Lipid-Coated Nanobubbles in Plants
Abstract
1. Introduction
2. What Is the Evidence for Nanobubbles in Plants?
3. What Is the Evidence for Polar Lipid Coatings of Nanobubbles in Xylem Sap?
4. How Do Nanobubbles Form in Xylem?
5. What Physical Conditions Do Nanobubbles Experience in Xylem?
6. How Do Nanobubbles Respond to Changes in Pressure?
7. How Can Nanobubbles Persist in Bulk Solution without Coalescing?
8. How Do Nanobubbles in Xylem Affect Plants?
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schenk, H.J.; Steppe, K.; Jansen, S. Nanobubbles: A new paradigm for air-seeding in xylem. Trends Plant Sci. 2015, 20, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Seddon, J.R.T.; Lohse, D.; Ducker, W.A.; Craig, V.S.J. A deliberation on nanobubbles at surfaces and in bulk. ChemPhysChem 2012, 13, 2179–2187. [Google Scholar] [CrossRef] [PubMed]
- Epstein, P.S.; Plesset, M.S. On the stability of gas bubbles in liquid-gas solutions. J. Chem. Phys. 1950, 18, 1505–1509. [Google Scholar] [CrossRef]
- Duncan, P.B.; Needham, D. Test of the Epstein-Plesset model for gas microparticle dissolution in aqueous media: Effect of surface tension and gas undersaturation in solution. Langmuir 2004, 20, 2567–2578. [Google Scholar] [CrossRef]
- Ushikubo, F.Y.; Furukawa, T.; Nakagawa, R.; Enari, M.; Makino, Y.; Kawagoe, Y.; Shiina, T.; Oshita, S. Evidence of the existence and the stability of nano-bubbles in water. Colloid Surf. A 2010, 361, 31–37. [Google Scholar] [CrossRef]
- Ohgaki, K.; Khanh, N.Q.; Joden, Y.; Tsuji, A.; Nakagawa, T. Physicochemical approach to nanobubble solutions. Chem. Eng. Sci. 2010, 65, 1296–1300. [Google Scholar] [CrossRef]
- Jin, F.; Li, J.; Ye, X.; Wu, C. Effects of pH and ionic strength on the stability of nanobubbles in aqueous solutions of α-Cyclodextrin. J. Phys. Chem. B 2007, 111, 11745–11749. [Google Scholar] [CrossRef]
- Jin, F.; Ye, X.; Wu, C. Observation of kinetic and structural scalings during slow coalescence of nanobubbles in an aqueous solution. J. Phys. Chem. B 2007, 111, 13143–13146. [Google Scholar] [CrossRef]
