CoNi Alloys Encapsulated in N-Doped Carbon Nanotubes for Stabilizing Oxygen Electrocatalysis in Zinc–Air Battery
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of NiCo-MOF
2.2. Synthesis of Co-MOF and Ni-MOF
2.3. Preparation of NiCo@NCNTs/HN, NiCo/HN, Co@NCNTs/HN, and Ni@NCNTs/HN
3. Results
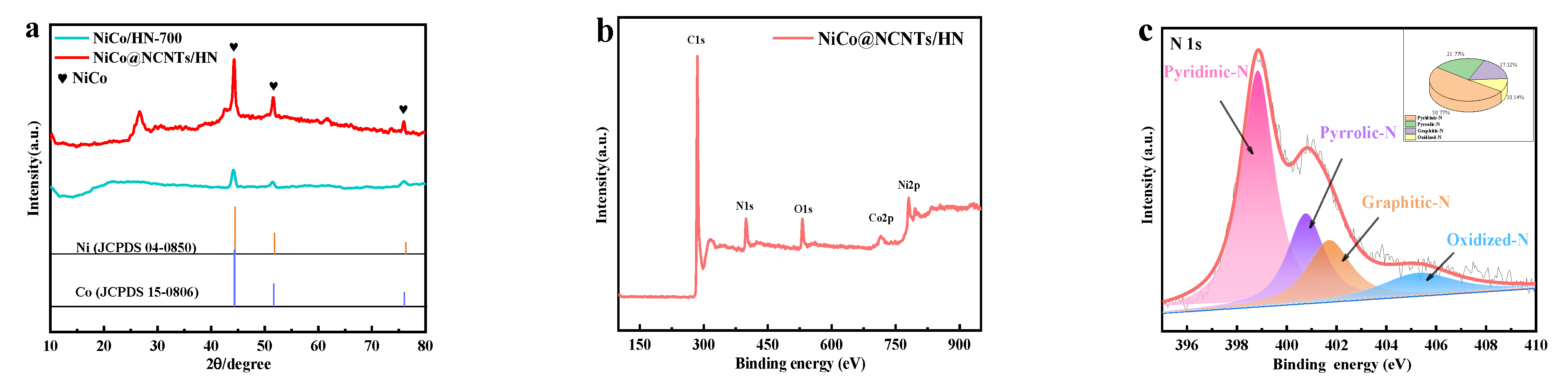
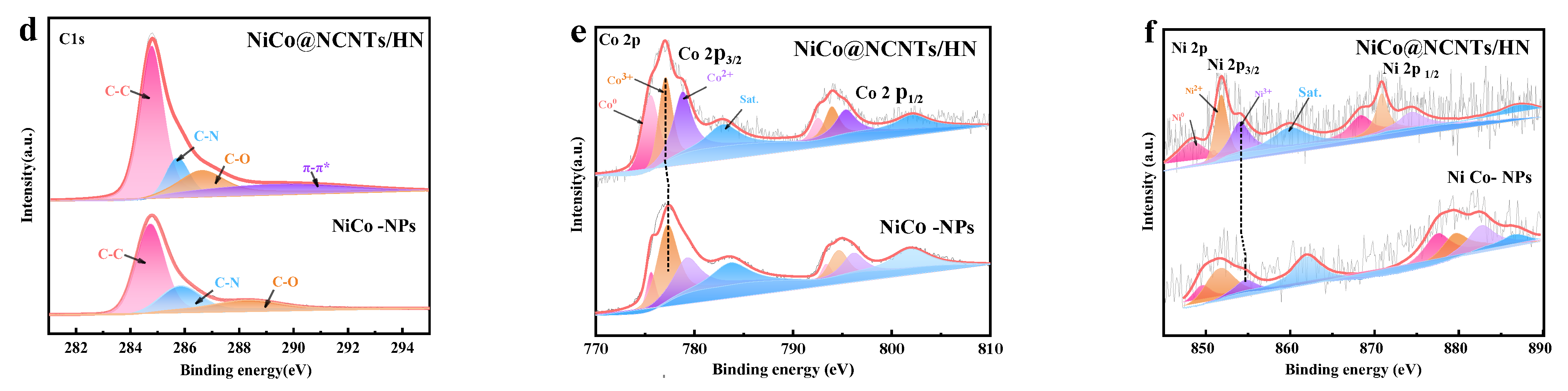
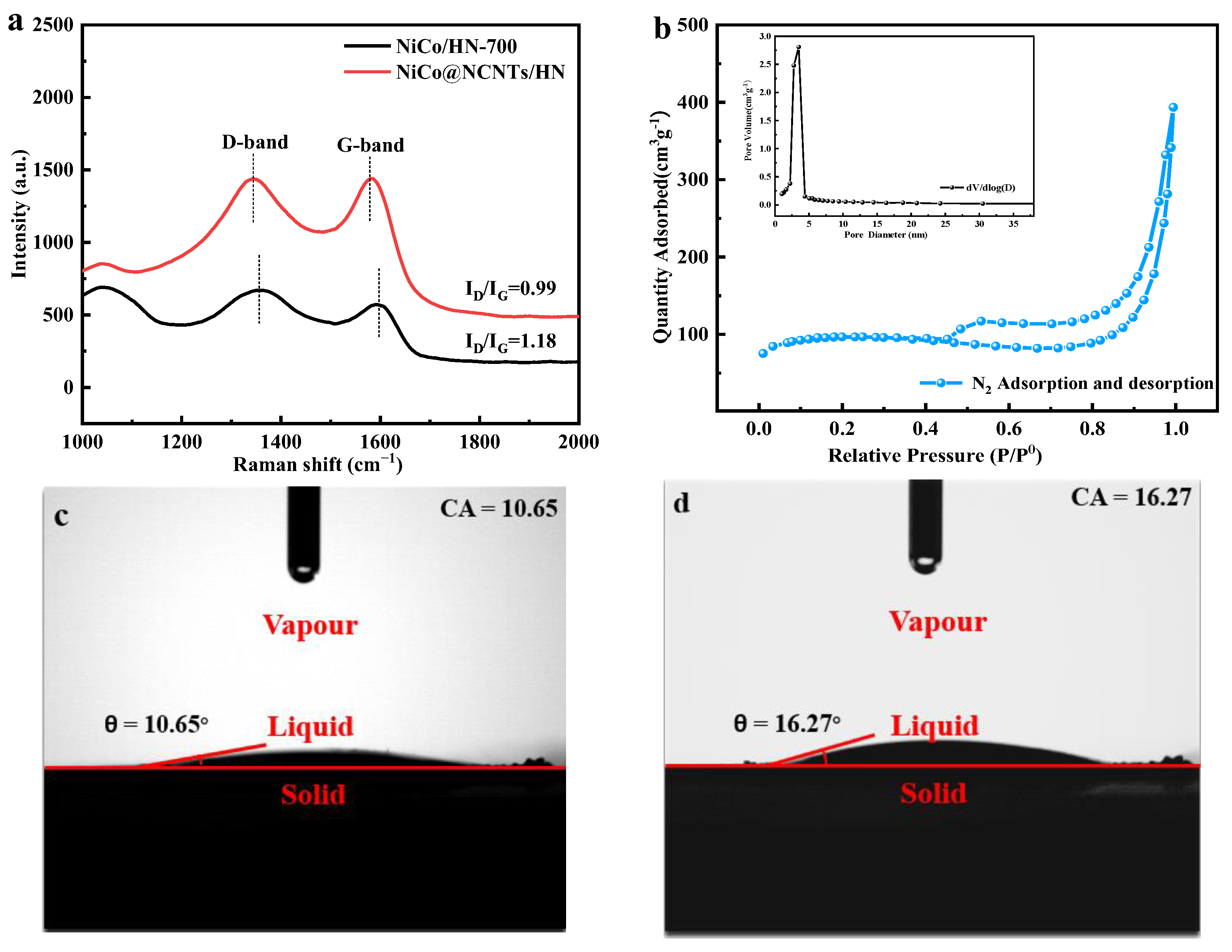
3.1. Structural and Compositional Analyses
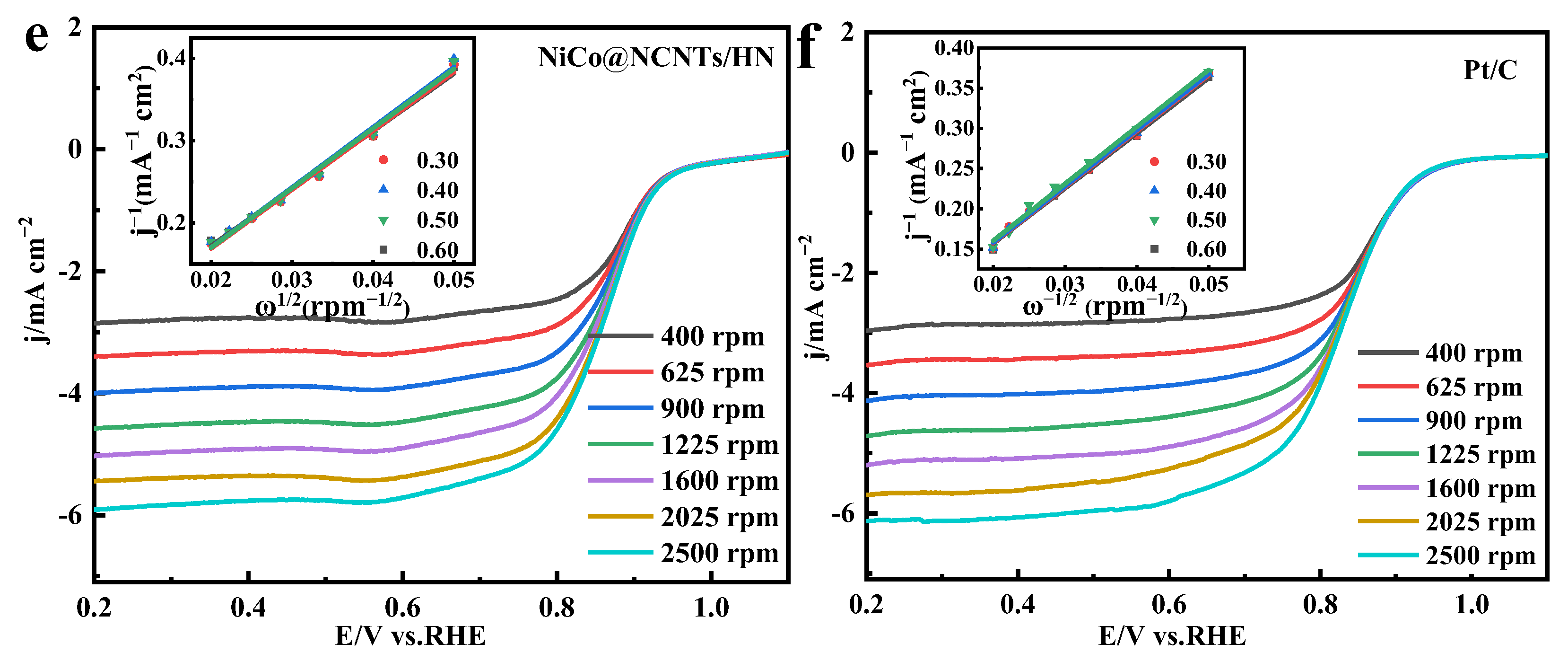
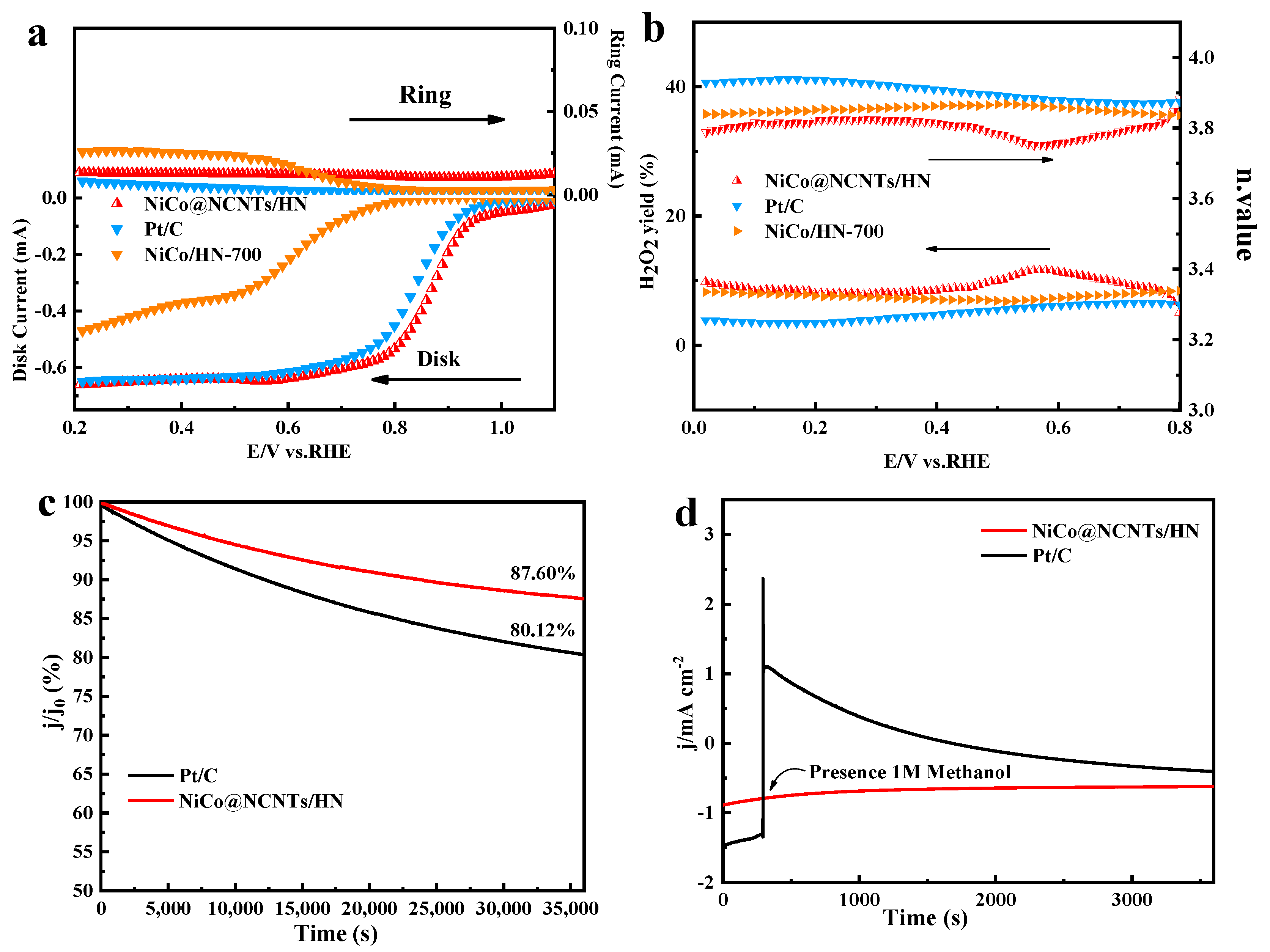
3.2. Electrocatalytic Activities of NiCo@NCNTs/HN Catalyst for ORR
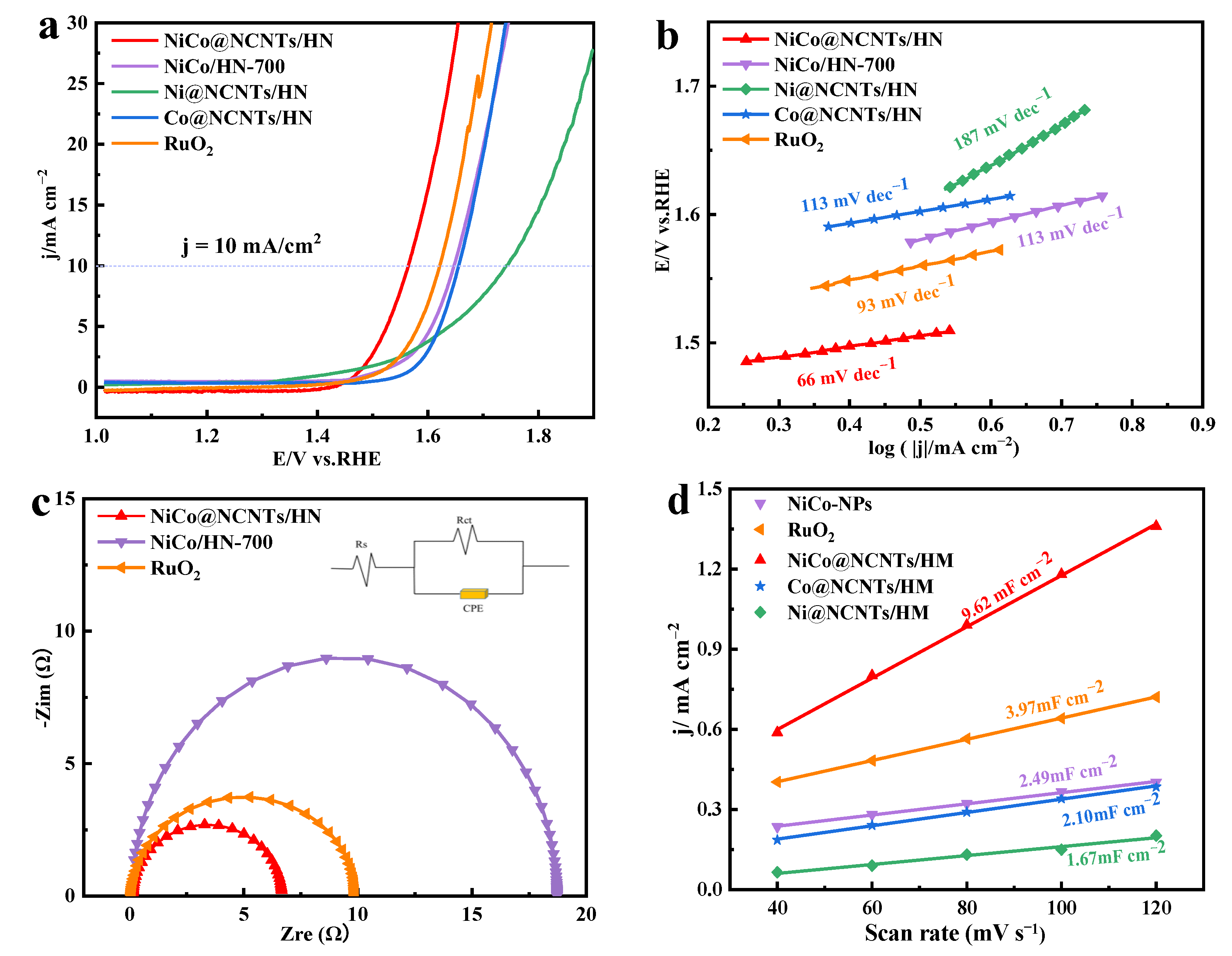
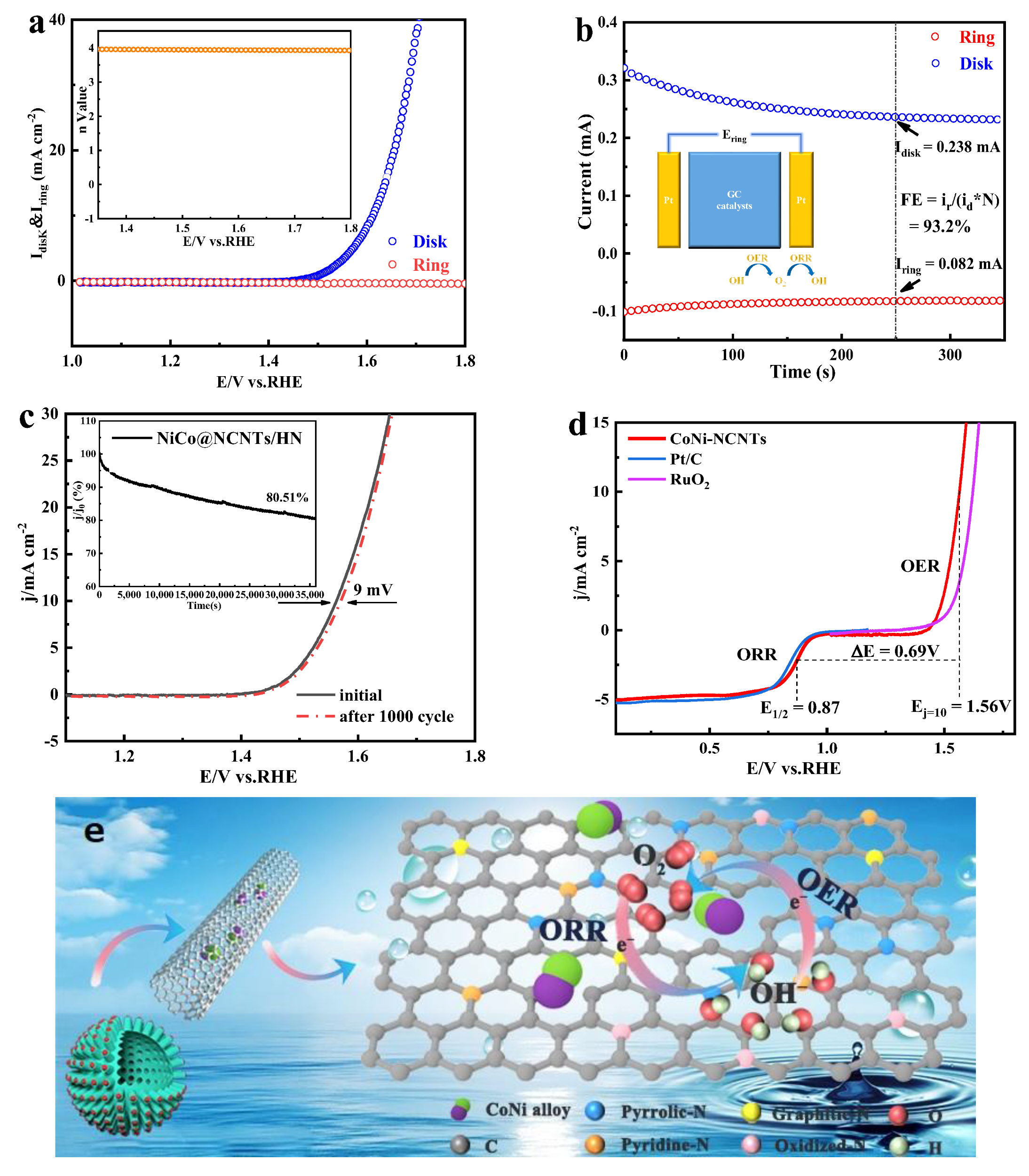
3.3. OER Performance on NiCo@NCNTs/HN Catalyst
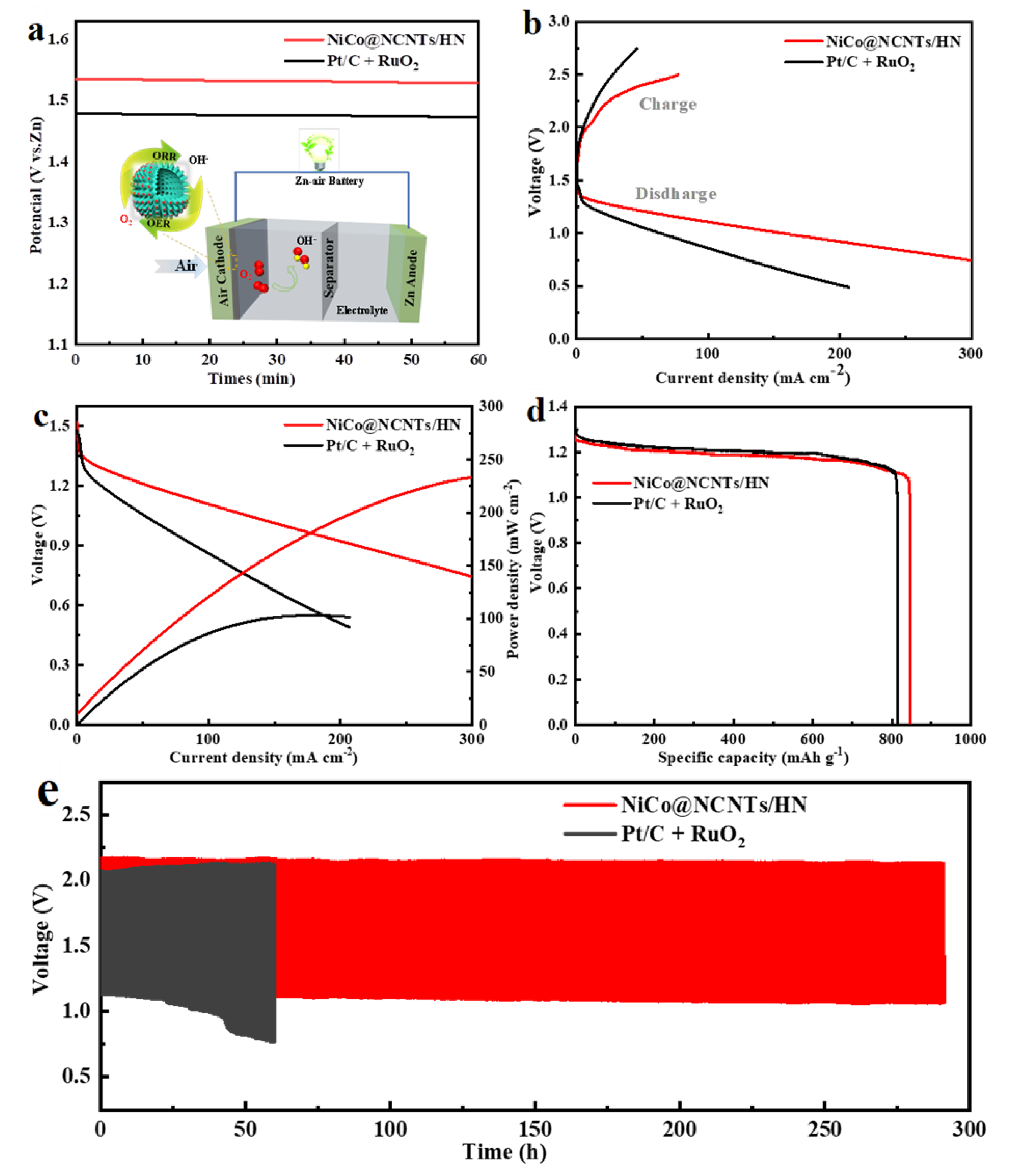
3.4. Application of NiCo@NCNTs/HN Catalyst in ZAB
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiao, M.; Wang, Y.; Wågberg, T.; Mamat, X.; Hu, X.; Zou, G.; Hu, G. Ni–Co bimetallic coordination effect for long lifetime rechargeable Zn–air battery. J. Energy Chem. 2020, 47, 146–154. [Google Scholar] [CrossRef]
- Li, X.; You, S.; Du, J.; Dai, Y.; Chen, H.; Cai, Z.; Ren, N.; Zou, J. ZIF-67-derived Co3O4@carbon protected by oxygen-buffering CeO2 as an efficient catalyst for boosting oxygen reduction/evolution reactions. J. Mater. Chem. A 2019, 7, 25853–25864. [Google Scholar] [CrossRef]
- Minakshi, M.; Sharma, N.; Ralph, D.; Appadoo, D.; Nallathamby, K. Synthesis and Characterization of Li(Co0.5Ni0.5)PO4 Cathode for Li-Ion Aqueous Battery Applications. Electrochem. Solid-State Lett. 2011, 14, A86–A89. [Google Scholar] [CrossRef]
- Guo, B.; Ju, Q.; Ma, R.; Li, Z.; Liu, Q.; Ai, F.; Yang, M.; Kaskel, S.; Luo, J.; Zhang, T.; et al. Mechanochemical synthesis of multi-site electrocatalysts as bifunctional zinc–air battery electrodes. J. Mater. Chem. A 2019, 7, 19355–19363. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; You, S.; Li, X.; Zhang, Y.; Cai, Z.; Liu, M.; Ren, N.; Zou, J. Fe3C/CoFe2O4 nanoparticles wrapped in one-dimensional MIL-53(Fe)-derived carbon nanofibers as efficient dual-function oxygen catalysts. Chem. Eng. J. 2021, 424, 130460. [Google Scholar] [CrossRef]
- Wang, Z.; Ang, J.; Liu, J.; Ma, X.; Kong, J.; Zhang, Y.; Yan, T.; Lu, X. FeNi alloys encapsulated in N-doped CNTs-tangled porous carbon fibers as highly efficient and durable bifunctional oxygen electrocatalyst for rechargeable zinc-air battery. Appl. Catal. B Environ. 2020, 263, 118344. [Google Scholar] [CrossRef]
- Zheng, X.; Cao, X.; Zeng, K.; Yan, J.; Sun, Z.; Rummeli, M.; Yang, R. A Self-Jet Vapor-Phase Growth of 3D FeNi@NCNT Clusters as Efficient Oxygen Electrocatalysts for Zinc-Air Batteries. Small 2021, 17, 2006183. [Google Scholar] [CrossRef]
- Xie, X.; Peng, H.; Sun, K.; Lei, X.; Zhu, R.; Zhang, Z.; Ma, G.; Lei, Z. Rational construction of FeNi3/N doped carbon nanotubes for high-performance and reversible oxygen catalysis reaction for rechargeable Zn-air battery. Chem. Eng. J. 2023, 452, 139253. [Google Scholar] [CrossRef]
- Go, Y.; Min, K.; An, H.; Kim, K.; Eun Shim, S.; Baeck, S.-H. Oxygen-vacancy-rich CoFe/CoFe2O4 embedded in N-doped hollow carbon spheres as a highly efficient bifunctional electrocatalyst for Zn–air batteries. Chem. Eng. J. 2022, 448, 137665. [Google Scholar] [CrossRef]
