Improvement in Switching Characteristics and Bias Stability of Solution-Processed Zinc–Tin Oxide Thin Film Transistors via Simple Low-Pressure Thermal Annealing Treatment
Abstract
1. Introduction
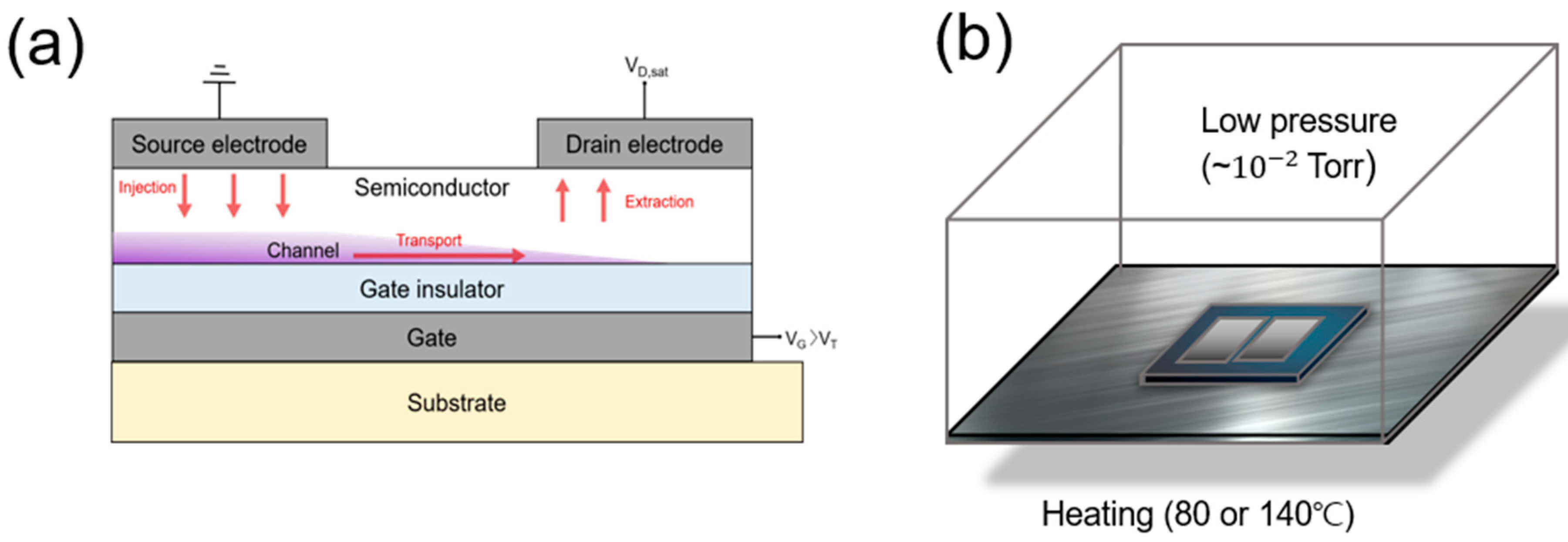
2. Materials and Methods
3. Results and Discussion
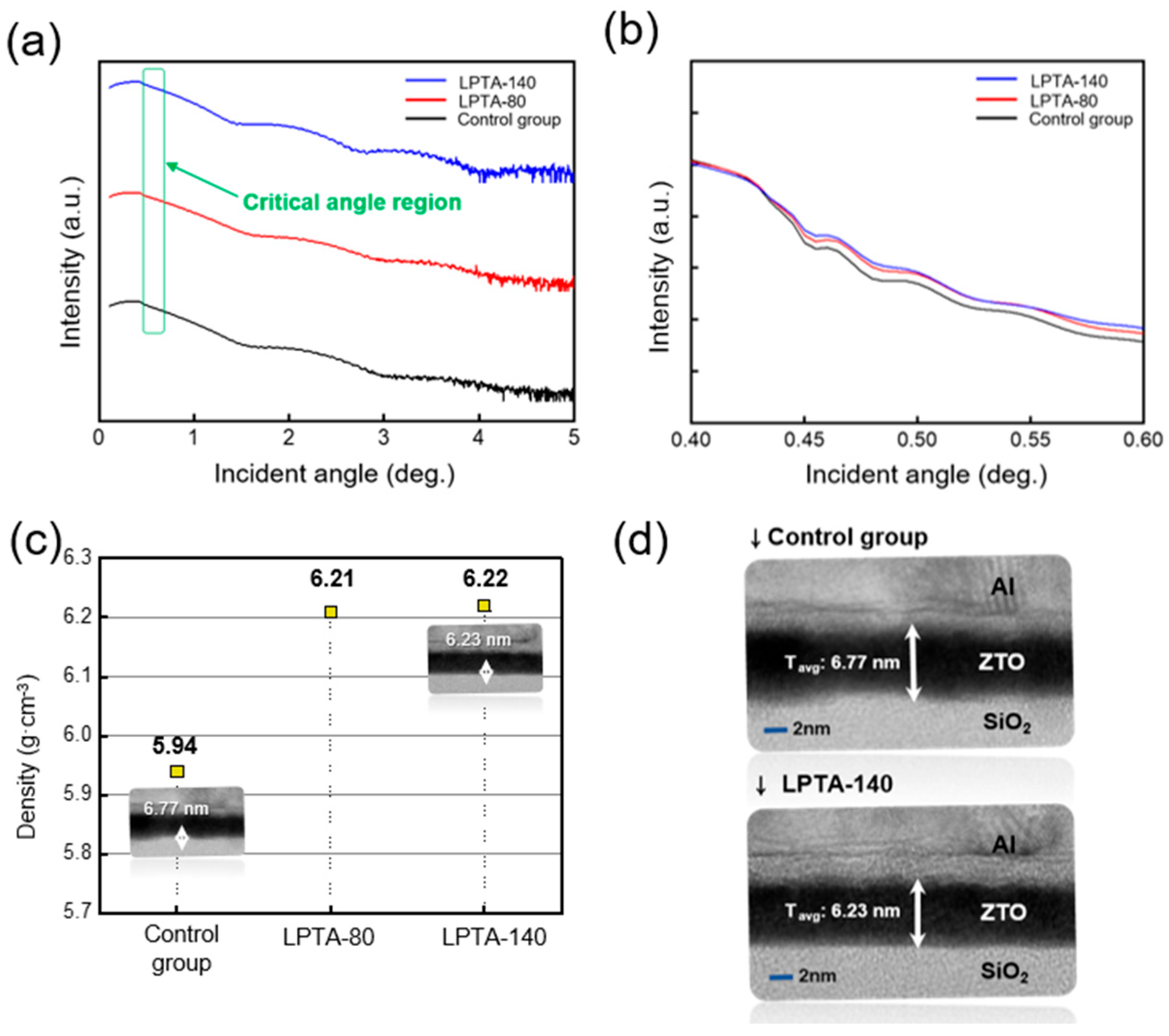
3.1. Physical Properties and Water Contact Angles of ZTO Thin Films
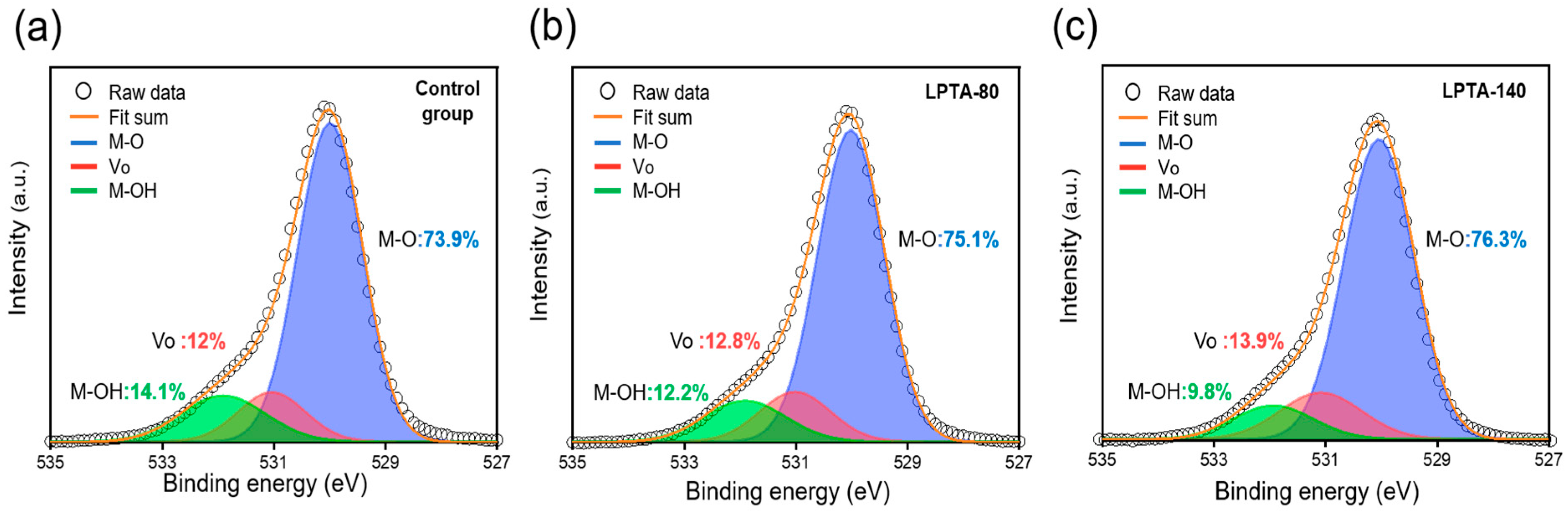
3.2. Chemical Properties of ZTO Thin Films
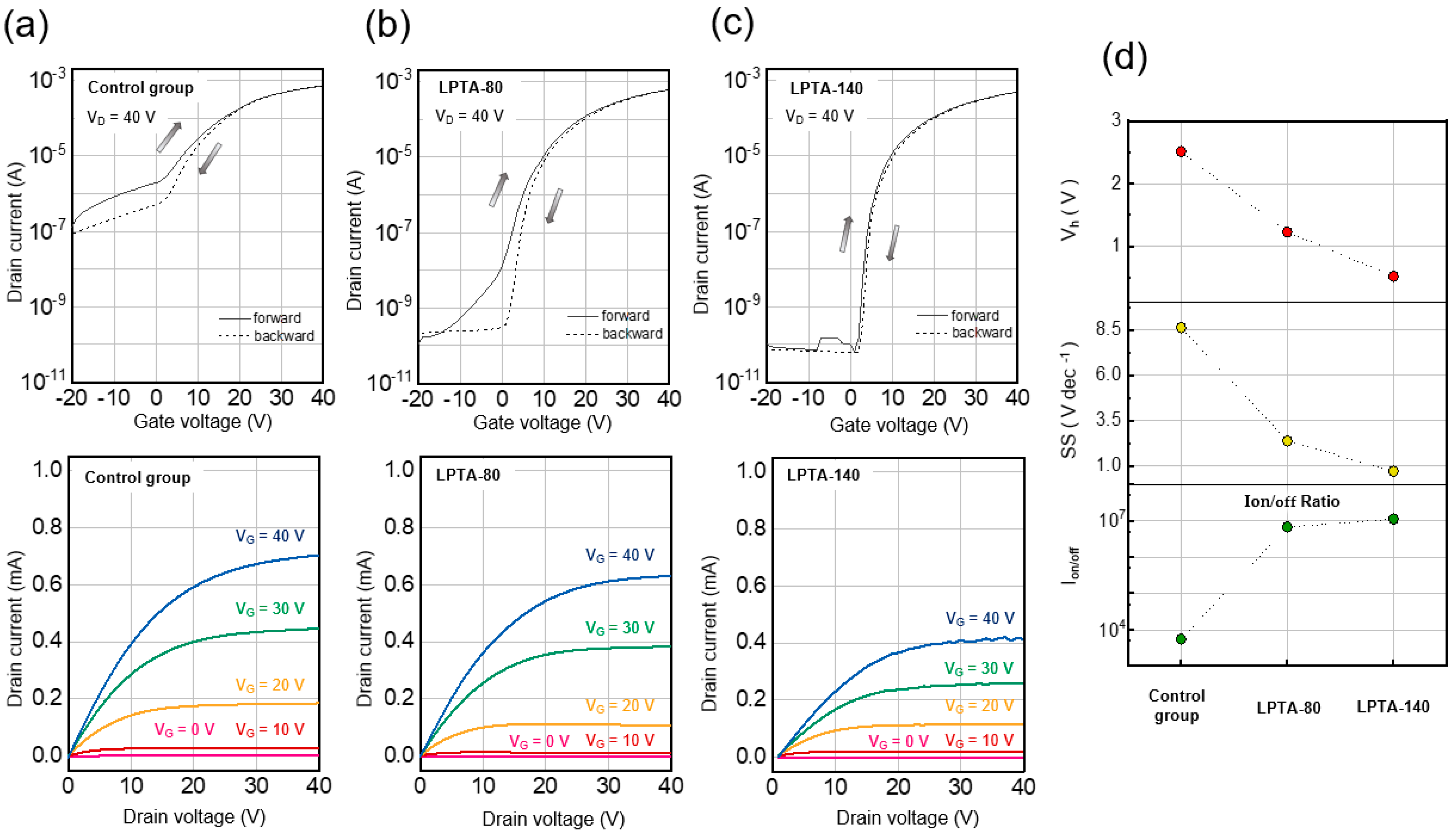
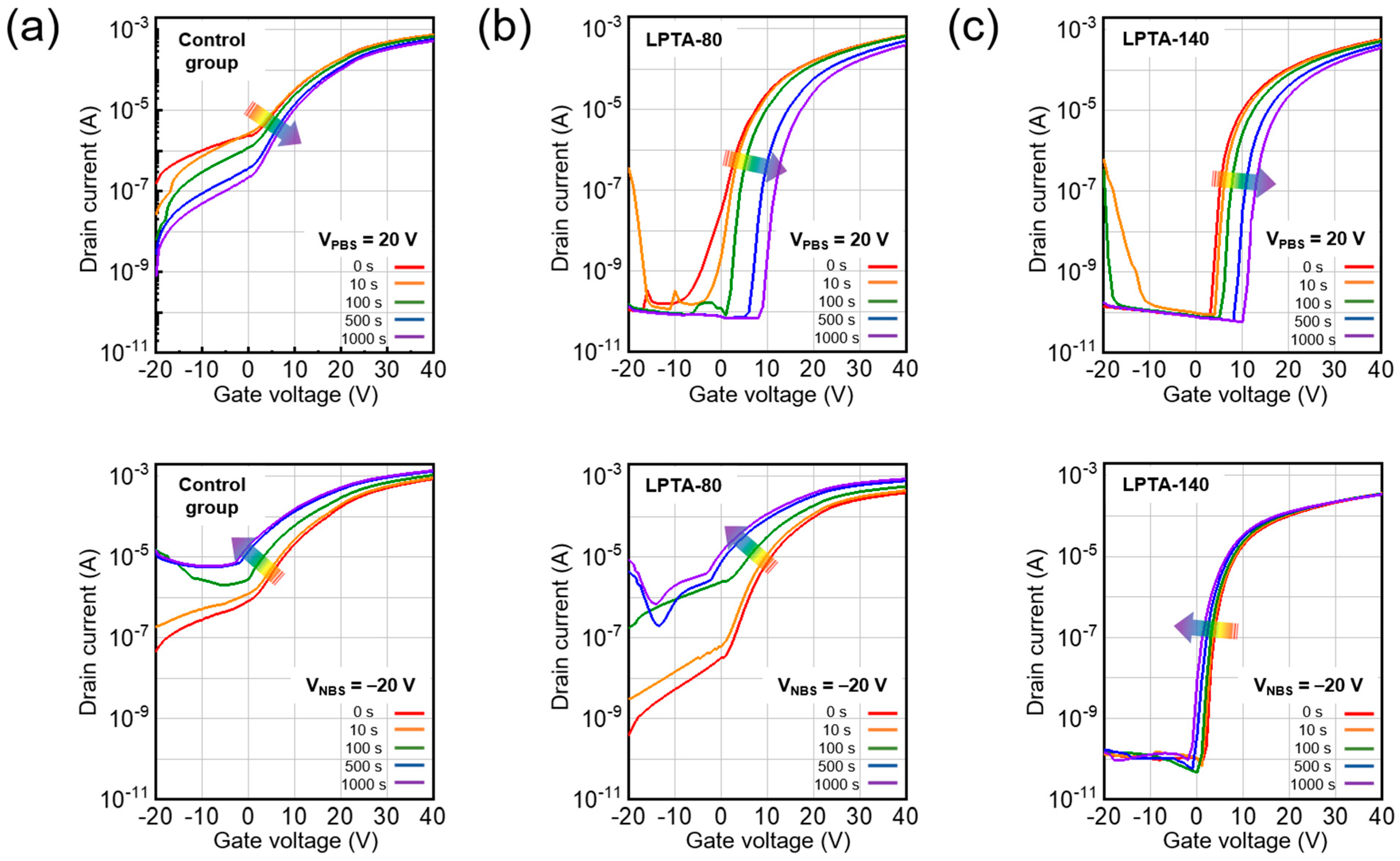
3.3. Effects of Low-Pressure Thermal Annealing on Electrical Characteristics in ZTO TFTs
3.4. Effects of Low-Pressure Thermal Annealing on D2D Uniformity in ZTO TFTs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fortunato, E.; Barquinha, P. Martins, Oxide semiconductor thin-film transistors: A review of recent advances. Adv. Mater. 2012, 24, 2945–2986. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, T.; Hosono, H. Material characteristics and applications of transparent amorphous oxide semiconductors. NPG Asia Mater. 2010, 2, 15–22. [Google Scholar] [CrossRef]
- Nathan, S.; Lee, S.; Jeon, J. Robertson, Amorphous oxide semiconductor TFTs for displays and imaging. IEEE/OSA J. Disp. Technol. 2014, 10, 917–927. [Google Scholar] [CrossRef]
