Quantitative In Situ Monitoring of Cu-Atom Release by Cu2O Nanocatalysts under Photocatalytic CO2 Reduction Conditions: New Insights into the Photocorrosion Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Flame Spray Pyrolysis (FSP) Synthesis of CuO and Cu2O Nanoparticles
2.2. Characterization of Materials
2.3. Electron Paramagnetic Resonance Spectroscopy (EPR)
2.4. Analytical Cu2+ Leaching Study by Anodic Stripping Voltammetry (ASV)
3. Results
3.1. Cu2+ Ion Release under CO2-Photoreduction Conditions
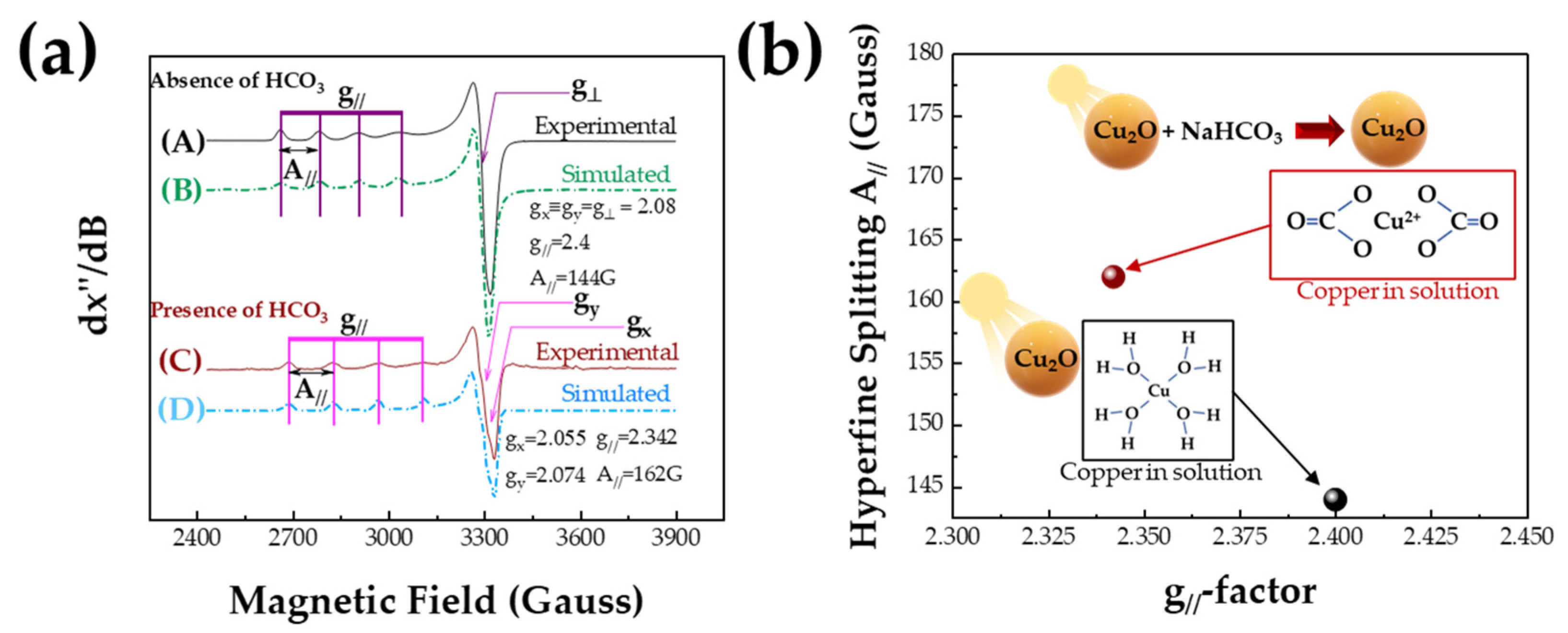
3.2. EPR Spectroscopy
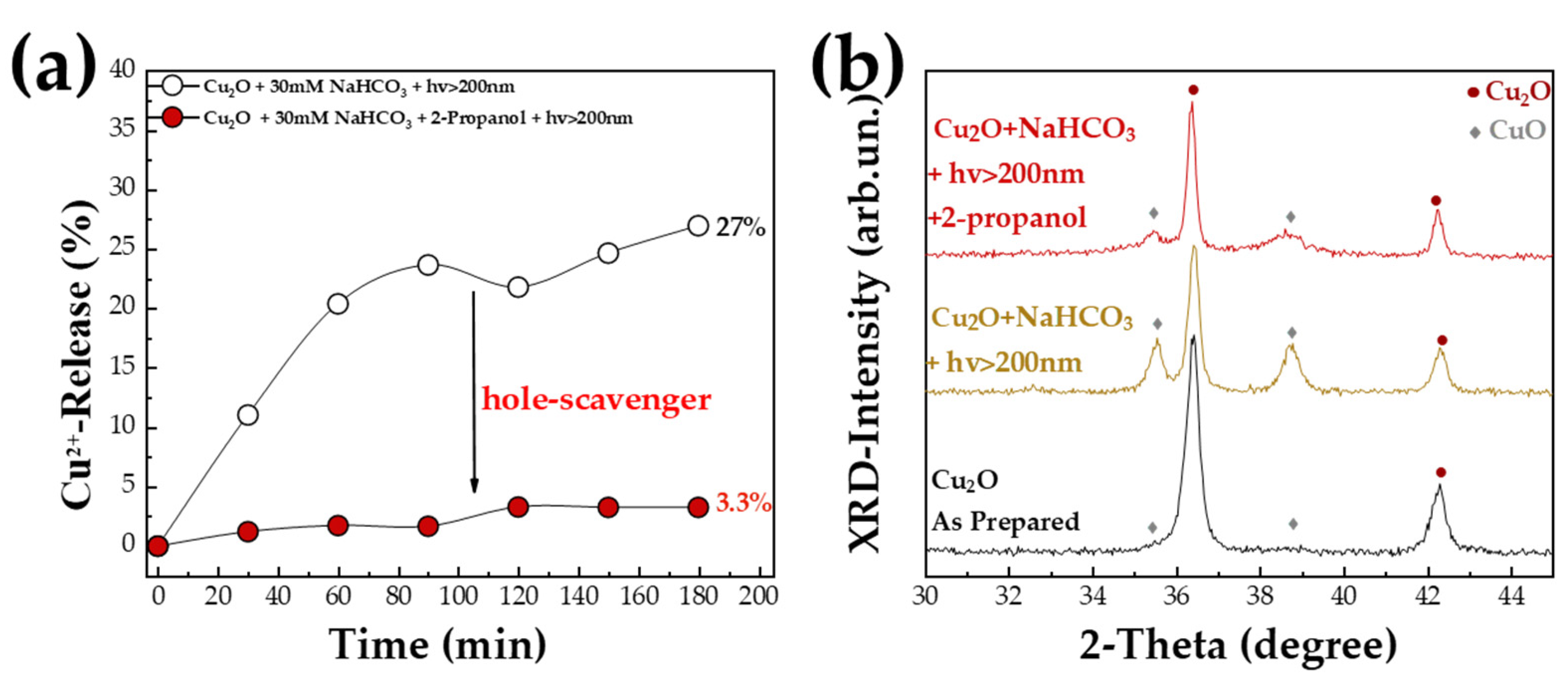
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Domen, K. Particulate Photocatalysts for Light-Driven Water Splitting: Mechanisms, Challenges, and Design Strategies. Chem. Rev. 2020, 120, 919–985. [Google Scholar] [CrossRef] [PubMed]
- Marschall, R. Semiconductor Composites: Strategies for Enhancing Charge Carrier Separation to Improve Photocatalytic Activity. Adv. Funct. Mater. 2014, 24, 2421–2440. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, L.; Cheng, H.; Zhu, Y. Significantly Enhanced Photocatalytic Performance of ZnO via Graphene Hybridization and the Mechanism Study. Appl. Catal. B Environ. 2011, 101, 382–387. [Google Scholar] [CrossRef]
- Ng, C.; Ng, Y.H.; Iwase, A.; Amal, R. Influence of Annealing Temperature of WO3 in Photoelectrochemical Conversion and Energy Storage for Water Splitting. ACS Appl. Mater. Interfaces 2013, 5, 5269–5275. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wu, X.; Ma, Y.; Gong, L.; Qu, F.; Fan, H. Porous SnO2 Nanowire Bundles for Photocatalyst and Li Ion Battery Applications. CrystEngComm 2011, 13, 3506–3510. [Google Scholar] [CrossRef]
- Zong, X.; Yan, H.; Wu, G.; Ma, G.; Wen, F.; Wang, L.; Li, C. Enhancement of Photocatalytic H2 Evolution on CdS by Loading MoS2 as Cocatalyst under Visible Light Irradiation. J. Am. Chem. Soc. 2008, 130, 7176–7177. [Google Scholar] [CrossRef]