- Fan, X.; Du, X.; Pang, Q.; Zhang, S.; Liu, Z.; Yue, X. In Situ Construction of Bifunctional N-Doped Carbon-Anchored Co Nanoparticles for OER and ORR. ACS Appl. Mater. Interfaces 2022, 14, 8549–8556. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, C.H.; Zheng, H.; Li, R.; Zhou, K. Oxygen-Rich Cobalt–Nitrogen–Carbon Porous Nanosheets for Bifunctional Oxygen Electrocatalysis. Adv. Funct. Mater. 2022, 32, 23. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, J.; Hu, Y.; Jin, Z.; Hu, K.; Reddy, K.; Yuan, Q.; Lin, X.; Qiu, H. Theoretically Revealed and Experimentally Demonstrated Synergistic Electronic Interaction of CoFe Dual-Metal Sites on N-doped Carbon for Boosting Both Oxygen Reduction and Evolution Reactions. Nano Lett. 2022, 22, 3392–3399. [Google Scholar] [CrossRef]
- Guo, Y.; Tang, J.; Henzie, J.; Jiang, B.; Qian, H.; Wang, Z.; Tan, H.; Bando, Y.; Yamauchi, Y. Assembly of hollow mesoporous nanoarchitectures composed of ultrafine Mo2C nanoparticles on N-doped carbon nanosheets for efficient electrocatalytic reduction of oxygen. Mater. Horiz. 2017, 4, 1171–1177. [Google Scholar] [CrossRef]
- Singh, H.; Marley-Hines, M.; Chakravarty, S.; Nath, M. Multi-walled carbon nanotube supported manganese selenide as a highly active bifunctional OER and ORR electrocatalyst. J. Mater. Chem. A 2022, 10, 6772–6784. [Google Scholar] [CrossRef]
- Xu, X.; Xie, J.; Liu, B.; Wang, R.; Liu, M.; Zhang, J.; Liu, J.; Cai, Z.; Zou, J. PBA-derived FeCo alloy with core-shell structure embedded in 2D N-doped ultrathin carbon sheets as a bifunctional catalyst for rechargeable Zn-air batteries. Appl. Catal. B Environ. 2022, 316, 121687. [Google Scholar] [CrossRef]
- Ma, X.; Chai, H.; Cao, Y.; Xu, J.; Wang, Y.; Dong, H.; Jia, D.; Zhou, W. An effective bifunctional electrocatalysts: Controlled growth of CoFe alloy nanoparticles supported on N-doped carbon nanotubes. J. Colloid Interface Sci. 2018, 514, 656–663. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, H.; Zhu, J.; Zhang, H. Indium-based bimetallic clusters anchored onto silicon-doped graphene as efficient multifunctional electrocatalysts for ORR, OER, and HER. Chem. Eng. J. 2023, 451, 138998. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Li, Z.; Zhao, N.; Xie, Y.; Du, Y.; Xuan, J.; Xiong, D.; Zhou, J.; Cai, L.; et al. CoNi nanoalloy-Co-N4 composite active sites embedded in hierarchical porous carbon as bi-functional catalysts for flexible Zn-air battery. Nano Energy 2022, 99, 107325. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, W.; Jiang, Z.; Tian, X.; Wang, G.; Chen, G.; Jiang, Z. NiFe nanoparticles supported on N-doped graphene hollow spheres entangled with self-grown N-doped carbon nanotubes for liquid electrolyte/flexible all-solid-state rechargeable zinc–air batteries. J. Mater. Chem. A 2022, 10, 12616–12631. [Google Scholar] [CrossRef]
- Kundu, A.; Samanta, A.; Raj, C. Hierarchical Hollow MOF-Derived Bamboo-like N-doped Carbon Nanotube-Encapsulated Co0.25Ni0.75 Alloy: An Efficient Bifunctional Oxygen Electrocatalyst for Zinc–Air Battery. ACS Appl. Mater. Interfaces 2021, 13, 30486–30496. [Google Scholar] [CrossRef]
- Wang, H.; Su, S.; Yu, T.; Meng, C.; Zhou, H.; Zhao, W.; Yan, S.; Bian, T.; Yuan, A. FeNi/NiFe2O4 hybrids confined in N-doped carbon sponge derived from Hofmann-type MOFs for oxygen electrocatalysis. Appl. Surf. Sci. 2022, 596, 153522. [Google Scholar] [CrossRef]
- Chae, S.; Muthurasu, A.; Kim, T.; Kim, J.; Khil, M.; Lee, M.; Kim, H.; Lee, J.; Kim, H. Templated fabrication of perfectly aligned metal-organic framework-supported iron-doped copper-cobalt selenide nanostructure on hollow carbon nanofibers for an efficient trifunctional electrode material. Appl. Catal. B Environ. 2021, 293, 120209. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Lin, R.; Tu, G.; Wang, J.; Li, Z. MOF-derived hollow β-FeOOH polyhedra anchored with α-Ni(OH)2 nanosheets as efficient electrocatalysts for oxygen evolution. Electrochim. Acta 2019, 301, 258–266. [Google Scholar] [CrossRef]