- Kamiya, T.; Nomura, K.; Hosono, H. Present status of amorphous In-Ga-Zn-O thin-film transistors. Sci. Technol. Adv. Mater. 2010, 11, 044305. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Ohta, H.; Takagi, A.; Kamiya, T.; Hirano, M.; Hosono, H. Room-temperature fabrication of transparent flexible thin-film transistors using amorphous oxide semiconductors. Nature 2004, 432, 488. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, J.; Yang, L.; Qu, M.; Qi, D.C.; Zhang, K.H.L. Wide Bandgap Oxide Semiconductors: From Materials Physics to Optoelectronic Devices. Adv. Mater. 2021, 33, 2006230. [Google Scholar] [CrossRef]
- Fortunato, E.M.C.; Pereira, L.M.N.; Barquinha, P.M.C.; Rego, A.M.B.D.; Goņalves, G.; Vil, A.; Morante, J.R.; Martins, R.F.P. High mobility indium free amorphous oxide thin film transistors. Appl. Phys. Lett. 2008, 92, 10–13. [Google Scholar] [CrossRef]
- Fernandes, C.; Santa, A.; Santos, Â.; Bahubalindruni, P.; Deuermeier, J.; Martins, R.; Fortunato, E.; Barquinha, P. A Sustainable Approach to Flexible Electronics with Zinc-Tin Oxide Thin-Film Transistors. Adv. Electron. Mater. 2018, 4, 1800032. [Google Scholar] [CrossRef]