- Creux, P.; Lachaise, J.; Graciaa, A.; Beattie, J.K. Specific cation effects at the hydroxide-charged air/water interface. J. Phys. Chem. C 2007, 111, 3753–3755. [Google Scholar] [CrossRef]
- Liu, M.Y.; Beattie, J.K.; Gray-Weale, A. The surface relaxation of water. J. Phys. Chem. B 2012, 116, 8981–8988. [Google Scholar] [CrossRef]
- Browne, C.; Tabor, R.F.; Chan, D.Y.C.; Dagastine, R.R.; Ashokkumar, M.; Grieser, F. Bubble coalescence during acoustic cavitation in aqueous electrolyte solutions. Langmuir 2011, 27, 12025–12032. [Google Scholar] [CrossRef] [PubMed]
- Weijs, J.H.; Seddon, J.R.T.; Lohse, D. Diffusive shielding stabilizes bulk nanobubble clusters. ChemPhysChem 2012, 13, 2197–2204. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.L.; Lozano, M.M.; Borden, M.A. Physical chemistry of experimental models for lipid shells of medical microbubbles. Bubble Sci. Eng. Techn. 2009, 1, 18–30. [Google Scholar] [CrossRef]
- Kwan, J.J.; Borden, M.A. Lipid monolayer collapse and microbubble stability. Adv. Colloid Interface Sci. 2012, 183, 82–99. [Google Scholar] [CrossRef]
- Kwan, J.J.; Borden, M.A. Microbubble dissolution in a multigas environment. Langmuir 2010, 26, 6542–6548. [Google Scholar] [CrossRef] [PubMed]
- Schenk, H.J.; Espino, S.; Visser, A.; Esser, B.K. Dissolved atmospheric gas in xylem sap measured with membrane inlet mass spectrometry. Plant Cell Environ. 2016, 39, 944–950. [Google Scholar] [CrossRef]
- Gallego-Urrea, J.A.; Tuoriniemi, J.; Hassellöv, M. Applications of particle-tracking analysis to the determination of size distributions and concentrations of nanoparticles in environmental, biological and food samples. TrAC Trends Anal. Chem. 2011, 30, 473–483. [Google Scholar] [CrossRef]
- Schenk, H.J.; Espino, S.; Romo, D.M.; Nima, N.; Do, A.Y.T.; Michaud, J.M.; Papahadjopoulos-Sternberg, B.; Yang, J.; Zuo, Y.; Steppe, K.; et al. Xylem surfactants introduce a new element to the cohesion-tension theory. Plant Physiol. 2017, 173, 1177–1196. [Google Scholar] [CrossRef]
