Porous Hierarchical Ni/Mg/Al Layered Double Hydroxide for Adsorption of Methyl Orange from Aqueous Solution
Abstract
1. Introduction
2. Experimental
2.1. Materials
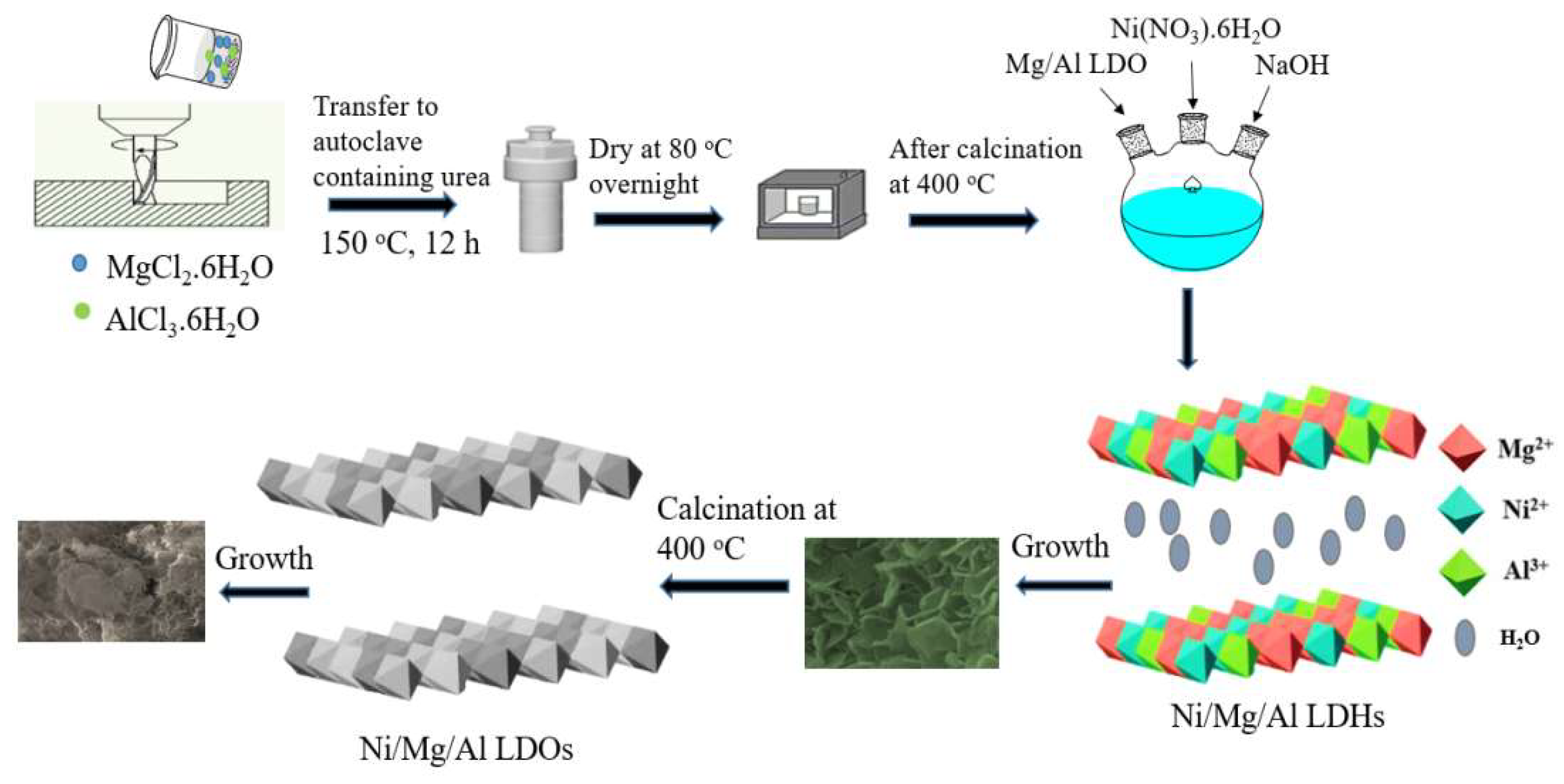
2.2. Synthesis
2.3. Characterizations
2.4. Batch Adsorption Experiment
2.5. Adsorption Kinetics
2.6. Adsorption Isotherm
2.7. Regeneration Cycle
3. Results and Discussion
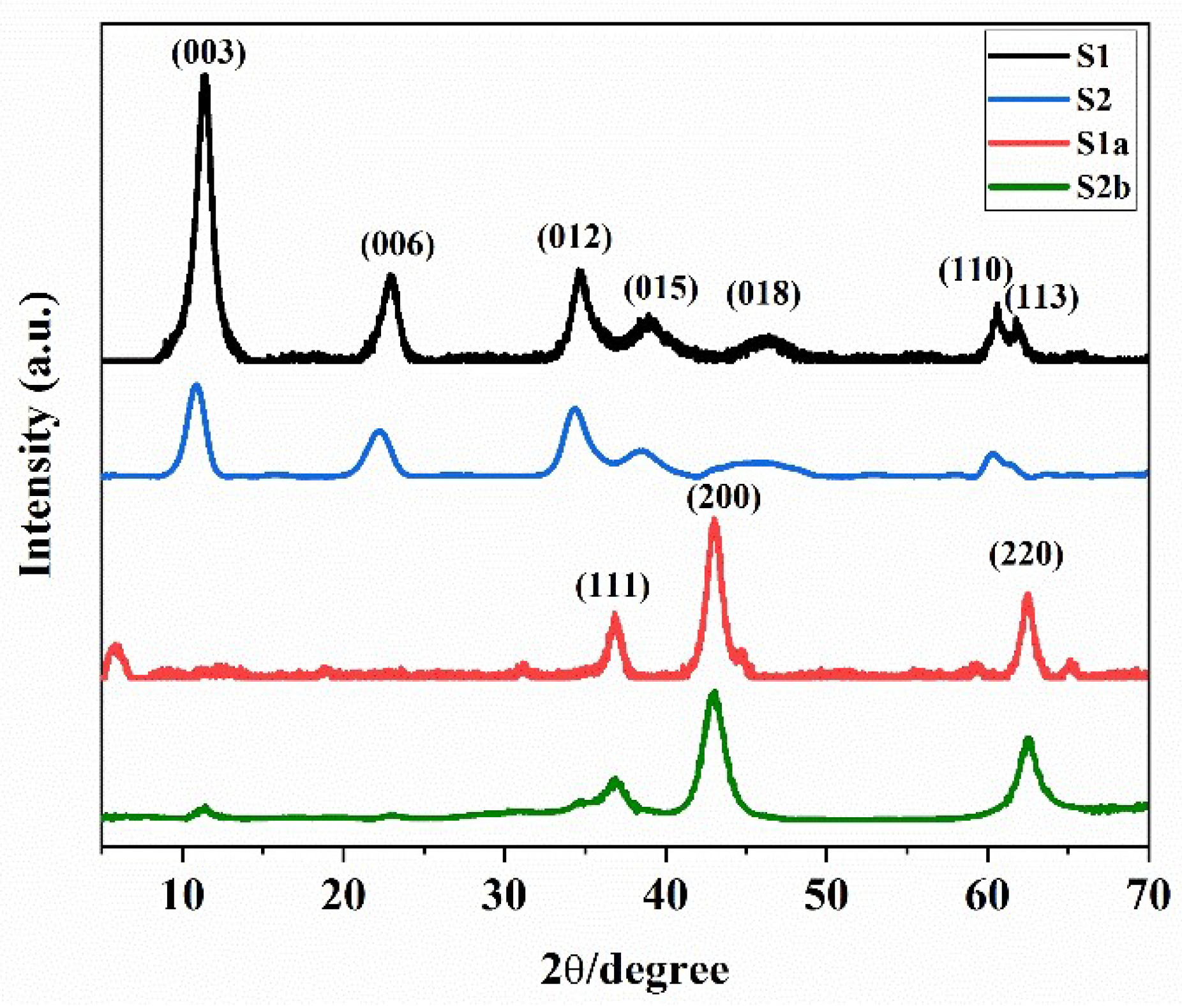
3.1. Crystal Structure
3.2. Morphology and Elemental Composition
3.3. Pore Properties
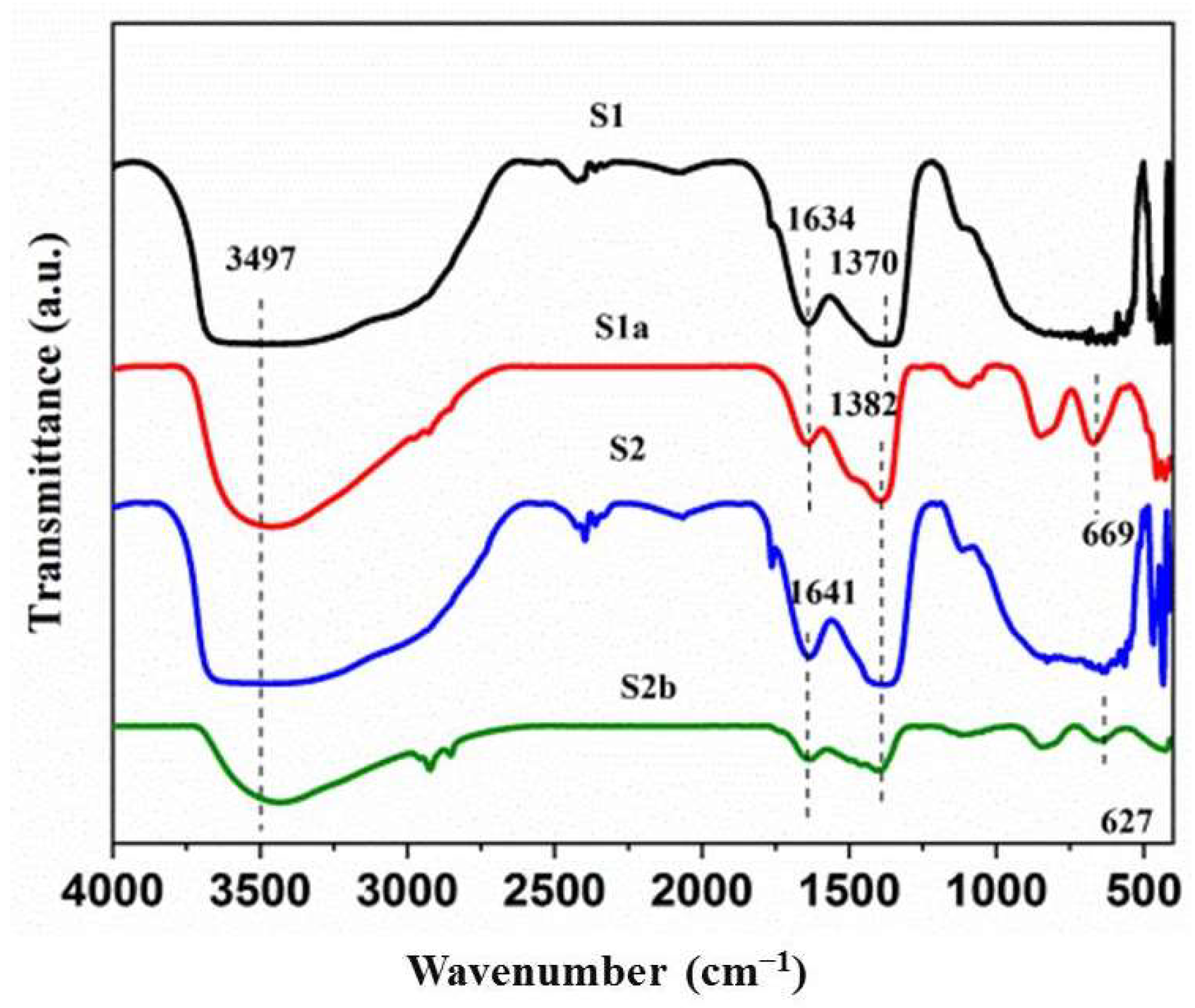
3.4. FTIR Spectra
3.5. Adsorption of Methyl Orange
3.5.1. Effect of Initial pH on Dye Adsorption
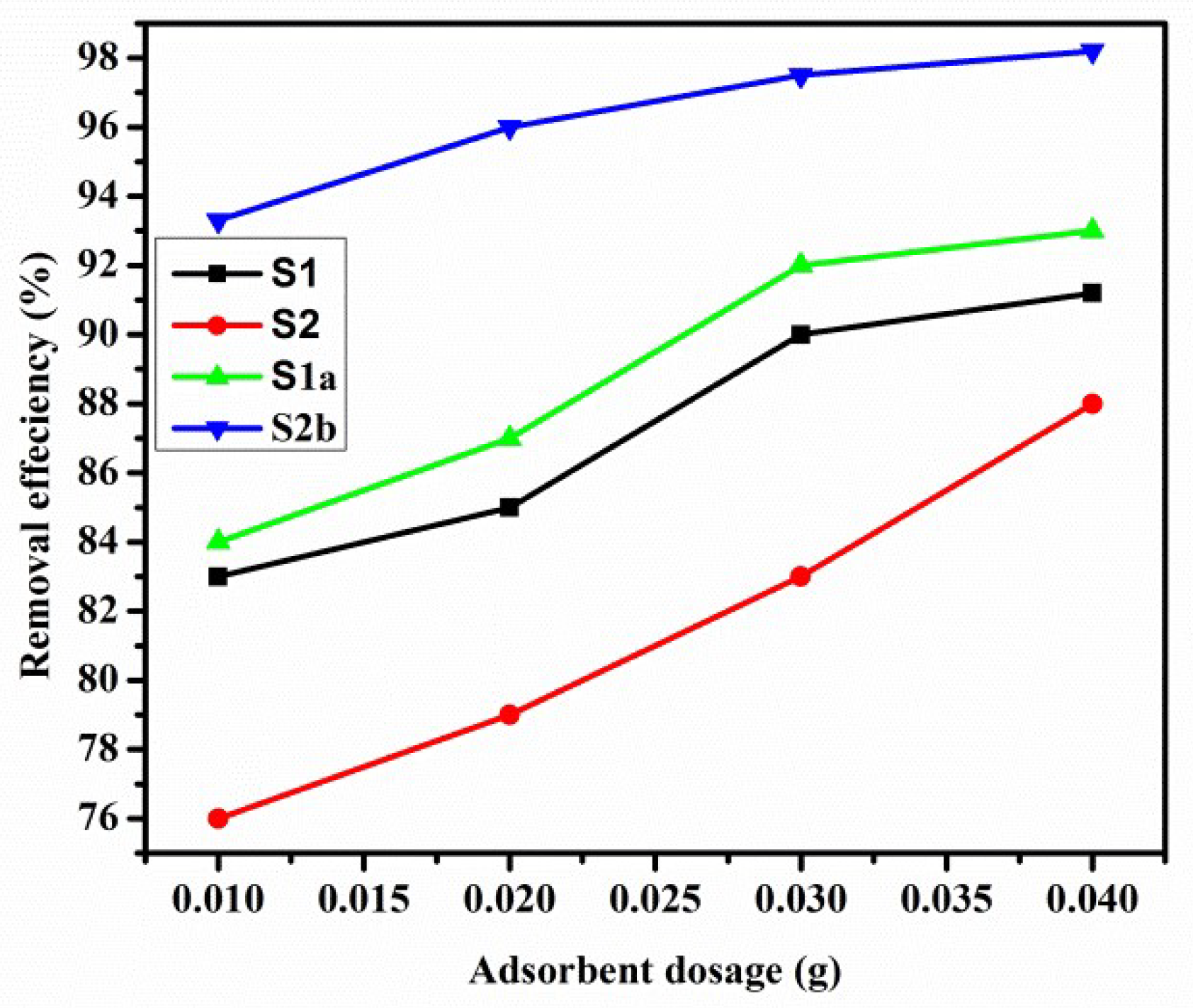
3.5.2. Effect of Dosage
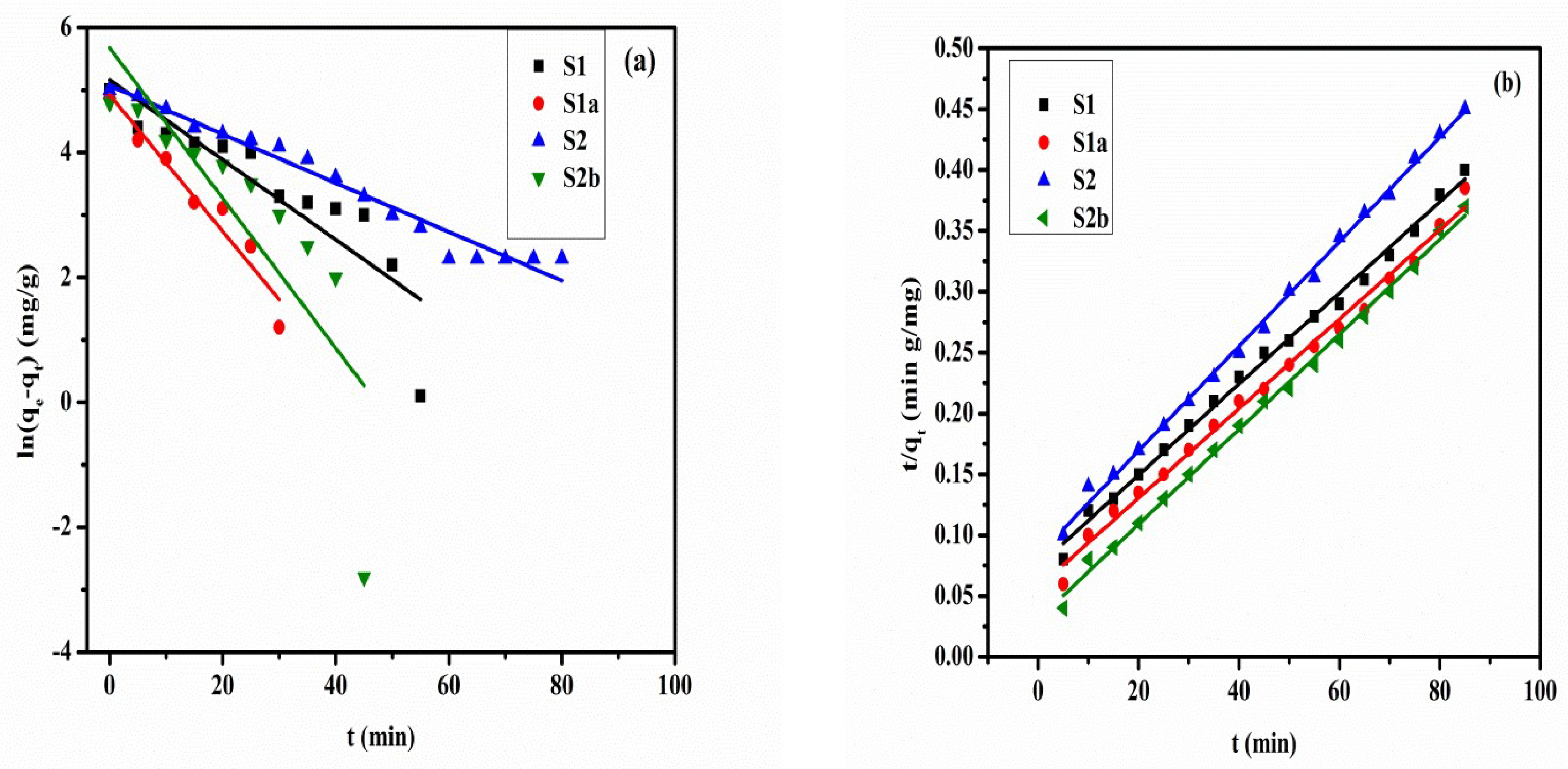
3.6. Adsorption Kinetics
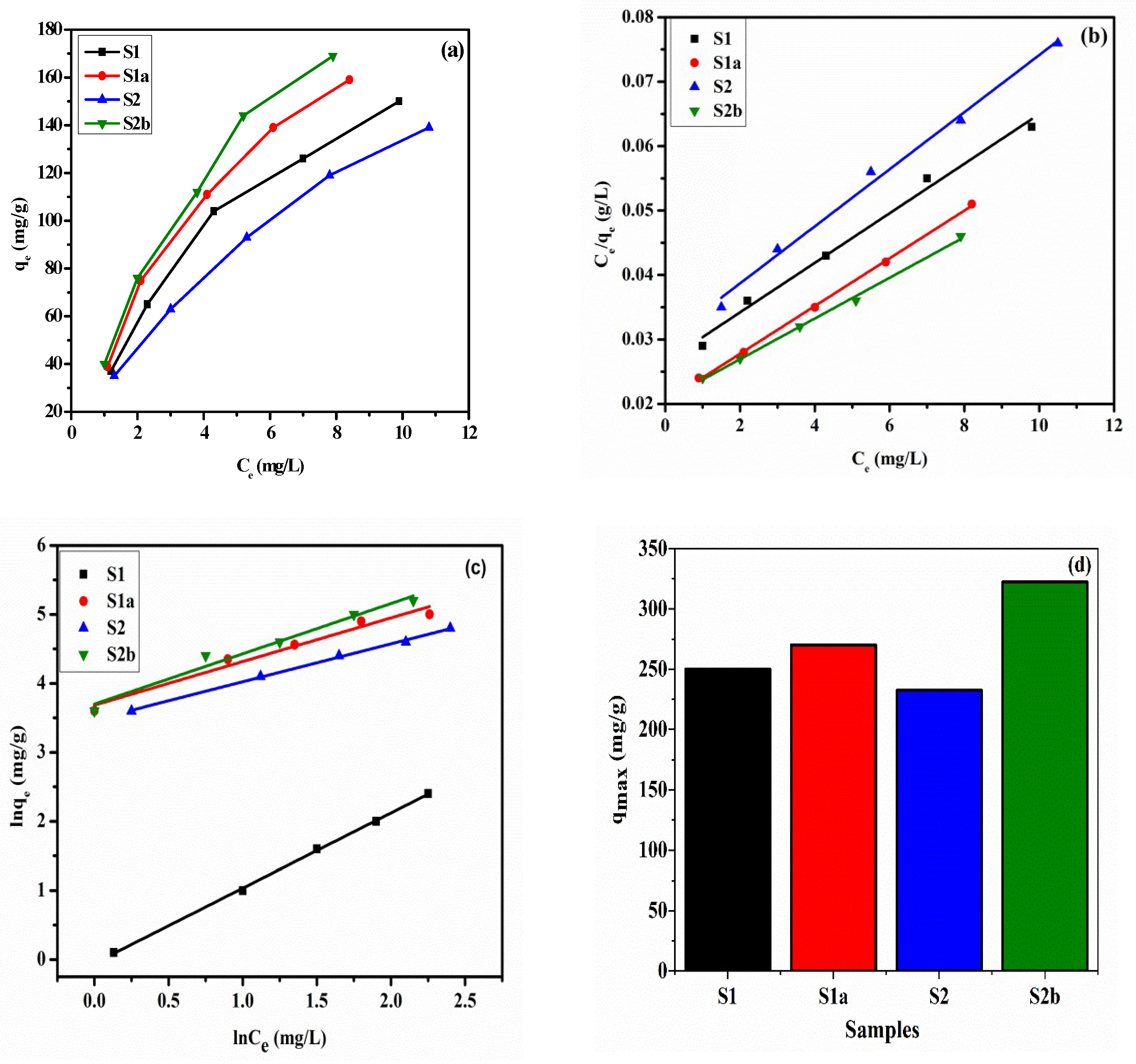
3.7. Adsorption Isotherm
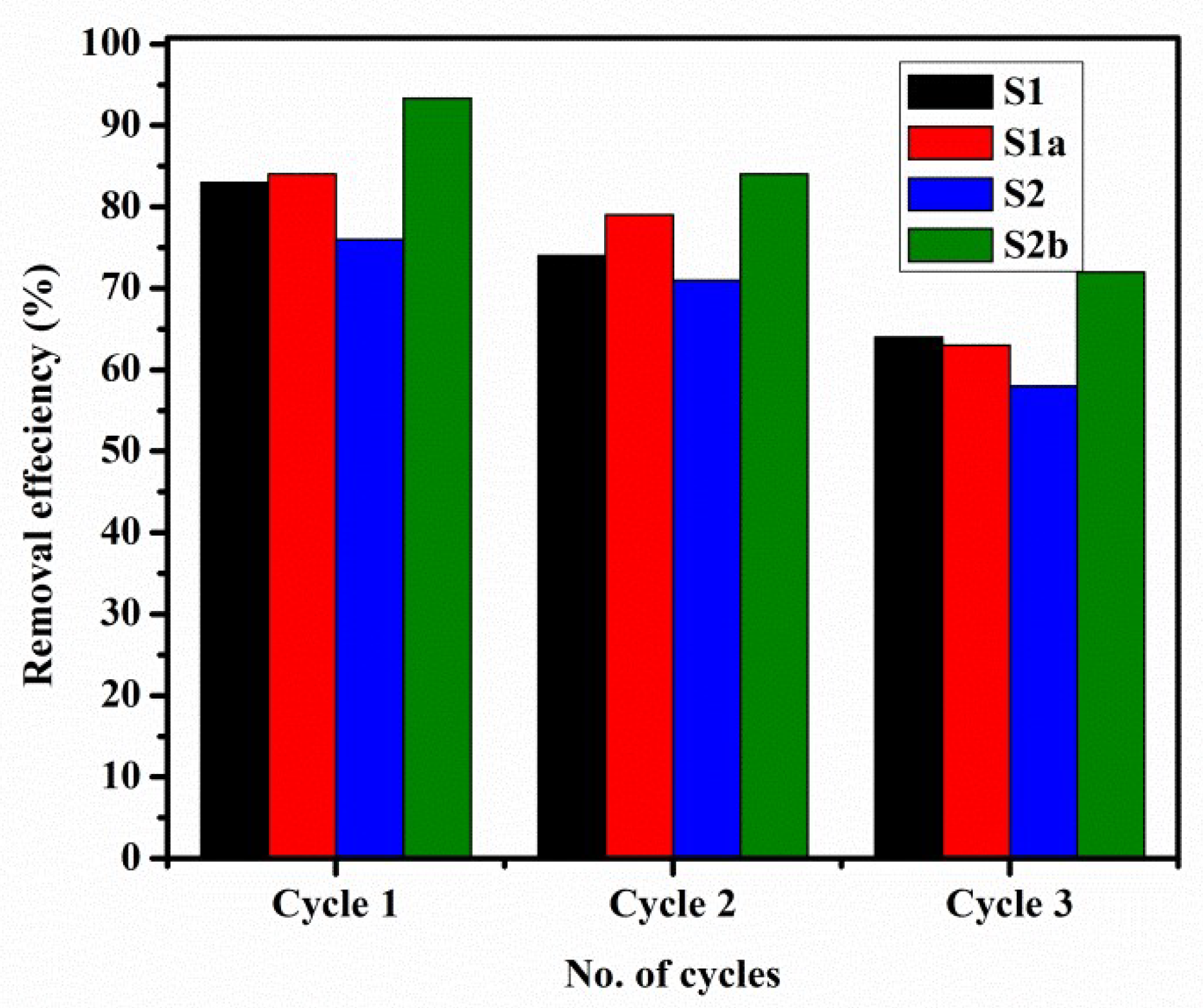
3.8. Regeneration Cycles
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ni, Z.M.; Xia, S.J.; Wang, L.G.; Xing, F.F.; Pan, G.X. Treatment of Methyl Orange by Calcined Layered Double Hydroxides in Aqueous Solution: Adsorption Property and Kinetic Studies. J. Colloid Interface Sci. 2007, 316, 284–291. [Google Scholar] [CrossRef]
- Islam, A.; Hwa, S.; Taufiq-yap, Y.H.; Huey, C.; Vo, D.N.; Lokman, M.; Hasan, M.; Khan, M.A.R.; Nur, A.S.M.; Awual, R. Resources, Conservation & Recycling Step towards the Sustainable Toxic Dyes Removal and Recycling from Aqueous Solution- A Comprehensive Review. Resour. Conserv. Recycl. 2021, 175, 105849. [Google Scholar] [CrossRef]
- Teo, S.H.; Ng, C.H.; Islam, A.; Abdulkareem-Alsultan, G.; Joseph, C.G.; Janaun, J.; Taufiq-Yap, Y.H.; Khandaker, S.; Islam, G.J.; Znad, H.; et al. Sustainable Toxic Dyes Removal with Advanced Materials for Clean Water Production: A Comprehensive Review. J. Clean. Prod. 2022, 332, 130039. [Google Scholar] [CrossRef]
