Construction of 2D/2D Mesoporous WO3/CeO2 Laminated Heterojunctions for Optimized Photocatalytic Performance
Abstract
1. Introduction
2. Experimental
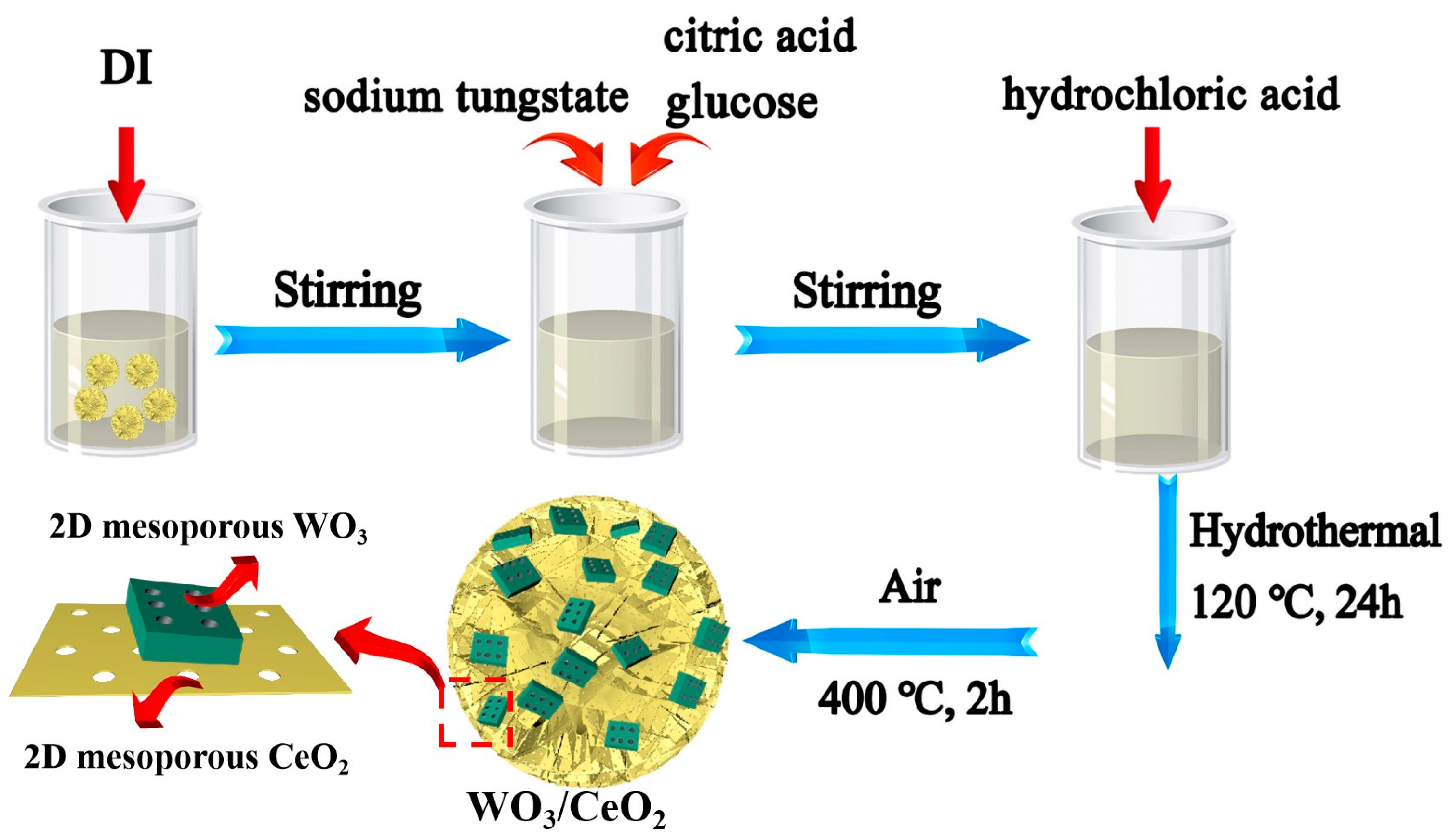
2.1. Synthesis of Mesoporous CeO2 Nanosheets, WO3 Nanoplates, and WO3/CeO2 Composites
2.2. Characterizations
2.3. Photocatalytic Activity Measurements
3. Results and Discussion
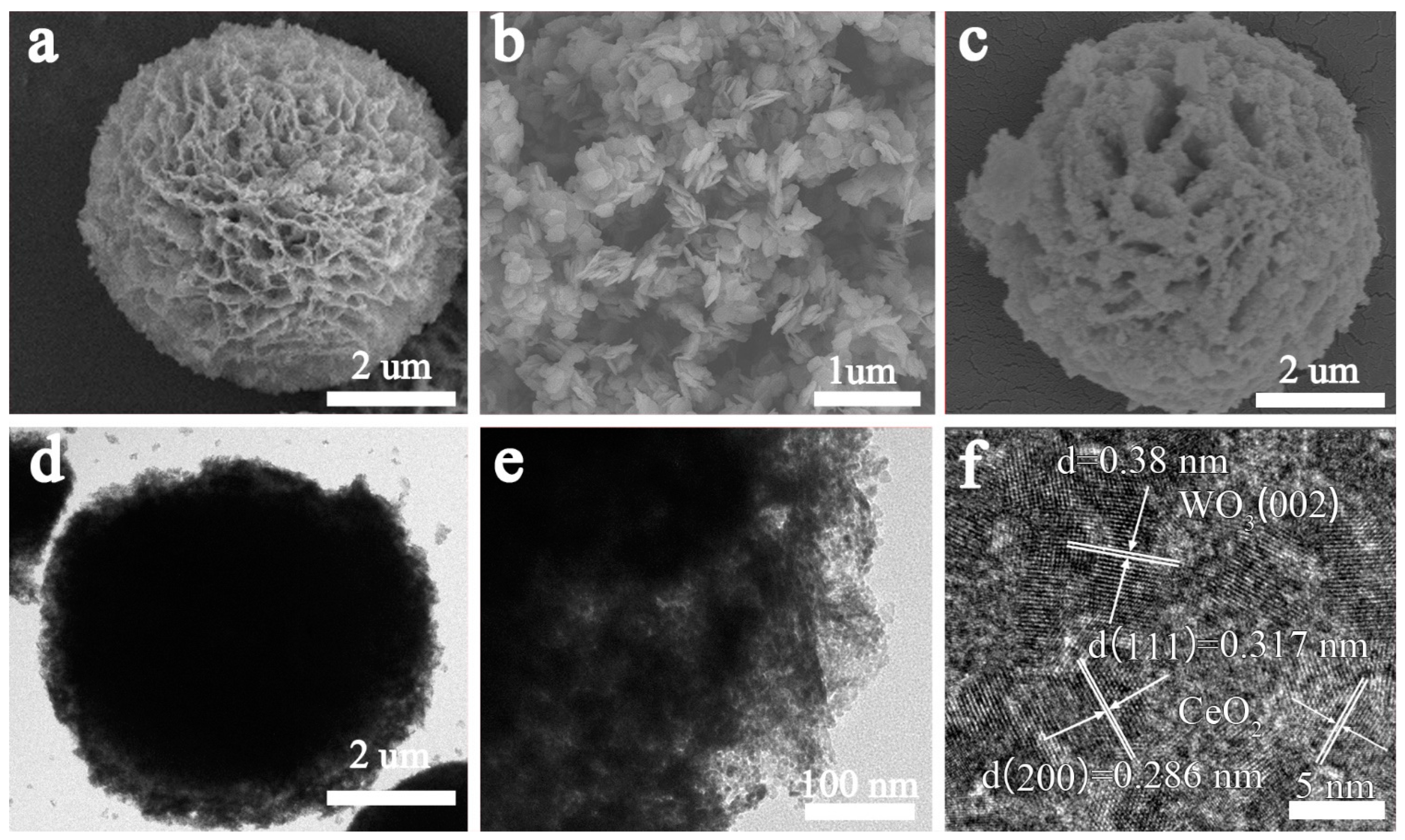
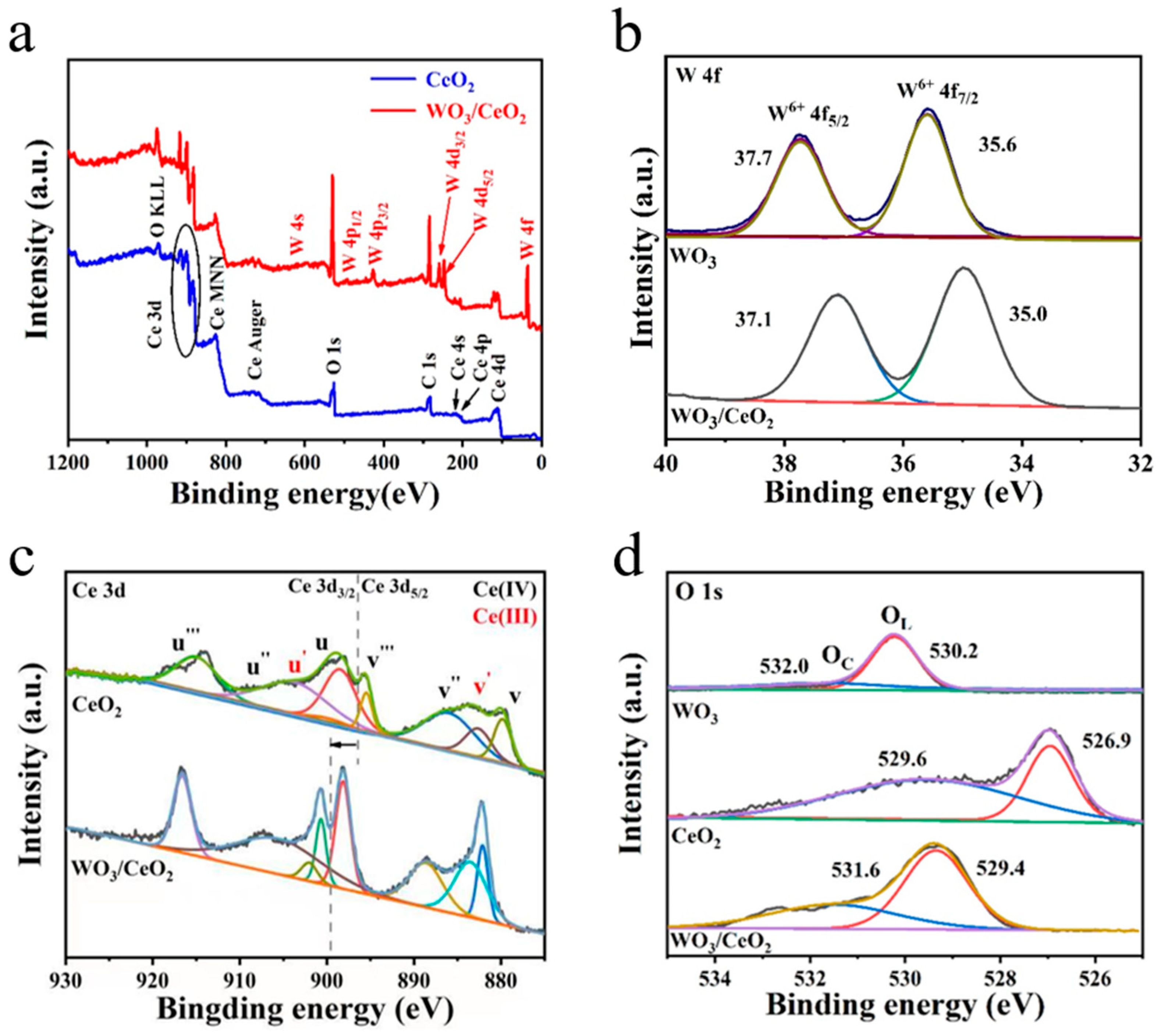
3.1. Morphology and Microstructure
3.2. Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Zhuan, R.; Chu, L. The occurrence, distribution and degradation of antibiotics by ionizing radiation: An overview. Sci. Total Environ. 2019, 646, 1385–1397. [Google Scholar] [CrossRef] [PubMed]
- Zainab, S.M.; Junaid, M.; Xu, N.; Malik, R.N. Antibiotics and antibiotic resistant genes (ARGs) in groundwater: A global review on dissemination, sources, interactions, environmental and human health risks. Water Res. 2020, 187, 116455. [Google Scholar] [CrossRef] [PubMed]
- Phoon, B.L.; Ong, C.C.; Saheed, M.S.M.; Show, P.L.; Chang, J.S.; Ling, T.C.; Lam, S.S.; Juan, J.C. Conventional and emerging technologies for removal of antibiotics from wastewater. J. Hazard. Mater. 2020, 400, 122961. [Google Scholar] [CrossRef]
- Oberoi, A.S.; Jia, Y.; Zhang, H.; Khanal, S.K.; Lu, H. Insights into the Fate and Removal of Antibiotics in Engineered Biological Treatment Systems: A Critical Review. Environ. Sci. Technol. 2019, 53, 7234–7264. [Google Scholar] [CrossRef] [PubMed]
- Saadati, F.; Keramati, N.; Ghazi, M.M. Influence of parameters on the photocatalytic degradation of tetracycline in wastewater: A review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 757–782. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Calvete, M.J.F.; Piccirillo, G.; Vinagreiro, C.S.; Pereira, M.M. Hybrid materials for heterogeneous photocatalytic degradation of antibiotics. Coord. Chem. Rev. 2019, 395, 63–85. [Google Scholar] [CrossRef]
