Mechanochemical Pretreated Mn+1AXn (MAX) Phase to Synthesize 2D-Ti3C2Tx MXene Sheets for High-Performance Supercapacitors
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Experiment
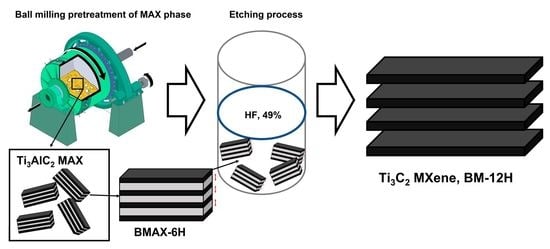
2.2.1. Ball Milling Procedure
2.2.2. Preparation of Ti3C2 MXene
2.3. Material Characterization
2.4. Electrochemical Studies
Fabrication of Coin-Type Symmetric Supercapacitors in Organic Electrolytes
3. Results and Discussion
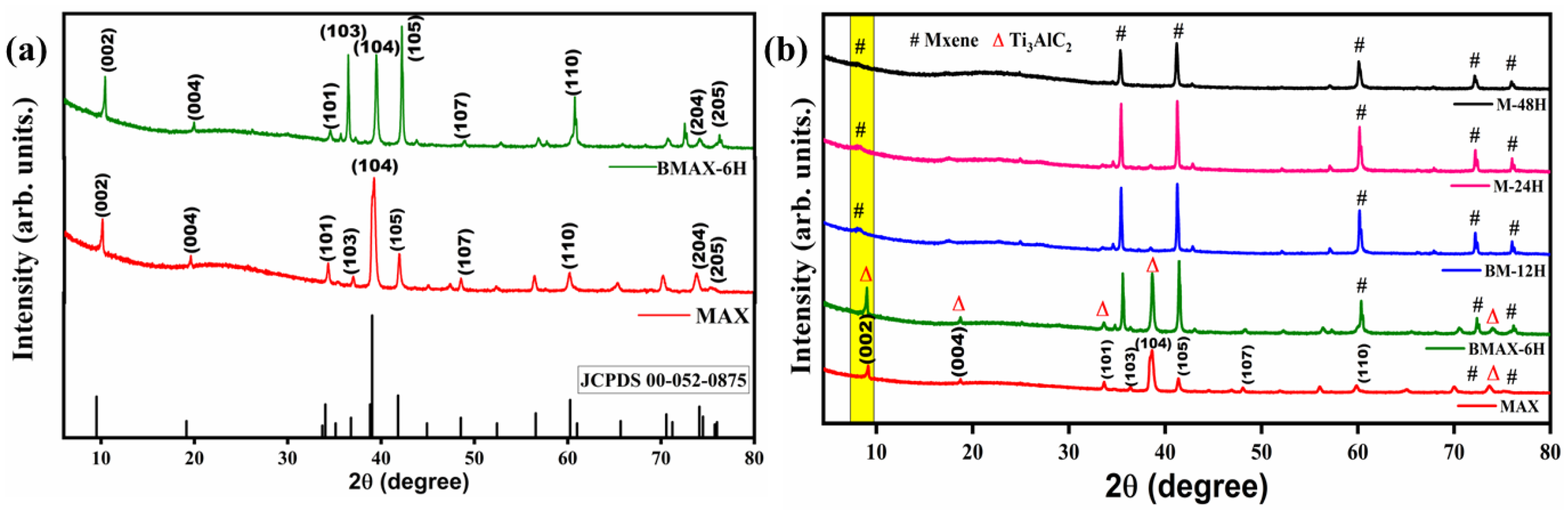
3.1. X-ray Diffraction(XRD) Analysis
3.2. Morphological and Elemental Analysis
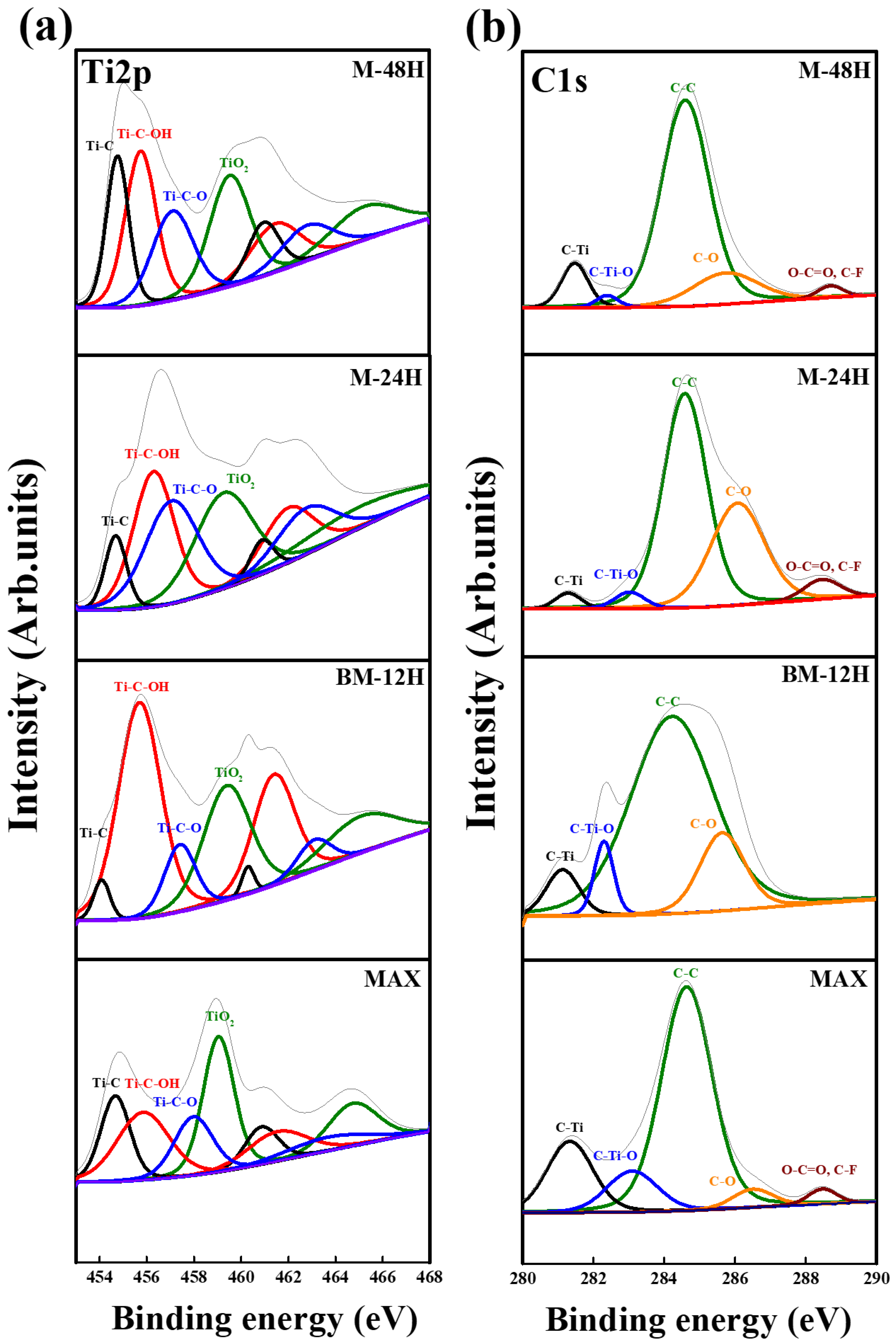
3.3. XPS Analysis
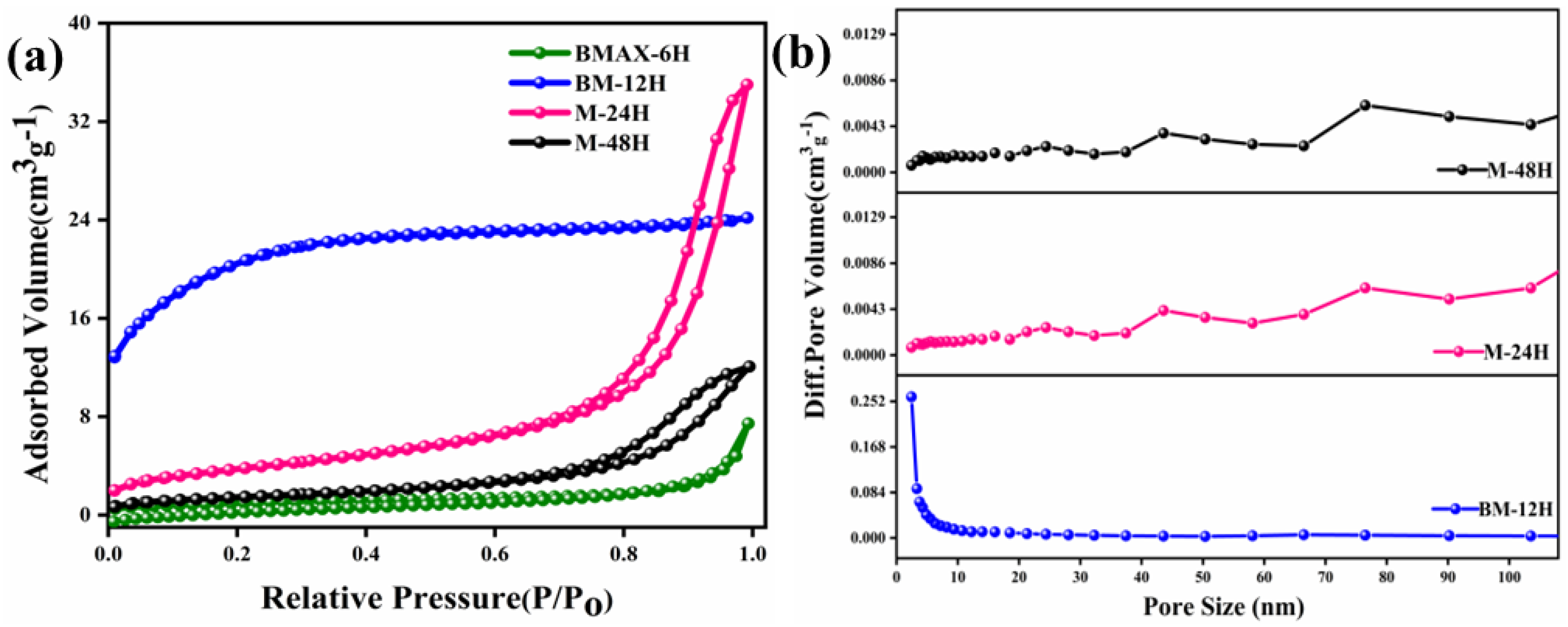
3.4. Surface Area Analysis
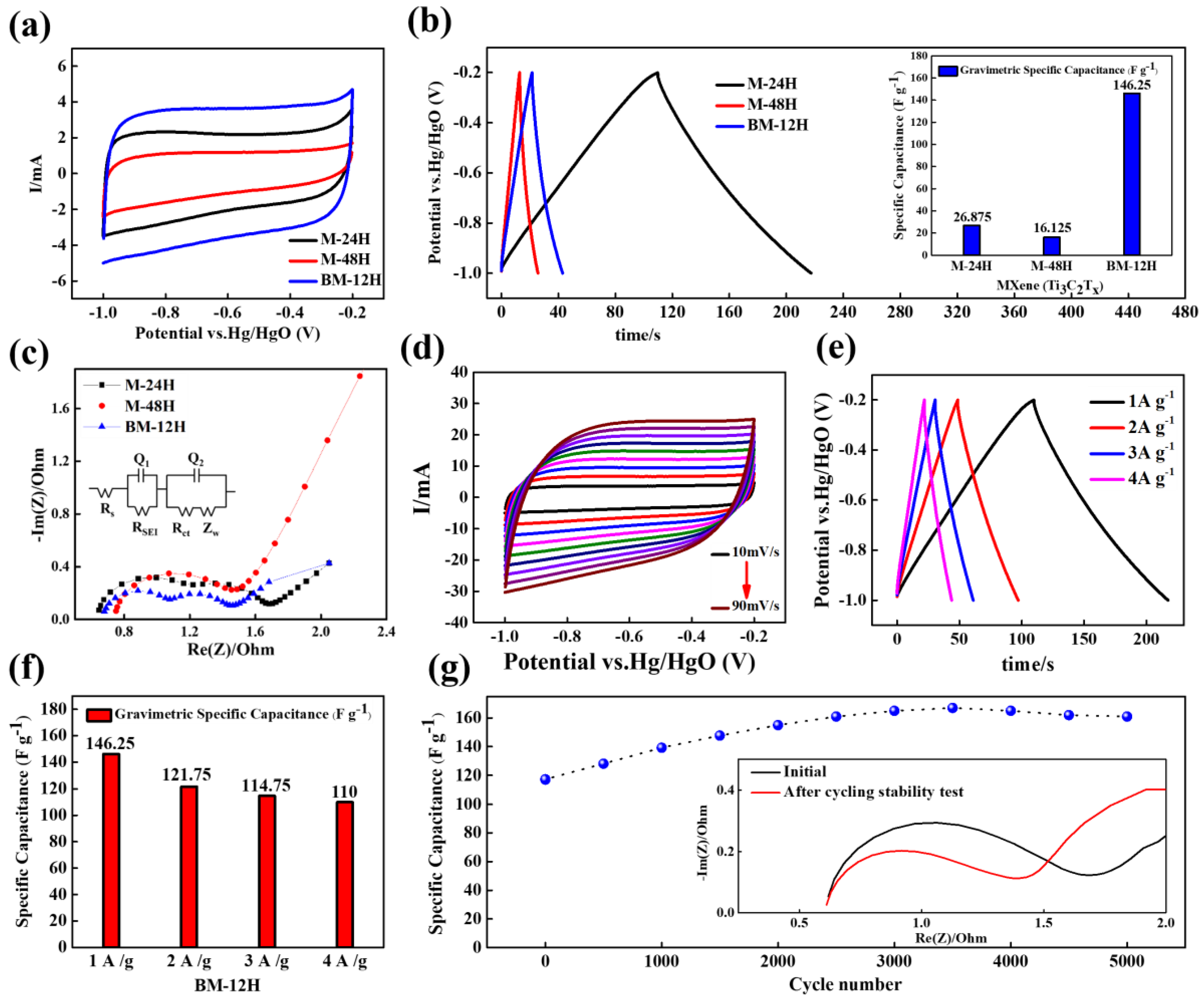
3.5. Electrochemical Analysis
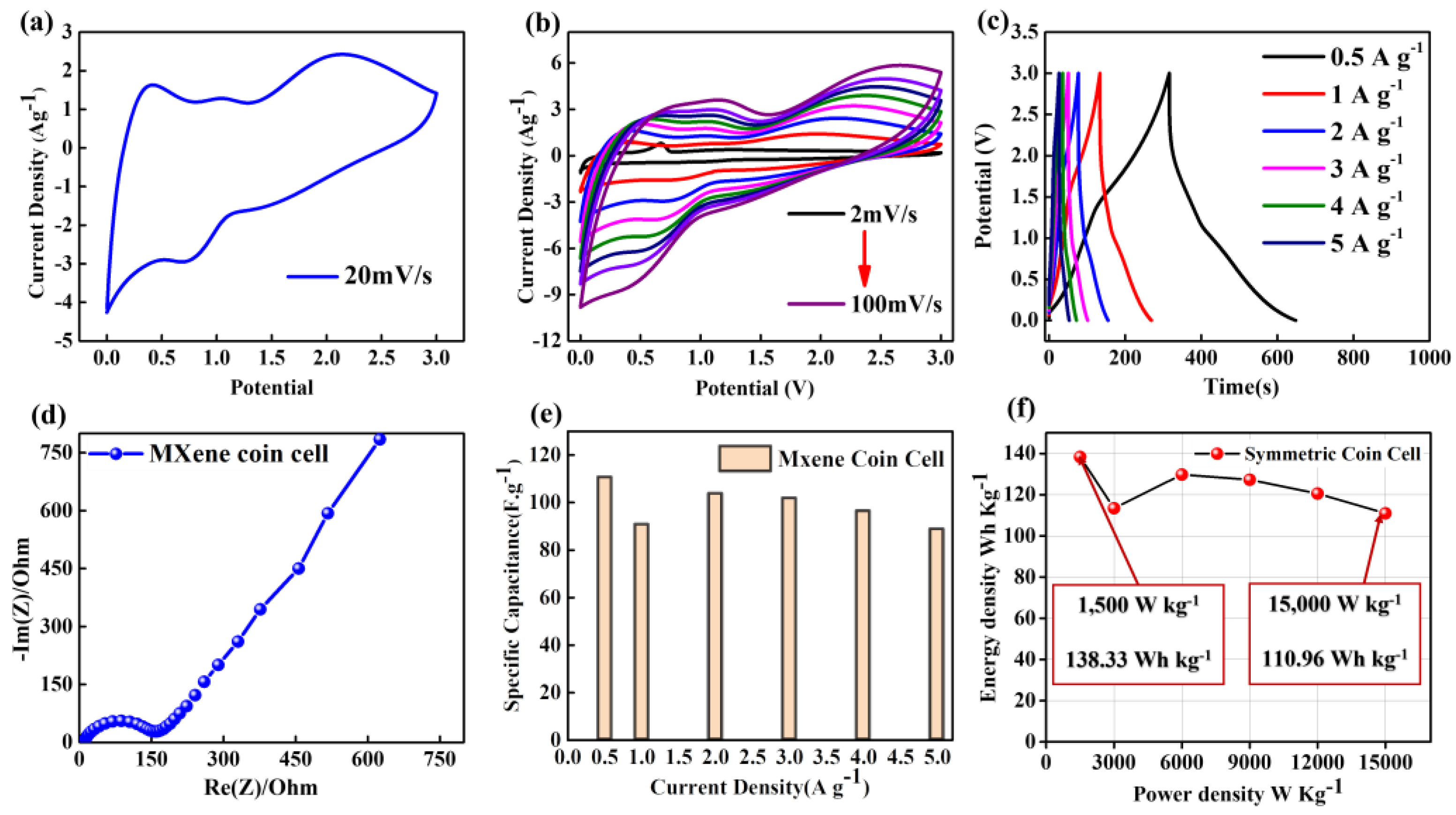
3.6. Fabrication of Symmetrical Coin Cell
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dutta, A.; Mitra, S.; Basak, M.; Banerjee, T. A Comprehensive Review on Batteries and Supercapacitors: Development and Challenges since Their Inception. Energy Storage 2022, 5, e339. [Google Scholar] [CrossRef]
- Poonam; Sharma, K.; Arora, A.; Tripathi, S.K. Review of Supercapacitors: Materials and Devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Cuce, E.; Harjunowibowo, D.; Cuce, P.M. Renewable and Sustainable Energy Saving Strategies for Greenhouse Systems: A Comprehensive Review. Renew. Sustain. Energy Rev. 2016, 64, 34–59. [Google Scholar] [CrossRef]
- Saidur, R.; Islam, M.R.; Rahim, N.A.; Solangi, K.H. A Review on Global Wind Energy Policy. Renew. Sustain. Energy Rev. 2010, 14, 1744–1762. [Google Scholar] [CrossRef]
- Timilsina, G.R.; Kurdgelashvili, L.; Narbel, P.A. Solar Energy: Markets, Economics and Policies. Renew. Sustain. Energy Rev. 2012, 16, 449–465. [Google Scholar] [CrossRef]
- Neill, S.P.; Hashemi, M.R.; Lewis, M.J. Tidal Energy Leasing and Tidal Phasing. Renew. Energy 2016, 85, 580–587. [Google Scholar] [CrossRef][Green Version]
- Islam, M.R.; Mekhilef, S.; Saidur, R. Progress and Recent Trends of Wind Energy Technology. Renew. Sustain. Energy Rev. 2013, 21, 456–468. [Google Scholar] [CrossRef]
- Gür, T.M. Review of Electrical Energy Storage Technologies, Materials and Systems: Challenges and Prospects for Large-Scale Grid Storage. Energy Environ. Sci. 2018, 11, 2696–2767. [Google Scholar] [CrossRef]
- Lain, M.J.; Kendrick, E. Understanding the Limitations of Lithium Ion Batteries at High Rates. J. Power Sources 2021, 493, 229690. [Google Scholar] [CrossRef]
- Marco, M.T.; Son, S.B.; Colclasure, A.M.; Shkrob, I.A.; Trask, S.E.; Bloom, I.D.; Abraham, D.P. How Fast Can a Li-Ion Battery Be Charged? Determination of Limiting Fast Charging Conditions. ACS Appl. Energy Mater. 2021, 4, 1063–1068. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, X.; Wang, Z.; Sun, F.; Dorrell, D.G. A Review of Supercapacitor Modeling, Estimation, and Applications: A Control/Management Perspective. Renew. Sustain. Energy Rev. 2018, 81, 1868–1878. [Google Scholar] [CrossRef]