- Guan, X.; Schenk, H.J.; Roth, M.R.; Welti, R.; Werner, J.; Kaack, L.; Trabi, C.L.; Jansen, S. Nanoparticles are linked to polar lipids in xylem sap of temperate angiosperm species. Tree Physiol. 2022, 42, 2003–2019. [Google Scholar] [CrossRef]
- D’Arrigo, J.S. Stable Gas-in-Liquid Emulsions: Production in Natural Waters and Artificial Media, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2003; 323p. [Google Scholar]
- Schenk, H.J.; Michaud, J.M.; Mocko, K.; Espino, S.; Melendres, T.; Roth, M.R.; Welti, R.; Kaack, L.; Jansen, S. Lipids in xylem sap of woody plants across the angiosperm phylogeny. Plant J. 2021, 105, 1477–1494. [Google Scholar] [CrossRef]
- Buhtz, A.; Kolasa, A.; Arlt, K.; Walz, C.; Kehr, J. Xylem sap protein composition is conserved among different plant species. Planta 2004, 219, 610–618. [Google Scholar] [CrossRef]
- Dafoe, N.J.; Constabel, C.P. Proteomic analysis of hybrid poplar xylem sap. Phytochemistry 2009, 70, 856–863. [Google Scholar] [CrossRef]
- Djordjevic, M.A.; Oakes, M.; Li, D.X.; Hwang, C.H.; Hocart, C.H.; Gresshoff, P.M. The Glycine max xylem sap and apoplast proteome. J. Proteome Res. 2007, 6, 3771–3779. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, H.B.; Natarajan, S.S.; Bennett, J.O.; Sicher, R.C. Protein and metabolite composition of xylem sap from field-grown soybeans (Glycine max). Planta 2011, 233, 921–931. [Google Scholar] [CrossRef]
- Ligat, L.; Lauber, E.; Albenne, C.; Clemente, H.S.; Valot, B.; Zivy, M.; Pont-Lezica, R.; Arlat, M.; Jamet, E. Analysis of the xylem sap proteome of Brassica oleracea reveals a high content in secreted proteins. Proteomics 2011, 11, 1798–1813. [Google Scholar] [CrossRef]
- Rep, M.; Dekker, H.L.; Vossen, J.H.; de Boer, A.D.; Houterman, P.M.; de Koster, C.G.; Cornelissen, B.J.C. A tomato xylem sap protein represents a new family of small cysteine-rich proteins with structural similarity to lipid transfer proteins. FEBS Lett. 2003, 534, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Kanduč, M.; Schneck, E.; Loche, P.; Jansen, S.; Schenk, H.J.; Netz, R.R. Cavitation in lipid bilayers poses strict negative pressure stability limit in biological liquids. Proc. Natl. Acad. Sci. USA 2020, 117, 10733–10739. [Google Scholar] [CrossRef]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef]