- Bayan, E.M.; Pustovaya, L.E.; Volkova, M.G. Recent advances in TiO2-based materials for photocatalytic degradation of antibiotics in aqueous systems. Environ. Technol. Innov. 2021, 24, 101822. [Google Scholar] [CrossRef]
- Qin, K.; Zhao, Q.; Yu, H.; Xia, X.; Li, J.; He, S.; Wei, L.; An, T. review of bismuth-based photocatalysts for antibiotic degradation: Insight into the photocatalytic degradation performance, pathways and relevant mechanisms. Environ. Res. 2021, 199, 111360. [Google Scholar] [CrossRef]
- Suyana, P.; Ganguly, P.; Nair, B.N.; Pillai, S.C.; Hareesh, U.S. Structural and compositional tuning in g-C3N4 based systems for photocatalytic antibiotic degradation. Chem. Eng. J. Adv. 2021, 8, 100148. [Google Scholar] [CrossRef]
- Li, D.; Shi, W. Recent developments in visible-light photocatalytic degradation of antibiotics. Chin. J. Catal. 2016, 37, 792–799. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Zeng, L.; Zhu, M. Recent progress on the removal of antibiotic pollutants using photocatalytic oxidation process. Crit. Rev. Environ. Sci. Technol. 2021, 52, 1401–1448. [Google Scholar] [CrossRef]
- Dutta, V.; Sharma, S.; Raizada, P.; Thakur, V.K.; Khan, A.A.P.; Saini, V.; Asiri, A.M.; Singh, P. An overview on WO3 based photocatalyst for environmental remediation. J. Environ. Chem. Eng. 2021, 9, 105018. [Google Scholar] [CrossRef]
- Kim, H.; Yoo, H.-Y.; Hong, S.; Lee, S.; Lee, S.; Park, B.-S.; Park, H.; Lee, C.; Lee, J. Effects of inorganic oxidants on kinetics and mechanisms of WO3-mediated photocatalytic degradation. Appl. Catal. B Environ. 2015, 162, 515–523. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nam, S.-N.; Son, J.; Oh, J. Tungsten Trioxide (WO3)-assisted Photocatalytic Degradation of Amoxicillin by Simulated Solar Irradiation. Sci. Rep. 2019, 9, 9349. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Liu, L.; Ai, F. Enhanced Photocatalytic Degradation of Methylene Blue by WO3 Nanoparticles Under NIR Light Irradiation. Front. Chem. 2021, 9, 683765. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jin, A.; Jia, Y.; Xia, T.; Deng, C.; Zhu, M.; Chen, C.; Chen, X. Synergy of adsorption and visible-light photocatalytic degradation of methylene blue by a bifunctional Z-scheme heterojunction of WO3/g-C3N4. Appl. Surf. Sci. 2017, 405, 359–371. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, J.; Yu, S.; Zhou, Y.; Yan, X. Ag loaded WO3 nanoplates for efficient photocatalytic degradation of sulfanilamide and their bactericidal effect under visible light irradiation. J. Hazard. Mater. 2016, 318, 407–416. [Google Scholar] [CrossRef]
- Isari, A.A.; Mehregan, M.; Mehregan, S.; Hayati, F.; Kalantary, R.R.; Kakavandi, B. Sono-photocatalytic degradation of tetracycline and pharmaceutical wastewater using WO3/CNT heterojunction nanocomposite under US and visible light irradiations: A novel hybrid system. J. Hazard. Mater. 2020, 390, 122050. [Google Scholar] [CrossRef]
- Xiao, Y.; Tan, S.; Wang, D.; Wu, J.; Jia, T.; Liu, Q.; Qi, Y.; Qi, X.; He, P.; Zhou, M. CeO2/BiOIO3 heterojunction with oxygen vacancies and Ce4+/Ce3+ redox centers synergistically enhanced photocatalytic removal heavy metal. Appl. Surf. Sci. 2020, 530, 147116. [Google Scholar] [CrossRef]
- Shen, C.-H.; Chen, Y.; Xu, X.-J.; Li, X.-Y.; Wen, X.-J.; Liu, Z.-T.; Xing, R.; Guo, H.; Fei, Z.-H. Efficient photocatalytic H2 evolution and Cr(VI) reduction under visible light using a novel Z-scheme SnIn4S8/CeO2 heterojunction photocatalysts. J. Hazard. Mater. 2021, 416, 126217. [Google Scholar] [CrossRef]
- Li, T.; Yin, J.; Sun, D.; Zhang, M.; Pang, H.; Xu, L.; Zhang, Y.; Yang, J.; Tang, Y.; Xue, J. Manipulation of Mott−Schottky Ni/CeO2 Heterojunctions into N-Doped Carbon Nanofibers for High-Efficiency Electrochemical Water Splitting. Small 2022, 18, 2106592. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wang, H.; Liu, J.; Liu, X.; Song, X.; Zhou, W.; Zhang, J.; Huo, P. Enhanced degradation of polyethylene terephthalate plastics by CdS/CeO2 heterojunction photocatalyst activated peroxymonosulfate. J. Hazard. Mater. 