- Naskar, P.; Maiti, A.; Chakraborty, P.; Kundu, D.; Biswas, B.; Banerjee, A. Chemical Supercapacitors: A Review Focusing on Metallic Compounds and Conducting Polymers. J. Mater. Chem. A Mater. 2021, 9, 1970–2017. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, S.; Mitchell, J.B.; Wang, R.; Zhan, C.; Jiang, D.E.; Presser, V.; Augustyn, V. Pseudocapacitance: From Fundamental Understanding to High Power Energy Storage Materials. Chem. Rev. 2020, 120, 6738–6782. [Google Scholar] [CrossRef]
- Gopi, C.V.V.M.; Sambasivam, S.; Raghavendra, K.V.G.; Vinodh, R.; Obaidat, I.M.; Kim, H.J. Facile Synthesis of Hierarchical Flower-like NiMoO4-CoMoO4 Nanosheet Arrays on Nickel Foam as an Efficient Electrode for High Rate Hybrid Supercapacitors. J. Energy Storage 2020, 30, 101550. [Google Scholar] [CrossRef]
- Selvaraj, A.R.; Raja, I.S.; Chinnadurai, D.; Rajendiran, R.; Cho, I.; Han, D.W.; Prabakar, K. Electrospun One Dimensional (1D) Pseudocapacitive Nanorods Embedded Carbon Nanofiber as Positrode and Graphene Wrapped Carbon Nanofiber as Negatrode for Enhanced Electrochemical Energy Storage. J. Energy Storage 2022, 46, 103731. [Google Scholar] [CrossRef]
- Hsu, S.Y.; Hsu, F.H.; Chen, J.L.; Cheng, Y.S.; Chen, J.M.; Lu, K.T. The Supercapacitor Electrode Properties and Energy Storage Mechanism of Binary Transition Metal Sulfide MnCo2S4compared with Oxide MnCo2O4 studied Using: In Situ Quick X-ray Absorption Spectroscopy. Mater. Chem. Front. 2021, 5, 4937–4949. [Google Scholar] [CrossRef]
- Wang, T.; Chen, H.C.; Yu, F.; Zhao, X.S.; Wang, H. Boosting the Cycling Stability of Transition Metal Compounds-Based Supercapacitors. Energy Storage Mater. 2019, 16, 545–573. [Google Scholar] [CrossRef][Green Version]
- Cui, M.; Meng, X. Overview of Transition Metal-Based Composite Materials for Supercapacitor Electrodes. Nanoscale Adv. 2020, 2, 5516–5528. [Google Scholar] [CrossRef]
- He, R.; Huang, X.; Feng, L. Recent Progress in Transition-Metal Sulfide Catalyst Regulation for Improved Oxygen Evolution Reaction. Energy Fuels 2022, 36, 6675–6694. [Google Scholar] [CrossRef]
- Chen, M.; Hu, Y.; Liang, K.; Zhao, Z.; Luo, Y.; Luo, S.; Ma, J. Interface Engineering Triggered by Carbon Nanotube-Supported Multiple Sulfides for Boosting Oxygen Evolution. Nanoscale 2021, 13, 18763–18772. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Hu, Q.; Zheng, M.; Chi, Y.; Qin, X.; Pang, H.; Xu, Q.; Verger, L.; Natu, V.; Carey, M.; et al. MXenes: An Introduction of Their Synthesis, Select Properties, and Applications. Trends Chem. 2018, 28, 656–669. [Google Scholar]
- Tomy, M.; Rajappan, A.A.; Vm, V.; Suryabai, X.T. Emergence of Novel 2D Materials for High-Performance Supercapacitor Electrode Applications: A Brief Review. Energy Fuels 2021, 35, 19881–19900. [Google Scholar] [CrossRef]
- Ni, H.; Wang, J.; Wu, A. Optical Bistability in Aperiodic Multilayer Composed of Graphene and Thue-Morse Lattices. Optik 2021, 242, 167163. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, D. Giant Spatial Goos–Hänchen Shifts in a Non-Hermitian Dielectric Slab Sandwiched by Graphene. Optik 2021, 242, 167332. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D Metal Carbides and Nitrides (MXenes) for Energy Storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, H.; Hu, T.; Fan, B.; Wang, X.; Li, Z. Emerging 2D MXenes for Supercapacitors: Status, Challenges and Prospects. Chem. Soc. Rev. 2020, 49, 6666–6693. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Peng, T.; Qin, X.; Zhang, Q.; Liu, T.; Dai, W.; Chen, B.; Yu, H.; Shi, S. Recent Advances in 2D MXenes: Preparation, Intercalation and Applications in Flexible Devices. J. Mater. Chem. A Mater. 2021, 9, 14147–14171. [Google Scholar] [CrossRef]