- Ding, J.; Sun, Q.; Zhong, L.; Wang, X.; Chai, L.; Li, Q.; Li, T.-T.; Hu, Y.; Qian, J.; Huang, S. Thermal conversion of hollow nickel-organic framework into bimetallic FeNi3 alloy embedded in carbon materials as efficient oer electrocatalyst. Electrochim. Acta 2020, 354, 136716. [Google Scholar] [CrossRef]
- Zhang, S.; Guan, B.Y.; Lou, X. Co–Fe Alloy/N-Doped Carbon Hollow Spheres Derived from Dual Metal–Organic Frameworks for Enhanced Electrocatalytic Oxygen Reduction. Small 2019, 15, 1805324. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, M.; Zhang, Y.; Jin, Z.; Peng, Z.; Liu, C.; Chen, S.; Ge, J.; Xing, W. Metal–Organic Framework-Induced Synthesis of Ultrasmall Encased NiFe Nanoparticles Coupling with Graphene as an Efficient Oxygen Electrode for a Rechargeable Zn–Air Battery. ACS Catal. 2016, 6, 6335–6342. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Wang, R.; Hong, M. Structural Evolution from Metal–Organic Framework to Hybrids of Nitrogen-Doped Porous Carbon and Carbon Nanotubes for Enhanced Oxygen Reduction Activity. Chem. Mater. 2015, 27, 7610–7618. [Google Scholar] [CrossRef]
- Wu, L.; Wang, Z.; Long, Y.; Li, J.; Liu, Y.; Wang, Q.; Wang, X.; Song, S.; Liu, X.; Zhang, H. Multishelled NixCo3-xO4 Hollow Microspheres Derived from Bimetal-Organic Frameworks as Anode Materials for High-Performance Lithium-Ion Batteries. Small 2017, 13, 1604270. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Z.; Han, W.; Zhang, Q.; Bai, Z.; Chen, Z.; Li, G.; Wang, X. Bimetallic CoNi Alloy Nanoparticles Embedded in Pomegranate-like Nitrogen-Doped Carbon Spheres for Electrocatalytic Oxygen Reduction and Evolution. ACS Appl. Nano Mater. 2020, 3, 1354–1362. [Google Scholar] [CrossRef]
- Wan, W.; Liu, X.; Li, H.; Peng, X.; Xi, D.; Luo, J. 3D carbon framework-supported CoNi nanoparticles as bifunctional oxygen electrocatalyst for rechargeable Zn-air batteries. Appl. Catal. B Environ. 2019, 240, 193–200. [Google Scholar] [CrossRef]
- Nam, G.; Son, Y.; Park, S.; Jeon, W.; Jang, H.; Park, J.; Chae, S.; Yoo, Y.; Ryu, J.; Kim, M.; et al. A Ternary Ni(46) Co(40) Fe(14) Nanoalloy-Based Oxygen Electrocatalyst for Highly Efficient Rechargeable Zinc-Air Batteries. Adv. Mater. 2018, 30, 1803372. [Google Scholar] [CrossRef]
- Chen, D.; Sun, Q.; Han, C.; Guo, Y.; Huang, Q.; Goddard, W.; Qian, J. Enhanced oxygen evolution catalyzed by in situ formed Fe-doped Ni oxyhydroxides in carbon nanotubes. J. Mater. Chem. A 2022, 10, 16007–16015. [Google Scholar] [CrossRef]
- Tang, Y.; Lei, Y.; Li, G.; Fu, T.; Xiang, Y.; Sha, J.; Yang, H.; Yu, P.; Si, Y.; Guo, C. Positive regulation of active sites for oxygen evolution reactions by encapsulating NiFe2O4 nanoparticles in N-doped carbon nanotubes in situ to construct efficient bifunctional oxygen catalysts for rechargeable Zn–air batteries. J. Mater. Chem. A 2022, 10, 5305–5316. [Google Scholar] [CrossRef]
- Mao, J.; Liu, P.; Li, J.; Yan, J.; Ye, S.; Song, W. Accelerated intermediate conversion through nickel doping into mesoporous Co-N/C nanopolyhedron for efficient ORR. J. Energy Chem. 2022, 73, 240–247. [Google Scholar] [CrossRef]
- Qiang, F.; Feng, J.; Wang, H.; Yu, J.; Shi, J.; Huang, M.; Shi, Z.; Liu, S.; Li, P.; Dong, L. Oxygen Engineering Enables N-Doped Porous Carbon Nanofibers as Oxygen Reduction/Evolution Reaction Electrocatalysts for Flexible Zinc–Air Batteries. ACS Catal. 2022, 12, 4002–4015. [Google Scholar] [CrossRef]
- Sheng, K.; Yi, Q.; Chen, A.; Wang, Y.; Yan, Y.; Nie, H.; Zhou, X. CoNi Nanoparticles Supported on N-Doped Bifunctional Hollow Carbon Composites as High-Performance ORR/OER Catalysts for Rechargeable Zn-Air Batteries. ACS Appl. Mater. Interfaces 2021, 13, 45394–45405. [Google Scholar] [CrossRef]