2023, 452, 131375. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, X.; Wei, Q.; He, P.; Cheng, Z.; Wu, J.; Qi, Y.; Sun, Y.; Song, J.; Yang, W. 3D/0D Cu3SnS4/CeO2 Heterojunction Photocatalyst with Dual Redox Pairs Synergistically Promotes the Photocatalytic Reduction of CO2. Energy Fuels 2022, 36, 7763–7774. [Google Scholar] [CrossRef]
- Bahadoran, A.; Ramakrishna, S.; Masudy-Panah, S.; De Lile, J.R.; Sadeghi, B.; Li, J.; Gu, J.; Liu, Q. Novel S-scheme WO3/CeO2 heterojunction with enhanced photocatalytic degradation of sulfamerazine under visible light irradiation. Appl. Surf. Sci. 2021, 568, 150957. [Google Scholar] [CrossRef]
- Channei, D.; Chansaenpak, K.; Phanichphant, S.; Jannoey, P.; Khanitchaidecha, W.; Nakaruk, A. Synthesis and Characterization of WO3/CeO2 Heterostructured Nanoparticles for Photodegradation of Indigo Carmine Dye. ACS Omega 2021, 6, 19771–19777. [Google Scholar] [CrossRef]
- Shandilya, P.; Sambyal, S.; Sharma, R.; Mandyal, P.; Fang, B. Properties, optimized morphologies, and advanced strategies for photocatalytic applications of WO3 based photocatalysts. J. Hazard. Mater. 2022, 428, 128218. [Google Scholar] [CrossRef]
- Niu, X.; Du, Y.; He, J.; Li, X.; Wen, G. Hydrothermal Synthesis of Co-Exposed-Faceted WO3 Nanocrystals with Enhanced Photocatalytic Performance. Nanomaterials 2022, 12, 2879. [Google Scholar] [CrossRef]
- Yang, D.; Xu, Y.; Pan, K.; Yu, C.; Wu, J.; Li, M.; Yang, F.; Qu, Y.; Zhou, W. Engineering surface oxygen vacancy of mesoporous CeO2 nanosheets assembled microspheres for boosting solar-driven photocatalytic performance. Chin. Chem. Lett. 2022, 33, 378–384. [Google Scholar] [CrossRef]
- Liu, C.; Mao, S.; Wang, H.; Wu, Y.; Wang, F.; Xia, M.; Chen, Q. Peroxymonosulfate-assisted for facilitating photocatalytic degradation performance of 2D/2D WO3/BiOBr S-scheme heterojunction. Chem. Eng. J. 2022, 430, 132806. [Google Scholar] [CrossRef]