- Tang, Y.; Ng, Y.H.; Yun, J.-H.; Amal, R. Fabrication of a CuInS2 Photoelectrode Using a Single-Step Electrodeposition with Controlled Calcination Atmosphere. RSC Adv. 2013, 4, 3278–3283. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, J.; Zhang, Y.; Li, Q.; Gong, J.R. Visible Light Photocatalytic H2-Production Activity of CuS/ZnS Porous Nanosheets Based on Photoinduced Interfacial Charge Transfer. Nano Lett. 2011, 11, 4774–4779. [Google Scholar] [CrossRef]
- Cao, S.; Yu, J. G-C3N4-Based Photocatalysts for Hydrogen Generation. J. Phys. Chem. Lett. 2014, 5, 2101–2107. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef]
- Rej, S.; Bisetto, M.; Naldoni, A.; Fornasiero, P. Well-Defined Cu2O Photocatalysts for Solar Fuels and Chemicals. J. Mater. Chem. A 2021, 9, 5915–5951. [Google Scholar] [CrossRef]
- Zindrou, A.; Belles, L.; Deligiannakis, Y. Cu-Based Materials as Photocatalysts for Solar Light Artificial Photosynthesis: Aspects of Engineering Performance, Stability, Selectivity. Solar 2023, 3, 87–112. [Google Scholar] [CrossRef]
- Dong, Z.; Zhou, J.; Zhang, Z.; Jiang, Y.; Zhou, R.; Yao, C. Construction of a p–n Type S-Scheme Heterojunction by Incorporating CsPbBr3 Nanocrystals into Mesoporous Cu2O Microspheres for Efficient CO2 Photoreduction. ACS Appl. Energy Mater. 2022, 5, 10076–10085. [Google Scholar] [CrossRef]
- Tang, Z.; He, W.; Wang, Y.; Wei, Y.; Yu, X.; Xiong, J.; Wang, X.; Zhang, X.; Zhao, Z.; Liu, J. Ternary Heterojunction in RGO-Coated Ag/Cu2O Catalysts for Boosting Selective Photocatalytic CO2 Reduction into CH4. Appl. Catal. B Environ. 2022, 311, 121371. [Google Scholar] [CrossRef]
- Wu, Y.A.; McNulty, I.; Liu, C.; Lau, K.C.; Liu, Q.; Paulikas, A.P.; Sun, C.-J.; Cai, Z.; Guest, J.R.; Ren, Y.; et al. Facet-Dependent Active Sites of a Single Cu2O Particle Photocatalyst for CO2 Reduction to Methanol. Nat. Energy 2019, 4, 957–968. [Google Scholar] [CrossRef]