- Khin, M.M.; Nair, A.S.; Babu, V.J.; Murugan, R.; Ramakrishna, S. A Review on Nanomaterials for Environmental Remediation. Energy Environ. Sci. 2012, 5, 8075–8109. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I.; Saleh, T.A.; Nayak, A.; Agarwal, S. Chemical Treatment Technologies for Waste-Water Recycling—An Overview. RSC Adv. 2012, 2, 6380–6388. [Google Scholar] [CrossRef]
- Shu, J.; Wang, Z.; Huang, Y.; Huang, N.; Ren, C.; Zhang, W. Adsorption Removal of Congo Red from Aqueous Solution by Polyhedral Cu2O Nanoparticles: Kinetics, Isotherms, Thermodynamics and Mechanism Analysis. J. Alloys Compd. 2015, 633, 338–346. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, J.; Cai, W.; Zhao, R.; Yuan, J. Hierarchically Porous NiAl-LDH Nanoparticles as Highly Efficient Adsorbent for p-Nitrophenol from Water. Appl. Surf. Sci. 2015, 349, 897–903. [Google Scholar] [CrossRef]
- Xu, P.; Han, H.; Zhuang, H.; Hou, B.; Jia, S.; Xu, C.; Wang, D. Advanced Treatment of Biologically Pretreated Coal Gasification Wastewater by a Novel Integration of Heterogeneous Fenton Oxidation and Biological Process. Bioresour. Technol. 2015, 182, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Hu, S.Z.; Li, F.Y.; Fan, Z.P.; Wang, F.; Zhang, J. Synthesis of CL Doped G-C 3 N 4 with Enhanced Photocatalytic Activity under Visible Light. Asian J. Chem. 2014, 26, 8543–8546. [Google Scholar] [CrossRef]
- Qi, L.; Yu, J.; Jaroniec, M. Enhanced and Suppressed Effects of Ionic Liquid on the Photocatalytic Activity of TiO2. Adsorption 2013, 19, 557–561. [Google Scholar] [CrossRef]
- Mólgora, C.C.; Domínguez, A.M.; Avila, E.M.; Drogui, P.; Buelna, G. Removal of Arsenic from Drinking Water: A Comparative Study between Electrocoagulation-Microfiltration and Chemical Coagulation-Microfiltration Processes. Sep. Purif. Technol. 2013, 118, 645–651. [Google Scholar] [CrossRef]