- Dang, W.; Wang, W.; Yang, Y.; Wang, Y.; Huang, J.; Fang, X.; Wu, L.; Rong, Z.; Chen, X.; Li, X.; et al. One-step hydrothermal synthesis of 2D WO3 nanoplates@ graphene nanocomposite with superior anode performance for lithium ion battery. Electrochim. Acta 2019, 313, 99–108. [Google Scholar] [CrossRef]
- Wan, J.; Tao, F.; Shi, Y.; Shi, Z.; Liu, Y.; Wu, G.; Kan, J.; Zhou, R. Designed preparation of nano rod shaped CeO2-MnO catalysts with different Ce/Mn ratios and its highly efficient catalytic performance for chlorobenzene complete oxidation: New insights into structure–activity correlations. Chem. Eng. J. 2022, 433, 133788. [Google Scholar] [CrossRef]
- Li, H.; Bian, Z.; Zhu, J.; Huo, Y.; Li, H.; Lu, Y. Mesoporous Au/TiO2 Nanocomposites with Enhanced Photocatalytic Activity. J. Am. Chem. Soc. 2007, 129, 4538–4539. [Google Scholar] [CrossRef]
- Lo, A.-Y.; Taghipour, F. Ordered mesoporous photocatalysts for CO2 photoreduction. J. Mater. Chem. A 2021, 9, 26430–26453. [Google Scholar] [CrossRef]
- Tok, A.I.Y.; Boey, F.Y.C.; Dong, Z.; Sun, X.L. Hydrothermal synthesis of CeO2 nano-particles. J. Mater. Process. Technol. 2007, 190, 217–222. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Wang, J.-J.; Li, P.; Tan, Z.-Y.; Zeng, J.-H.; He, Y.-R.; Habibul, N. Removal of U(VI) from aqueous solution via photocatalytic reduction over WO3/g-C3N4 composite under visible light. Chem. Eng. J. 2022, 428, 131209. [Google Scholar] [CrossRef]
- Li, P.; Wang, B.; Qin, C.; Han, C.; Sun, L.; Wang, Y. Band-gap-tunable CeO2 nanoparticles for room-temperature NH3 gas sensors. Ceram. Int. 2020, 46, 19232–19240. [Google Scholar] [CrossRef]
- Kwong, W.L.; Savvides, N.; Sorrell, C.C. Electrodeposited nanostructured WO3 thin films for photoelectrochemical applications. Electrochim. Acta 2012, 75, 371–380. [Google Scholar] [CrossRef]
- Liu, R.; Li, H.; Duan, L.; Shen, H.; Zhang, Y.; Zhao, X. In situ synthesis and enhanced visible light photocatalytic activity of C-TiO2 microspheres/carbon quantum dots. Ceram. Int. 2017, 43, 8648–8654. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Yin, S. Oxygen vacancies confined in SnO2 nanoparticles for desirable electronic structure and enhanced visible light photocatalytic activity. Appl. Surf. Sci. 2017, 420, 399–406. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef]
