Mesoporous-Structure MOF-14-Based QCM p-Xylene Gas Sensor
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of MOF-14 with Microporous Structure
2.3. Synthesis of MOF-14 with Mesoporous Structure
2.4. Fabrication of QCM Sensors and Measuring Equipment
2.5. Characterization
3. Results
3.1. Characterization of MOF-14
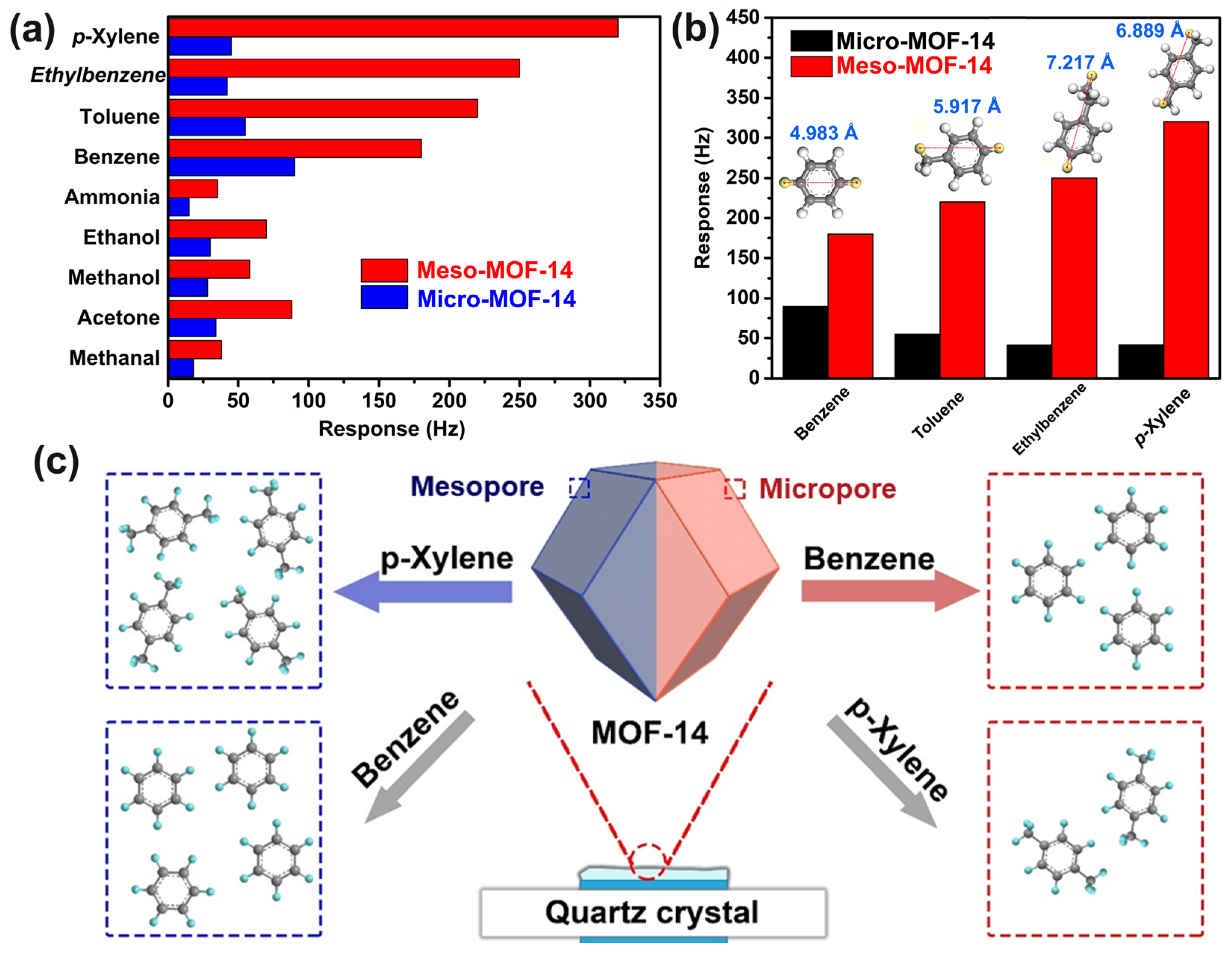
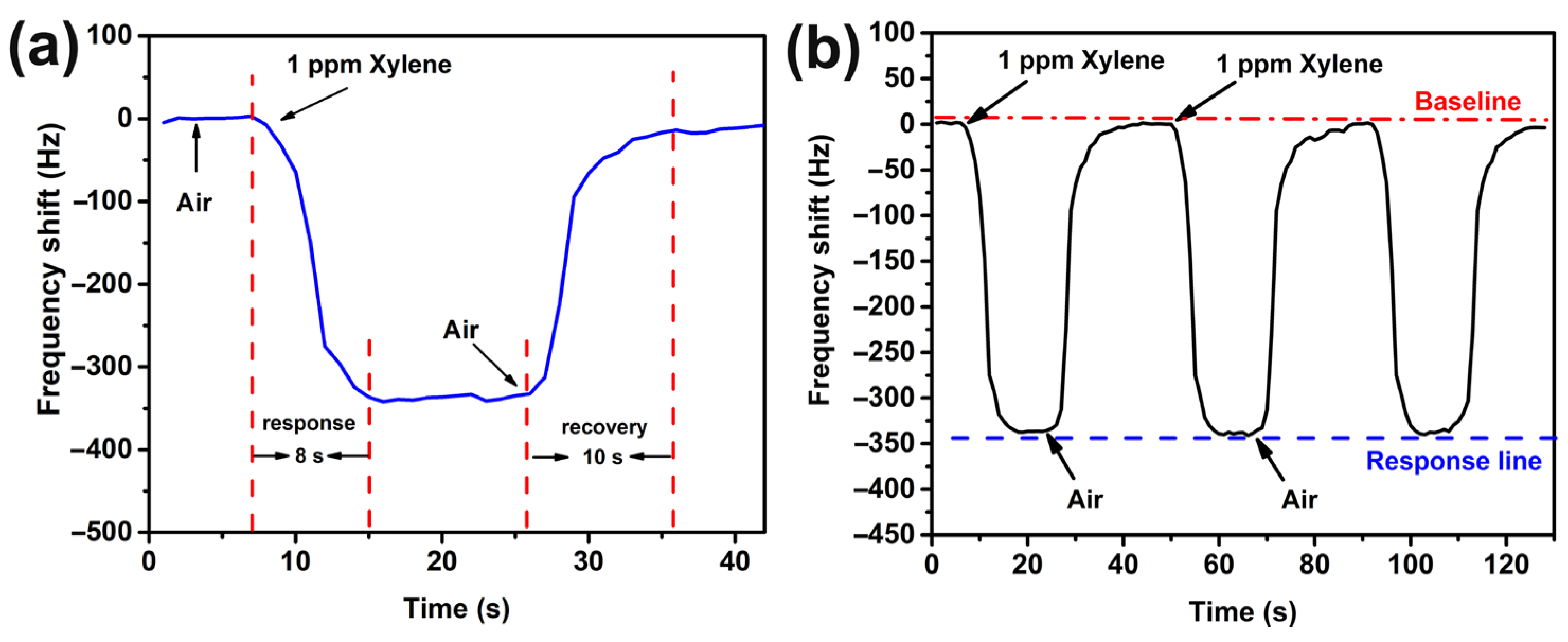
3.2. Sensing Performance of MOF-14-Based QCM Sensors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.-F.; Li, N.; Jiang, G.-B.; Liu, J.-M.; Jönsson, J.A.; Wen, M.-J. Disposable ionic liquid coating for headspace solid-phase microextraction of benzene, toluene, ethylbenzene, and xylenes in paints followed by gas chromatography–flame ionization detection. J. Chromatogr. A 2005, 1066, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Torad, N.L.; Kim, J.; Kim, M.; Lim, H.; Na, J.; Alshehri, S.M.; Ahamad, T.; Yamauchi, Y.; Eguchi, M.; Ding, B.; et al. Nanoarchitectured porous carbons derived from ZIFs toward highly sensitive and selective QCM sensor for hazardous aromatic vapors. J. Hazard. Mater. 2020, 405, 124248. [Google Scholar] [CrossRef] [PubMed]
- Benignus, V.A. Health effects of toluene: A review. Neurotoxicology 1981, 2, 567–588. [Google Scholar] [PubMed]
- Singha, N.; Neogi, S.; Pramanik, B.; Das, S.; Dasgupta, A.; Ghosh, R.; Das, D. Ultrafast, highly sensitive, and selective detection of p-xylene at room temperature by peptide-hydrogel-based composite material. ACS Appl. Polym. Mater. 2019, 1, 2267–2272. [Google Scholar] [CrossRef]