- Ghosh, S.; Bera, S.; Sardar, S.; Pal, S.; Camargo, F.V.A.; D’Andrea, C.; Cerullo, G. Role of Efficient Charge Transfer at the Interface between Mixed-Phase Copper-Cuprous Oxide and Conducting Polymer Nanostructures for Photocatalytic Water Splitting. ACS Appl. Mater. Interfaces 2023, 15, 18867–18877. [Google Scholar] [CrossRef]
- Chen, J.-L.; Liu, M.-M.; Xie, S.-Y.; Yue, L.-J.; Gong, F.-L.; Chai, K.-M.; Zhang, Y.-H. Cu2O-Loaded TiO2 Heterojunction Composites for Enhanced Photocatalytic H2 Production. J. Mol. Struct. 2022, 1247, 131294. [Google Scholar] [CrossRef]
- Toe, C.Y.; Zheng, Z.; Wu, H.; Scott, J.; Amal, R.; Ng, Y.H. Photocorrosion of Cuprous Oxide in Hydrogen Production: Rationalising Self-Oxidation or Self-Reduction. Angew. Chem. Int. Ed. 2018, 57, 13613–13617. [Google Scholar] [CrossRef]
- Toe, C.Y.; Scott, J.; Amal, R.; Ng, Y.H. Recent Advances in Suppressing the Photocorrosion of Cuprous Oxide for Photocatalytic and Photoelectrochemical Energy Conversion. J. Photochem. Photobiol. C Photochem. Rev. 2019, 40, 191–211. [Google Scholar] [CrossRef]
- Chen, S.; Wang, L.-W. Thermodynamic Oxidation and Reduction Potentials of Photocatalytic Semiconductors in Aqueous Solution. Chem. Mater. 2012, 24, 3659–3666. [Google Scholar] [CrossRef]
- Wu, L.; Tsui, L.; Swami, N.; Zangari, G. Photoelectrochemical Stability of Electrodeposited Cu2O Films. J. Phys. Chem. C 2010, 114, 11551–11556. [Google Scholar] [CrossRef]
- Yang, Y.; Han, J.; Ning, X.; Su, J.; Shi, J.; Cao, W.; Xu, W. Photoelectrochemical Stability Improvement of Cuprous Oxide (Cu2O) Thin Films in Aqueous Solution. Int. J. Energy Res. 2016, 40, 112–123. [Google Scholar] [CrossRef]
- Wu, F.; Banerjee, S.; Li, H.; Myung, Y.; Banerjee, P. Indirect Phase Transformation of CuO to Cu2O on a Nanowire Surface. Langmuir 2016, 32, 4485–4493. [Google Scholar] [CrossRef]