- Li, H.; Yu, H.; Quan, X.; Chen, S.; Zhang, Y. Uncovering the Key Role of the Fermi Level of the Electron Mediator in a Z-Scheme Photocatalyst by Detecting the Charge Transfer Process of WO3-metal-gC3N4 (Metal = Cu, Ag, Au). ACS Appl. Mater. Interfaces 2016, 8, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-H.; Wen, X.-J.; Fei, Z.-H.; Liu, Z.-T.; Mu, Q.-M. Novel Z-scheme W18O49/CeO2 heterojunction for improved photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2020, 579, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-F.; Song, J.; Wang, X.; Pan, L.; Li, K.; Zhang, X.; Wang, L.; Zou, J.-J. Switching charge transfer of C3N4/W18O49 from type-II to Z-scheme by interfacial band bending for highly efficient photocatalytic hydrogen evolution. Nano Energy 2017, 40, 308–316. [Google Scholar] [CrossRef]
- Wang, H.; Liao, B.; Lu, T.; Ai, Y.; Liu, G. Enhanced visible-light photocatalytic degradation of tetracycline by a novel hollow BiOCl@CeO2 heterostructured microspheres: Structural characterization and reaction mechanism. J. Hazard. Mater. 2020, 385, 121552. [Google Scholar] [CrossRef]
- Lai, C.; Xu, F.; Zhang, M.; Li, B.; Liu, S.; Yi, H.; Li, L.; Qin, L.; Liu, X.; Fu, Y.; et al. Facile synthesis of CeO2/carbonate doped Bi2O2CO3 Z-scheme heterojunction for improved visible-light photocatalytic performance: Photodegradation of tetracycline and photocatalytic mechanism. J. Colloid Interface Sci. 2021, 588, 283–294. [Google Scholar] [CrossRef]
- Ye, Z.; Li, J.; Zhou, M.; Wang, H.; Ma, Y.; Huo, P.; Yu, L.; Yan, Y. Well-dispersed nebula-like ZnO/CeO2@HNTs heterostructure for efficient photocatalytic degradation of tetracycline. Chem. Eng. J. 2016, 304, 917–933. [Google Scholar] [CrossRef]