- Zhu, M.-Y.; Zhang, L.-X.; Yin, J.; Chen, J.-J.; Bie, L.-J.; Fahlman, B.D. Physisorption induced p-xylene gas-sensing performance of (C4H9NH3)2PbI4 layered perovskite. Sens. Actuators B Chem. 2018, 282, 659–664. [Google Scholar] [CrossRef]
- Kim, T.H.; Kwak, C.H.; Lee, J.H. NiO/NiWO4 composite yolk-shell spheres with nanoscale nio outer layer for ultrasen-sitive and selective detection of sub-ppm-level p-xylene. ACS Appl. Mater. Interfaces 2017, 9, 32034–32043. [Google Scholar] [CrossRef]
- Loomis, D.; Guyton, K.Z.; Grosse, Y.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Vilahur, N.; Mattock, H.; Straif, K. Carcinogenicity of benzene. Lancet Oncol. 2017, 18, S147020451730832X. [Google Scholar] [CrossRef]
- Potter, D.W.; Pawliszyn, J. Detection of substituted benzenes in water at the pg/ml level using solid-phase microextraction and gas chromatography—Ion trap mass spectrometry. J. Chromatogr. A 1992, 625, 247–255. [Google Scholar] [CrossRef]
- Schweigkofler, M.; Niessner, R. Determination of siloxanes and voc in landfill gas and sewage gas by canister sampling and GC-MS/AES analysis. Environ. Sci. Technol. 1999, 33, 3680–3685. [Google Scholar] [CrossRef]
- Xia, C.; Zhang, S.; Sun, D.; Jiang, B.; Wang, W.; Xin, X. Coassembly of Mixed Weakley-Type Polyoxometalates to Novel Nanoflowers with Tunable Fluorescence for the Detection of Toluene. Langmuir 2018, 34, 6367–6375. [Google Scholar] [CrossRef]