- Li, K.; Liang, M.; Wang, H.; Wang, X.; Huang, Y.; Coelho, J.; Pinilla, S.; Zhang, Y.; Qi, F.; Nicolosi, V.; et al. 3D MXene Architectures for Efficient Energy Storage and Conversion. Adv. Funct. Mater. 2020, 30, 2000842. [Google Scholar] [CrossRef]
- Hou, C.; Huang, C.; Yu, H.; Shi, S. Surface-Engineered Ti3C2Tx with Tunable Work Functions for Highly Efficient Polymer Solar Cells. Small 2022, 18, 2201046. [Google Scholar] [CrossRef]
- Khazaei, M.; Ranjbar, A.; Esfarjani, K.; Bogdanovski, D.; Dronskowski, R.; Yunoki, S. Insights into Exfoliation Possibility of MAX Phases to MXenes. Phys. Chem. Chem. Phys. 2018, 20, 8579–8592. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Benchakar, M.; Loupias, L.; Garnero, C.; Bilyk, T.; Morais, C.; Canaff, C.; Guignard, N.; Morisset, S.; Pazniak, H.; Hurand, S.; et al. One MAX Phase, Different MXenes: A Guideline to Understand the Crucial Role of Etching Conditions on Ti3C2Tx Surface Chemistry. Appl. Surf. Sci. 2020, 530, 147209. [Google Scholar] [CrossRef]
- Xue, N.; Li, X.; Zhang, M.; Han, L.; Liu, Y.; Tao, X. Chemical-Combined Ball-Milling Synthesis of Fluorine-Free Porous MXene for High-Performance Lithium Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 10234–10241. [Google Scholar] [CrossRef]
- Tian, S.; Cheng, G.; Tang, Z.; Sha, F.; Xuan, Z.; Ding, G. Fabrication of Two-Dimensional Ti3C2Tx MXenes by Ball Milling Pretreatment and Mild Etchant and Their Microstructure. Ceram. Int. 2020, 46, 28949–28954. [Google Scholar] [CrossRef]
- Tang, H.; Hu, Q.; Zheng, M.; Chi, Y.; Qin, X.; Pang, H.; Xu, Q. MXene–2D Layered Electrode Materials for Energy Storage. Prog. Nat. Sci. Mater. Int. 2018, 28, 133–147. [Google Scholar] [CrossRef]
- Xu, J.; Peng, T.; Zhang, Q.; Zheng, H.; Yu, H.; Shi, S. Intercalation Effects on the Electrochemical Properties of Ti3C2TxMXene Nanosheets for High-Performance Supercapacitors. ACS Appl. Nano Mater. 2022, 5, 8794–8803. [Google Scholar] [CrossRef]
- Wu, Y.; Nie, P.; Wang, J.; Dou, H.; Zhang, X. Few-Layer MXenes Delaminated via High-Energy Mechanical Milling for Enhanced Sodium-Ion Batteries Performance. ACS Appl. Mater. Interfaces 2017, 9, 39610–39617. [Google Scholar] [CrossRef]
- Shao, H.; Lin, Z.; Xu, K.; Taberna, P.-L.; Simon, P. Electrochemical Study of Pseudocapacitive Behavior of Ti3C2Tx MXene Material in Aqueous Electrolytes. Energy Storage Mater. 2019, 18, 456–461. [Google Scholar] [CrossRef][Green Version]
- Yang, L.; Dall’Agnese, Y.; Hantanasirisakul, K.; Shuck, C.E.; Maleski, K.; Alhabeb, M.; Chen, G.; Gao, Y.; Sanehira, Y.; Jena, A.K.; et al. SnO2-Ti3C2 MXene Electron Transport Layers for Perovskite Solar Cells. J. Mater. Chem. A Mater. 2019, 7, 5635–5642. [Google Scholar] [CrossRef]
- Agresti, A.; Pazniak, A.; Pescetelli, S.; di Vito, A.; Rossi, D.; Pecchia, A.; Auf der Maur, M.; Liedl, A.; Larciprete, R.; Kuznetsov, D.V.; et al. Titanium-Carbide MXenes for Work Function and Interface Engineering in Perovskite Solar Cells. Nat. Mater. 2019, 18, 1228–1234. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Du, R.; Li, Z.; Song, H.; Chao, Z.; Zu, D.; Chong, D.; Gao, N.; Li, C. Rationally Designed CdS/Ti3C2 MXene Electrocatalysts for Efficient CO2 Reduction in Aqueous Electrolyte. Ceram. Int. 2021, 47, 28321–28327. [Google Scholar] [CrossRef]