- Zhang, K.; Gu, S.; Wu, Y.; Fan, Q.; Zhu, C. Preparation of pyramidal SnO/CeO2 nano-heterojunctions with enhanced photocatalytic activity for degradation of tetracycline. Nanotechnology 2020, 31, 215702. [Google Scholar] [CrossRef]
- Wen, X.-J.; Niu, C.-G.; Ruan, M.; Zhang, L.; Zeng, G.-M. AgI nanoparticles-decorated CeO2 microsheets photocatalyst for the degradation of organic dye and tetracycline under visible-light irradiation. J. Colloid Interface Sci. 2017, 497, 368–377. [Google Scholar] [CrossRef]
- Chen, C.; Wang, D.; Li, Y.; Huang, H.; Ke, Y. ‘Flower-like AgBr/CeO2 Z-scheme heterojunction photocatalyst with enhanced visible light photocatalytic and antibacterial activities. Appl. Surf. Sci. 2021, 565, 150534. [Google Scholar] [CrossRef]
- Shen, J.; Shen, J.; Zhang, W.; Yu, X.; Tang, H.; Zhang, M.; Zulfiqar; Liu, Q. Built-in electric field induced CeO2/Ti3C2-MXene Schottky-junction for coupled photocatalytic tetracycline degradation and CO2 reduction. Ceram. Int. 2019, 45, 24146–24153. [Google Scholar] [CrossRef]
- Su, F.; Li, P.; Huang, J.; Gu, M.; Liu, Z.; Xu, Y. Photocatalytic degradation of organic dye and tetracycline by ternary Ag2O/AgBr–CeO2 photocatalyst under visible-light irradiation. Sci. Rep. 2021, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Zhu, Q.; Huang, J.; Liu, J.; Tan, G.; Sun, C.; Li, T.; Liu, S.; Li, Y.; Wang, H.; et al. Visually resolving the direct Z-scheme heterojunction in CdS@ZnIn2S4 hollow cubes for photocatalytic evolution of H2 and H2O2 from pure water. Appl. Catal. B Environ. 2021, 293, 120213. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Z.; Du, P.; Ning, X.; Wang, Y.; Zhang, D.; Liu, J.; Zhang, S.; Lu, X. Embedding Ultrasmall Au Clusters into the Pores of a Covalent Organic Framework for Enhanced Photostability and Photocatalytic Performance. Angew. Chem. Int. Ed. 2020, 59, 6082–6089. [Google Scholar] [CrossRef]
- Feng, C.; Wu, Z.P.; Huang, K.W.; Ye, J.; Zhang, H. Surface Modification of 2D Photocatalysts for Solar Energy Conversion. Adv. Mater. 2022, 34, e2200180. [Google Scholar] [CrossRef]