- Lim, S.J.; Kim, T.H.; Kim, J.Y.; Shin, I.H.; Kwak, H.S. Enhanced Treatment of Swine Wastewater by Electron Beam Irradiation and Ion-Exchange Biological Reactor. Sep. Purif. Technol. 2016, 157, 72–79. [Google Scholar] [CrossRef]
- Selvasembian, R.; Gwenzi, W.; Chaukura, N.; Mthembu, S. Recent Advances in the Polyurethane-Based Adsorbents for the Decontamination of Hazardous Wastewater Pollutants. J. Hazard. Mater. 2021, 417, 125960. [Google Scholar] [CrossRef]
- Mckay, G. Adsorption of Dyestuffs from Aqueous Solutions with Activated Carbon I: Equilibrium and Batch Contact-Time Studies. J. Chem. Technol. Biotechnol. 1982, 32, 759–772. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, S.; Zhao, G.; Wang, Q.; Wang, X. Adsorption of Polycyclic Aromatic Hydrocarbons on Graphene Oxides and Reduced Graphene Oxides. Chem. Asian J. 2013, 8, 2755–2761. [Google Scholar] [CrossRef]
- Janoš, P.; Buchtová, H.; Rýznarová, M. Sorption of Dyes from Aqueous Solutions onto Fly Ash. Water Res. 2003, 37, 4938–4944. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, J.; Deng, Y.; Tian, Y.; Zhang, G.; Liao, J.; Yang, J.; Yang, Y.; Liu, N.; Sun, Q. Uranium(VI) Adsorption from Aqueous Solutions by Microorganism-Graphene Oxide Composites via an Immobilization Approach; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; ISBN 8628854126. [Google Scholar]
- Zakir, M. Adsorption of Lead (II) and Copper (II) Ions on Rice Husk Activated Carbon under Sonication 1. Int. Symp. Chem. Bioprocess Eng. 2013, 23, 25–28. [Google Scholar]
- Pavan, P.C.; Crepaldi, E.L.; Valim, J.B. Sorption of Anionic Surfactants on Layered Double Hydroxides. J. Colloid Interface Sci. 2000, 229, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Shan, R.; Yan, L.; Yang, Y.; Yang, K.; Yu, S.; Yu, H.; Zhu, B.; Du, B. Highly Efficient Removal of Three Red Dyes by Adsorption onto Mg-Al-Layered Double Hydroxide. J. Ind. Eng. Chem. 2015, 21, 561–568. [Google Scholar] [CrossRef]
