Removal of Acetaminophen Drug from Wastewater by Fe3O4 and ZSM-5 Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Batch Adsorption Studies
2.3. Internal Validation Method
2.4. The Batch Adsorption Study
2.5. Adsorption Isotherms
3. Results and Discussions
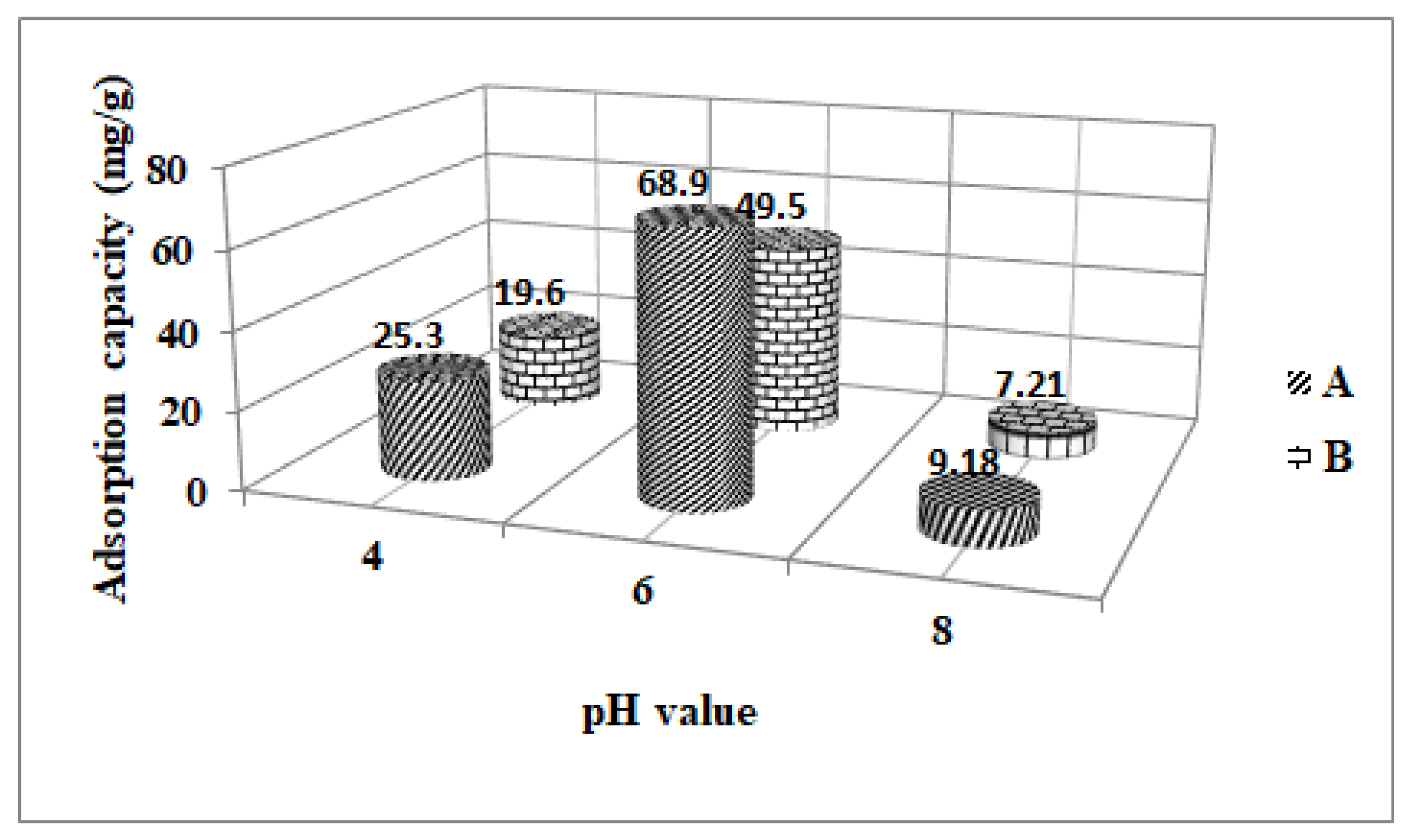
3.1. The Effect of Solution pH on the Quantity of Acetaminophen Removed by Fe3O4 and ZSM-5
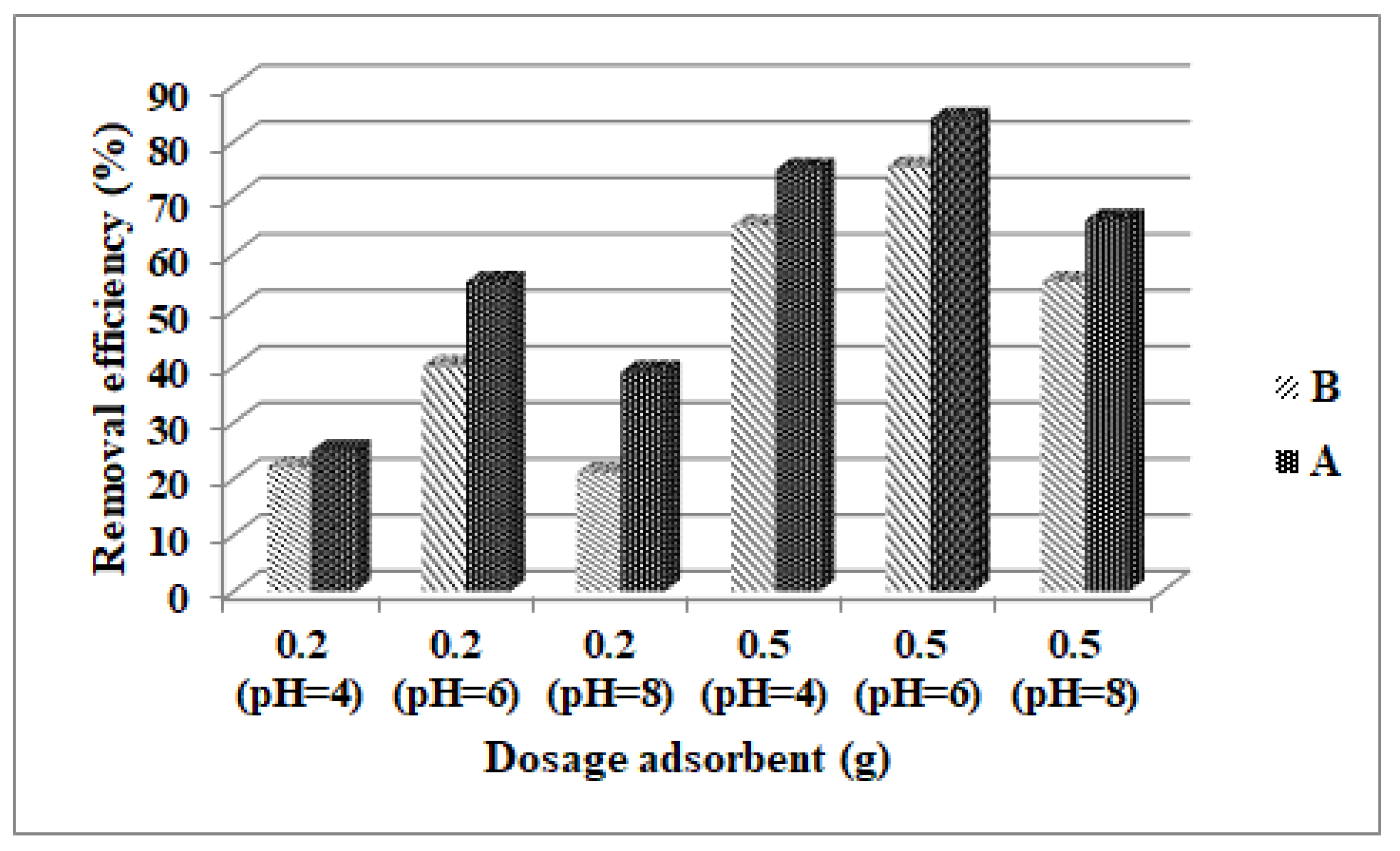
3.2. The Effect of Dosage Adsorbent on the Removal Efficiency of Fe3O4 and ZSM-5
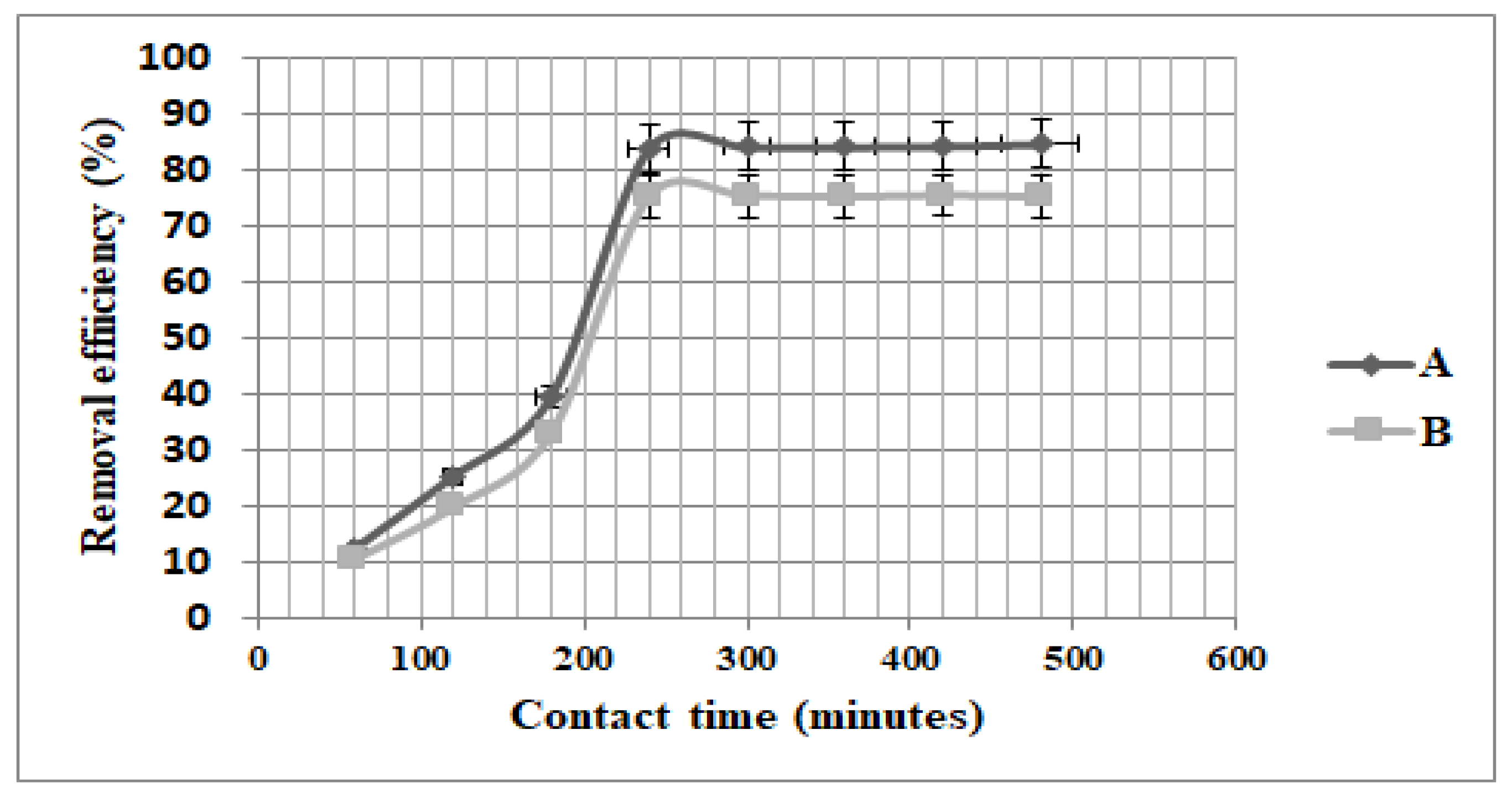
3.3. The Effect of Contact Time on the Removal Efficiency of Fe3O4 and ZSM-5
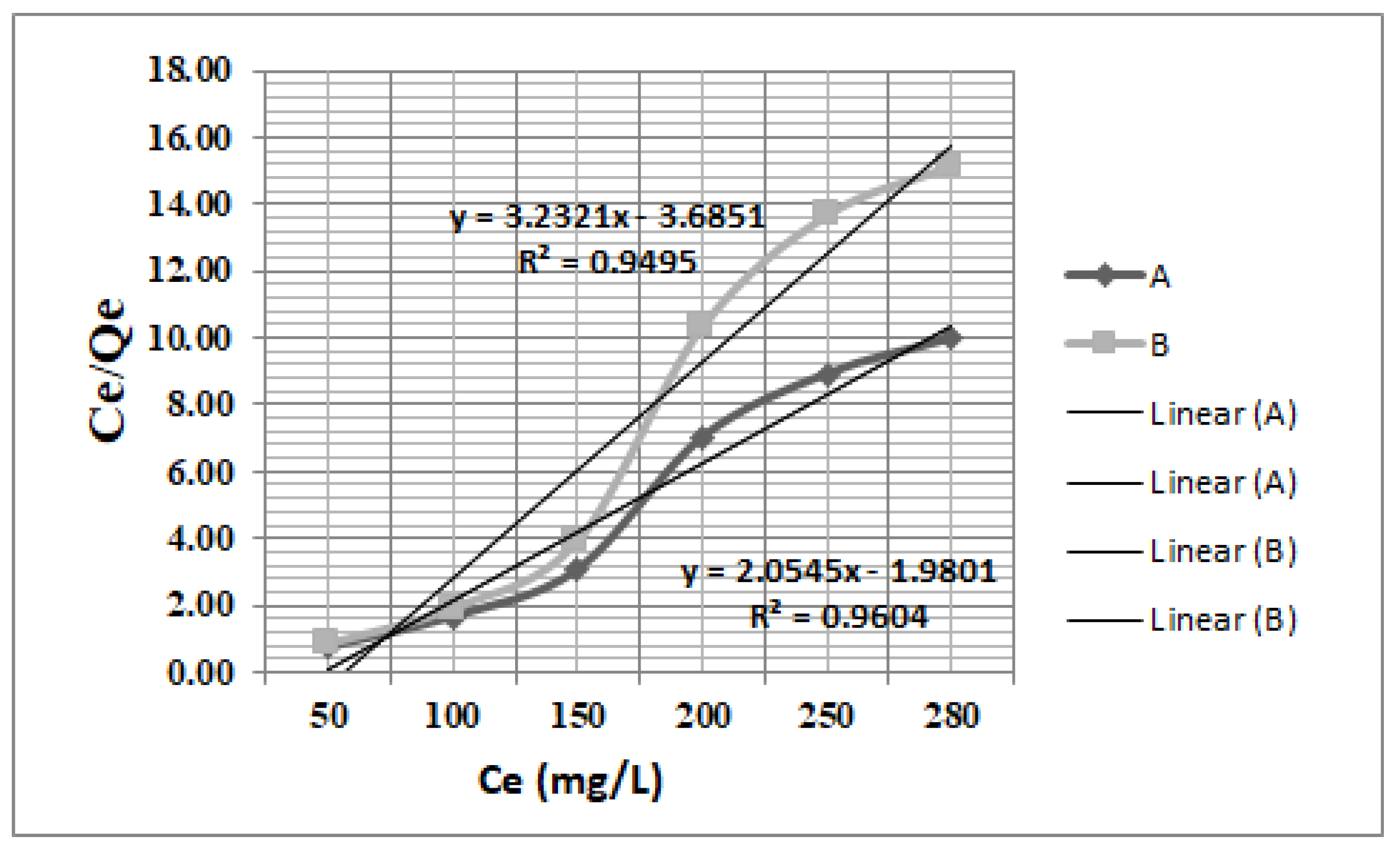
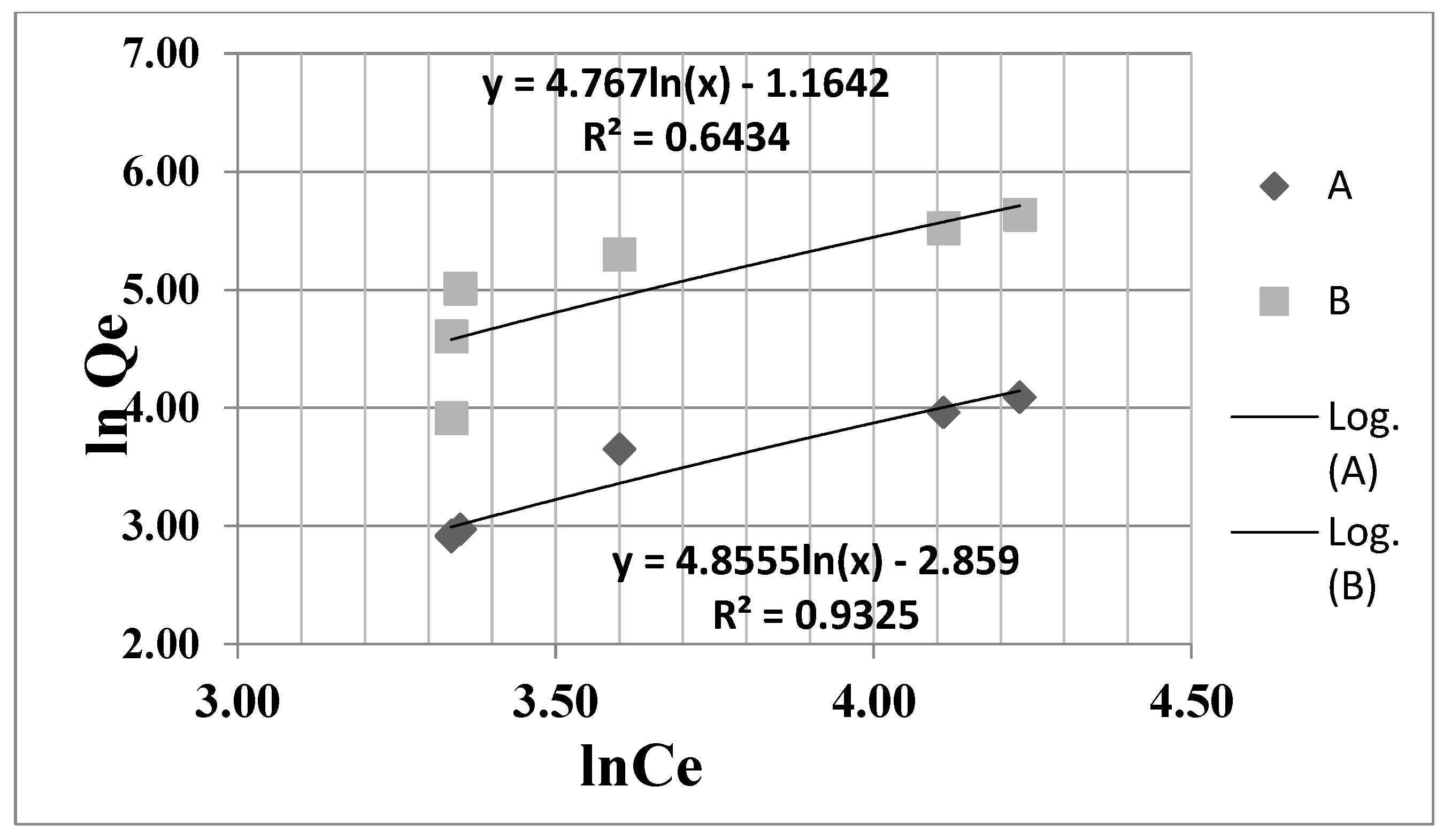
3.4. Langmuir and Freundlich Adsorption Isotherms
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gotore, O.; Munodawafa, A.; Rameshprabu, R. Biochar derived from non-customized matamba fruit shell as an adsorbent for wastewater treatment. J. Bioresour. Bioprod. 2022, 7, 109–115. [Google Scholar]
- Al Arsh, B. New generation nano-adsorbents for the removal of emerging pollutants in water. J. Mol. Liq. 2018, 261, 583–593. [Google Scholar]