- Li, J.; Kang, Y.; Wei, W.; Li, X.; Lei, Z.; Liu, P. Well-dispersed ultrafine CoFe nanoalloy decorated N-doped hollow carbon microspheres for rechargeable/flexible Zn-air batteries. Chem. Eng. J. 2021, 407, 127961. [Google Scholar] [CrossRef]
- Li, J.; Qian, J.; Chen, X.; Zeng, X.; Li, L.; Ouyang, B.; Kan, E.; Zhang, W. Three-dimensional hierarchical graphitic carbon encapsulated CoNi alloy/N-doped CNTs/carbon nanofibers as an efficient multifunctional electrocatalyst for high-performance microbial fuel cells. Compos. Part B Eng. 2022, 231, 109573. [Google Scholar] [CrossRef]
- Ha, Y.; Shi, L.; Yan, X.; Chen, Z.; Li, Y.; Xu, W.; Wu, R. Multifunctional Electrocatalysis on a Porous N-Doped NiCo(2)O(4)@C Nanonetwork. ACS Appl. Mater. Interfaces 2019, 11, 45546–45553. [Google Scholar] [CrossRef]
- Fu, Y.; Yu, H.; Jiang, C.; Zhang, T.; Zhan, R.; Li, X.; Li, J.; Tian, J.; Yang, R. NiCo Alloy Nanoparticles Decorated on N-Doped Carbon Nanofibers as Highly Active and Durable Oxygen Electrocatalyst. Adv. Funct. Mater. 2018, 28, 1705094. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Y.; Zhang, Y.; Xia, S.; Yu, J.; Ding, B. Direct Magnetic Reinforcement of Electrocatalytic ORR/OER with Electromagnetic Induction of Magnetic Catalysts. Adv. Mater. 2021, 33, 2007525. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zai, S.; Zhou, Y.; Du, L.; Jiang, Q. Fe3C-Co Nanoparticles Encapsulated in a Hierarchical Structure of N-Doped Carbon as a Multifunctional Electrocatalyst for ORR, OER, and HER. Adv. Funct. Mater. 2019, 29, 1901949. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Yu, P.; Tian, C.; Sun, F.; Ma, J.; Li, W.; Fu, H. A Stable Bifunctional Catalyst for Rechargeable Zinc-Air Batteries: Iron-Cobalt Nanoparticles Embedded in a Nitrogen-Doped 3D Carbon Matrix. Angew. Chem. Int. Ed. Engl. 2018, 57, 16166–16170. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ling, X.; Wang, Y.; Ma, T.; Zhong, C.; Hu, W.; Deng, Y. Generation of Nanoparticle, Atomic-Cluster, and Single-Atom Cobalt Catalysts from Zeolitic Imidazole Frameworks by Spatial Isolation and Their Use in Zinc-Air Batteries. Angew. Chem. Int. Ed. Engl. 2019, 58, 5359–5364. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Wang, Y.; Ye, T.; Wu, J.; Chen, Z.; Li, J.; Lei, Y.; Wang, D.; Li, Y. Engineering Dual Single-Atom Sites on 2D Ultrathin N-doped Carbon Nanosheets Attaining Ultra-Low-Temperature Zinc-Air Battery. Angew. Chem. Int. Ed. Engl. 2022, 61, 202115219. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, B.; You, S.; Li, Y.; Zhang, Y.; Wang, D.; Tang, B.; Sun, Y.; Zou, J. Three-dimensional Ni3Se4 flowers integrated with ultrathin carbon layer with strong electronic interactions for boosting oxygen reduction/evolution reactions. Chem. Eng. J. 2022, 430, 132720. [Google Scholar] [CrossRef]
- Lee, S.; Theerthagiri, J.; Nithyadharseni, P.; Arunachalam, P.; Balaji, D.; Madan Kumar, A.; Madhavan, J.; Mittal, V.; Choi, M. Heteroatom-doped graphene-based materials for sustainable energy applications: A review. Renew. Sustain. Energy Rev. 2021, 143, 110849. [Google Scholar] [CrossRef]
- Du, C.; Huang, H.; Wu, Y.; Wu, S.; Song, W. Ultra-efficient electrocatalytic hydrogen evolution at one-step carbonization generated molybdenum carbide nanosheets/N-doped carbon. Nanoscale 2016, 8, 16251–16258. [Google Scholar] [CrossRef]
- Xie, X.; Shang, L.; Shi, R.; Waterhouse, G.; Zhao, J.; Zhang, T. Tubular assemblies of N-doped carbon nanotubes loaded with NiFe alloy nanoparticles as efficient bifunctional catalysts for rechargeable zinc-air batteries. Nanoscale 2020, 12, 13129–13136. [Google Scholar] [CrossRef]