- Jiang, Y.; Tian, M.; Wang, H.; Wei, C.; Sun, Z.; Rummeli, M.H.; Strasser, P.; Sun, J.; Yang, R. Mildly Oxidized MXene (Ti3C2, Nb2C, and V2C) Electrocatalyst via a Generic Strategy Enables Longevous Li-O2 Battery under a High Rate. ACS Nano 2021, 15, 19640–19650. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Lian, R.; Li, H.; Zhang, Y.; Gong, Z.; Zhu, K.; Ye, K.; Yan, J.; Wang, G.; Gao, Y.; et al. Induction of Planar Sodium Growth on MXene (Ti3C2Tx)-Modified Carbon Cloth Hosts for Flexible Sodium Metal Anodes. ACS Nano 2020, 14, 8744–8753. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Wang, P.; Ye, K.; Zhu, K.; Yan, J.; Wang, G.; Chen, G.; Cao, D. MXene-Modified Conductive Framework as a Universal Current Collector for Dendrite-Free Lithium and Zinc Metal Anode. J. Colloid Interface Sci. 2022, 625, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Dong, G.; Zhang, M.; Wang, D.; Chen, Y.; Cao, D.; Zhu, K.; Chen, G. Surface-Engineered Ti3C2TX MXene Enabling Rapid Sodium/Potassium Ion Storage. 2D Mater. 2023, 10, 014005. [Google Scholar] [CrossRef]
- Li, R.; Zhang, L.; Shi, L.; Wang, P. MXene Ti3C2: An Effective 2D Light-to-Heat Conversion Material. ACS Nano 2017, 11, 3752–3759. [Google Scholar] [CrossRef][Green Version]
- Zhao, J.; Yang, Y.; Yang, C.; Tian, Y.; Han, Y.; Liu, J.; Yin, X.; Que, W. A Hydrophobic Surface Enabled Salt-Blocking 2D Ti3C2 MXene Membrane for Efficient and Stable Solar Desalination. J. Mater. Chem. A Mater. 2018, 6, 16196–16204. [Google Scholar] [CrossRef]
- Wu, M.; He, M.; Hu, Q.; Wu, Q.; Sun, G.; Xie, L.; Zhang, Z.; Zhu, Z.; Zhou, A. Ti3C2 MXene-Based Sensors with High Selectivity for NH3 Detection at Room Temperature. ACS Sens. 2019, 4, 2763–2770. [Google Scholar] [CrossRef]
- Kalambate, P.K.; Dhanjai; Sinha, A.; Li, Y.; Shen, Y.; Huang, Y. An Electrochemical Sensor for Ifosfamide, Acetaminophen, Domperidone, and Sumatriptan Based on Self-Assembled MXene/MWCNT/Chitosan Nanocomposite Thin Film. Microchim. Acta 2020, 187, 402. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Y.; Zhang, Y.; Meng, H.; Xu, Y.; Han, Y.; Wang, Z.; Dong, Y.; Zhang, X. Highly Safe and Ionothermal Synthesis of Ti3C2 MXene with Expanded Interlayer Spacing for Enhanced Lithium Storage. J. Energy Chem. 2020, 47, 203–209. [Google Scholar] [CrossRef]
- Huang, X.; Wu, P. A Facile, High-Yield, and Freeze-and-Thaw-Assisted Approach to Fabricate MXene with Plentiful Wrinkles and Its Application in On-Chip Micro-Supercapacitors. Adv. Funct. Mater. 2020, 30, 1910048. [Google Scholar] [CrossRef]
- Cho, I.; Selvaraj, A.R.; Bak, J.; Kim, H.; Prabakar, K. Anomalous Increase in Specific Capacitance in MXene during Galvanostatic Cycling Studies. J. Energy Storage 2022, 53, 105207. [Google Scholar] [CrossRef]
- Malaki, M.; Maleki, A.; Varma, R.S. MXenes and Ultrasonication. J. Mater. Chem. A Mater. 2019, 7, 10843–10857. [Google Scholar] [CrossRef]