- Ocampo-Perez, R.; Aguilar-Madera, C.G.; Diaz-Blancas, V. 3D modeling of overall ad-sorption rate of acetaminophen on activated carbon pellets. Chem. Eng. J. 2017, 321, 510–520. [Google Scholar] [CrossRef]
- Saucier, C.; Karthickeyan, P.; Ranjithkumar, V.; Lima, E.C.; Dosreis, G.S.; Brum, I.A.S. Efficient removal of amoxicillin and paracetamol from aqueous solutions using magnetic activated carbon. Environ. Sci. Pollut. Res. 2017, 24, 5918–5932. [Google Scholar] [CrossRef] [PubMed]
- Dutta, M.; Das, U.; Mondal, S.; Bhattachriya, S.; Khatun, R.; Bagal, R. Adsorption of acetaminophen by using tea waste derived activated carbon. Int. J. Environ. Sci. 2015, 6, 270–281. [Google Scholar]
- Tiwari, D.K.; Behari, J.; Sen, P. Application of Nanoparticles in Waste Water Treatment. World Appl. Sci. J. 2008, 3, 417–433. [Google Scholar]
- Somma, S.; Reverchon, E.; Baldino, L. Water Purification of Classical and Emerging Organic Pollutants: An Extensive Review. Chem. Eng. J. 2021, 5, 47. [Google Scholar] [CrossRef]
- Morone, A.; Mulay, P.; Kamble, S.P. Removal of pharmaceutical and personal care products from wastewater using advanced materials. PPCP Waste Manag. Treat. Technol. 2019, 8, 173–212. [Google Scholar]
