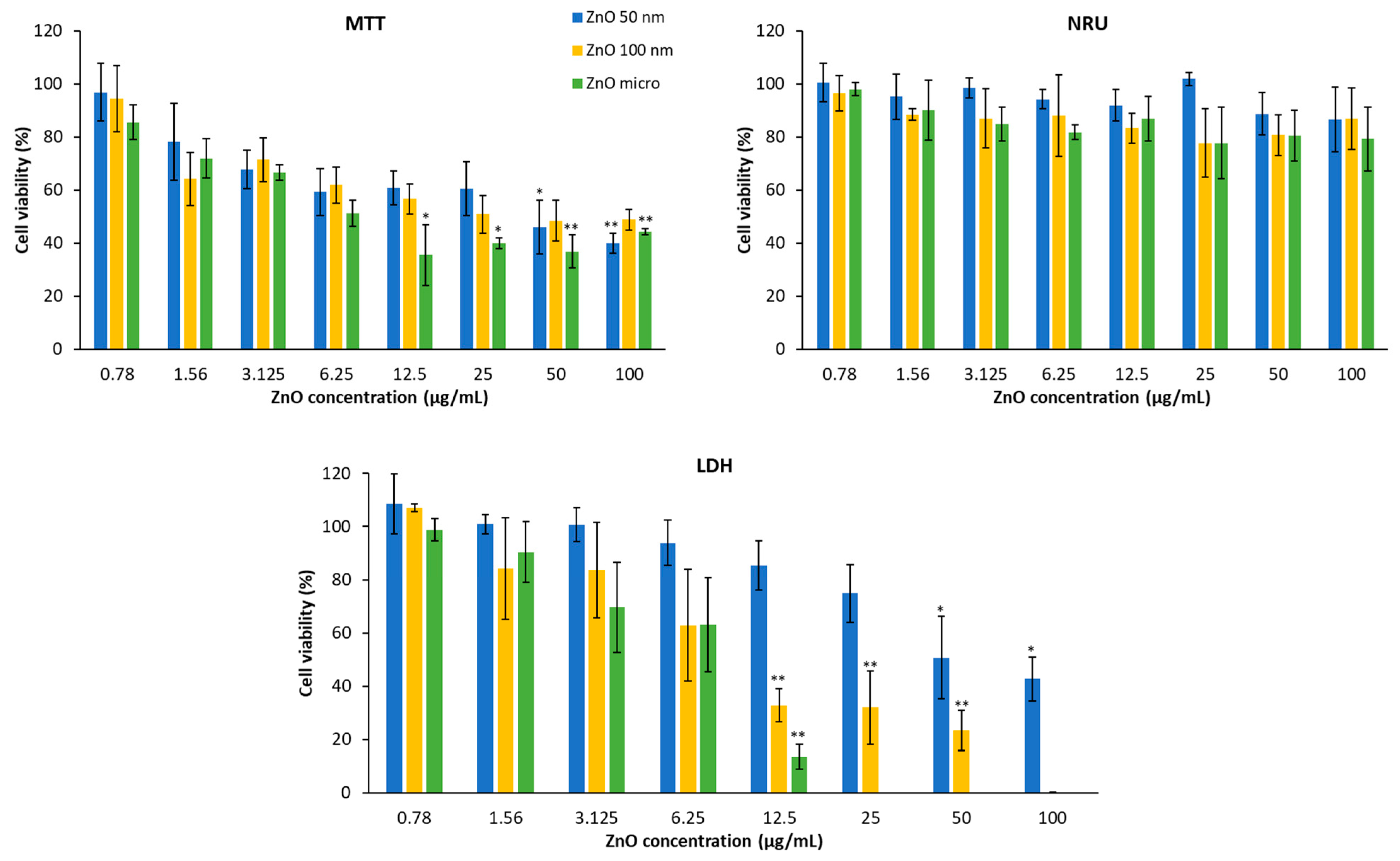
| ZnO Particles | 2 h 1 | 24 h 1 |
|---|---|---|
| 50 nm | 1014.9 ± 90.5 | 2165.0 ± 237.6 $$ |
| 100 nm | 940.5 ± 72.4 | 1354.0 ± 62.2 $ |
| Micro | 721.1 ± 12.3 | 916.0 ± 43.2 $ |
| ZnO Particles | 2 h 1 | 24 h 1 | ||||
|---|---|---|---|---|---|---|
| BSA | Fibrinogen | DMEM 2 | BSA | Fibrinogen | DMEM 2 | |
| 50 nm | 806.5 ± 52.6 ** | 30.0 ± 7.2 ** | 772.2 ± 34.1 ** | 995.9 ± 171.3 **,$ | 25.6 ± 0.2 ** | 1199.0 ± 188.2 **,$$ |
| 100 nm | 709.5 ± 15.8 ** | 53.3 ± 3.6 ** | 614.90 ± 12.7 ** | 1334.5 ± 262.3 $$ | 173.9 ± 50.1 ** | 827.5 ± 102.0 **,$ |
| Micro | 625.9 ± 104.7 * | 120.4 ± 78.8 ** | 470.30 ± 20.6 ** | 1802.0 ± 49.5 **,$$ | 214.0 ± 91.1 ** | 507.6 ± 36.2 ** |
| Incubation Media | ZnO | Al2O3 |
|---|---|---|
| 0.9% NaCl pH 5.7 pH 7.4 | Adsorption No adsorption | No adsorption No adsorption |
| PBS pH 5.7 pH 7.4 | No adsorption No adsorption | No adsorption No adsorption |
| Tris–maleate pH 5.7 pH 7.4 | Adsorption Adsorption | No adsorption No adsorption |
| ZnO Particles | HaCaT | A549 | ||
|---|---|---|---|---|
| MTT 1 | LDH | MTT | LDH | |
| 50 nm | 68.7 ± 5.9 | 38.9 ± 1.6 | 33.1 ± 1.7 | 91.5 ± 9.3 |
| 100 nm | 13.1 ± 1.4 | 16.1 ± 4.7 | 42.9 ± 5.6 | 10.3 ± 0.3 |
| Micro | 11.3 ± 1.0 | 6.2 ± 1.3 | 10.4 ± 0.2 | 6.3 ± 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitjans, M.; Marics, L.; Bilbao, M.; Maddaleno, A.S.; Piñero, J.J.; Vinardell, M.P. Size Matters? A Comprehensive In Vitro Study of the Impact of Particle Size on the Toxicity of ZnO. Nanomaterials 2023, 13, 1800. https://doi.org/10.3390/nano13111800
Mitjans M, Marics L, Bilbao M, Maddaleno AS, Piñero JJ, Vinardell MP. Size Matters? A Comprehensive In Vitro Study of the Impact of Particle Size on the Toxicity of ZnO. Nanomaterials. 2023; 13(11):1800. https://doi.org/10.3390/nano13111800
Chicago/Turabian StyleMitjans, Montserrat, Laura Marics, Marc Bilbao, Adriana S. Maddaleno, Juan José Piñero, and M. Pilar Vinardell. 2023. "Size Matters? A Comprehensive In Vitro Study of the Impact of Particle Size on the Toxicity of ZnO" Nanomaterials 13, no. 11: 1800. https://doi.org/10.3390/nano13111800
APA StyleMitjans, M., Marics, L., Bilbao, M., Maddaleno, A. S., Piñero, J. J., & Vinardell, M. P. (2023). Size Matters? A Comprehensive In Vitro Study of the Impact of Particle Size on the Toxicity of ZnO. Nanomaterials, 13(11), 1800. https://doi.org/10.3390/nano13111800