- Parthiban, S.; Kwon, J.Y. Role of dopants as a carrier suppressor and strong oxygen binder in amorphous indium-oxide-based field effect transistor. J. Mater. Res. 2014, 29, 1585–1596. [Google Scholar] [CrossRef]
- Kim, S.J.; Yoon, S.; Kim, H.J. Review of solution-processed oxide thin-film transistors. Jpn. J. Appl. Phys. 2014, 53, 02BA02. [Google Scholar] [CrossRef]
- Park, J.W.; Kang, B.H.; Kim, H.J. A Review of Low-Temperature Solution-Processed Metal Oxide Thin-Film Transistors for Flexible Electronics. Adv. Funct. Mater. 2020, 30, 1904632. [Google Scholar] [CrossRef]
- Jeong, S.; Moon, J. Low-temperature, solution-processed metal oxide thin film transistors. J. Mater. Chem. 2012, 22, 1243–1250. [Google Scholar] [CrossRef]
- Glynn, C.; O’Dwyer, C. Solution Processable Metal Oxide Thin Film Deposition and Material Growth for Electronic and Photonic Devices. Adv. Mater. Interfaces 2017, 4, 1600610. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, H.; Kim, I.D. Overview of electroceramic materials for oxide semiconductor thin film transistors. J. Electroceramics. 2014, 32, 117–140. [Google Scholar] [CrossRef]