- Meng, F.L.; Li, X.Z.; Yuan, Z.Y.; Lei, Y.F.; Qi, T.Y.; Li, J. Ppb-level xylene gas sensors based on Co3O4 nanoparticle-coated reduced graphene oxide (RGO) nanosheets operating at low temperature. IEEE Trans. Instrum. Meas. 2021, 70, 9511510. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, Y.; Jiang, Y.; Xu, M.; Jiang, J. Wearable electrochemical gas sensor for methanol leakage detection. Microchem. J. 2023, 190, 108715. [Google Scholar] [CrossRef]
- Weber, I.C.; Rüedi, P.; Šot, P.; Güntner, A.T.; Pratsinis, S.E. Handheld Device for Selective Benzene Sensing over Toluene and Xylene. Adv. Sci. 2022, 9, 34837486. [Google Scholar] [CrossRef]
- Mhanna, M.; Sy, M.; Arfaj, A.; Llamas, J.; Farooq, A. Laser-based selective BTEX sensing using deep neural networks. Opt. Lett. 2022, 47, 3247–3250. [Google Scholar] [CrossRef]
- Dutta, K.; Chattopadhyay, P.P.; Lu, C.W.; Ho, M.S.; Bhattacharyya, P. A highly sensitive BTX sensor based on electro-chemically derived wall connected TiO2 nanotubes. Appl. Surf. Sci. 2015, 354, 353–361. [Google Scholar] [CrossRef]
- Joshi, N.; da Silva, L.F.; Jadhav, H.S.; Shimizu, F.M.; Suman, P.H.; M’peko, J.-C.; Orlandi, M.O.; Gil Seo, J.; Mastelaro, V.R.; Oliveira, O.N. Yolk-shelled ZnCo2O4 microspheres: Surface properties and gas sensing application. Sens. Actuators B Chem. 2018, 257, 906–915. [Google Scholar] [CrossRef]
- Koo, W.-T.; Choi, S.-J.; Jang, J.-S.; Kim, I.-D. Metal-Organic Framework Templated Synthesis of Ultrasmall Catalyst Loaded ZnO/ZnCo2O4 Hollow Spheres for Enhanced Gas Sensing Properties. Sci. Rep. 2017, 7, 45074. [Google Scholar] [CrossRef]
- Speller, N.C.; Siraj, N.; McCarter, K.S.; Vaughan, S.; Warner, I.M. QCM virtual sensor array: Vapor identification and molecular weight approximation. Sens. Actuators B Chem. 2017, 246, 952–960. [Google Scholar] [CrossRef]