- Liu, J.; He, T.; Wang, Q.; Zhou, Z.; Zhang, Y.; Wu, H.; Li, Q.; Zheng, J.; Sun, Z.; Lei, Y.; et al. Confining ultrasmall bimetallic alloys in porous N–carbon for use as scalable and sustainable electrocatalysts for rechargeable Zn–air batteries. J. Mater. Chem. A 2019, 7, 12451–12456. [Google Scholar] [CrossRef]
- Zhao, J.; He, Y.; Chen, Z.; Zheng, X.; Han, X.; Rao, D.; Zhong, C.; Hu, W.; Deng, Y. Engineering the Surface Metal Active Sites of Nickel Cobalt Oxide Nanoplates toward Enhanced Oxygen Electrocatalysis for Zn-Air Battery. ACS Appl. Mater. Interfaces 2019, 11, 4915–4921. [Google Scholar] [CrossRef]
- Xiao, J.; Kuang, Q.; Yang, S.; Xiao, F.; Wang, S.; Guo, L. Surface structure dependent electrocatalytic activity of Co3O4 anchored on graphene sheets toward oxygen reduction reaction. Sci. Rep. 2013, 3, 2300. [Google Scholar] [CrossRef]
- Liu, S.; Hu, L.; Xu, X.; Al-Ghamdi, A.; Fang, X. Nickel Cobaltite Nanostructures for Photoelectric and Catalytic Applications. Small 2015, 11, 4267–4283. [Google Scholar] [CrossRef]
- Huang, S.; Geng, Y.; Xia, J.; Chen, D.; Lu, J. NiCo Alloy Nanoparticles on a N/C Dual-Doped Matrix as a Cathode Catalyst for Improved Microbial Fuel Cell Performance. Small 2022, 18, 2106355. [Google Scholar] [CrossRef]
- Hou, Y.; Cui, S.; Wen, Z.; Guo, X.; Feng, X.; Chen, J. Strongly Coupled 3D Hybrids of N-doped Porous Carbon Nanosheet/CoNi Alloy-Encapsulated Carbon Nanotubes for Enhanced Electrocatalysis. Small 2015, 11, 5940–5948. [Google Scholar] [CrossRef]
- Singh, K.; Razmjooei, F.; Yu, J. Active sites and factors influencing them for efficient oxygen reduction reaction in metal-N coordinated pyrolyzed and non-pyrolyzed catalysts: A review. J. Mater. Chem. A 2017, 5, 20095–20119. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Z.; Lei, Z.; Yu, L.; Wu, W.; Wang, Z.; Cheng, N. Electronic modulation optimizes OH* intermediate adsorption on Co-Nx-C sites via coupling CoNi alloy in hollow carbon nanopolyhedron toward efficient reversible oxygen electrocatalysis. Appl. Catal. B Environ. 2022, 304, 121006. [Google Scholar] [CrossRef]
- Xie, D.; Yu, D.; Hao, Y.; Han, S.; Li, G.; Wu, X.; Hu, F.; Li, L.; Chen, H.Y.; Liao, Y.F.; et al. Dual-Active Sites Engineering of N-Doped Hollow Carbon Nanocubes Confining Bimetal Alloys as Bifunctional Oxygen Electrocatalysts for Flexible Metal-Air Batteries. Small 2021, 17, 2007239. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, J.; Peng, Y.; Jung, C.; Fisher, A.; Wang, X. Design of Efficient Bifunctional Oxygen Reduction/Evolution Electrocatalyst: Recent Advances and Perspectives. Adv. Energy Mater. 2017, 7, 1700544. [Google Scholar] [CrossRef]
- Ma, J.; Liu, B.; Wang, R.; Sun, Z.; Zhang, Y.; Sun, Y.; Cai, Z.; Li, Y.; Zou, J. Single-Cu-atoms anchored on 3D macro-porous carbon matrix as efficient catalyst for oxygen reduction and Pt co-catalyst for methanol oxidation. Chin. Chem. Lett. 2022, 33, 2585–2589. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H.; Huang, C.; Ye, P.; Luo, X.; Ning, J.; Zhong, Y.; Hu, Y. Trifunctional electrocatalyst of N-doped graphitic carbon nanosheets encapsulated with CoFe alloy nanocrystals: The key roles of bimetal components and high-content graphitic-N. Appl. Catal. B Environ. 2021, 298, 120512. [Google Scholar] [CrossRef]
- Feng, X.; Jiao, Q.; Chen, W.; Dang, Y.; Dai, Z.; Suib, S.L.; Zhang, J.; Zhao, Y.; Li, H.; Feng, C. Cactus-like NiCo2S4@NiFe LDH hollow spheres as an effective oxygen bifunctional electrocatalyst in alkaline solution. Appl. Catal. B Environ. 2021, 286, 119869. [Google Scholar] [CrossRef]