- Pirvu, F.; Covaliu-Mierlă, C.I.; Paun, I.; Paraschiv, G.; Vasile, I. Treatment of Wastewater Containing Nonsteroidal Anti-Inflammatory Drugs Using Activated Carbon Material. Materials 2022, 15, 559. [Google Scholar] [CrossRef]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging pollutants in water: A. review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef]
- Lindqvist, N.; Tuhkanen, T.; Kronberg, L. Occurrence of acidic pharmaceuticals in raw and treated sew-ages and in receiving waters. Water Res. 2005, 39, 2219–2228. [Google Scholar] [CrossRef] [PubMed]
- Deninger, M.J.; Schoenwald, R.D. Uptake of Acetaminophen, indomethacin and ketoprofen into isolated rabbit parietal cells. J. Pharm. Sci. 2000, 52, 501–519. [Google Scholar]
- Katal, R.; Farahani, M.H.D.A.; Jiang Yong, H. Degradation of acetaminophen in a photocatalytic (batch and continuous system) and photo electrocatalytic process by application of faceted-TiO2. Sep. Purif. Technol. 2020, 230, 115859. [Google Scholar] [CrossRef]
- Wang, L.; Bian, Z. Photocatalytic degradation of paracetamol on Pd-BiVO4 under visible light irradiation. Chemosphere 2020, 239, 124815. [Google Scholar] [CrossRef] [PubMed]