- Lin, X.; Liang, Y.; Lu, Z.; Lou, H.; Zhang, X.; Liu, S.; Zheng, B.; Liu, R.; Fu, R.; Wu, D. Mechanochemistry: A Green, Activation-Free and Top-Down Strategy to High-Surface-Area Carbon Materials. ACS Sustain. Chem. Eng. 2017, 5, 8535–8540. [Google Scholar] [CrossRef]
- Szczesniak, B.; Borysiuk, S.; Choma, J.; Jaroniec, M. Mechanochemical Synthesis of Highly Porous Materials. Mater. Horiz. 2020, 7, 1457–1473. [Google Scholar] [CrossRef]
- Wang, J.; Hu, Y.; Yang, B.; Wang, X.; Qin, J.; Cao, M. Mechanochemistry-Induced Biaxial Compressive Strain Engineering in MXenes for Boosting Lithium Storage Kinetics. Nano Energy 2021, 87, 106053. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Wang, D.; Mao, Z. Defect Engineering to Boost the Lithium-Ion Storage Performance of Ti3C2TX MXene Induced by Plasma-Assisted Mechanochemistry. ACS Appl. Energy Mater. 2021, 4, 10280–10289. [Google Scholar] [CrossRef]
- Kumar, G.R.; Jayasankar, K.; Das, S.K.; Dash, T.; Dash, A.; Jena, B.K.; Mishra, B.K. Shear-Force-Dominated Dual-Drive Planetary Ball Milling for the Scalable Production of Graphene and Its Electrocatalytic Application with Pd Nanostructures. RSC Adv. 2016, 6, 20067–20073. [Google Scholar] [CrossRef]
- von Treifeldt, J.E.; Firestein, K.L.; Fernando, J.F.S.; Zhang, C.; Siriwardena, D.P.; Lewis, C.E.M.; Golberg, D.V. The Effect of Ti3AlC2 MAX Phase Synthetic History on the Structure and Electrochemical Properties of Resultant Ti3C2 MXenes. Mater. Des. 2021, 199, 109403. [Google Scholar] [CrossRef]
- Su, X.; Zhang, J.; Mu, H.; Zhao, J.; Wang, Z.; Zhao, Z.; Han, C.; Ye, Z. Effects of Etching Temperature and Ball Milling on the Preparation and Capacitance of Ti3C2 MXene. J. Alloys Compd. 2018, 752, 32–39. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Liu, Y.; Hu, W. Effect of HF Etching on Titanium Carbide (Ti3C2Tx) Microstructure and Its Capacitive Properties. Chem. Eng. J. 2023, 452, 139512. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, Z.; Sui, X.; Li, Y.; Liu, X.; Zhang, Y. Ti3AlC2 MAX and Ti3C2 MXene Quantum Sheets for Record-High Optical Nonlinearity. J. Phys. Chem. Lett. 2022, 13, 3929–3936. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Luo, D.; Zhang, Y.; Zhang, Z.; Wang, J.; Cui, G.; Wang, X.; Yu, A.; Chen, Z. Hierarchically Porous Ti3C2MXene with Tunable Active Edges and Unsaturated Coordination Bonds for Superior Lithium-Sulfur Batteries. ACS Nano 2021, 15, 19457–19467. [Google Scholar] [CrossRef] [PubMed]
- Natu, V.; Benchakar, M.; Canaff, C.; Habrioux, A.; Célérier, S.; Barsoum, M.W. A Critical Analysis of the X-Ray Photoelectron Spectra of Ti3C2Tz MXenes. Matter 2021, 4, 1224–1251. [Google Scholar] [CrossRef]
- Hu, M.; Hu, T.; Li, Z.; Yang, Y.; Cheng, R.; Yang, J.; Cui, C.; Wang, X. Surface Functional Groups and Interlayer Water Determine the Electrochemical Capacitance of Ti3C2Tx MXene. ACS Nano 2018, 12, 3578–3586. [Google Scholar] [CrossRef]
- Huang, K.; Li, C.; Li, H.; Ren, G.; Wang, L.; Wang, W.; Meng, X. Photocatalytic Applications of Two-Dimensional Ti3C2MXenes: A Review. ACS Appl. Nano Mater. 2020, 3, 9581–9603. [Google Scholar] [CrossRef]
- Halim, J.; Cook, K.M.; Naguib, M.; Eklund, P.; Gogotsi, Y.; Rosen, J.; Barsoum, M.W. X-ray Photoelectron Spectroscopy of Select Multi-Layered Transition Metal Carbides (MXenes). Appl. Surf. Sci. 2016, 362, 406–417. [Google Scholar] [CrossRef][Green Version]