- Ogimoto, Y.; Selyanchyn, R.; Takahara, N.; Wakamatsu, S.; Lee, S.-W. Detection of ammonia in human breath using quartz crystal microbalance sensors with functionalized mesoporous SiO2 nanoparticle films. Sens. Actuators B Chem. 2015, 215, 428–436. [Google Scholar] [CrossRef]
- Hao, R.; Wang, D.; Zhang, X.; Zuo, G.; Wei, H.; Yang, R.; Zhang, Z.; Cheng, Z.; Guo, Y.; Cui, Z.; et al. Rapid detection of Bacillus anthracis using monoclonal antibody functionalized QCM sensor. Biosens. Bioelectron. 2009, 24, 1330–1335. [Google Scholar] [CrossRef]
- Henry, T.; Martins, P.; Eustache, E.; Servet, B.; Divay, L.; Jouanne, P.; Grasset, P.; Dudon, J.-P.; Hugonnot, P.; Fleury-Frenette, K. Assessment of atomic layer deposited TiO2 photocatalytic self-cleaning by quartz crystal microbalance. J. Vac. Sci. Technol. A 2020, 38, 043404. [Google Scholar] [CrossRef]
- Gao, N.; Li, H.-Y.; Zhang, W.; Zhang, Y.; Zeng, Y.; Zhixiang, H.; Liu, J.; Jiang, J.; Miao, L.; Yi, F.; et al. QCM-based humidity sensor and sensing properties employing colloidal SnO2 nanowires. Sens. Actuators B Chem. 2019, 293, 129–135. [Google Scholar] [CrossRef]
- Wang, L.; Gao, J.; Xu, J. QCM formaldehyde sensing materials: Design and sensing mechanism. Sens. Actuators B Chem. 2019, 293, 71–82. [Google Scholar] [CrossRef]
- Wang, L.; Xu, J.; Wang, X.; Cheng, Z.; Xu, J. Facile preparation of N-rich functional polymer with porous framework as QCM sensing material for rapid humidity detection. Sens. Actuators B Chem. 2019, 288, 289–297. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von schwingquarzen zur wägung dünner schichten und zur mikrowägung. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Lv, Y.; Xu, P.; Yu, H.; Xu, J.; Li, X. Ni-MOF-74 as sensing material for resonant-gravimetric detection of ppb-level CO. Sens. Actuators B Chem. 2018, 262, 562–569. [Google Scholar] [CrossRef]
- Xu, T.; Xu, P.; Zheng, D.; Yu, H.; Li, X. Metal–Organic Frameworks for Resonant-Gravimetric Detection of Trace-Level Xylene Molecules. Anal. Chem. 2016, 88, 12234–12240. [Google Scholar] [CrossRef]
- Homayoonnia, S.; Zeinali, S. Design and fabrication of capacitive nanosensor based on MOF nanoparticles as sensing layer for VOCs detection. Sens. Actuators B Chem. 2016, 237, 776–786. [Google Scholar] [CrossRef]
- Xu, F.; Sun, L.; Huang, P.; Sun, Y.; Zheng, Q.; Zou, Y.; Chu, H.; Yan, E.; Zhang, H.; Wang, J.; et al. A pyridine vapor sensor based on metal-organic framework-modified quartz crystal microbalance. Sens. Actuators B Chem. 2018, 254, 872–877. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, J.-M.; Jin, L.-N.; Sun, W.-Y. Controlled Synthesis of Porous Coordination-Polymer Microcrystals with Definite Morphologies and Sizes under Mild Conditions. Chem. A Eur. J. 2014, 20, 14783–14789. [Google Scholar] [CrossRef]
- Chen, C.; Li, B.; Zhou, L.; Xia, Z.; Feng, N.; Ding, J.; Wang, L.; Wan, H.; Guan, G. Synthesis of Hierarchically Structured Hybrid Materials by Controlled Self-Assembly of Metal–Organic Framework with Mesoporous Silica for CO2 Adsorption. ACS Appl. Mater. Interfaces 2017, 9, 23060–23071. [Google Scholar] [CrossRef]