- Covaliu, I.C.; Neamtu, J.; Georgescu, G.; Malaeru, T.; Cristea, C.; Jitaru, I. Synthesis and characterization of ferrites (Fe3O4/CuFe2O4)—Calcium alginate hybrids for magnetic resonance imaging. Dig. J. Nanomater. Biostruct. 2011, 6, 245–252. [Google Scholar]
- Covaliu, C.; Paraschiv, G.; Vasile, E.; Matei, E. Nanostructured zeolites for heavy metals removal from wastewater. In Proceedings of the Applied Nanotechnology and Nanoscience International Conference, Rome, Italy, 18–20 October 2017; p. 151. Available online: https://premc.org/doc/ANNIC2017/ANNIC2017_Book_Of_Abstracts.pdf (accessed on 20 October 2022).
- Jayasree, P.; Remya, N. Photocatalytic degradation of paracetamol using aluminosilicate supported TiO2. Water Sci. Technol. 2020, 82, 2114–2124. [Google Scholar] [CrossRef]
- Tepe, O.; Tunç, Z.; Yildiz, B.; Sahin, M. Efficient removal of paracetamol by manganese oxide octahedral molecular sieves (OMS-2) and persulfate. Water Air Soil Pollut. 2020, 231, 238. [Google Scholar] [CrossRef]
