Promotion Effects of Ultrafine Bubbles/Nanobubbles on Seed Germination
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Germination
2.1.1. Air UFBs for the Use of Seed Germination and Its Measuring Device
2.1.2. Seed Material
2.1.3. Germination Test
2.1.4. Analysis of Germination Process
2.2. Evaluation of ROS in UFB Water
2.2.1. Oxygen UFBs for ROS Detection
2.2.2. Electron Spin Resonance (ESR) Measurement
3. Results and Discussion
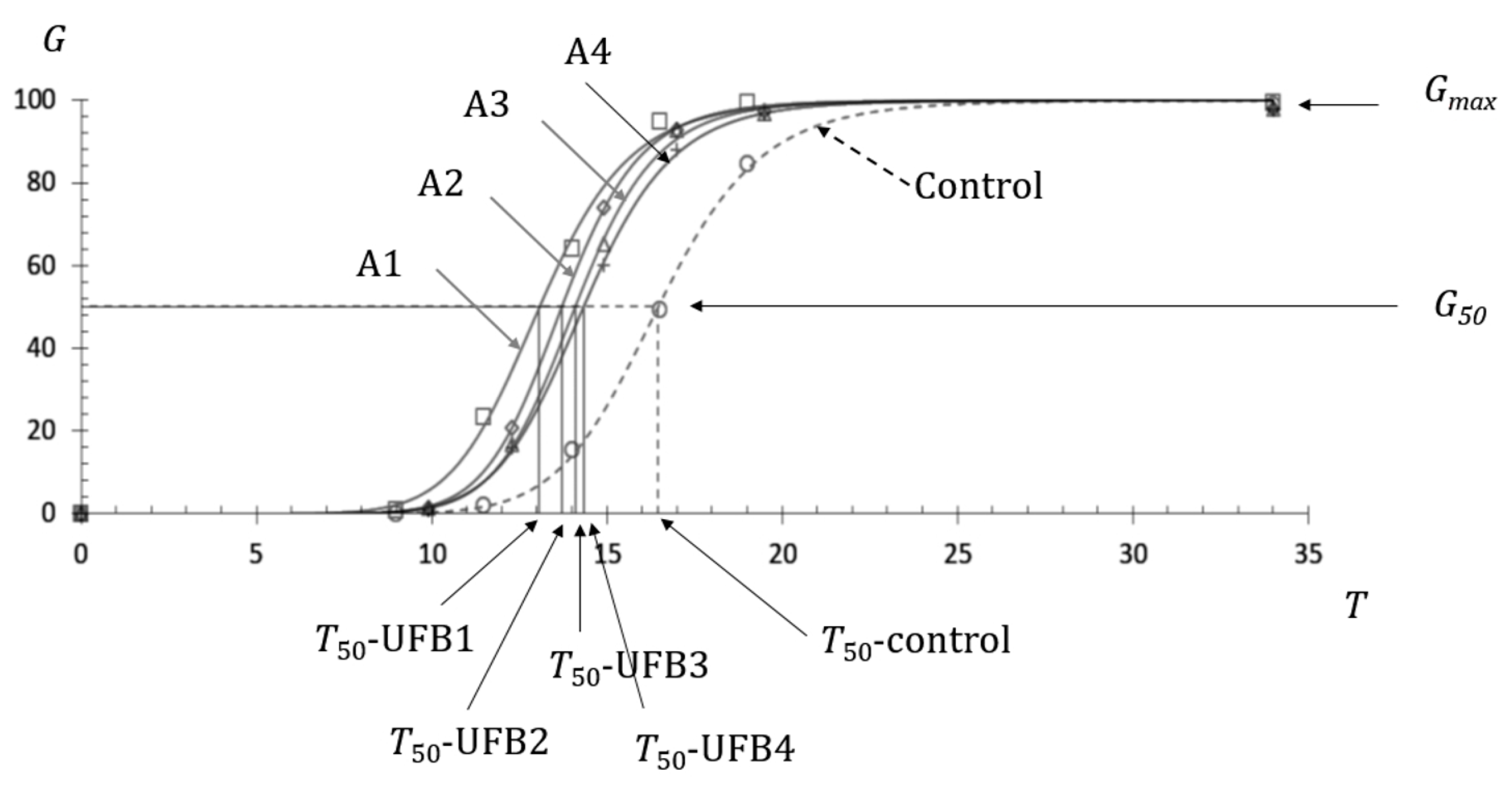
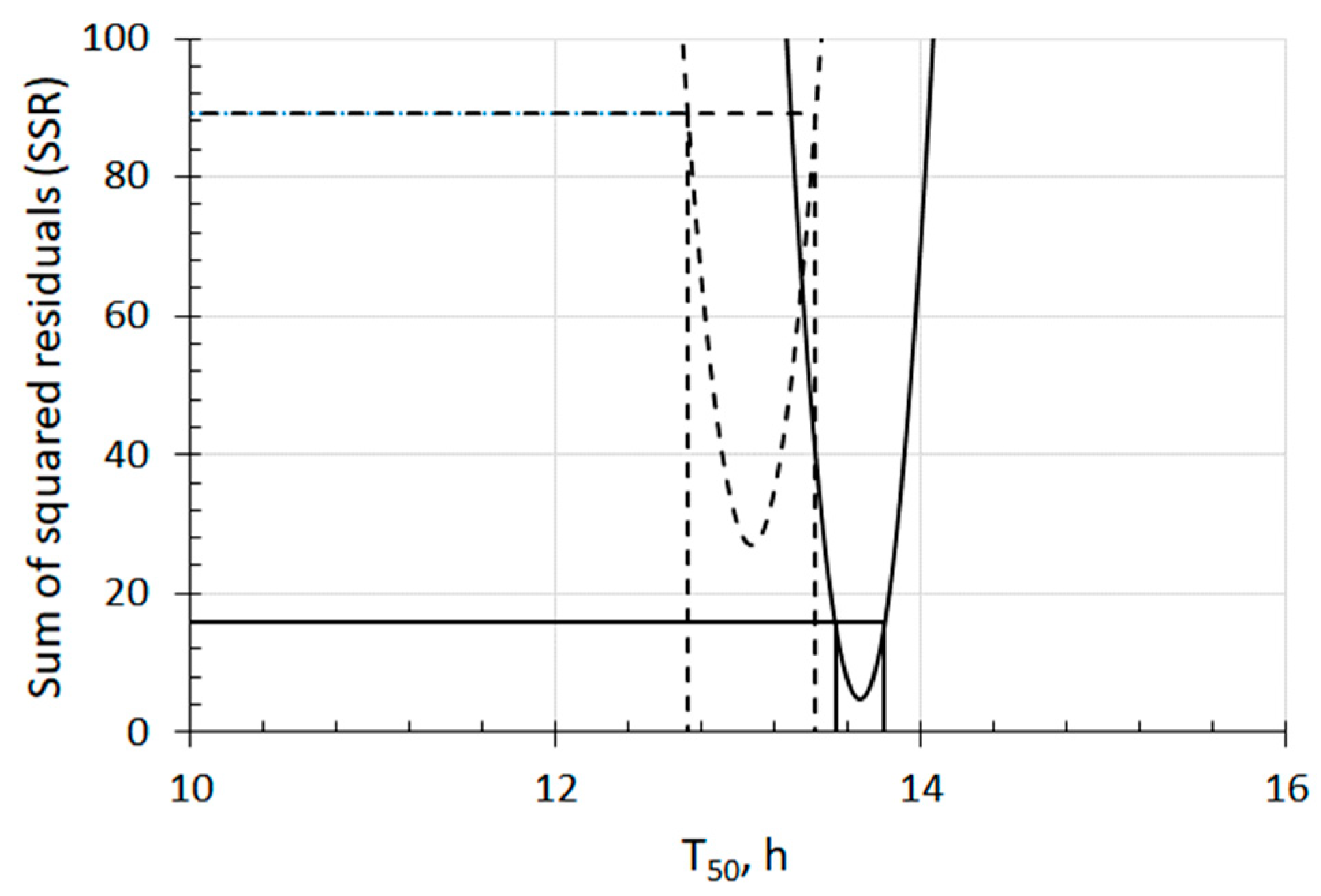
3.1. Fundamental Aspect of the Effect of UFBs on Seed Germination