- Li, W.; Lu, B.; Gan, L.; Tian, N.; Zhang, P.; Yan, W.; Chen, W.; Chen, Y.; Zhou, Z.; Sun, S. High activity and durability of carbon-supported core-shell PtP @Pt/C catalyst for oxygen reduction reaction. Chin. J. Catal. 2021, 42, 2173–2180. [Google Scholar] [CrossRef]
- Li, S.; Chen, W.; Pan, H.; Cao, Y.; Jiang, Z.; Tian, X.; Hao, X.; Maiyalagan, T.; Jiang, Z. FeCo Alloy Nanoparticles Coated by an Ultrathin N-Doped Carbon Layer and Encapsulated in Carbon Nanotubes as a Highly Efficient Bifunctional Air Electrode for Rechargeable Zn-Air Batteries. ACS Sustain. Chem. Eng. 2019, 7, 8530–8541. [Google Scholar] [CrossRef]
- Sikdar, N.; Konkena, B.; Masa, J.; Schuhmann, W.; Maji, T.K. Co3O4@Co/NCNT Nanostructure Derived from a Dicyanamide-Based Metal-Organic Framework as an Efficient Bi-functional Electrocatalyst for Oxygen Reduction and Evolution Reactions. Chemistry 2017, 23, 18049–18056. [Google Scholar] [CrossRef]
- Liu, L.; Yan, F.; Li, K.; Zhu, C.; Xie, Y.; Zhang, X.; Chen, Y. Ultrasmall FeNi3N particles with an exposed active (110) surface anchored on nitrogen-doped graphene for multifunctional electrocatalysts. J. Mater. Chem. A 2019, 7, 1083–1091. [Google Scholar] [CrossRef]
- Chhetri, K.; Muthurasu, A.; Dahal, B.; Kim, T.; Mukhiya, T.; Chae, S.H.; Ko, T.H.; Choi, Y.C.; Kim, H.Y. Engineering the abundant heterointerfaces of integrated bimetallic sulfide-coupled 2D MOF-derived mesoporous CoS2 nanoarray hybrids for electrocatalytic water splitting. Mater. Today Nano 2022, 17, 2588–8420. [Google Scholar] [CrossRef]
- Du, P.; Hu, K.; Lyu, J.; Li, H.; Lin, X.; Xie, G.; Liu, X.; Ito, Y.; Qiu, H. Anchoring Mo single atoms/clusters and N on edge-rich nanoporous holey graphene as bifunctional air electrode in Zn−air batteries. Appl. Catal. B 2020, 276, 119172. [Google Scholar] [CrossRef]
- Muthurasu, A.; Sampath, P.; Ko, T.H.; Lohani, P.C.; Pathak, I.; Acharya, D.; Chhetri, K.; Kim, D.H.; Kim, H.Y. Partial selenium surface modulation of metal organic framework assisted cobalt sulfide hollow spheres for high performance bifunctional oxygen electrocatalysis and rechargeable zinc-air batteries. Appl. Catal. B Environ. 2023, 330, 122523. [Google Scholar] [CrossRef]
- Yin, J.; Li, Y.; Lv, F.; Fan, Q.; Zhao, Y.; Zhang, Q.; Wang, W.; Cheng, F.; Xi, P.; Guo, S. NiO/CoN Porous Nanowires as Efficient Bifunctional Catalysts for Zn-Air Batteries. ACS Nano 2017, 11, 2275–2283. [Google Scholar] [CrossRef]
- Li, M.; Li, H.; Jiang, X.; Jiang, M.; Zhan, X.; Fu, G.; Lee, J.-M.; Tang, Y. Gd-induced electronic structure engineering of a NiFe-layered double hydroxide for efficient oxygen evolution. J. Mater. Chem. A 2021, 9, 2999–3006. [Google Scholar] [CrossRef]
- Wang, B.; Ye, Y.; Xu, L.; Quan, Y.; Wei, W.; Zhu, W.; Li, H.; Xia, J. Space-Confined Yolk-Shell Construction of Fe3O4 Nanoparticles Inside N-Doped Hollow Mesoporous Carbon Spheres as Bifunctional Electrocatalysts for Long-Term Rechargeable Zinc–Air Batteries. Adv. Funct. Mater. 2020, 30, 2005834. [Google Scholar] [CrossRef]
- Sharma, P.; Minakshi Sundaram, M.; Watcharatharapong, T.; Laird, D.; Euchner, H.; Ahuja, R. Zn Metal Atom Doping on the Surface Plane of One-Dimesional NiMoO4 Nanorods with Improved Redox Chemistry. ACS Appl. Mater. Interfaces 2020, 12, 44815–44829. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, J.; Duan, W.; He, G.; Tang, Z. NiFe Alloyed Nanoparticles Encapsulated in Nitrogen Doped Carbon Nanotubes for Bifunctional Electrocatalysis Toward Rechargeable Zn-Air Batteries. ChemCatChem 2019, 11, 5994–6001. [Google Scholar] [CrossRef]