- Periyasamy, S.; Muthuchamy, M. Electrochemical oxidation of paracetamol in water by graphite anode: Effect of pH, electrolyte concentration and current density. J. Environ. Chem. Eng. 2018, 6, 7358–7367. [Google Scholar] [CrossRef]
- Elsayed, A.E.; Osman, D.I.; Attia, S.K.; Ahmed, H.M.; Shoukry, E.M.; Mostafa, Y.M.; Taman, A.R. A study on the removal characteristics of organic and inorganic pollutants from wastewater by low cost biosorbent. Egypt. J. Chem. 2020, 63, 1429–1442. [Google Scholar] [CrossRef]
- Krishnan, K.A.; Anirudhan, T. Removal of cadmium (II) from aqueous solutions by steam-activated sul-phurised carbon prepared from sugar-cane bagasse pith: Kinetics and equilibrium studies. Water SA 2003, 29, 147–156. [Google Scholar] [CrossRef]
- Gusain, R.; Kumar, N.; Ray, S.S. Recent advances in carbon nanomaterial-based adsorbents for water purification. Coord. Chem. Rev. 2020, 405, 213111. [Google Scholar] [CrossRef]
- Ghane, G.I. Atmospheric Leaching of Enargite in Iron Sulfate Solutions Catalyzed by Activated Carbon. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Cananda, 2011. [Google Scholar]
- Matsui, M.; Kiyozumi, Y.; Yamamoto, T.; Mizushina, Y.; Mizukami, F.; Sakaguchi, K. Selective adsorption of biopolymers on zeolites. Chem. A Eur. J. 2001, 7, 1555–1560. [Google Scholar] [CrossRef]