- Reed, A.; Stone, C.; Roh, K.; Song, H.W.; Wang, X.; Liu, M.; Lee, S. The role of third cation doping on phase stability, carrier transport and carrier suppression in amorphous oxide semiconductors. J. Mater. Chem. C 2020, 8, 13798–13810. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Seo, J.S.; Yun, J.M.; Park, H.J.; Yang, S.; Park, S.H.K.; Bae, B.S. An “aqueous route” for the fabrication of low-temperature-processable oxide flexible transparent thin-film transistors on plastic substrates. NPG Asia Mater. 2013, 5, e45. [Google Scholar] [CrossRef]
- Rim, Y.S.; Lim, H.S.; Kim, H.J. Low-temperature metal-oxide thin-film transistors formed by directly photopatternable and combustible solution synthesis. ACS Appl. Mater. Interfaces 2013, 5, 3565–3571. [Google Scholar] [CrossRef]
- Kim, K.; Seo, D.; Kwon, S.; Jeon, Y.; Hwang, Z.; Wang, J.; Park, S.; Lee, J.; Jang, I.; Man, X.; et al. Viable strategy to minimize trap states of patterned oxide thin films for both exceptional electrical performance and uniformity in sol–gel processed transistors. Chem. Eng. J. 2022, 441, 135833. [Google Scholar] [CrossRef]
- Tappertzhofen, S.; Waser, R.; Valov, I. Impact of the Counter-Electrode Material on RedoxProcesses in Resistive Switching Memories. ChemElectroChem 2014, 1, 1287. [Google Scholar] [CrossRef]