- Choat, B.; Cobb, A.R.; Jansen, S. Structure and function of bordered pits: New discoveries and impacts on whole-plant hydraulic function. New Phytol. 2008, 177, 608–626. [Google Scholar] [CrossRef] [PubMed]
- Jansen, S.; Choat, B.; Pletsers, A. Morphological variation of intervessel pit membranes and implications to xylem function in angiosperms. Amer. J. Bot. 2009, 96, 409–419. [Google Scholar] [CrossRef]
- Kaack, L.; Altaner, C.M.; Carmesin, C.; Diaz, A.; Holler, M.; Kranz, C.; Neusser, G.; Odstrcil, M.; Schenk, H.J.; Schmidt, V.; et al. Function and three-dimensional structure of intervessel pit membranes in angiosperms: A review. IAWA J. 2019, 40, 673–702. [Google Scholar] [CrossRef]
- Zhang, Y.; Carmesin, C.; Kaack, L.; Klepsch, M.M.; Kotowska, M.; Matei, T.; Schenk, H.J.; Weber, M.; Walther, P.; Schmidt, V.; et al. High porosity with tiny pore constrictions and unbending pathways characterise the 3D structure of intervessel pit membranes in angiosperm xylem. Plant Cell Environ. 2020, 43, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Caupin, F.; Cole, M.W.; Balibar, S.; Treiner, J. Absolute limit for the capillary rise of a fluid. EPL 2008, 82, 1–6. [Google Scholar] [CrossRef]
- Meyra, A.G.; Kuz, V.A.; Zarragoicoechea, G.J. Geometrical and physicochemical considerations of the pit membrane in relation to air seeding: The pit membrane as a capillary valve. Tree Physiol. 2007, 27, 1401–1405. [Google Scholar] [CrossRef] [PubMed]
- Jansen, S.; Klepsch, M.; Li, S.; Kotowska, M.M.; Schiele, S.; Zhang, Y.; Schenk, H.J. Challenges in understanding air-seeding in angiosperm xylem. Acta Hort. 2018, 1222, 13–20. [Google Scholar] [CrossRef]
- Schenk, H.J.; Espino, S.; Rich-Cavazos, S.M.; Jansen, S. From the sap’s perspective: The nature of vessel surfaces in angiosperm xylem. Amer. J. Bot. 2018, 105, 174–187. [Google Scholar] [CrossRef]
- Yang, J.; Michaud, J.M.; Jansen, S.; Schenk, H.J.; Zuo, Y.Y. Dynamic surface tension of xylem sap lipids. Tree Physiol. 2020, 40, 433–444. [Google Scholar] [CrossRef]