- Lu, L.; Li, J.; Ng, D.H.L.; Yang, P.; Song, P.; Zuo, M. Synthesis of Novel Hierarchically Porous Fe3O4@MgAl–LDH Magnetic Microspheres and Its Superb Adsorption Properties of Dye from Water. J. Ind. Eng. Chem. 2017, 46, 315–323. [Google Scholar] [CrossRef]
- El Gaini, L.; Lakraimi, M.; Sebbar, E.; Meghea, A.; Bakasse, M. Removal of Indigo Carmine Dye from Water to Mg-Al-CO3-Calcined Layered Double Hydroxides. J. Hazard. Mater. 2009, 161, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, P.; Narayanan, S.K. Effect of Hydrothermal Carbonization Reaction Parameters On. Environ. Prog. Sustain. Energy 2014, 33, 676–680. [Google Scholar]
- Ahmed, I.M.; Gasser, M.S. Adsorption Study of Anionic Reactive Dye from Aqueous Solution to Mg-Fe-CO 3 Layered Double Hydroxide (LDH). Appl. Surf. Sci. 2012, 259, 650–656. [Google Scholar] [CrossRef]
- Kowalik, P.; Konkol, M.; Kondracka, M.; Próchniak, W.; Bicki, R.; Wiercioch, P. Memory Effect of the CuZnAl-LDH Derived Catalyst Precursor—In Situ XRD Studies. Appl. Catal. A Gen. 2013, 464–465, 339–347. [Google Scholar] [CrossRef]
- Chagas, L.H.; De Carvalho, G.S.G.; Do Carmo, W.R.; San Gil, R.A.S.; Chiaro, S.S.X.; Leitão, A.A.; Diniz, R.; De Sena, L.A.; Achete, C.A. MgCoAl and NiCoAl LDHs Synthesized by the Hydrothermal Urea Hydrolysis Method: Structural Characterization and Thermal Decomposition. Mater. Res. Bull. 2015, 64, 207–215. [Google Scholar] [CrossRef]
- Peng, C.; Dai, J.; Yu, J.; Yin, J. Calcined Mg-Fe Layered Double Hydroxide as an Absorber for the Removal of Methyl Orange Calcined Mg-Fe Layered Double Hydroxide as an Absorber for the Removal of Methyl Orange. Aip Adv. 2015, 5, 057138. [Google Scholar] [CrossRef]
- Lei, C.; Zhu, X.; Zhu, B.; Jiang, C.; Le, Y.; Yu, J. Superb Adsorption Capacity of Hierarchical Calcined Ni/Mg/Al Layered Double Hydroxides for Congo Red and Cr(VI) Ions. J. Hazard. Mater. 2017, 321, 801–811. [Google Scholar] [CrossRef]