- Brezonik, P.L.; Brauner, P.A.; Stumm, W. Trace Metal Analysis by Anodic Stripping Voltammetry: Effect of Sorption by Natural and Model Organic Compounds. Water Res. 1976, 10, 605–612. [Google Scholar] [CrossRef]
- Pilbrow, J.R. Transition Ion Electron Paramagnetic Resonance; Clarendon Press: Oxford, UK, 1990; ISBN 978-0-19-855214-7. [Google Scholar]
- Tada, S.; Otsuka, F.; Fujiwara, K.; Moularas, C.; Deligiannakis, Y.; Kinoshita, Y.; Uchida, S.; Honma, T.; Nishijima, M.; Kikuchi, R. Development of CO2-to-Methanol Hydrogenation Catalyst by Focusing on the Coordination Structure of the Cu Species in Spinel-Type Oxide Mg1–XCuxAl2O4. ACS Catal. 2020, 10, 15186–15194. [Google Scholar] [CrossRef]
- Naatz, H.; Manshian, B.B.; Rios Luci, C.; Tsikourkitoudi, V.; Deligiannakis, Y.; Birkenstock, J.; Pokhrel, S.; Mädler, L.; Soenen, S.J. Model-Based Nanoengineered Pharmacokinetics of Iron-Doped Copper Oxide for Nanomedical Applications. Angew. Chem. 2020, 132, 1844–1852. [Google Scholar] [CrossRef]
- Papadas, I.T.; Kosma, C.; Deligiannakis, Y. Ternary [Al2O3–Electrolyte–Cu2+] Species: EPR Spectroscopy and Surface Complexation Modeling. J. Colloid Interface Sci. 2009, 339, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Grigoropoulou, G.; Christoforidis, K.C.; Louloudi, M.; Deligiannakis, Y. Structure-Catalytic Function Relationship of SiO2-Immobilized Mononuclear Cu Complexes: An EPR Study. Langmuir 2007, 23, 10407–10418. [Google Scholar] [CrossRef]
- Zois, D.; Vartzouma, C.; Deligiannakis, Y.; Hadjiliadis, N.; Casella, L.; Monzani, E.; Louloudi, M. Active Catalytic Centers in Silica-Supported Cu(II) and Mn(II) Biomimetic Complexes: Correlation between Catalytic and EPR Data. J. Mol. Catal. A Chem. 2007, 261, 306–317. [Google Scholar] [CrossRef]
- Locatelli, C.; Torsi, G. Simultaneous Square Wave Anodic Stripping Voltammetric Determination of Cr, Pb, Sn, Sb, Cu, Zn in Presence of Reciprocal Interference: Application to Meal Matrices. Microchem. J. 2004, 78, 175–180. [Google Scholar] [CrossRef]