- Lee, S.; Kim, D.H.; Needham, D. Equilibrium and dynamic interfacial tension measurements at microscopic interfaces using a micropipet technique. 2. Dynamics of phospholipid monolayer formation and equilibrium tensions at water-air interface. Langmuir 2001, 17, 5544–5550. [Google Scholar] [CrossRef]
- Kovscek, A.R.; Radke, C.J. Gas bubble snap-off under pressure-driven flow in constricted noncircular capillaries. Colloid Surf. A 1996, 117, 55–76. [Google Scholar] [CrossRef]
- Kovscek, A.R.; Tang, G.Q.; Radke, C.J. Verification of Roof snap off as a foam-generation mechanism in porous media at steady state. Colloid Surf. A 2007, 302, 251–260. [Google Scholar] [CrossRef]
- Berg, S.; Ott, H.; Klapp, S.A.; Schwing, A.; Neiteler, R.; Brussee, N.; Makurat, A.; Leu, L.; Enzmann, F.; Schwarz, J.-O.; et al. Real-time 3D imaging of Haines jumps in porous media flow. Proc. Natl. Acad. Sci. USA 2013, 110, 3755–3759. [Google Scholar] [CrossRef]
- Moebius, F.; Canone, D.; Or, D. Characteristics of acoustic emissions induced by fluid front displacement in porous media. Water Resour. Res. 2012, 48, W11507. [Google Scholar] [CrossRef]
- Choat, B.; Jansen, S.; Zwieniecki, M.A.; Smets, E.; Holbrook, N.M. Changes in pit membrane porosity due to deflection and stretching: The role of vestured pits. J. Exp. Bot. 2004, 55, 1569–1575. [Google Scholar] [CrossRef]
- Davitt, K.; Rolley, E.; Caupin, F.; Arvengas, A.; Balibar, S. Equation of state of water under negative pressure. J. Chem. Phys. 2010, 133, 174507. [Google Scholar] [CrossRef]
- Vincent, O.; Marmottant, P.; Quinto-Su, P.A.; Ohl, C.-D. Birth and growth of cavitation bubbles within water under tension confined in a simple synthetic tree. Phys. Rev. Let. 2012, 108, 184502. [Google Scholar] [CrossRef]
- Brennen, C.E. Cavitation and Bubble Dynamics; Cambridge University Press: Cambridge, UK, 2014; 263p. [Google Scholar]
- Dixon, H.H.; Joly, J. On the ascent of sap. Philos. Trans. R. Soc. Lond. B 1895, 186, 563–576. [Google Scholar]
- Jansen, S.; Schenk, H.J. On the ascent of sap in the presence of bubbles. Amer. J. Bot. 2015, 102, 1561–1563. [Google Scholar] [CrossRef]