- Zhang, L.; Xiong, Z.; Li, L.; Burt, R.; Zhao, X.S. Uptake and Degradation of Orange II by Zinc Aluminum Layered Double Oxides. J. Colloid Interface Sci. 2016, 469, 224–230. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Huong Le, T.X.; Bechelany, M.; Esposito, G.; Van Hullebusch, E.D.; Oturan, M.A.; Cretin, M. A Hierarchical CoFe-Layered Double Hydroxide Modified Carbon-Felt Cathode for Heterogeneous Electro-Fenton Process. J. Mater. Chem. A 2017, 5, 3655–3666. [Google Scholar] [CrossRef]
- Liu, M.; Xu, J.; Cheng, B.; Ho, W.; Yu, J. Synthesis and Adsorption Performance of Mg(OH) 2 Hexagonal Nanosheet-Graphene Oxide Composites. Appl. Surf. Sci. 2015, 332, 121–129. [Google Scholar] [CrossRef]
- Aurich, A.; Hofmann, J.; Oltrogge, R.; Wecks, M.; Gläser, R.; Blömer, L.; Mauersberger, S.; Müller, R.A.; Sicker, D.; Giannis, A. Improved Isolation of Microbiologically Produced (2R,3S)-Isocitric Acid by Adsorption on Activated Carbon and Recovery with Methanol. Org. Process Res. Dev. 2017, 21, 866–870. [Google Scholar] [CrossRef]
- Yu, L.; Xue, W.; Cui, L.; Xing, W.; Cao, X.; Li, H. Use of Hydroxypropyl-β-Cyclodextrin/Polyethylene Glycol 400, Modified Fe3O4 Nanoparticles for Congo Red Removal. Int. J. Biol. Macromol. 2014, 64, 233–239. [Google Scholar] [CrossRef] [PubMed]
- El Hassani, K.; Beakou, B.H.; Kalnina, D.; Oukani, E.; Anouar, A. Effect of Morphological Properties of Layered Double Hydroxides on Adsorption of Azo Dye Methyl Orange: A Comparative Study. Appl. Clay Sci. 2017, 140, 124–131. [Google Scholar] [CrossRef]
- Sun, Z.; Lin, C.; Zheng, J.; Wang, L.; Zhang, J.; Xu, F.; Hou, J. Fabrication and Characterization of Hierarchical Mg/Ni/Al Layered Double Hydroxide Framework on Aluminum Foam. Mater. Lett. 2013, 113, 83–86. [Google Scholar] [CrossRef]
- Crepaldi, E.L.; Tronto, J.; Cardoso, L.P.; Valim, J.B. Sorption of Terephthalate Anions by Calcined and Uncalcined Hydrotalcite-like Compounds. Colloids Surfaces A Physicochem. Eng. Asp. 2002, 211, 103–114. [Google Scholar] [CrossRef]