- Solakidou, M.; Giannakas, A.; Georgiou, Y.; Boukos, N.; Louloudi, M.; Deligiannakis, Y. Efficient Photocatalytic Water-Splitting Performance by Ternary CdS/Pt-N-TiO2 and CdS/Pt-N,F-TiO2: Interplay between CdS Photo Corrosion and TiO2-Dopping. Appl. Catal. B Environ. 2019, 254, 194–205. [Google Scholar] [CrossRef]
- Teoh, W.Y.; Amal, R.; Mädler, L. Flame Spray Pyrolysis: An Enabling Technology for Nanoparticles Design and Fabrication. Nanoscale 2010, 2, 1324. [Google Scholar] [CrossRef]
- Strobel, R.; Pratsinis, S.E. Flame Aerosol Synthesis of Smart Nanostructured Materials. J. Mater. Chem. 2007, 17, 4743. [Google Scholar] [CrossRef]
- Waser, O.; Groehn, A.J.; Eggersdorfer, M.L.; Pratsinis, S.E. Air Entrainment During Flame Aerosol Synthesis of Nanoparticles. Aerosol Sci. Technol. 2014, 48, 1195–1206. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, Z.; Yan, K.; Zhao, H.; Zhang, J. One-Step Synthesis of CuO–Cu2O Heterojunction by Flame Spray Pyrolysis for Cathodic Photoelectrochemical Sensing of l-Cysteine. ACS Appl. Mater. Interfaces 2017, 9, 40452–40460. [Google Scholar] [CrossRef]
- Athanassiou, E.K.; Grass, R.N.; Stark, W.J. Large-Scale Production of Carbon-Coated Copper Nanoparticles for Sensor Applications. Nanotechnology 2006, 17, 1668–1673. [Google Scholar] [CrossRef]
- Belles, L.; Moularas, C.; Smykała, S.; Deligiannakis, Y. Flame Spray Pyrolysis Co3O4/CoO as Highly-Efficient Nanocatalyst for Oxygen Reduction Reaction. Nanomaterials 2021, 11, 925. [Google Scholar] [CrossRef]
- Psathas, P.; Moularas, C.; Smykała, S.; Deligiannakis, Y. Highly Crystalline Nanosized NaTaO3/NiO Heterojunctions Engineered by Double-Nozzle Flame Spray Pyrolysis for Solar-to-H2 Conversion: Toward Industrial-Scale Synthesis. ACS Appl. Nano Mater. 2023, 6, 2658–2671. [Google Scholar] [CrossRef]