3.2. Negative Effect on Seed Germination Caused by Excess Number Concentration
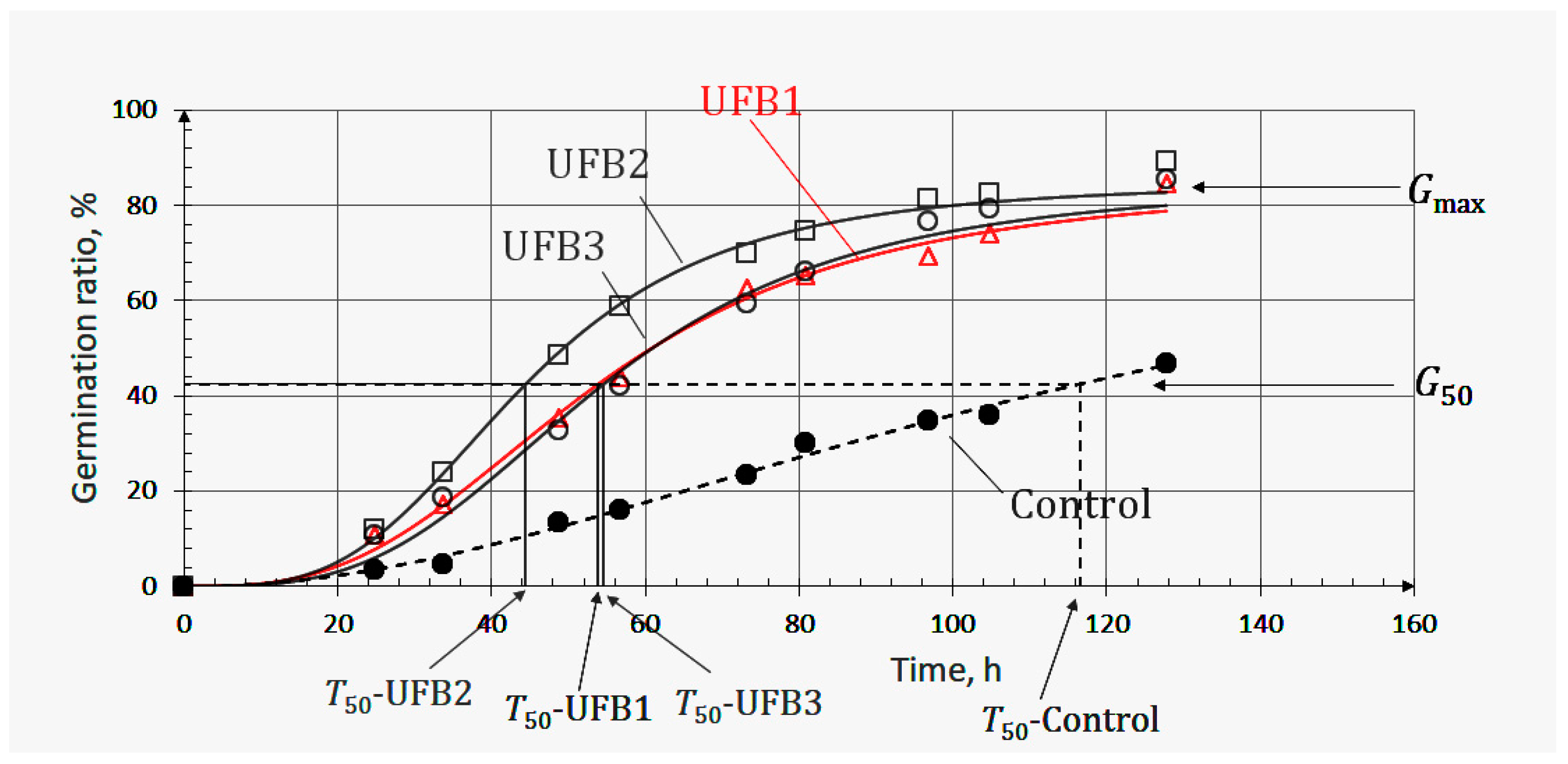
3.3. A Possible Factor Promoting Seed Germination
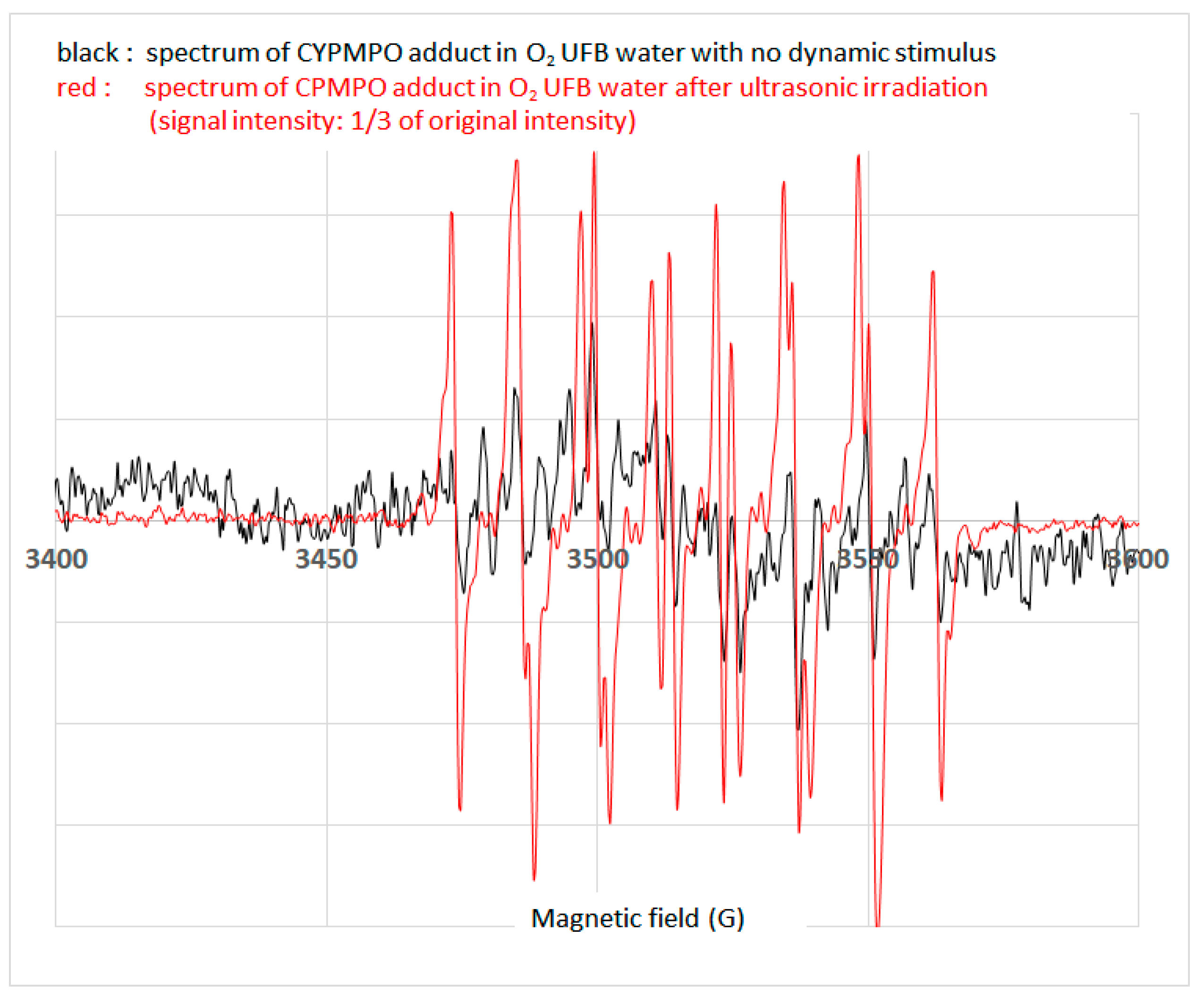
3.4. Detection of ROS in O2 UFB Water
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ISO20480-1:2017; Fine bubble technology—General principles for usage and measurement of fine bubbles—Part 1: Terminology. ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/obp/ui/#iso:std:iso:20480:-1:ed-1:v1:en (accessed on 22 February 2023).