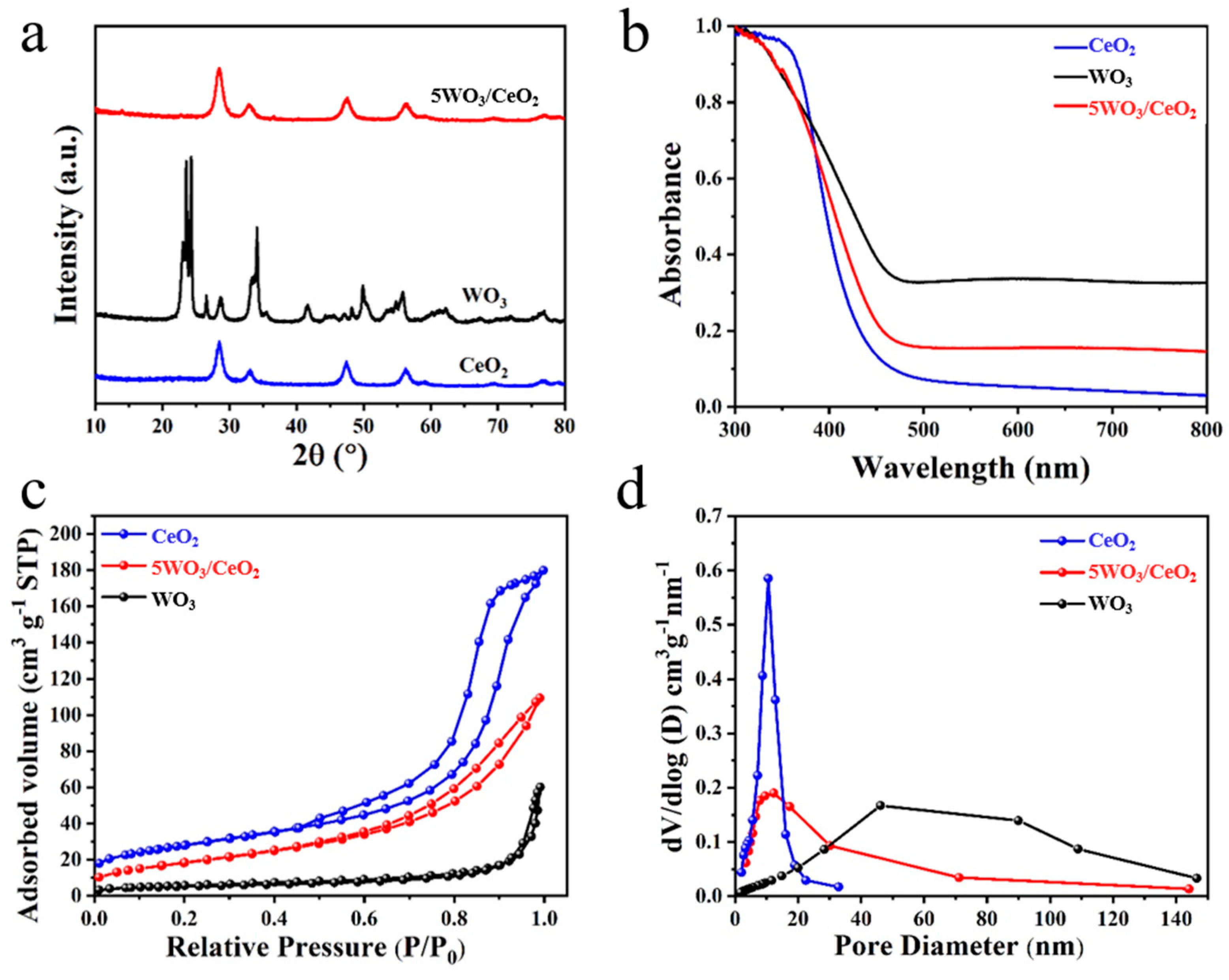

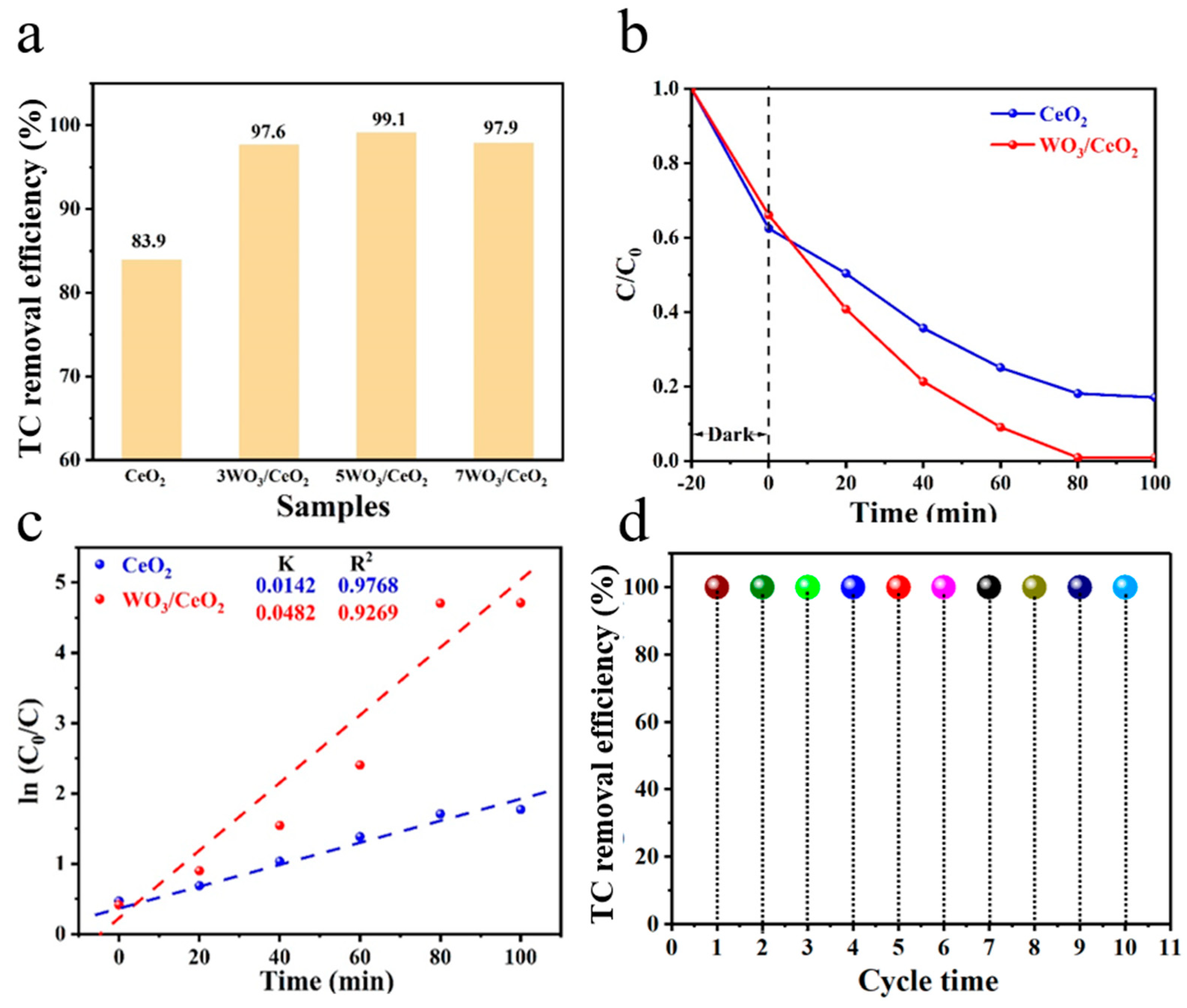

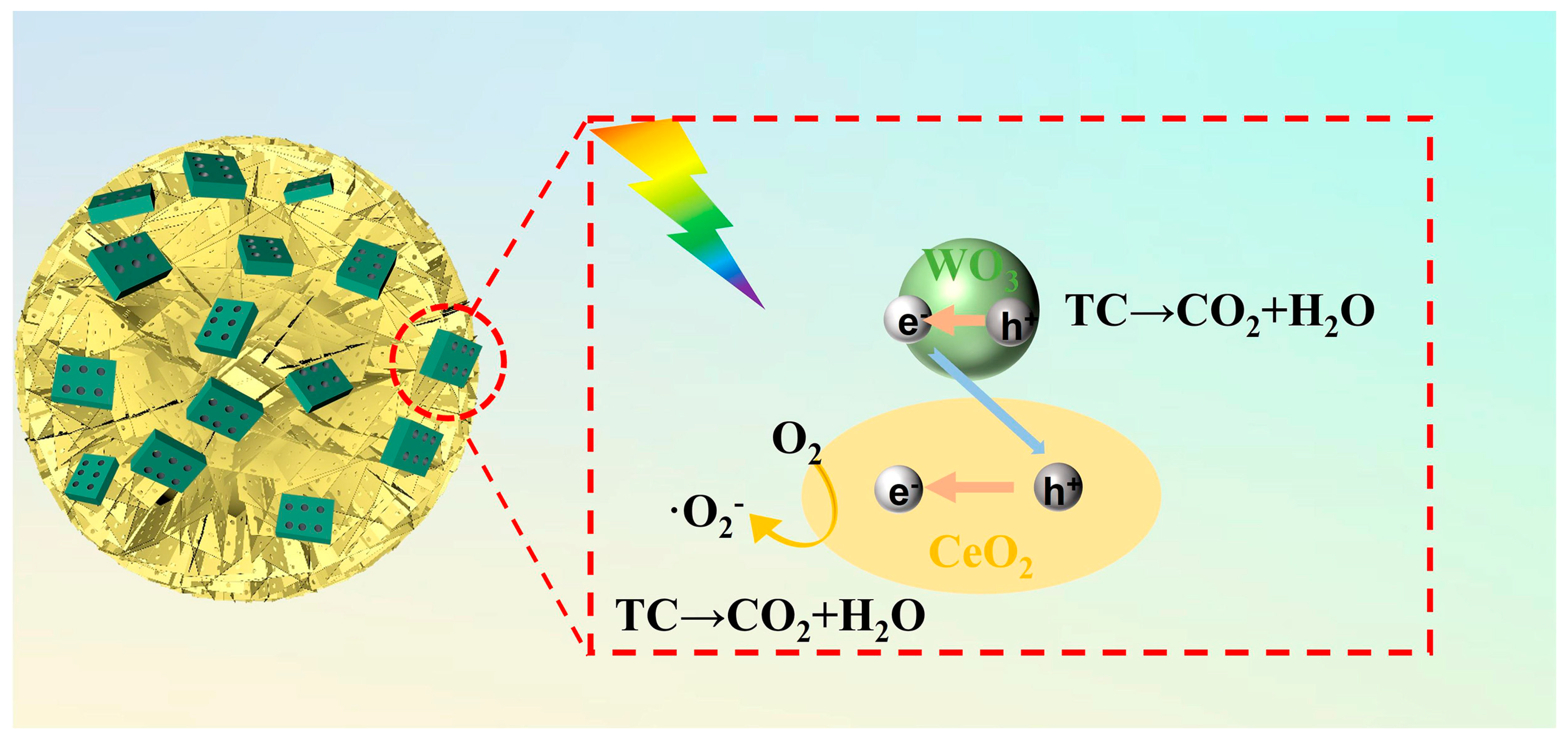
| Catalyst | D (nm) | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (nm) |
|---|---|---|---|---|
| CeO2 | 19.3 | 100.3 | 0.21 | 9.3 |
| WO3 | 20.1 | 22.0 | 0.20 | 44.7 |
| Photocatalyst | Time (min) | Removal Efficiency (%) | Number of Cycling Test | Ref. |
|---|---|---|---|---|
| BiOCl@CeO2 | 120 | 90 | 4 | [45] |
| CeO2/carbonate doped Bi2O2CO3 | 90 | 79.5 | 4 | [46] |
| ZnO/CeO2@Halloysite nanotubes | 90 | 94 | 4 | [47] |
| SnO/CeO2 | 140 | 99 | 5 | [48] |
| AgI/CeO2 | 60 | 94.34 | not studied | [49] |
| AgBr/CeO2 | 120 | 77.8 | not studied | [50] |
| CeO2/Ti3C2-MXene | 60 | 80.2 | 4 | [51] |
| Ag2O/AgBr–CeO2 | 60 | 93.68 | 4 | [52] |
| 2D/2D mesoporous WO3/CeO2 | 80 | 99.1 | 10 | this study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Yang, D.; Mou, Y.; Liao, L.; Wang, S.; Guo, L.; Wang, X.; Li, Z.; Zhou, W. Construction of 2D/2D Mesoporous WO3/CeO2 Laminated Heterojunctions for Optimized Photocatalytic Performance. Nanomaterials 2023, 13, 1798. https://doi.org/10.3390/nano13111798
Wang W, Yang D, Mou Y, Liao L, Wang S, Guo L, Wang X, Li Z, Zhou W. Construction of 2D/2D Mesoporous WO3/CeO2 Laminated Heterojunctions for Optimized Photocatalytic Performance. Nanomaterials. 2023; 13(11):1798. https://doi.org/10.3390/nano13111798
Chicago/Turabian StyleWang, Wenjie, Decai Yang, Yifan Mou, Lijun Liao, Shijie Wang, Liping Guo, Xuepeng Wang, Zhenzi Li, and Wei Zhou. 2023. "Construction of 2D/2D Mesoporous WO3/CeO2 Laminated Heterojunctions for Optimized Photocatalytic Performance" Nanomaterials 13, no. 11: 1798. https://doi.org/10.3390/nano13111798
APA StyleWang, W., Yang, D., Mou, Y., Liao, L., Wang, S., Guo, L., Wang, X., Li, Z., & Zhou, W. (2023). Construction of 2D/2D Mesoporous WO3/CeO2 Laminated Heterojunctions for Optimized Photocatalytic Performance. Nanomaterials, 13(11), 1798. https://doi.org/10.3390/nano13111798