- Liu, X.; Xu, H.; Ning, H.; Lu, K.; Zhang, H.; Zhang, X.; Yao, R.; Fang, Z.; Lu, X.; Peng, J. Induced Nano-Scale Self-Formed Metal-Oxide Interlayer in Amorphous Silicon Tin Oxide Thin Film Transistors. Sci. Rep. 2018, 8, 4160. [Google Scholar] [CrossRef] [PubMed]
- Rim, Y.S.; Jeong, W.H.; Kim, D.L.; Lim, H.S.; Kim, K.M.; Kim, H.J. Simultaneous modification of pyrolysis and densification for low-temperature solution-processed flexible oxide thin-film transistors. J. Mater. Chem. 2012, 22, 12491–12497. [Google Scholar] [CrossRef]
- Duca, M.D.; Plosceanu, C.L.; Pop, T. Surface modifications of polyvinylidene fluoride (PVDF) under rf Ar plasma. Polym. Degrad. Stab. 1998, 61, 65–72. [Google Scholar] [CrossRef]
- Ahmad, D.; Van Den Boogaert, I.; Miller, J.; Presswell, R.; Jouhara, H. Hydrophilic and hydrophobic materials and their applications. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 2686–2725. [Google Scholar] [CrossRef]
- Kim, H.J.; Tak, Y.J.; Park, S.P.; Na, J.W.; Kim, Y.G.; Hong, S.; Kim, P.H.; Kim, G.T.; Kim, B.K.; Kim, H.J. The self-activated radical doping effects on the catalyzed surface of amorphous metal oxide films. Sci. Rep. 2017, 7, 12469. [Google Scholar] [CrossRef]