- Psathas, P.; Zindrou, A.; Papachristodoulou, C.; Boukos, N.; Deligiannakis, Y. In Tandem Control of La-Doping and CuO-Heterojunction on SrTiO3 Perovskite by Double-Nozzle Flame Spray Pyrolysis: Selective H2 vs. CH4 Photocatalytic Production from H2O/CH3OH. Nanomaterials 2023, 13, 482. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A Graphical User Interface for the Rietveld Refinement Program BGMN. J. Appl. Cryst. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Stoll, S.; Schweiger, A. EasySpin, a Comprehensive Software Package for Spectral Simulation and Analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Cuppett, J.D.; Duncan, S.E.; Dietrich, A.M. Evaluation of Copper Speciation and Water Quality Factors That Affect Aqueous Copper Tasting Response. Chem. Senses 2006, 31, 689–697. [Google Scholar] [CrossRef]
- Koirala, R.; Pratsinis, S.E.; Baiker, A. Synthesis of Catalytic Materials in Flames: Opportunities and Challenges. Chem. Soc. Rev. 2016, 45, 3053–3068. [Google Scholar] [CrossRef]
- Athanassiou, E.K.; Grass, R.N.; Stark, W.J. Chemical Aerosol Engineering as a Novel Tool for Material Science: From Oxides to Salt and Metal Nanoparticles. Aerosol Sci. Technol. 2010, 44, 161–172. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Chen, Q.; Zhou, Y.-J.; Li, H.-M.; Fu, J.-W.; Liu, M. Cu-Based Bimetallic Catalysts for CO2 Reduction Reaction. Adv. Sens. Energy Mater. 2022, 1, 100023. [Google Scholar] [CrossRef]
- Ali, S.; Razzaq, A.; Kim, H.; In, S.-I. Activity, Selectivity, and Stability of Earth-Abundant CuO/Cu2O/Cu0-Based Photocatalysts toward CO2 Reduction. Chem. Eng. J. 2022, 429, 131579. [Google Scholar] [CrossRef]
- Pedersen, O.; Colmer, T.; Sand-Jensen, K. Underwater Photosynthesis of Submerged Plants—Recent Advances and Methods. Front. Plant Sci. 2013, 4, 140. [Google Scholar] [CrossRef]