- Du, J.; You, S.; Li, X.; Tang, B.; Jiang, B.; Yu, Y.; Cai, Z.; Ren, N.; Zou, J. In Situ Crystallization of Active NiOOH/CoOOH Heterostructures with Hydroxide Ion Adsorption Sites on Velutipes-like CoSe/NiSe Nanorods as Catalysts for Oxygen Evolution and Cocatalysts for Methanol Oxidation. ACS Appl. Mater. Interfaces 2020, 12, 686–697. [Google Scholar] [CrossRef]
- Guo, Y.; Yuan, P.; Zhang, J.; Xia, H.; Cheng, F.; Zhou, M.; Li, J.; Qiao, Y.; Mu, S.; Xu, Q. Co2P-CoN double active centers confined in N-doped carbon nanotube: Heterostructural engineering for trifunctional catalysis toward HER, ORR, OER, and Zn-air batteries driven water splitting. Adv. Funct. Mater. 2018, 28, 1805641. [Google Scholar] [CrossRef]
- Jian, H.; Gu, J.; Zheng, X.; Liu, M.; Qiu, X.; Wang, L.; Li, W.; Chen, Z.; Ji, X.; Li, J. Defect-rich and ultrathin N doped carbon nanosheets as advanced trifunctional metal-free electrocatalysts for the ORR, OER and HER. Energy Environ. Sci. 2019, 12, 322–333. [Google Scholar] [CrossRef]
- Anantharaj, S.; Karthik, P.; Kundu, S. Self-assembled IrO2 nanoparticles on a DNA scaffold with enhanced catalytic and oxygen evolution reaction (OER) activities. J. Mater. Chem. A 2015, 3, 24463–24478. [Google Scholar] [CrossRef]
- Li, T.; He, Z.; Liu, X.; Jiang, M.; Liao, Q.; Ding, R.; Liu, S.; Zhao, C.; Guo, W.; Zhang, S.; et al. Interface interaction of Ag-CeO2-Co3O4 facilitate ORR/OER activity for Zn-air battery. Surf. Interfaces 2022, 33, 102270. [Google Scholar] [CrossRef]
- Aulia, S.; Lin, Y.; Chang, L.; Wang, Y.; Lin, M.; Ho, K.; Yeh, M. Oxygen Plasma-Activated NiFe Prussian Blue Analogues Interconnected N-Doped Carbon Nanotubes as a Bifunctional Electrocatalyst for a Rechargeable Zinc–Air Battery. ACS Appl. Energy Mater. 2022, 5, 9801–9810. [Google Scholar] [CrossRef]
- Wang, Y.; Gan, R.; Zhao, S.; Ma, W.; Zhang, X.; Song, Y.; Ma, C.; Shi, J. B, N, F tri-doped lignin-derived carbon nanofibers as an efficient metal-free bifunctional electrocatalyst for ORR and OER in rechargeable liquid/solid-state Zn-air batteries, Appl. Surf. Sci. 2022, 598, 153891. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Q.; Liu, S.; Wang, E. Manganese-doped cobalt spinel oxide as bifunctional oxygen electrocatalyst toward high-stable rechargeable Zn-air battery. Electrochim. Acta 2023, 437, 141477. [Google Scholar] [CrossRef]
- Yang, T.; Hu, X.; Zheng, W.; Li, Z.; Wu, D.; Lu, G.; Zhao, Q.; Yang, Z.; Wang, R.; Xu, C. Rational Design of an FeCo2O4@FeCo2S4 Heterostructure as an Efficient Bifunctional Electrocatalyst for Zn–Air Batteries. ACS Appl. Energy Mater. 2022, 5, 9742–9749. [Google Scholar] [CrossRef]
- Liu, X.; Zang, J.; Song, C.S.; Gao, H.; Zhou, S.; Wang, Y. A hybrid of Co3O4 nanoparticles coupled with B, Co/N-codoped C@B4C as an efficient bifunctional catalyst for oxygen reduction and oxygen evolution reactions. Int. J. Hydrogen Energy 2023, 48, 542–552. [Google Scholar] [CrossRef]
- Liu, Z.; Wan, J.; Li, M.; Shi, Z.; Liu, J.; Tang, Y. Synthesis of Co/CeO2/C hetero-particles with abundant oxygen-vacancies supported by carbon aerogels for ORR and OER. Nanoscale 2022, 14, 1997–2003. [Google Scholar] [CrossRef]
- Mondal, R.; Ratnawat, H.; Mukherjee, S.; Gupta, A.; Singh, P. Investigation of the Role of Sr and Development of Superior Sr-Doped Hexagonal BaCoO3−δ Perovskite Bifunctional OER/ORR Catalysts in Alkaline Media. Energy Fuels 2022, 36, 3219–3228. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, F.; Zhang, Y.; Luo, X.; Chen, L.; Shi, Y. ZnS modified N, S dual-doped interconnected porous carbon derived from dye sludge waste as high-efficient ORR/OER catalyst for rechargeable zinc-air battery. J. Colloid Interface Sci. 2022, 616, 659–667. [Google Scholar] [CrossRef]