- Cho, S.W.; Kim, D.E.; Kim, K.S.; Jung, S.H.; Cho, H.K. Towards environmentally stable solution-processed oxide thin-film transistors: A rare-metal-free oxide-based semiconductor/insulator heterostructure and chemically stable multi-stacking. J. Mater. Chem. C 2017, 5, 10498–10508. [Google Scholar] [CrossRef]
- Jeon, J.K.; Um, J.G.; Lee, S.; Jang, J. Control of O-H bonds at a-IGZO/SiO2 interface by long time thermal annealing for highly stable oxide TFT. AIP Adv. 2017, 7, 125110. [Google Scholar] [CrossRef]
- Kamiya, T.; Hosono, H. (Invited) Roles of Hydrogen in Amorphous Oxide Semiconductor. ECS Trans. 2013, 54, 103–113. [Google Scholar] [CrossRef]
- Kamiya, T.; Nomura, K.; Hosono, H. Subgap states, doping and defect formation energies in amorphous oxide semiconductor a-InGaZnO 4 studied by density functional theory. Phys. Status Solidi Appl. Mater. Sci. 2010, 207, 1698–1703. [Google Scholar] [CrossRef]
- Tak, Y.J.; Park, S.P.; Jung, T.S.; Lee, H.; Kim, W.G.; Park, J.W.; Kim, H.J. Reduction of activation temperature at 150 °C for IGZO films with improved electrical performance via UV-thermal treatment. J. Inf. Disp. 2016, 17, 73–78. [Google Scholar] [CrossRef]