- Ushikubo, F.Y.; Furukawa, T.; Nakagawa, R.; Enari, M.; Makino, Y.; Kawagoe, Y.; Shiina, T.; Oshita, S. Evidence of the existence of and the stability of nano-bubbles in water. Colloids Surf. A 2010, 361, 31–37. [Google Scholar] [CrossRef]
- Uchida, T.; Oshita, S.; Ohmori, M.; Tsuno, T.; Soejima, K.; Shinozaki, S.; Take, Y.; Mitsuta, K. Transmission electron microscope observations of nanobubbles and their capture of impurities in wastewater. Nanoscale Res. Lett. 2011, 6, 295. [Google Scholar] [CrossRef] [PubMed]
- Alheshibri, M.; Qian, J.; Jehannin, M.; Craig, V.S.J. A History of nanobubbles. Langmuir 2016, 32, 11086–11100. [Google Scholar] [CrossRef]
- Wang, X.; Lei, Z.; Shimizu, K.; Zhang, Z.; Lee, D.J. Recent advancements in nanobubble water technology and its application in energy recovery from organic solid wastes towards a greater environmental friendliness of anaerobic digestion system. Renew. Sustain. Energy Rev. 2021, 145, 111074. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Chen, C.W.; Tseng, Y.S.; Kuo, C.H.; Wu, C.H.; Di Dong, C. Advances in micro- and nano bubbles technology for application in biochemical processes. Environ. Technol. Innov. 2021, 23, 101729. [Google Scholar] [CrossRef]
- Terasaka, K.; Yasui, K.; Kanematsu, W.; Aya, N. (Eds.) Ultrafine Bubbles; Jenny Stanford: Singapore, 2022. [Google Scholar]
- Park, J.S.; Kurata, K. Application of microbubbles to hydroponics solution promotes lettuce growth. HortTechnology 2009, 19, 212–215. [Google Scholar] [CrossRef]
- Park, J.S.; Ohashi, K.; Kurata, K.; Lee, J.W. Promotion of lettuce growth by application of microbubbles in nutrient solution using different rates of electrical conductivity and under periodic intermittent generation in a deep flow technique culture system. Eur. J. Hortic. Sci. 2010, 75, 198–203. [Google Scholar]
- Kobayashi, N.; Yamaji, K. Leaf lettuce (Lactuca sativa L. ‘L-121′) growth in hydroponics with different nutrient solutions used to generate ultrafine bubbles. J. Plant Nutr. 2021, 45, 816–827. [Google Scholar] [CrossRef]
- Ebina, K.; Shi, K.; Hirao, M.; Hashimoto, J.; Kawato, Y.; Kaneshiro, S.; Morimoto, T.; Koizumi, K.; Yoshikawa, H. Oxygen and air nanobubble water solution promote the growth of plants, fishes, and mice. PLoS ONE 2013, 8, e65339. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Sun, J.; Dai, H.; Zhang, B.; Xiang, W.; Hu, Z.; Li, P.; Yang, J.; Zhang, W. Nanobubbles promote nutrient utilization and plant growth in rice by upregulating nutrient uptake genes and stimulating growth hormone production. Sci. Total Environ. 2021, 800, 149627. [Google Scholar] [CrossRef]
- Liu, S.; Kawagoe, Y.; Makino, Y.; Oshita, S. Effects of nanobubbles on the physicochemical properties of water: The basis for peculiar properties of water containing nanobubbles. Chem. Eng. Sci. 2013, 93, 250–256. [Google Scholar] [CrossRef]
- Liu, S.; Oshita, S.; Makino, Y.; Wang, Q.; Kawagoe, Y.; Uchida, T. Oxidative capacity of nanobubbles and its effect on seed germination. ACS Sustain. Chem. Eng. 2016, 4, 1347–1353. [Google Scholar] [CrossRef]