- Chen, Y.; Shui, Z.; Chen, W.; Chen, G. Chloride Binding of Synthetic Ca-Al-NO3 LDHs in Hardened Cement Paste. Constr. Build. Mater. 2015, 93, 1051–1058. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Abelló, S.; Pérez-Ramírez, J. Steam Activation of Mg-Al Hydrotalcite. Influence on the Properties of the Derived Mixed Oxides. Microporous Mesoporous Mater. 2006, 96, 102–108. [Google Scholar] [CrossRef]
- Extremera, R.; Pavlovic, I.; Pérez, M.R.; Barriga, C. Removal of Acid Orange 10 by Calcined Mg/Al Layered Double Hydroxides from Water and Recovery of the Adsorbed Dye. Chem. Eng. J. 2012, 213, 392–400. [Google Scholar] [CrossRef]
- dos Santos, R.M.M.; Gonçalves, R.G.L.; Constantino, V.R.L.; da Costa, L.M.; da Silva, L.H.M.; Tronto, J.; Pinto, F.G. Removal of Acid Green 68:1 from Aqueous Solutions by Calcined and Uncalcined Layered Double Hydroxides. Appl. Clay Sci. 2013, 80–81, 189–195. [Google Scholar] [CrossRef]
- Pourfaraj, R.; Fatemi, S.J.; Kazemi, S.Y.; Biparva, P. Synthesis of Hexagonal Mesoporous MgAl LDH Nanoplatelets Adsorbent for the Effective Adsorption of Brilliant Yellow. J. Colloid Interface Sci. 2017, 508, 65–74. [Google Scholar] [CrossRef]
- Zubair, M.; Jarrah, N.; Khalid, A.; Saood, M. Starch-NiFe-Layered Double Hydroxide Composites: Ef Fi Cient Removal of Methyl Orange from Aqueous Phase. J. Mol. Liq. 2018, 249, 254–264. [Google Scholar] [CrossRef]
- Huang, R.; Liu, Q.; Huo, J.; Yang, B. Adsorption of Methyl Orange onto Protonated Cross-Linked Chitosan. Arab. J. Chem. 2017, 10, 24–32. [Google Scholar] [CrossRef]
- Abdelkader, N.B.; Bentouami, A.; Derriche, Z.; Bettahar, N.; Ménorval, L. De Synthesis and Characterization of Mg–Fe Layer Double Hydroxides and Its Application on Adsorption of Orange G from Aqueous Solution. Chem. Eng. J. 2011, 169, 231–238. [Google Scholar] [CrossRef]