- Shah, S.A.; Habib, T.; Gao, H.; Gao, P.; Sun, W.; Green, M.J.; Radovic, M. Template-Free 3D Titanium Carbide (Ti3C2Tx) MXene Particles Crumpled by Capillary Forces. Chem. Commun. 2017, 53, 400–403. [Google Scholar] [CrossRef]
- Caffrey, N.M. Effect of Mixed Surface Terminations on the Structural and Electrochemical Properties of Two-Dimensional Ti3C2T2 and V2CT2 MXenes Multilayers. Nanoscale 2018, 10, 13520–13530. [Google Scholar] [CrossRef][Green Version]
- Ma, Y.; Lv, X.; Xiong, D.; Zhao, X.; Zhang, Z. Catalytic Degradation of Ranitidine Using Novel Magnetic Ti3C2-Based MXene Nanosheets Modified with Nanoscale Zero-Valent Iron Particles. Appl. Catal. B 2021, 284, 119720. [Google Scholar] [CrossRef]
- Haddadi, S.A.; Hu, S.; Ghaderi, S.; Ghanbari, A.; Ahmadipour, M.; Pung, S.Y.; Li, S.; Feilizadeh, M.; Arjmand, M. Amino-Functionalized MXene Nanosheets Doped with Ce(III) as Potent Nanocontainers toward Self-Healing Epoxy Nanocomposite Coating for Corrosion Protection of Mild Steel. ACS Appl. Mater. Interfaces 2021, 13, 42074–42093. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wei, Y.; Wang, Y.; Chen, H.; Caro, J.; Wang, H. A Two-Dimensional Lamellar Membrane: MXene Nanosheet Stacks. Angew. Chem. 2017, 129, 1851–1855. [Google Scholar] [CrossRef]
- Pazniak, A.; Bazhin, P.; Shplis, N.; Kolesnikov, E.; Shchetinin, I.; Komissarov, A.; Polcak, J.; Stolin, A.; Kuznetsov, D. Ti3C2Tx MXene Characterization Produced from SHS-Ground Ti3AlC2. Mater. Des. 2019, 183, 108143. [Google Scholar] [CrossRef]
- Chertopalov, S.; Mochalin, V.N. Environment-Sensitive Photoresponse of Spontaneously Partially Oxidized Ti3C2 MXene Thin Films. ACS Nano 2018, 12, 6109–6116. [Google Scholar] [CrossRef]
- Khazaei, M.; Arai, M.; Sasaki, T.; Ranjbar, A.; Liang, Y.; Yunoki, S. OH-Terminated Two-Dimensional Transition Metal Carbides and Nitrides as Ultralow Work Function Materials. Phys. Rev. B Condens. Matter. Mater. Phys. 2015, 92, 075411. [Google Scholar] [CrossRef][Green Version]
- Schultz, T.; Frey, N.C.; Hantanasirisakul, K.; Park, S.; May, S.J.; Shenoy, V.B.; Gogotsi, Y.; Koch, N. Surface Termination Dependent Work Function and Electronic Properties of Ti3C2Tx MXene. Chem. Mater. 2019, 31, 6590–6597. [Google Scholar] [CrossRef][Green Version]
- Hart, J.L.; Hantanasirisakul, K.; Lang, A.C.; Anasori, B.; Pinto, D.; Pivak, Y.; van Omme, J.T.; May, S.J.; Gogotsi, Y.; Taheri, M.L. Control of MXenes’ Electronic Properties through Termination and Intercalation. Nat. Commun. 2019, 10, 522. [Google Scholar] [CrossRef][Green Version]
- Li, B.; Zhao, Z.; Gao, F.; Wang, X.; Qiu, J. Mesoporous Microspheres Composed of Carbon-Coated TiO2 Nanocrystals with Exposed {001} Facets for Improved Visible Light Photocatalytic Activity. Appl. Catal. B 2014, 147, 958–964. [Google Scholar] [CrossRef]
- Peng, C.; Wang, H.; Yu, H.; Peng, F. (111) TiO2-x/Ti3C2: Synergy of Active Facets, Interfacial Charge Transfer and Ti3+ Doping for Enhance Photocatalytic Activity. Mater. Res. Bull. 2017, 89, 16–25. [Google Scholar] [CrossRef]
- Selvaraj, A.R.; Muthusamy, A.; Inho-Cho; Kim, H.J.; Senthil, K.; Prabakar, K. Ultrahigh Surface Area Biomass Derived 3D Hierarchical Porous Carbon Nanosheet Electrodes for High Energy Density Supercapacitors. Carbon 2021, 174, 463–474. [Google Scholar] [CrossRef]