- Liu, S.; Oshita, S.; Kawabata, S.; Makino, Y.; Yoshimoto, T. Identification of ROS produced by nanobubbles and their positive and negative effects on vegetable seed germination. Langmuir 2016, 32, 11295–11302. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Oshita, S.; Kawabata, S.; Thuyet, D.Q. Nanobubble water’s promotion effect of barley (Hordeum vulgare L.) sprouts supported by RNA-Seq analysis. Langmuir 2017, 33, 12478–12486. [Google Scholar] [CrossRef]
- Oshita, S.; Kamijo, Y.; Pham, T.Q.A.; Yoshimura, M.; Sotome, I.; Kameya, H.; Fujita, T.; Liu, S. Number concentration of ultrafince bubble being effective in promoting barley seed. Jpn. J. Multiph. Flow 2020, 34, 194–204. [Google Scholar] [CrossRef]
- Ahmed, A.K.A.; Shi, X.; Hua, L.; Manzueta, L.; Qing, W.; Marhaba, T.; Zhang, W. Influences of air, oxygen, nitrogen, and carbon dioxide nanobubbles on seed germination and plant growth. J. Agric. Food Chen. 2018, 66, 5117–5124. [Google Scholar] [CrossRef]
- Siregar, I.; Muharam, K.F.; Purwanto, A.; Sudrajat, D.J. Seed germination characteristics in different storage time of Gmelina arborea treated with ultrafine bubbles priming. Biodiversitas 2020, 21, 4558–4564. [Google Scholar] [CrossRef]
- Yasui, K.; Tuziuti, T.; Kanematsu, W. Extreme conditions in a dissolving air nanobubble. Phys. Rev. E 2016, 94, 013106. [Google Scholar] [CrossRef]
- Yasui, K.; Tuziuti, T.; Kanematsu, W. Mysteries of buble nanobubbles (ultrafine bubbles); stability and radical formation. Ultrason. Sonochem. 2018, 48, 259–266. [Google Scholar] [CrossRef]
- Yasui, K.; Tuziuti, T.; Kanematsu, W. Mechanism of OH radical production from ozon bubbles in water after stopping cavitation. Ultrason. Sonochem. 2019, 58, 104707. [Google Scholar] [CrossRef]
- Yasui, K. On some aspects of nanobubble-containing systems. Nanomaterials 2022, 12, 2175. [Google Scholar] [CrossRef] [PubMed]
- Akar, T.; Avci, M.; Dusunceli, F. Barley: Post-Harvest Operations. 2004. Available online: https://www.fao.org/fileadmin/user_upload/inpho/docs/Post_Harvest_Compendium_-_BARLEY.pdf (accessed on 22 February 2023).
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-response analysis using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef] [PubMed]
- IDEC Corporation. High Density Fine Bubble Liquid Generation Method and High Density Fine Bubble Liquid Generation Apparatus. JP Patent JP5715272B2, 20 March 2015. [Google Scholar]
- Bailly, C.; El-Maarouf-Bouteau, H.; Corvineau, F. From intracellular signaling networks to cell death: The dual role of reactive oxygen species in seed physiology. Comptes Rendus Biol. 2008, 331, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Sarath, G.; Hou, G.; Baird, L.M.; Mitchell, R.B. Reactive oxygen species, ABA and nitric oxide interactions on the gemination of warm-season C4-grasses. Planta 2007, 226, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Tawaratsumida, T.; Kondo, K.; Kasa, S.; Sakamoto, M.; Aoki, N.; Zheng, S.; Yuasa, T.; Iwaya-Inoue, M. Reactive oxygen species are involved in gibberellin/abscisic acid signaling in barley aleurone cells. Plant Physiol. 2012, 158, 1705–1714. Available online: https://www.plantphysiol.org/cgi/doi/10.1104/pp.111.192740 (accessed on 15 March 2015).