- Schenk, H.J.; Jansen, S.; Hölttä, T. Positive pressure in xylem and its role in hydraulic function (Tansley review). New Phytol. 2021, 230, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Quick, D.D.; Espino, S.; Morua, M.G.; Schenk, H.J. Effects of thermal gradients in sapwood on stem psychrometry. Acta Hort. 2018, 1197, 23–29. [Google Scholar] [CrossRef]
- Javanainen, M.; Lamberg, A.; Cwiklik, L.; Vattulainen, I.; Ollila, O.H.S. Atomistic model for nearly quantitative simulations of Langmuir monolayers. Langmuir 2018, 34, 2565–2572. [Google Scholar] [CrossRef] [PubMed]
- Baoukina, S.; Mendez-Villuendas, E.; Tieleman, D.P. Molecular view of phase coexistence in lipid monolayers. J. Amer. Chem. Soc. 2012, 134, 17543–17553. [Google Scholar] [CrossRef]
- Debenedetti, P.G.; Reiss, H. Reversible work of formation of an embryo of a new phase within a uniform macroscopic mother phase. J. Chem. Phys. 1998, 108, 5498–5505. [Google Scholar] [CrossRef]
- Ingram, S.; Salmon, Y.; Lintunen, A.; Hölttä, T.; Vesala, T.; Vehkamäki, H. Dynamic surface tension enhances the stability of nanobubbles in xylem sap. Front. Plant Sci. 2021, 12, 3044. [Google Scholar] [CrossRef] [PubMed]
- Menzl, G.; Dellago, C. Effect of entropy on the nucleation of cavitation bubbles in water under tension. J. Chem. Phys. 2016, 145, 211918. [Google Scholar] [CrossRef]
- Manning, G.S. On the thermodynamic stability of bubbles, immiscible droplets, and cavities. Phys. Chem. Chem. Phys. 2020, 22, 17523–17531. [Google Scholar] [CrossRef] [PubMed]
- Vehmas, T.; Makkonen, L. Metastable nanobubbles. ACS Omega 2021, 6, 8021–8027. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Wang, R.; Tang, J.; Yang, L.; Feng, Z.; Xu, C.; Yang, F.; Gu, N. Dynamic tracking of bulk nanobubbles from microbubbles shrinkage to collapse. Colloid Surf. A 2020, 589, 124430. [Google Scholar] [CrossRef]
- Cipcigan, F.; Sokhan, V.; Martyna, G.; Crain, J. Structure and hydrogen bonding at the limits of liquid water stability. Sci. Rep. 2018, 8, 1718. [Google Scholar] [CrossRef]