- Zheng, Y.; Guo, J.; Ning, D.; Huang, Y.; Lei, W.; Li, J.; Li, J.; Schuck, G.; Shen, J.; Guo, Y.; et al. Design of metal-organic frameworks for improving pseudo-solid-state magnesium-ion electrolytes: Open metal sites, isoreticular expansion, and framework topology. J. Mater. Sci. Technol. 2023, 144, 15–27. [Google Scholar] [CrossRef]
- Pérez, J.M.; Morales-Cámara, S.; García-Salas, F.M.; Ruiz-Cuevas, N.; López-Vargas, M.E.; Choquesillo-Lazarte, D.; Cepeda, J.; García, J.A.; Abdelkader-Fernández, V.K.; Rodríguez-Diéguez, A.; et al. Metal–Organic Frameworks Based on a Janus-Head Biquinoline Ligand as Catalysts in the Transformation of Carbonyl Compounds into Cyanohydrins and Alcohols. Cryst. Growth Des. 2022, 22, 7395–7404. [Google Scholar] [CrossRef]
- Fasana, C.D.; Gonzalez, F.G.; Wade, J.W.; Weeks, A.M.; Dhanapala, B.D.; Anderson, M.E. Tuning interfacial interactions for bottom-up assembly of surface-anchored metal-organic frameworks to tailor film morphology and pattern surface features. Aggregate 2022, 3, e241. [Google Scholar] [CrossRef]
- Wang, Z.; Ge, L.; Feng, D.; Jiang, Z.; Wang, H.; Li, M.; Lin, R.; Zhu, Z. Crystal Facet Engineering of Copper-Based Metal–Organic Frameworks with Inorganic Modulators. Cryst. Growth Des. 2021, 21, 926–934. [Google Scholar] [CrossRef]
- Ma, Z.; Yuan, T.; Fan, Y.; Wang, L.; Duan, Z.; Du, W.; Zhang, D.; Xu, J. A benzene vapor sensor based on a metal-organic framework-modified quartz crystal microbalance. Sens. Actuators B Chem. 2020, 311, 127365. [Google Scholar] [CrossRef]
- Cao, Y.; Fan, Y.; Ma, Z.; Cheng, Z.; Xiang, Q.; Duan, Z.; Xu, J. Urea-functionalized SBA-15 hybrids: Post-grafting synthesis, high-performance organophosphorus sensing and their response mechanism. Sens. Actuators B Chem. 2018, 273, 1162–1169. [Google Scholar] [CrossRef]