- Liu, Y.; Ye, N.; Liu, R.; Chen, M.; Zhang, J. H2O2 mediates the regulation of ABA catabolism and GA biosynthesis in Arabidopsis seed dormancy and germination. J. Exp. Bot. 2010, 61, 2979–2990. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Koda, Y.; Zheng, S.; Yuasa, T.; Iwaya-Inoue, M. Regulation of soybean seed germination through ethylene production in response to reactive oxygen species. Ann. Bot. 2013, 111, 95–102. [Google Scholar] [CrossRef]
- Kameya, H. Evaluation of hydroxyl radical and alkyl-oxy radical scavenging activity of coffee by ESR spin trapping method. J. Food Sci. Eng. 2017, 7, 305–311. [Google Scholar]
- Oowada, S.; Endo, N.; Kameya, H.; Shimmei, M.; Kotake, Y. Multiple free-radical scavenging capacity in serum. J. Clin. Biochem. Nutr. 2012, 51, 117–121. [Google Scholar] [CrossRef]
- Fang, X.; Mark, G.; Von Sonntag, C. OH radical formation by ultrasound in aqueous solutions Part I: The chemistry underlying the terephthalate dosimeter. Ultrason. Sonochem. 1996, 3, 57–63. [Google Scholar] [CrossRef]
- Gogate, P.R.; Shirgaonkar, I.Z.; Senthilkumar, P.; Vichare, N.P.; Pandit, A.B. Cavitation reactors: Efficiency assessment using a model reaction. AIChE J. 2001, 47, 2526–2538. [Google Scholar] [CrossRef]
- Donoghue, M.A.; Xu, X.; Bernlohr, D.A.; Arraga, E.A. Capillary electrophoretic analysis of hydroxyl radicals produced by respiring mitochondria. Anal. Bioanal. Chem. 2013, 405, 6053–6060. [Google Scholar] [CrossRef] [PubMed]
- Petri, B.G.; Watts, R.J.; Teel, A.L.; Huling, S.G.; Brown, R.A. Fundamentals of ISCO using hydrogen peroxide. In In Situ Cemical Oxidation for Groundwater Remediation; Siegrist, R.L., Crimi, M., Simpkin, T.J., Eds.; Springer Science+Business Media: New York, NY, USA, 2011; pp. 33–88. [Google Scholar]
| UFB1 | UFB2 | UFB3 | UFB4 | |
|---|---|---|---|---|
| Number concentration ± SD, mL−1 | 8.5 × 108 ± 3.6 × 107 | 5.0 × 108 ± 3.7 × 107 | 3.8 × 108 ± 4.8 × 107 | 1.4 × 108 ± 1.7 × 107 |
| Mean diameter ± SD, nm | 127.3 ± 4.7 | 123.5 ± 2.8 | 135.9 ± 4.1 | 132.5 ± 7.5 |
| T50, h | 13.1 | 13.7 | 14.1 | 14.3 |
| UFB1 | UFB2 | UFB3 | |
|---|---|---|---|
| Number concentration ± SD, mL−1 | 1.0 × 109 ± 4.4 × 107 | 7.4 × 108 ± 9.0 × 107 | 2.4 × 108 ± 3.3 × 107 |
| Mean diameter ± SD, nm | 136.8 ± 3.4 | 147.1 ± 5.4 | 143.6 ± 5.7 |
| T50, h | 53.9 | 44.4 | 54.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oshita, S.; Boerzhijin, S.; Kameya, H.; Yoshimura, M.; Sotome, I. Promotion Effects of Ultrafine Bubbles/Nanobubbles on Seed Germination. Nanomaterials 2023, 13, 1677. https://doi.org/10.3390/nano13101677
Oshita S, Boerzhijin S, Kameya H, Yoshimura M, Sotome I. Promotion Effects of Ultrafine Bubbles/Nanobubbles on Seed Germination. Nanomaterials. 2023; 13(10):1677. https://doi.org/10.3390/nano13101677
Chicago/Turabian StyleOshita, Seiichi, Surina Boerzhijin, Hiromi Kameya, Masatoshi Yoshimura, and Itaru Sotome. 2023. "Promotion Effects of Ultrafine Bubbles/Nanobubbles on Seed Germination" Nanomaterials 13, no. 10: 1677. https://doi.org/10.3390/nano13101677
APA StyleOshita, S., Boerzhijin, S., Kameya, H., Yoshimura, M., & Sotome, I. (2023). Promotion Effects of Ultrafine Bubbles/Nanobubbles on Seed Germination. Nanomaterials, 13(10), 1677. https://doi.org/10.3390/nano13101677