- Balucani, U.; Brodholt, J.P.; Vallauri, R. Analysis of the velocity autocorrelation function of water. J. Phys. Condens. Matter 1996, 8, 6139. [Google Scholar] [CrossRef]
- Chau, P.L.; Hardwick, A.J. A new order parameter for tetrahedral configurations. Mol. Phys. 1998, 93, 511–518. [Google Scholar] [CrossRef]
- Errington, J.R.; Debenedetti, P.G. Relationship between structural order and the anomalies of liquid water. Nature 2001, 409, 318–321. [Google Scholar] [CrossRef]
- Derjaguin, B.; Landau, L. Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Prog. Surf. Sci. 1993, 43, 30–59. [Google Scholar] [CrossRef]
- Verwey, E.; Overbeek, J.T.G. Theory of the stability of lyophobic colloids. J. Colloid Sci. 1955, 10, 224–225. [Google Scholar] [CrossRef]
- Butt, H.-J.; Kappl, M. Surface and Interfacial Forces, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2018; p. 456. [Google Scholar]
- Khaled Abdella Ahmed, A.; Sun, C.; Hua, L.; Zhang, Z.; Zhang, Y.; Marhaba, T.; Zhang, W. Colloidal properties of air, oxygen, and nitrogen nanobubbles in water: Effects of ionic strength, natural organic matters, and surfactants. Environ. Eng. Sci. 2018, 35, 720–727. [Google Scholar] [CrossRef]
- Jin, J.; Feng, Z.; Yang, F.; Gu, N. Bulk nanobubbles fabricated by repeated compression of microbubbles. Langmuir 2019, 35, 4238–4245. [Google Scholar] [CrossRef] [PubMed]
- Kooijman, E.E.; Chupin, V.; Fuller, N.L.; Kozlov, M.M.; de Kruijff, B.; Burger, K.N.J.; Rand, P.R. Spontaneous curvature of phosphatidic acid and lysophosphatidic acid. Biochemistry 2005, 44, 2097–2102. [Google Scholar] [CrossRef]
- Zhang, T.; Brantley, S.L.; Verreault, D.; Dhankani, R.; Corcelli, S.A.; Allen, H.C. Effect of pH and salt on surface pKa of phosphatidic acid monolayers. Langmuir 2018, 34, 530–539. [Google Scholar] [CrossRef]
- Marsh, D. Handbook of Lipid Bilayers, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Currier, S.F.; Mautner, H.G. On the mechanism of action of choline acetyltransferase. Proc. Natl. Acad. Sci. USA 1974, 71, 3355–3358. [Google Scholar] [CrossRef]