- Hu, Q.; Ma, Z.; Yang, J.; Gao, T.; Wu, Y.; Dong, Z.; Li, X.; Zeng, W.; Zhao, S.; Xu, J. Ultrathin PANI-Decorated, Highly Purified and Well Dispersed Array Cncs for Highly Sensitive HCHO Sensors. Chemosensors 2021, 9, 276. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, H.; Zheng, Q.; Xu, J.; Li, X. Amine-Functionalized SBA-15 with Uniform Morphology and Well-Defined Mesostructure for Highly Sensitive Chemosensors To Detect Formaldehyde Vapor. Langmuir 2012, 28, 7843–7850. [Google Scholar] [CrossRef]
- Wu, Y.; Peterson, V.K.; Luks, E.; Darwish, T.A.; Kepert, C.J. Interpenetration as a mechanism for negative thermal ex-pansion in the metal-organic framework Cu3(BTB)2(MOF-14). Angew. Chem. Int. Ed. 2014, 53, 5175–5178. [Google Scholar] [CrossRef]
- Zhang, D.; Fan, Y.; Li, G.; Du, W.; Li, R.; Liu, Y.; Cheng, Z.; Xu, J. Biomimetic synthesis of zeolitic imidazolate frameworks and their application in high performance acetone gas sensors. Sens. Actuators B Chem. 2020, 302, 127187. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Xiang, Q.; Chen, Y.; Duan, Z.; Xu, J. High performance formaldehyde detection based on a novel copper (II) complex functionalized QCM gas sensor. Sens. Actuators B Chem. 2017, 248, 820–828. [Google Scholar] [CrossRef]
- Yan, D.; Xu, P.; Xiang, Q.; Mou, H.; Xu, J.; Wen, W.; Li, X.; Zhang, Y. Polydopamine nanotubes: Bio-inspired synthesis, formaldehyde sensing properties and thermodynamic investigation. J. Mater. Chem. A 2016, 4, 3487–3493. [Google Scholar] [CrossRef]