- Crini, Ã.; Badot, P. Application of Chitosan, a Natural Aminopolysaccharide, for Dye Removal from Aqueous Solutions by Adsorption Processes Using Batch Studies: A Review of Recent Literature. Prog. Polym. Sci. 2008, 33, 399–447. [Google Scholar] [CrossRef]
- Chen, R.; Yu, J.; Xiao, W. Hierarchically Porous MnO2 Microspheres with Enhanced Adsorption Performance. J. Mater. Chem. A 2013, 1, 11682–11690. [Google Scholar] [CrossRef]
- Guo, Y.; Zhu, Z.; Qiu, Y.; Zhao, J. Enhanced Adsorption of Acid Brown 14 Dye on Calcined Mg/Fe Layered Double Hydroxide with Memory Effect. Chem. Eng. J. 2013, 219, 69–77. [Google Scholar] [CrossRef]
- Xiong, L.; Yang, Y.; Mai, J.; Sun, W.; Zhang, C.; Wei, D.; Chen, Q.; Ni, J. Adsorption Behavior of Methylene Blue onto Titanate Nanotubes. Chem. Eng. J. 2010, 156, 313–320. [Google Scholar] [CrossRef]
- Zahra, F.; Khalidi, A.; Elhalil, A. Characteristics and Mechanisms of Methyl Orange Sorption onto Zn/Al Layered Double Hydroxide Intercalated by Dodecyl Sulfate Anion. Sci. Afr. 2019, 6, e00216. [Google Scholar] [CrossRef]
- Mamat, M.; Abdullah, M.A.A.; Kadir, M.A.; Jaafar, A.M.; Kusrini, E. Preparation of Layered Double Hydroxides with Different Divalent Metals for the Adsorption of Methyl Orange Dye from Aqueous Solutions. Int. J. Technol. 2018, 9, 1103–1111. [Google Scholar] [CrossRef]


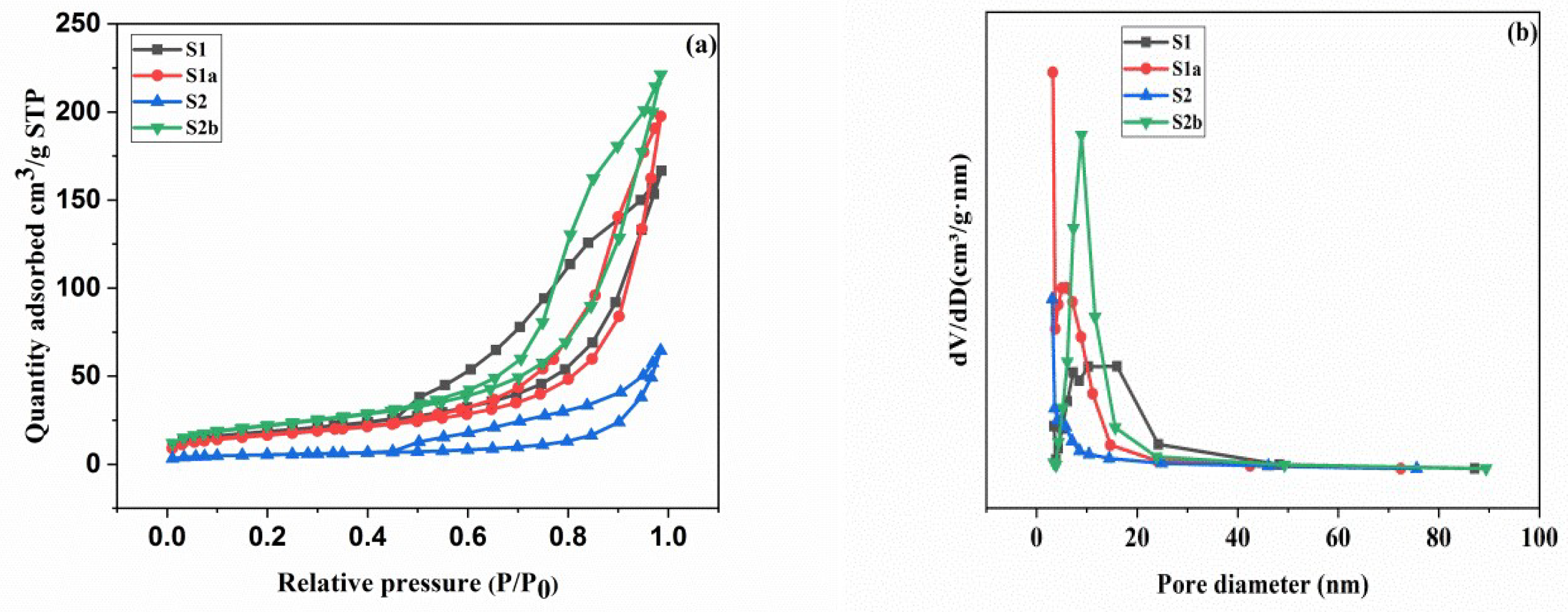


| Samples | dpore (nm) | Vpore (cm3/g) | SBET (m2/g) |
|---|---|---|---|
| S1 | 15.26 | 0.001 | 59 |
| S1a | 19.92 | 0.001 | 66 |
| S2 | 15.77 | 0.002 | 19 |
| S2b | 23.90 | 0.003 | 78 |
| Samples | qe,exp (mg/g) | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|---|
| qe,cal (mg/g) | k1 (×10−2 min−1) | R2 | qe,cal (mg/g) | k2 (×10−3 g/mg min) | R2 | ||
| S1 | 211.4 | 1.636 | 7.47 | 0.9043 | 246.0 | 2.51 | 0.970 |
| S1a | 220.6 | 1.646 | 7.54 | 0.7531 | 250.0 | 4.50 | 0.991 |
| S2 | 185.5 | 1.483 | 3.70 | 0.9700 | 222.2 | 2.82 | 0.972 |