- Yoshinaga, M.Y.; Kellermann, M.Y.; Valentine, D.L.; Valentine, R.C. Phospholipids and glycolipids mediate proton containment and circulation along the surface of energy-transducing membranes. Progr. Lid. Res. 2016, 64, 1–15. [Google Scholar] [CrossRef]
- Kalisch, B.; Dörmann, P.; Hölzl, G. DGDG and glycolipids in plants and algae. In Lipids in Plant and Algae Development; Nakamura, Y., L-Beisson, Y., Eds.; Subcellular biochemistry; Springer: Cham, Switzerland, 2016; pp. 51–83. [Google Scholar]
- Kobayashi, K. Role of membrane glycerolipids in photosynthesis, thylakoid biogenesis and chloroplast development. J. Plant Res. 2016, 129, 565–580. [Google Scholar] [CrossRef]
- Bacle, A.; Gautier, R.; Jackson, C.L.; Fuchs, P.F.J.; Vanni, S. Interdigitation between triglycerides and lipids modulates surface properties of lipid droplets. Biophys. J. 2017, 112, 1417–1430. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.M.; Katul, G.; Domec, J.-C. Catastrophic hydraulic failure and tipping points in plants. Plant Cell Environ. 2022, 45, 2231–2266. [Google Scholar] [CrossRef] [PubMed]
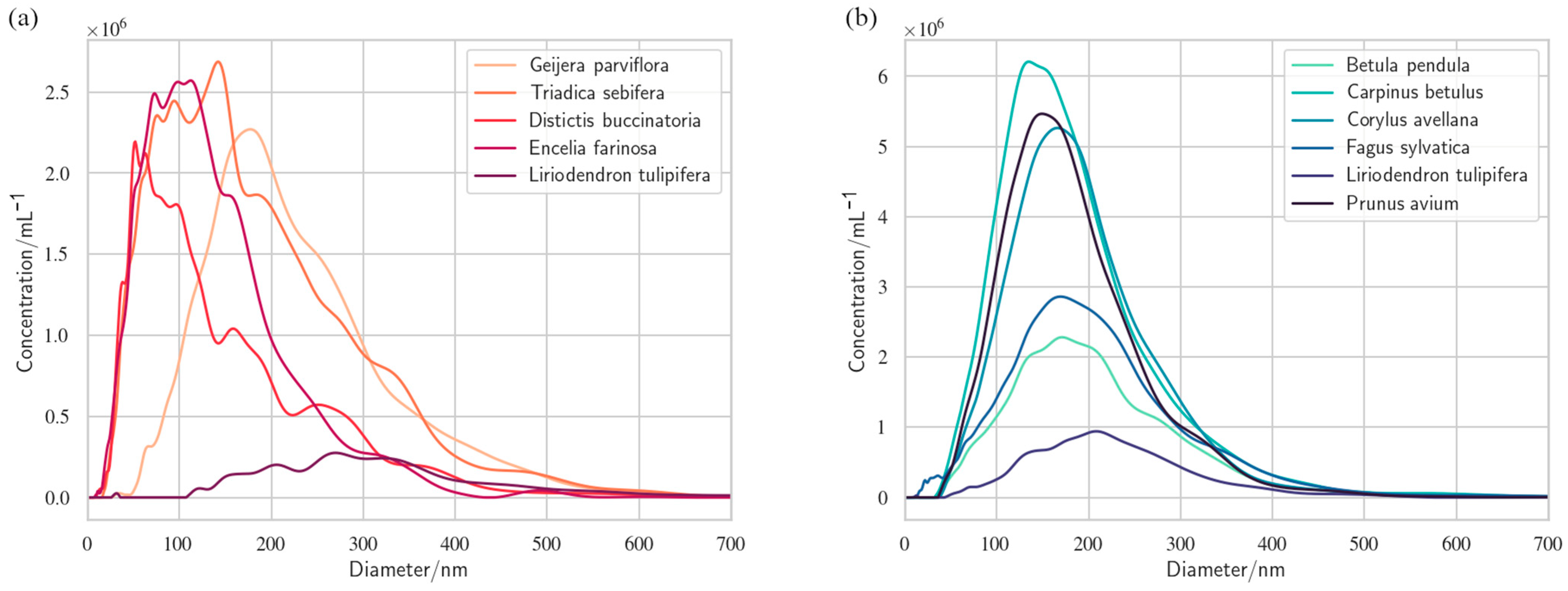

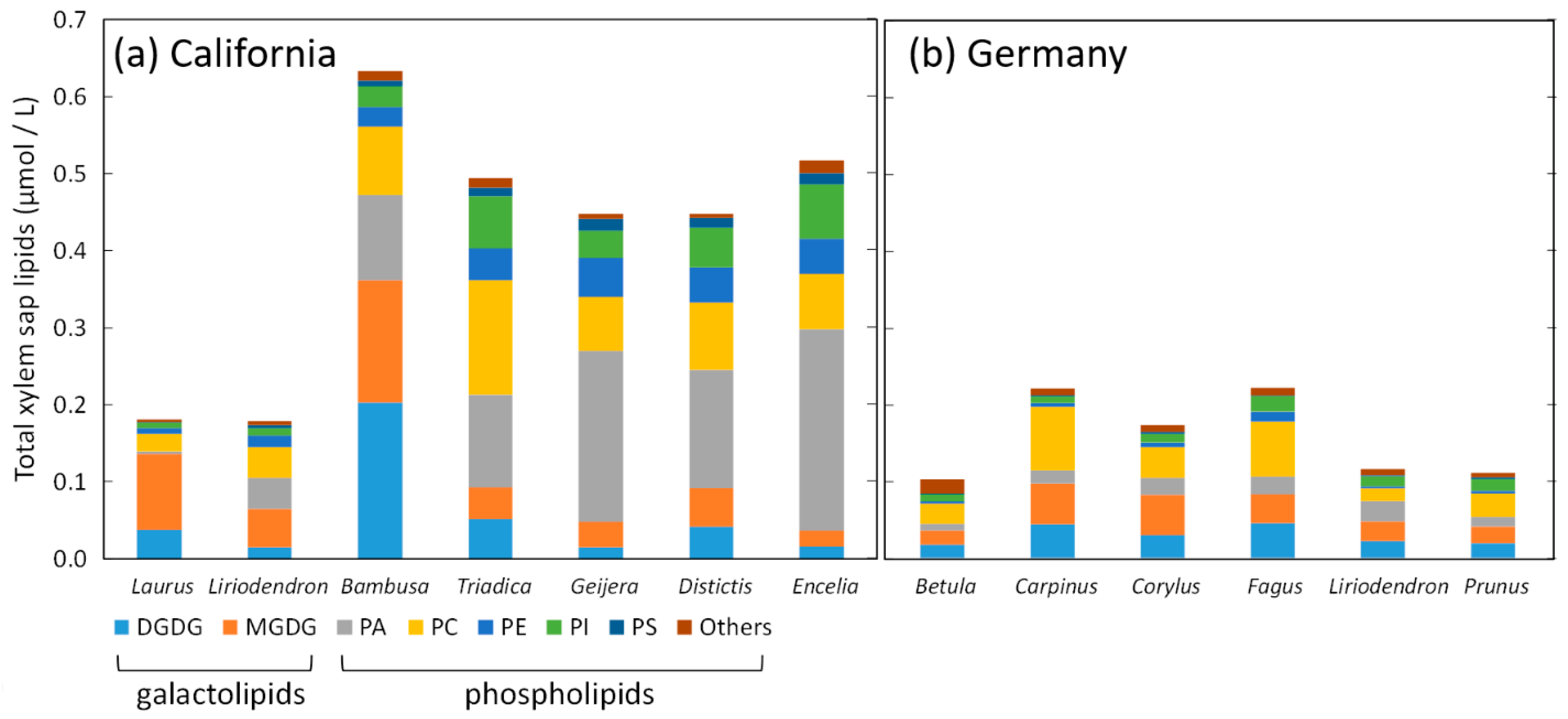
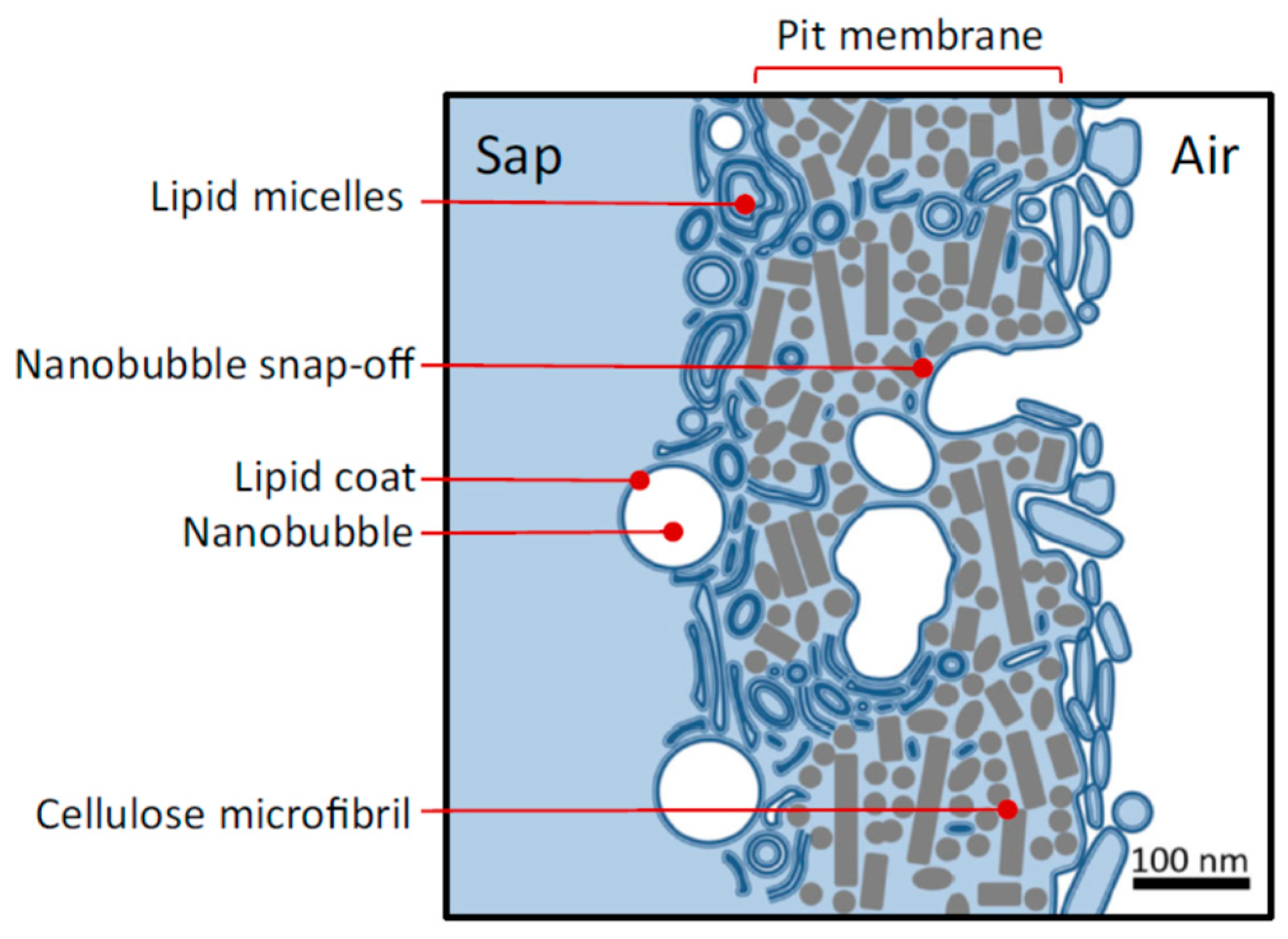
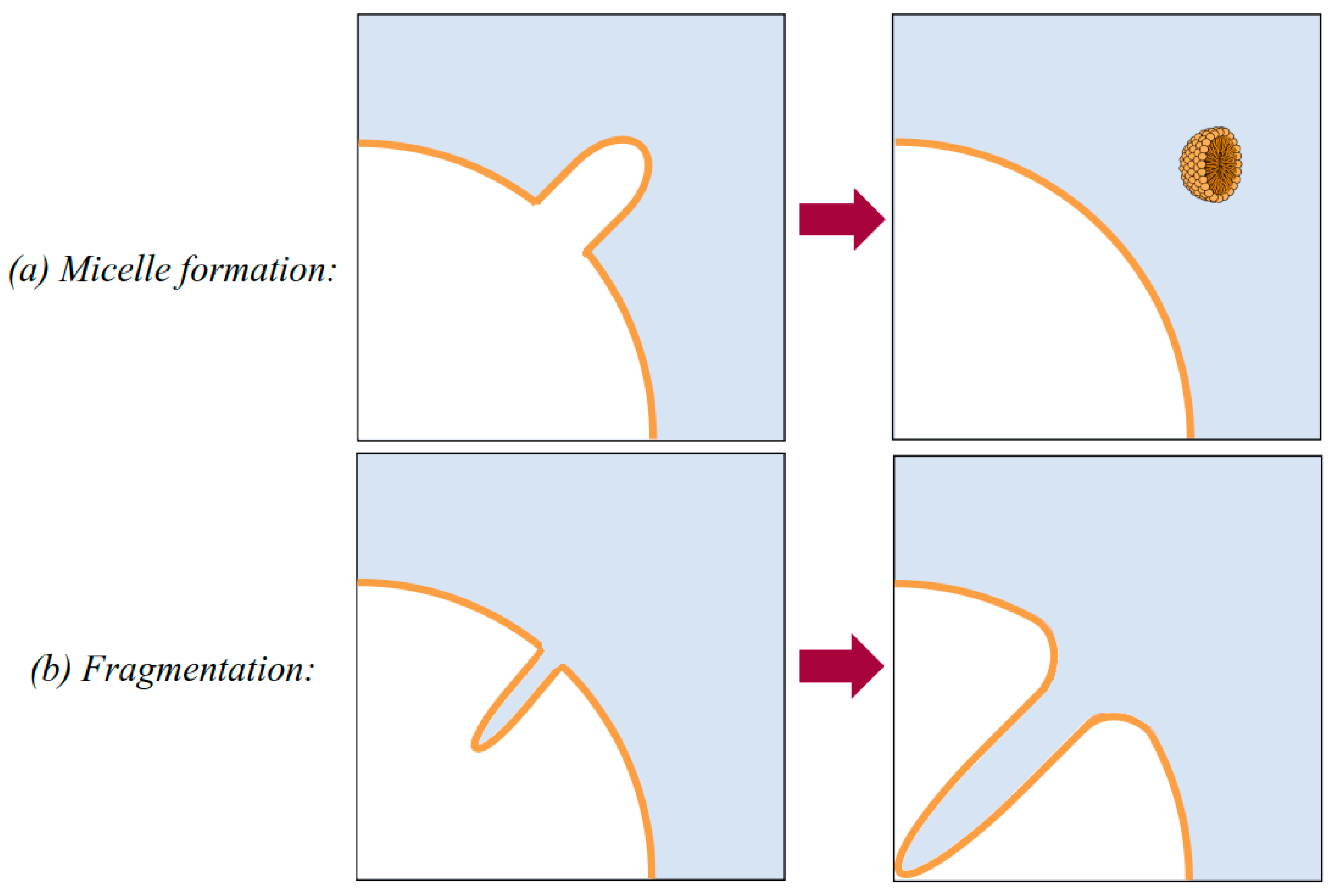

| Lipid | Phosphate pKa | Ammonium pKa |
|---|---|---|
| PS | 2.6 | 11.55 |
| PE | 1.7 | 9.8 |
| PC | 1.5 | 13.9 [72] |
| PI | 2.5 | n/a |
| PA | 3.2 [69], 11.5 [70] | n/a |
| PG | 3.5 | n/a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ingram, S.; Jansen, S.; Schenk, H.J. Lipid-Coated Nanobubbles in Plants. Nanomaterials 2023, 13, 1776. https://doi.org/10.3390/nano13111776
Ingram S, Jansen S, Schenk HJ. Lipid-Coated Nanobubbles in Plants. Nanomaterials. 2023; 13(11):1776. https://doi.org/10.3390/nano13111776
Chicago/Turabian StyleIngram, Stephen, Steven Jansen, and H. Jochen Schenk. 2023. "Lipid-Coated Nanobubbles in Plants" Nanomaterials 13, no. 11: 1776. https://doi.org/10.3390/nano13111776
APA StyleIngram, S., Jansen, S., & Schenk, H. J. (2023). Lipid-Coated Nanobubbles in Plants. Nanomaterials, 13(11), 1776. https://doi.org/10.3390/nano13111776