- Selvaraj, A.R.; Chinnadurai, D.; Cho, I.; Bak, J.S.; Prabakar, K. Bio-Waste Wood-Derived Porous Activated Carbon with Tuned Microporosity for High Performance Supercapacitors. J. Energy Storage 2022, 52, 104928. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Li, H.; Lin, S.; Ding, W.; Zhu, X.; Sheng, Z.; Wang, H.; Zhu, X.; Sun, Y. 2D/2D 1T-MoS2/Ti3C2 MXene Heterostructure with Excellent Supercapacitor Performance. Adv. Funct. Mater. 2020, 30, 0190302. [Google Scholar] [CrossRef]
- Cao, M.; Wang, F.; Wang, L.; Wu, W.; Lv, W.; Zhu, J. Room Temperature Oxidation of Ti3C2 MXene for Supercapacitor Electrodes. J. Electrochem. Soc. 2017, 164, A3933–A3942. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Wang, Y.; Peng, X. Homogeneously Assembling Like-Charged WS2 and GO Nanosheets Lamellar Composite Films by Filtration for Highly Efficient Lithium Ion Batteries. Nano Energy 2014, 7, 25–32. [Google Scholar] [CrossRef]
- Qiu, X.; Xiao, Z.; Wang, L.; Fan, L.Z. High Rate Integrated Quasi-Solid State Supercapacitors Based on Nitrogen-Enriched Active Carbon Fiber/Reduced Graphene Oxide Nanocomposite. Carbon 2018, 130, 196–205. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Ying, Y.; Wang, Y.; Peng, X. Binder-Free Layered Ti3C2/CNTs Nanocomposite Anodes with Enhanced Capacity and Long-Cycle Life for Lithium-Ion Batteries. Dalton Trans. 2015, 44, 7123–7126. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A Review of Electrolyte Materials and Compositions for Electrochemical Supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef]
- Pal, B.; Yang, S.; Ramesh, S.; Thangadurai, V.; Jose, R. Electrolyte Selection for Supercapacitive Devices: A Critical Review. Nanoscale Adv. 2019, 1, 3807–3835. [Google Scholar] [CrossRef][Green Version]
- Al-Temimy, A.; Anasori, B.; Mazzio, K.A.; Kronast, F.; Seredych, M.; Kurra, N.; Mawass, M.-A.; Raoux, S.; Gogotsi, Y.; Petit, T. Enhancement of Ti3C2 MXene Pseudocapacitance after Urea Intercalation Studied by Soft X-ray Absorption Spectroscopy. J. Phys. Chem. C 2020, 124, 5079–5086. [Google Scholar] [CrossRef]
- Dall’Agnese, Y.; Rozier, P.; Taberna, P.-L.; Gogotsi, Y.; Simon, P. Capacitance of Two-Dimensional Titanium Carbide (MXene) and MXene/Carbon Nanotube Composites in Organic Electrolytes. J. Power Sources 2016, 306, 510–515. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Wang, X.; Li, X.; Bai, Y.; Xiao, H.; Liu, Y.; Liu, R.; Yuan, G. Engineering 3D Ion Transport Channels for Flexible MXene Films with Superior Capacitive Performance. Adv. Funct. Mater. 2019, 29, 1900326. [Google Scholar] [CrossRef]
- Selvaraj, A.R.; Kim, H.J.; Senthil, K.; Prabakar, K. Cation Intercalated One-Dimensional Manganese Hydroxide Nanorods and Hierarchical Mesoporous Activated Carbon Nanosheets with Ultrahigh Capacitance Retention Asymmetric Supercapacitors. J. Colloid Interface Sci. 2020, 566, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kajiyama, S.; Iinuma, H.; Hosono, E.; Oro, S.; Moriguchi, I.; Okubo, M.; Yamada, A. Pseudocapacitance of MXene Nanosheets for High-Power Sodium-Ion Hybrid Capacitors. Nat. Commun. 2015, 6, 6544. [Google Scholar] [CrossRef][Green Version]