- Jebaslinhepzybai, B.T.; Partheeban, T.; Gavali, D.S.; Thapa, R.; Sasidharan, M. One-pot solvothermal synthesis of Co2P nanoparticles: An efficient HER and OER electrocatalysts. Int. J. Hydrogen Energy 2021, 46, 21924–21938. [Google Scholar] [CrossRef]
- Tu, T.; Zhou, X.; Zhang, P.; Tan, L.; Xu, Z.; Liu, M.; Li, W.; Kang, X.; Wu, Y.; Zheng, J. Co7Fe3 Nanoparticles Confined in N-Doped Carbon Nanocubes for Highly Efficient, Rechargeable Zinc–Air Batteries. ACS Sustain. Chem. Eng. 2022, 10, 8694–8703. [Google Scholar] [CrossRef]
- Gao, K.; Shen, M.; Duan, C.; Xiong, C.; Dai, L.; Zhao, W.; Lu, W.; Ding, S.; Ni, Y. Co-N-Doped Directional Multichannel PAN/CA-Based Electrospun Carbon Nanofibers as High-Efficiency Bifunctional Oxygen Electrocatalysts for Zn–Air Batteries. ACS Sustain. Chem. Eng. 2021, 9, 17068–17077. [Google Scholar] [CrossRef]
- He, Y.; Yang, X.; Li, Y.; Liu, L.; Guo, S.; Shu, C.; Liu, F.; Liu, Y.; Tan, Q.; Wu, G. Atomically Dispersed Fe–Co Dual Metal Sites as Bifunctional Oxygen Electrocatalysts for Rechargeable and Flexible Zn–Air Batteries. ACS Catal. 2022, 12, 1216–1227. [Google Scholar] [CrossRef]
- Wu, D.; Hu, X.; Yang, Z.; Yang, T.; Wen, J.; Lu, G.; Zhao, Q.; Li, Z.; Jiang, X.; Xu, C. NiFe LDH Anchoring on Fe/N-Doped Carbon Nanofibers as a Bifunctional Electrocatalyst for Rechargeable Zinc–Air Batteries. Ind. Eng. Chem. Res. 2022, 61, 7523–7528. [Google Scholar] [CrossRef]
- Li, W.; Wu, Y.; Chen, M.; Dai, P.; Jiang, T.; Zhou, S. Ultrathin nitrogen-doped defective carbon layer embedded with NiFe for solid zinc-air batteries. J. Alloy. Compd. 2022, 925, 166658. [Google Scholar] [CrossRef]
- Qin, T.; Ding, Y.; Zhang, R.; Gao, X.; Tang, Z.; Liu, Y.; Gao, D. Bifunctional CoO/CoS2 hierarchical nanospheres electrocatalyst for rechargeable Zn-Air battery. FlatChem 2022, 32, 100343. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Shi, K.; Zhu, Z.; Li, X.; Xu, H.; Gao, J. Bimetallic dispersion zeolitic imidazolate framework derived spherical porous bifunctional catalysts for liquid/solid Zn-Air batteries. J. Alloy. Compd. 2022, 925, 166680. [Google Scholar] [CrossRef]
- Kong, Q.; Lv, X.; Weng, C.; Ren, J.; Tian, W.; Yuan, Z. Curving Engineering of Hollow Concave-Shaped Rhombic Dodecahedrons of N-Doped Carbon Encapsulated with Fe-Doped Co/Co3O4 Nanoparticles for an Efficient Oxygen Reduction Reaction and Zn–Air Batteries. ACS Sustain. Chem. Eng. 2022, 10, 11441–11450. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, Y.; Xu, X.; Wang, X.; Liu, M.; Gao, T.; Liu, B.; Li, L.; Meng, X.; Gu, P.; Zou, J. CoNi Alloys Encapsulated in N-Doped Carbon Nanotubes for Stabilizing Oxygen Electrocatalysis in Zinc–Air Battery. Nanomaterials 2023, 13, 1788. https://doi.org/10.3390/nano13111788
Nie Y, Xu X, Wang X, Liu M, Gao T, Liu B, Li L, Meng X, Gu P, Zou J. CoNi Alloys Encapsulated in N-Doped Carbon Nanotubes for Stabilizing Oxygen Electrocatalysis in Zinc–Air Battery. Nanomaterials. 2023; 13(11):1788. https://doi.org/10.3390/nano13111788
Chicago/Turabian StyleNie, Yao, Xiaoqin Xu, Xinyu Wang, Mingyang Liu, Ting Gao, Bin Liu, Lixin Li, Xin Meng, Peng Gu, and Jinlong Zou. 2023. "CoNi Alloys Encapsulated in N-Doped Carbon Nanotubes for Stabilizing Oxygen Electrocatalysis in Zinc–Air Battery" Nanomaterials 13, no. 11: 1788. https://doi.org/10.3390/nano13111788
APA StyleNie, Y., Xu, X., Wang, X., Liu, M., Gao, T., Liu, B., Li, L., Meng, X., Gu, P., & Zou, J. (2023). CoNi Alloys Encapsulated in N-Doped Carbon Nanotubes for Stabilizing Oxygen Electrocatalysis in Zinc–Air Battery. Nanomaterials, 13(11), 1788. https://doi.org/10.3390/nano13111788