- Pathak, P.; Mandavgane, S.; Kulkarni, B.D. Fruit peel waste as a novel low-cost bio adsorbent. Rev. Chem. Eng. 2015, 31, 361–381. [Google Scholar] [CrossRef]
- MahmudI, M.; Arsad, S.; Amalia, M.C.; Rohmaningsih, H.A.; Prasetiya, F.S. An Alternative Activated Carbon from Agricultural Waste on Chromium. Removal. J. Ecol. Eng. 2020, 21, 1–9. [Google Scholar] [CrossRef]
- Jjagwe, J.; Wilberforce, O.P.; Menya, E.; Kalibbalac, H.M. Synthesis and Application of Granular Activated Carbon from Biomass Waste Materials for Water Treatment: A Review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Poudel, M.B.; Awasthi, G.P.; Kim, H.J. Novel insight into the adsorption of Cr(VI) and Pb(II) ions by MOF derived Co-Al layered double hydroxide @hematite nanorods on 3D porous carbon nanofiber network. J. Chem. Eng. 2021, 417, 129312. [Google Scholar] [CrossRef]
- ICH Topic Q 2 (R1), Validation of Analytical Procedures: Text and Methodology, EMA. CPMP/ICH/381/95. 2015. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-2-r1-validation-analytical-procedures-text-methodology-step-5_en.pdf (accessed on 20 October 2022).