| S2b | 228.3 | 1.719 | 6.70 | 0.8166 | 256.4 | 2.89 | 0.980 |
| Samples | Langmuir Isotherm Model | Freundlich Isotherm Model | ||||||
|---|---|---|---|---|---|---|---|---|
| qmax (mg/g) | KL (L/mg) | R2 | RL | qmax | KF (mg/g) (L/mg)1/n | n | R2 | |
| S1 | 250.0 | 0.1562 | 0.993 | 0.169–0.359 | 5.674 | 1.285 | 1.55 | 0.991 |
| S1a | 270.2 | 0.1787 | 0.999 | 0.156–0.528 | 5.130 | 1.320 | 1.54 | 0.988 |
| S2 | 232.5 | 0.1410 | 0.993 | 0.154–0.369 | 5.620 | 1.248 | 1.57 | 0.991 |
| S2b | 322.5 | 0.1527 | 0.993 | 0.171–0.567 | 5.813 | 1.331 | 1.41 | 0.985 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waheed, T.; Din, S.u.; Ming, L.; Ahmad, P.; Min, P.; Haq, S.; Khandaker, M.U.; Boukhris, I.; Faruque, M.R.I.; Rehman, F.U.; et al. Porous Hierarchical Ni/Mg/Al Layered Double Hydroxide for Adsorption of Methyl Orange from Aqueous Solution. Nanomaterials 2023, 13, 1943. https://doi.org/10.3390/nano13131943
Waheed T, Din Su, Ming L, Ahmad P, Min P, Haq S, Khandaker MU, Boukhris I, Faruque MRI, Rehman FU, et al. Porous Hierarchical Ni/Mg/Al Layered Double Hydroxide for Adsorption of Methyl Orange from Aqueous Solution. Nanomaterials. 2023; 13(13):1943. https://doi.org/10.3390/nano13131943
Chicago/Turabian StyleWaheed, Tayyaba, Salah ud Din, Lei Ming, Pervaiz Ahmad, Pu Min, Sirajul Haq, Mayeen Uddin Khandaker, Imed Boukhris, Mohammad Rashed Iqbal Faruque, Fazal Ur Rehman, and et al. 2023. "Porous Hierarchical Ni/Mg/Al Layered Double Hydroxide for Adsorption of Methyl Orange from Aqueous Solution" Nanomaterials 13, no. 13: 1943. https://doi.org/10.3390/nano13131943
APA StyleWaheed, T., Din, S. u., Ming, L., Ahmad, P., Min, P., Haq, S., Khandaker, M. U., Boukhris, I., Faruque, M. R. I., Rehman, F. U., & Din, I. U. (2023). Porous Hierarchical Ni/Mg/Al Layered Double Hydroxide for Adsorption of Methyl Orange from Aqueous Solution. Nanomaterials, 13(13), 1943. https://doi.org/10.3390/nano13131943