- Xu, P.; Yu, H.; Guo, S.; Li, X. Microgravimetric thermodynamic modeling for optimization of chemical sensing nano-materials. Anal. Chem. 2014, 86, 4178–4187. [Google Scholar] [CrossRef]
- Liu, N.; Fan, Y.; Ma, Z.; Lin, H.; Xu, J. Materials design and sensing mechanism of novel calix[6]arene composite for sensitively detecting amine drugs. Chin. Chem. Lett. 2020, 31, 2129–2132. [Google Scholar] [CrossRef]
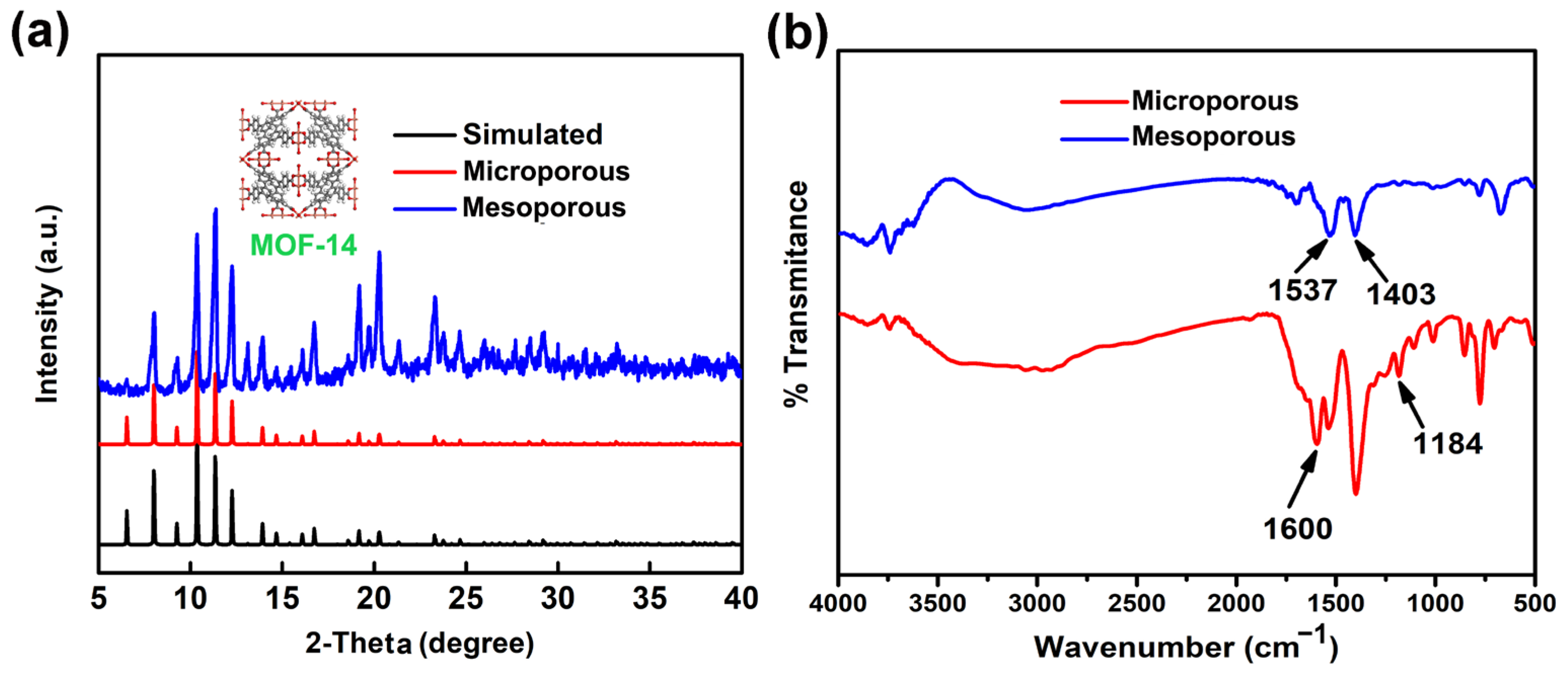
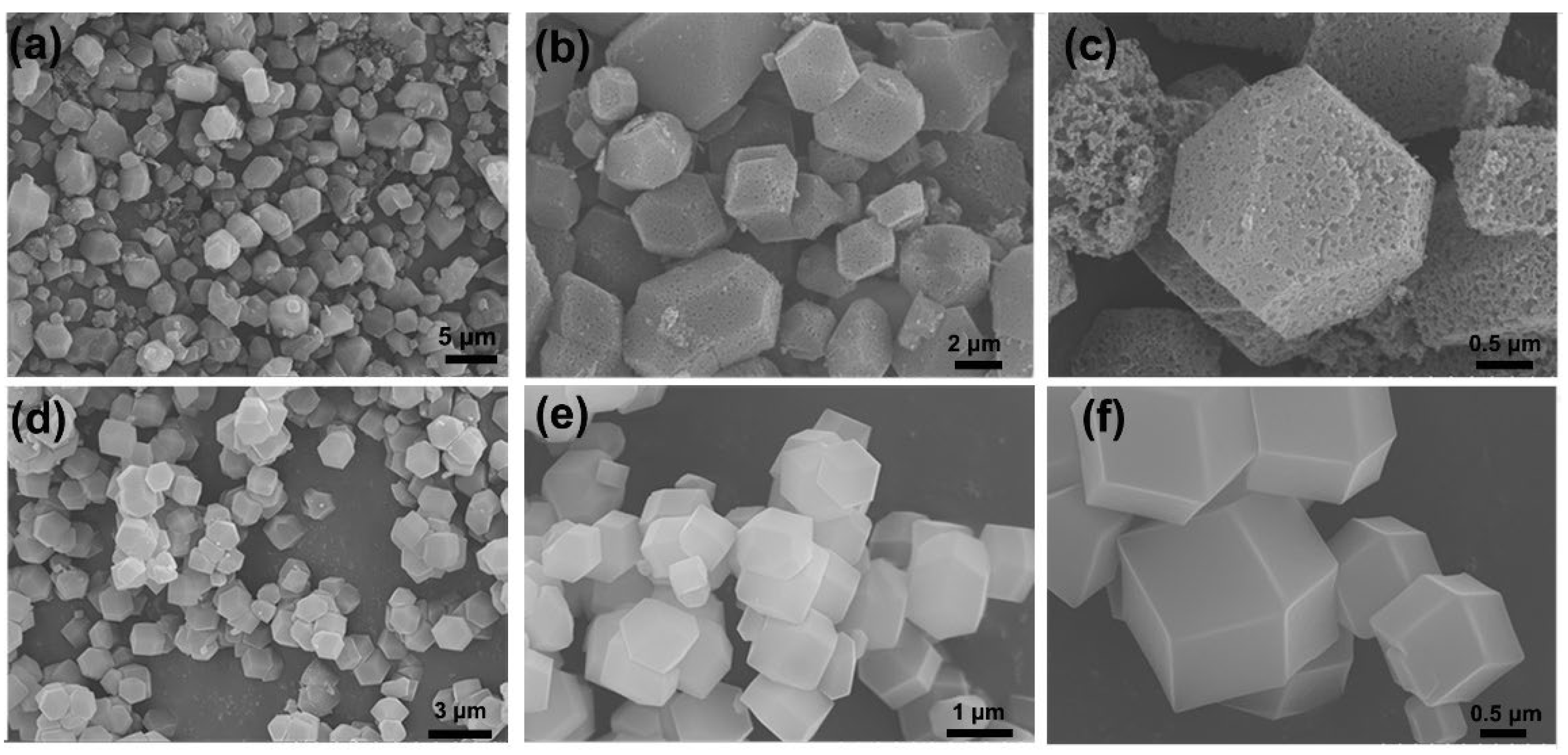
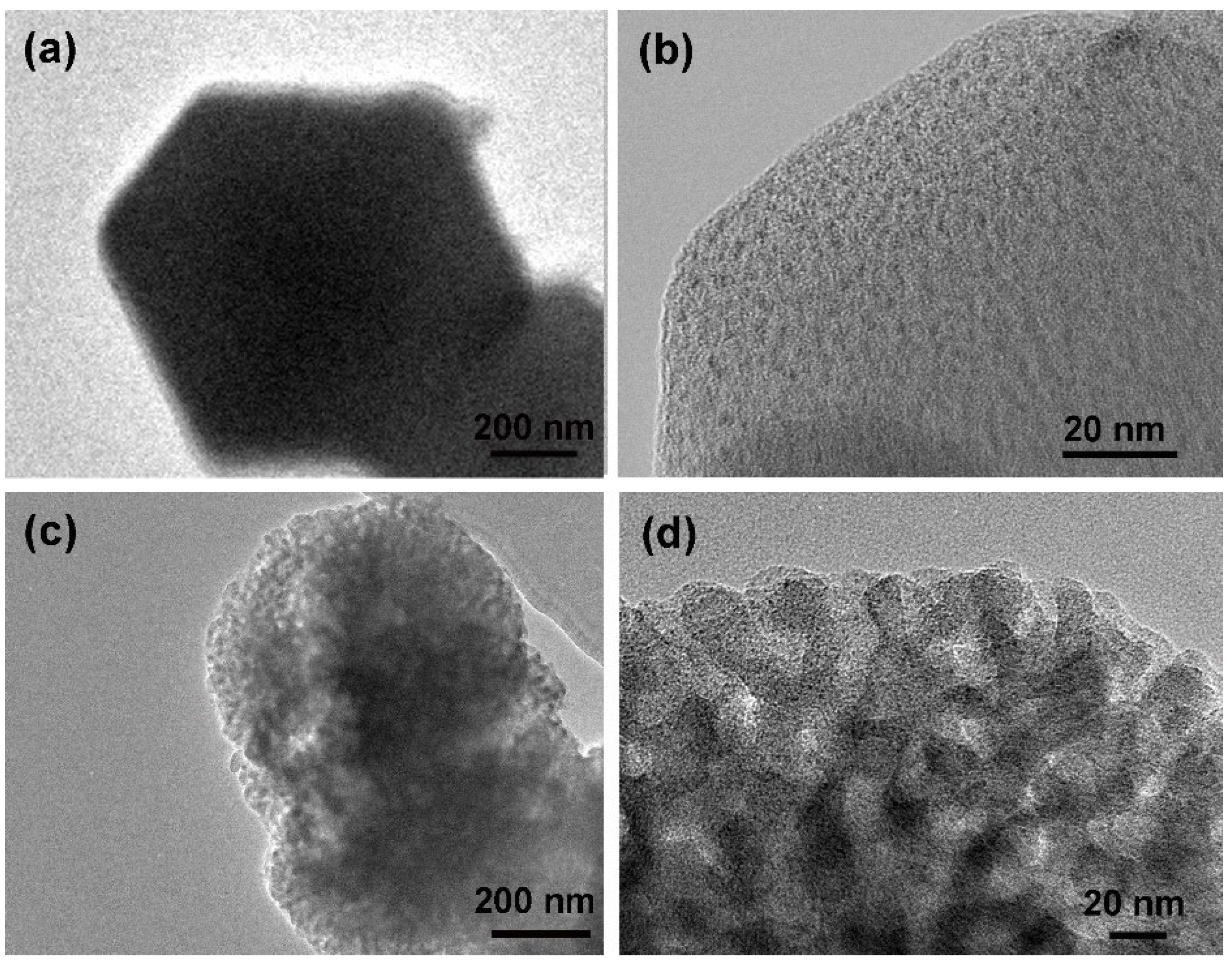
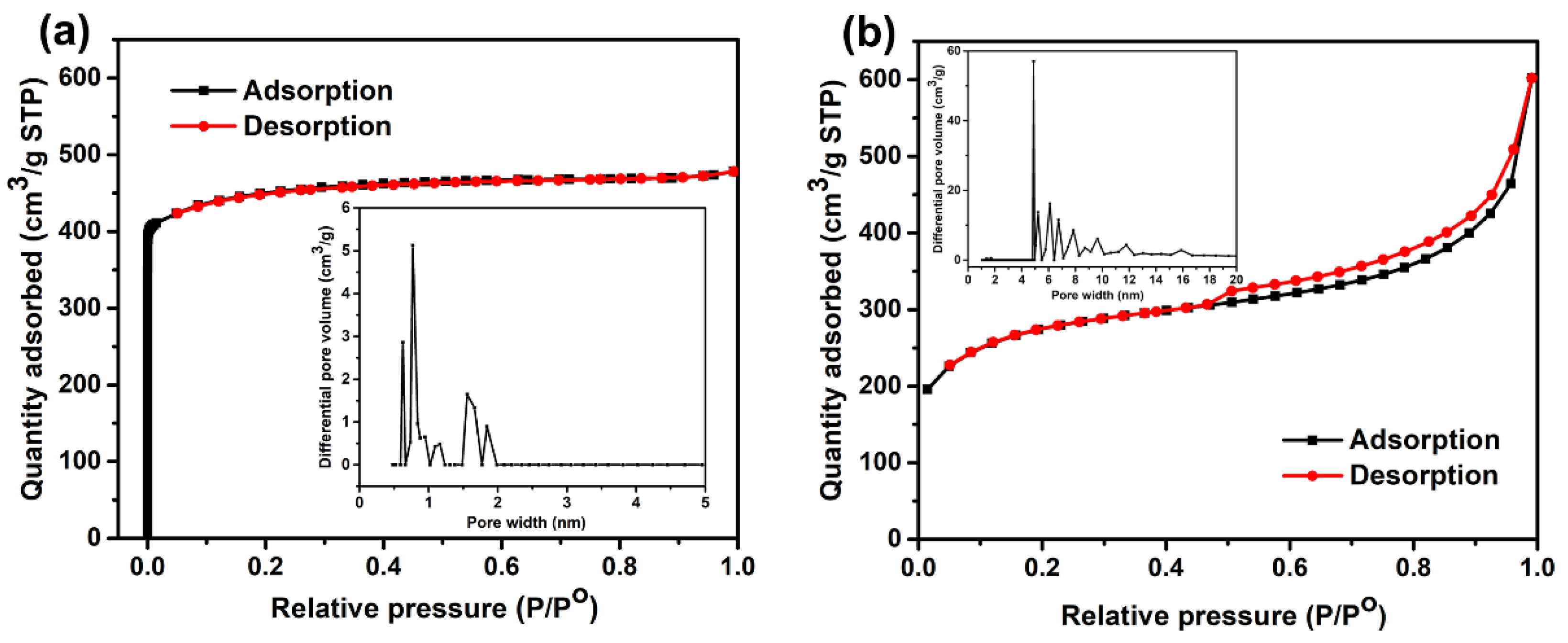

| Materials | SBET (m2/g) | SLangmuir (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|---|
| Meso-MOF-14 | 880 | 1063 | 0.93 | 4.23 |
| Micro-MOF-14 | 1237 | 1548 | 0.54 | 1.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Yuan, T.; Fan, Y.; Chen, Y.; Bai, Y.; Xu, J. Mesoporous-Structure MOF-14-Based QCM p-Xylene Gas Sensor. Nanomaterials 2023, 13, 1743. https://doi.org/10.3390/nano13111743
Ma Z, Yuan T, Fan Y, Chen Y, Bai Y, Xu J. Mesoporous-Structure MOF-14-Based QCM p-Xylene Gas Sensor. Nanomaterials. 2023; 13(11):1743. https://doi.org/10.3390/nano13111743
Chicago/Turabian StyleMa, Zhiheng, Tongwei Yuan, Yu Fan, Yang Chen, Yueling Bai, and Jiaqiang Xu. 2023. "Mesoporous-Structure MOF-14-Based QCM p-Xylene Gas Sensor" Nanomaterials 13, no. 11: 1743. https://doi.org/10.3390/nano13111743
APA StyleMa, Z., Yuan, T., Fan, Y., Chen, Y., Bai, Y., & Xu, J. (2023). Mesoporous-Structure MOF-14-Based QCM p-Xylene Gas Sensor. Nanomaterials, 13(11), 1743. https://doi.org/10.3390/nano13111743