- Pan, H.; Heagy, M.D. Photons to Formate: A Review on Photocatalytic Reduction of CO2 to Formic Acid. Nanomaterials 2020, 10, 2422. [Google Scholar] [CrossRef]
- Pan, H.; Chowdhury, S.; Premachandra, D.; Olguin, S.; Heagy, M.D. Semiconductor Photocatalysis of Bicarbonate to Solar Fuels: Formate Production from Copper (I) Oxide. ACS Sustain. Chem. Eng. 2018, 6, 1872–1880. [Google Scholar] [CrossRef]
- Hathaway, B.J.; Billing, D.E. The Electronic Properties and Stereochemistry of Mono-Nuclear Complexes of the Copper(II) Ion. Coord. Chem. Rev. 1970, 5, 143–207. [Google Scholar] [CrossRef]
- Peisach, J.; Blumberg, W.E. Structural Implications Derived from the Analysis of Electron Paramagnetic Resonance Spectra of Natural and Artificial Copper Proteins. Arch. Biochem. Biophys. 1974, 165, 691–708. [Google Scholar] [CrossRef]
- Conrad, F. Microwave-Assisted Synthesis of Nanostructured Gallium Oxide Materials. Ph.D. Thesis, University of Zurich, Zürich, Switzerland, 2012. [Google Scholar] [CrossRef]
- Sharpe, P.K.; Vickerman, J.C. Solid State Properties of Copper Containing Spinel Solid Solutions (CuxMg1–XAl2O4). J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1977, 73, 505. [Google Scholar] [CrossRef]
- Godiksen, A.; Vennestrøm, P.N.R.; Rasmussen, S.B.; Mossin, S. Identification and Quantification of Copper Sites in Zeolites by Electron Paramagnetic Resonance Spectroscopy. Top. Catal. 2017, 60, 13–29. [Google Scholar] [CrossRef]
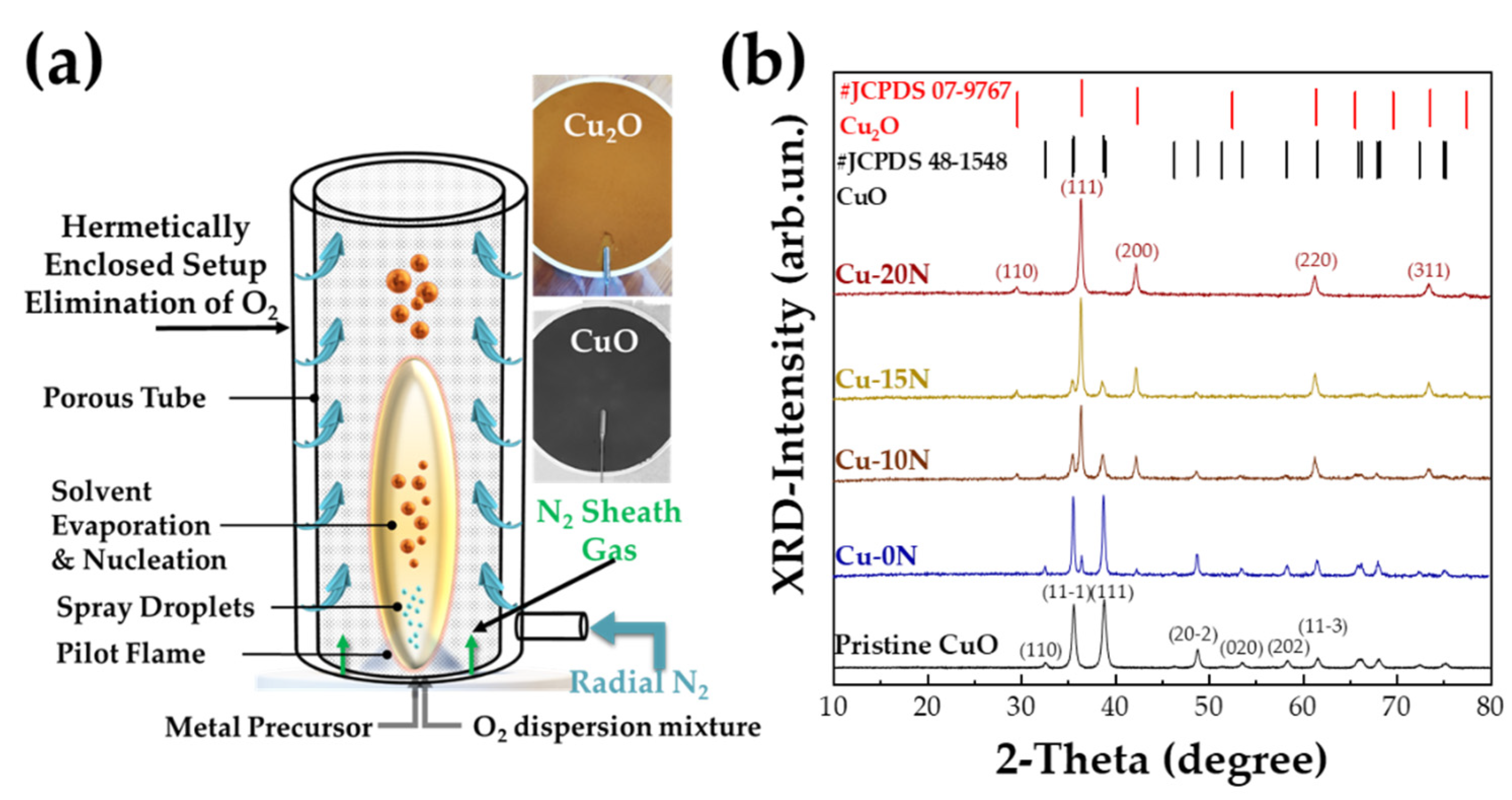
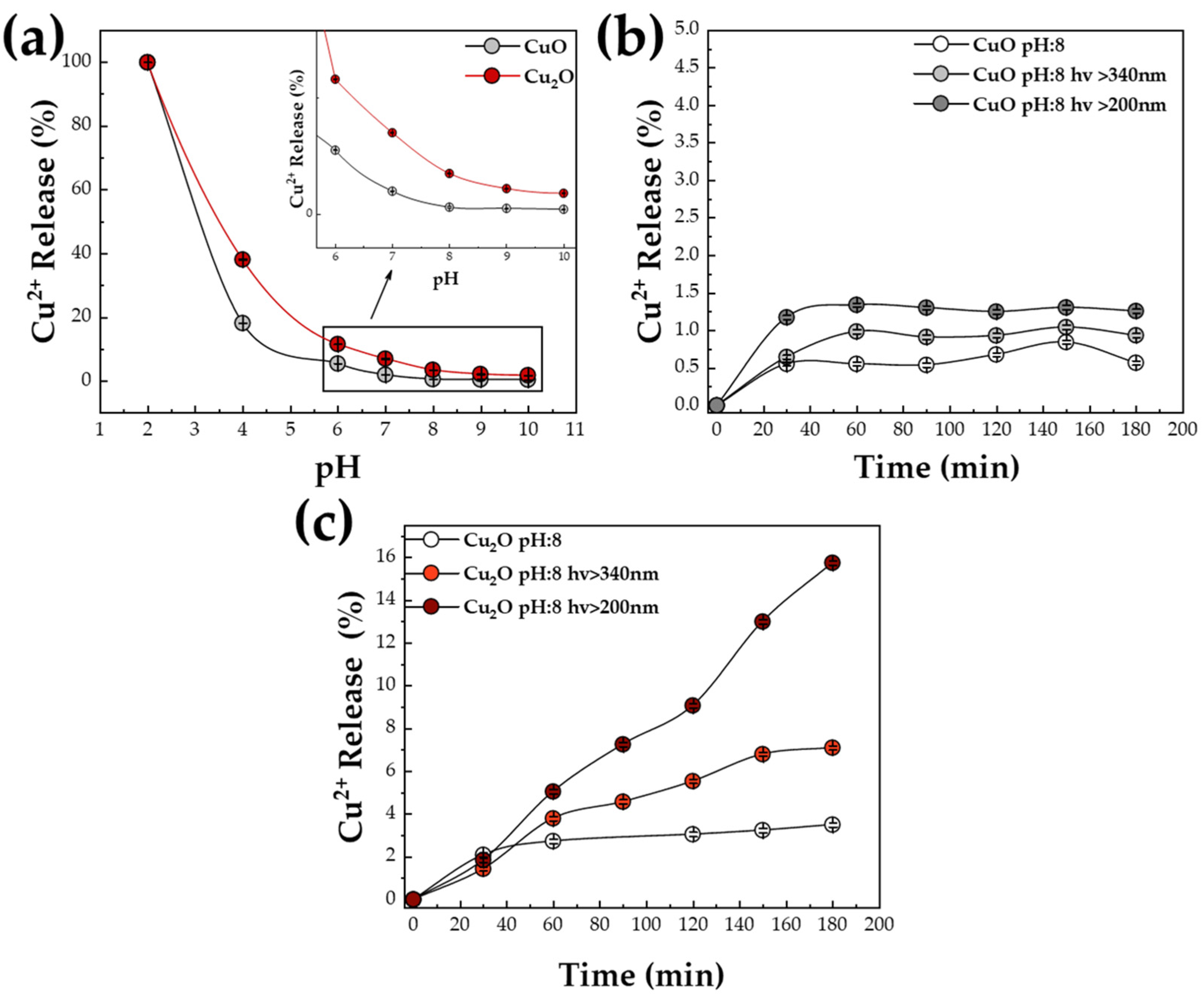

| Radial N2 (L min−1) | CuO (%) | Cu2O (%) | dXRD CuO (nm) | dXRD Cu2O (nm) | |
|---|---|---|---|---|---|
| Pristine CuO | - | 100 ± 1 | - | 20 ± 1 | - |
| Cu-0N | 0 | 90 ± 2 | 10 ± 2 | 29 ± 1 | 34 ± 1 |
| Cu-10N | 10 | 60 ± 2 | 40 ± 2 | 21 ± 1 | 30 ± 1 |
| Cu-15N | 15 | 40 ± 3 | 60 ± 2 | 22 ± 1 | 31 ± 1 |
| Cu-20N (Cu2O) | 20 | 5 ± 3 | 95 ± 2 | - | 25 ± 1 |
| g [gx, gy, gz] | Az = A//Gauss | Reference | |
|---|---|---|---|