- Srinivasan, S.; Jothibas, M.; Nesakumar, N. Enhancing Electric Double Layer Capacitance of Two-Dimensional Titanium Carbide (MXene) with Facile Synthesis and Accentuated Properties. Energy Fuels 2022, 36, 2811–2820. [Google Scholar] [CrossRef]
- Wu, W.; Wang, C.; Zhao, C.; Wei, D.; Zhu, J.; Xu, Y. Facile Strategy of Hollow Polyaniline Nanotubes Supported on Ti3C2-MXene Nanosheets for High-Performance Symmetric Supercapacitors. J. Colloid Interface Sci. 2020, 580, 601–613. [Google Scholar] [CrossRef]
- Vandeginste, V. A Review of Fabrication Technologies for Carbon Electrode-Based Micro-Supercapacitors. Appl. Sci. 2022, 12, 862. [Google Scholar] [CrossRef]
- Ma, J.; Cheng, Y.; Wang, L.; Dai, X.; Yu, F. Free-Standing Ti3C2Tx MXene Film as Binder-Free Electrode in Capacitive Deionization with an Ultrahigh Desalination Capacity. Chem. Eng. J. 2020, 384, 123329. [Google Scholar] [CrossRef]
- Srimuk, P.; Kaasik, F.; Krüner, B.; Tolosa, A.; Fleischmann, S.; Jäckel, N.; Tekeli, M.C.; Aslan, M.; Suss, M.E.; Presser, V. MXene as a Novel Intercalation-Type Pseudocapacitive Cathode and Anode for Capacitive Deionization. J. Mater. Chem. A Mater. 2016, 4, 18265–18271. [Google Scholar] [CrossRef][Green Version]
- Buczek, S.; Barsoum, M.L.; Uzun, S.; Kurra, N.; Andris, R.; Pomerantseva, E.; Mahmoud, K.A.; Gogotsi, Y. Rational Design of Titanium Carbide MXene Electrode Architectures for Hybrid Capacitive Deionization. Energy Environ. Mater. 2020, 3, 398–404. [Google Scholar] [CrossRef]
- Hou, W.; Sun, Y.; Zhang, Y.; Wang, T.; Wu, L.; Du, Y.; Zhong, W. Mixed-Dimensional Heterostructure of Few-Layer MXene Based Vertical Aligned MoS2 Nanosheets for Enhanced Supercapacitor Performance. J. Alloys Compd. 2021, 859, 157797. [Google Scholar] [CrossRef]

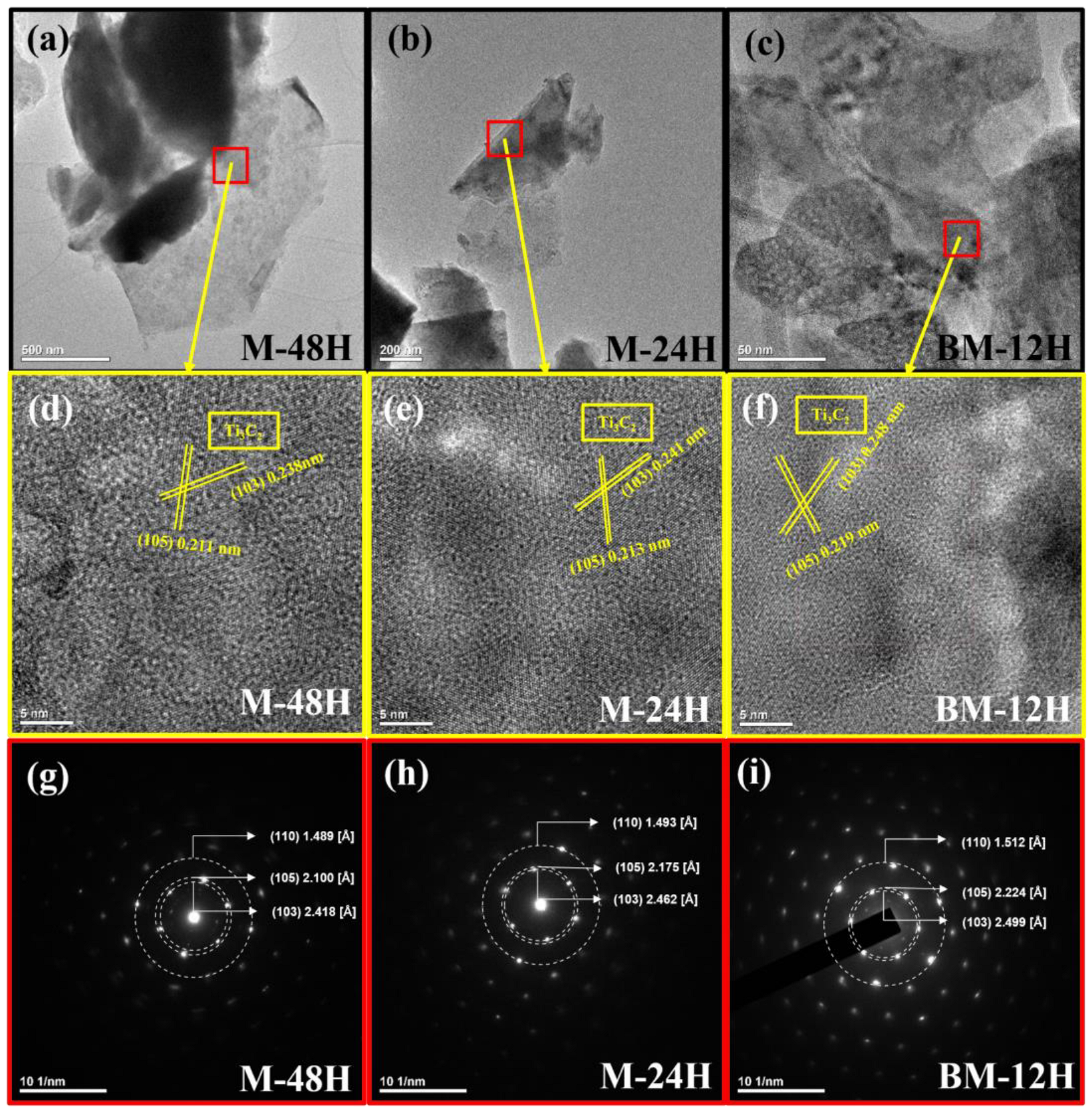
| (103) | (105) | (110) | ||||
|---|---|---|---|---|---|---|
| D-Spacing (Å) | Width of Lattice Pattern (nm) | D-Spacing (Å) | Width of Lattice Pattern (nm) | D-Spacing (Å) | Width of Lattice Pattern (nm) | |
| M-48H | 2.42 | 0.24 | 2.100 | 0.211 | 1.489 | - |
| M-24H | 2.46 | 0.24 | 2.175 | 0.213 | 1.493 | - |
| BM-12H | 2.5 | 0.25 | 2.224 | 0.219 | 1.512 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, I.; Selvaraj, A.R.; Bak, J.; Kim, H.; Prabakar, K. Mechanochemical Pretreated Mn+1AXn (MAX) Phase to Synthesize 2D-Ti3C2Tx MXene Sheets for High-Performance Supercapacitors. Nanomaterials 2023, 13, 1741. https://doi.org/10.3390/nano13111741
Cho I, Selvaraj AR, Bak J, Kim H, Prabakar K. Mechanochemical Pretreated Mn+1AXn (MAX) Phase to Synthesize 2D-Ti3C2Tx MXene Sheets for High-Performance Supercapacitors. Nanomaterials. 2023; 13(11):1741. https://doi.org/10.3390/nano13111741
Chicago/Turabian StyleCho, Inho, Aravindha Raja Selvaraj, Jinsoo Bak, Heeje Kim, and Kandasamy Prabakar. 2023. "Mechanochemical Pretreated Mn+1AXn (MAX) Phase to Synthesize 2D-Ti3C2Tx MXene Sheets for High-Performance Supercapacitors" Nanomaterials 13, no. 11: 1741. https://doi.org/10.3390/nano13111741
APA StyleCho, I., Selvaraj, A. R., Bak, J., Kim, H., & Prabakar, K. (2023). Mechanochemical Pretreated Mn+1AXn (MAX) Phase to Synthesize 2D-Ti3C2Tx MXene Sheets for High-Performance Supercapacitors. Nanomaterials, 13(11), 1741. https://doi.org/10.3390/nano13111741