- ISO/CEI: Guide 98-3/2008; Uncertainty of Measurement. Part 3. Guide to the Expression of Uncertainty in Measurement (GUM: 1995). International Organization for Standardization: Geneva, Switzerland, 2008. Available online: https://www.iso.org/standard/50461.html (accessed on 20 October 2022).

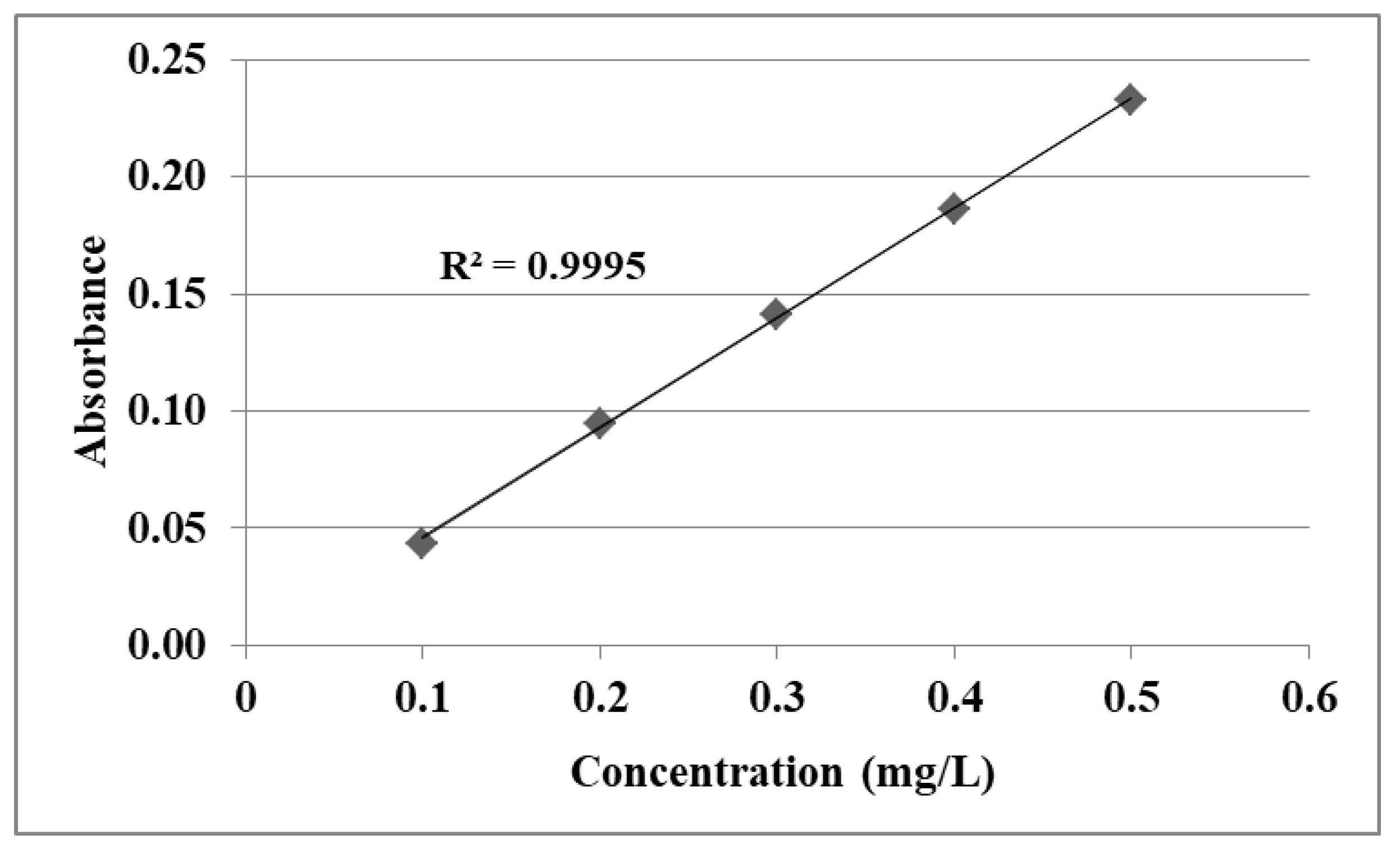
| Compound | Linearity Range (mg/L) | Regression Equations | R2 | λ (nm) |
|---|---|---|---|---|
| Acetaminophen dissolved in 0.1 M HCl | 0.10–0.50 | y = 0.4591x + 0.0004 | 0.9994 | 302 |
| No. | Acetaminophen | ||
|---|---|---|---|
| Performance Parameters | U.M. | Obtained Results | |
| 1 | Precision | mg/L | 0.03 |
| 2 | Detection limit (LOD) | mg/L | 0.04 |
| 3 | Quantification limit (LOQ) | mg/L | 0.12 |
| 4 | Extended uncertainty | % | 17.5 |
| Langmuir Equation | Observations |
|---|---|
| (3) | Ce—is the equilibrium concentration of acetaminophen, mg/L; qe—is the amount of acetaminophen adsorbed per gram of the asorbent at equilibrium mg/g; qmax—is the maximum quantity, mg/g; KL—is the Langmuir isotherm constant, L/mg; qmax and KL were obtained from the slope and intercept of the plots. |
| (4) | RL—of a dimensionless constant separation factor or equilibrium parameter RL—value indicates that the process is unfavorable if RL > 1; linear if RL = 1; favorable if 0 < RL < 1 and irreversible if RL = 0; KL is the Langmuir constant; C0—is the initial concentration, measured in mg/L. |
| Freundlich Equation | Observations |