| Cu2+ from Cu2O + hv (Xenon > 200 nm) | gx = gy = g̝⊥ = 2.08 gz = g// = 2.4 | 144 | This work |
| Cu2+ from Cu2O + hv (Xenon > 200 nm) + NaHCO3 | gx = 2.055 gy = 2.074 gz = g// = 2.342 | 162 | This work |
| Cu2+ + H2O (pH:2) | gx = 2.078 gy = 2.078 gz = g// = 2.42 | 126 | [29] |
| (Cu2+ in zeolites) Cu-CHA hydrated | gx = gy = g̝⊥ = 2.07 gz = g// = 2.394 | 157 | [57] |
| (Cu2+ in zeolites) Cu-MOR hydrated | gx = gy = g̝⊥ = 2.08 gz = g// = 2.4 | 154 | [57] |
| Material |
CuO (%) |
Cu2O (%) |
dXRD CuO (nm) |
dXRD Cu2O (nm) |
|---|---|---|---|---|
| Cu2O (Cu-20N) | 5 ± 3 | 95 ± 3 | - | 25 ± 1 |
| Cu2O + 30 mM NaHCO3 + hv > 200 nm | 60 ± 3 | 40 ± 3 | 17 ± 1 | 26 ± 1 |
| Cu2O + 30 mM NaHCO3 + hv > 200 nm + 2-propanol | 25 ± 3 | 75 ± 3 | 8 ± 1 | 33 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zindrou, A.; Deligiannakis, Y. Quantitative In Situ Monitoring of Cu-Atom Release by Cu2O Nanocatalysts under Photocatalytic CO2 Reduction Conditions: New Insights into the Photocorrosion Mechanism. Nanomaterials 2023, 13, 1773. https://doi.org/10.3390/nano13111773
Zindrou A, Deligiannakis Y. Quantitative In Situ Monitoring of Cu-Atom Release by Cu2O Nanocatalysts under Photocatalytic CO2 Reduction Conditions: New Insights into the Photocorrosion Mechanism. Nanomaterials. 2023; 13(11):1773. https://doi.org/10.3390/nano13111773
Chicago/Turabian StyleZindrou, Areti, and Yiannis Deligiannakis. 2023. "Quantitative In Situ Monitoring of Cu-Atom Release by Cu2O Nanocatalysts under Photocatalytic CO2 Reduction Conditions: New Insights into the Photocorrosion Mechanism" Nanomaterials 13, no. 11: 1773. https://doi.org/10.3390/nano13111773
APA StyleZindrou, A., & Deligiannakis, Y. (2023). Quantitative In Situ Monitoring of Cu-Atom Release by Cu2O Nanocatalysts under Photocatalytic CO2 Reduction Conditions: New Insights into the Photocorrosion Mechanism. Nanomaterials, 13(11), 1773. https://doi.org/10.3390/nano13111773