| (5) | KF is Freundlich constant which represents sorption capacity and n is the Freundlich constant that shows sorption intensity. KF and n were calculated from the intercepts and slopes of the Freundlich plots. Ce—is the equilibrium concentration of acetaminophen, mg/L; |
| Adsorbent Material | Langmuir Parameters | Freundlich Parameters | |||||
|---|---|---|---|---|---|---|---|
| Qmax (mg/g) | KL (L/mg) | RL | R2 | KF (L/g) | 1/n | R2 | |
| Fe3O4 | 68.9 | 0.149 | 0.79 | 0.9644 | 42.13 | 0.356 | 0.9325 |
| ZSM-5 | 49.5 | 0.103 | 0.86 | 0.9530 | 33.89 | 0.314 | 0.6434 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirvu, F.; Covaliu-Mierlă, C.I.; Catrina, G.A. Removal of Acetaminophen Drug from Wastewater by Fe3O4 and ZSM-5 Materials. Nanomaterials 2023, 13, 1745. https://doi.org/10.3390/nano13111745
Pirvu F, Covaliu-Mierlă CI, Catrina GA. Removal of Acetaminophen Drug from Wastewater by Fe3O4 and ZSM-5 Materials. Nanomaterials. 2023; 13(11):1745. https://doi.org/10.3390/nano13111745
Chicago/Turabian StylePirvu, Florinela, Cristina Ileana Covaliu-Mierlă, and Gina Alina Catrina. 2023. "Removal of Acetaminophen Drug from Wastewater by Fe3O4 and ZSM-5 Materials" Nanomaterials 13, no. 11: 1745. https://doi.org/10.3390/nano13111745
APA StylePirvu, F., Covaliu-Mierlă, C. I., & Catrina, G. A. (2023). Removal of Acetaminophen Drug from Wastewater by Fe3O4 and ZSM-5 Materials. Nanomaterials, 13(11), 1745. https://doi.org/10.3390/nano13111745