- Kyndiah, A.; Ablat, A.; Guyot-Reeb, S.; Schultz, T.; Zu, F.; Koch, N.; Amsalem, P.; Chiodini, S.; Alic, T.Y.; Topal, Y.; et al. A Multifunctional Interlayer for Solution Processed High Performance Indium Oxide Transistors. Sci. Rep. 2018, 8, 10946. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Kim, T.G.; Kim, Y.H.; Park, J. Improved device performance of solution-processed zinc–tin–oxide thin film transistor effects using graphene/Al electrode. J. Phys. D Appl. Phys. 2015, 48, 035101. [Google Scholar] [CrossRef]
- Sabri, M.M.; Jung, J.; Yoon, D.H.; Yoon, S.; Tak, Y.J.; Kim, H.J. Hydroxyl radical-assisted decomposition and oxidation in solution-processed indium oxide thin-film transistors. J. Mater. Chem. C 2015, 3, 7499–7505. [Google Scholar] [CrossRef]
- Jo, J.W.; Kim, K.H.; Kim, J.; Ban, S.G.; Kim, Y.H.; Park, S.K. High-Mobility and Hysteresis-Free Flexible Oxide Thin-Film Transistors and Circuits by Using Bilayer Sol-Gel Gate Dielectrics. ACS Appl. Mater. Interfaces 2018, 10, 2679–2687. [Google Scholar] [CrossRef]
- Trinh, T.T.; Nguyen, V.D.; Ryu, K.; Jang, K.; Lee, W.; Baek, S.; Raja, J.; Yi, J. Improvement in the performance of an InGaZnO thin-film transistor by controlling interface trap densities between the insulator and active layer. Semicond. Sci. Technol. 2011, 26, 085012. [Google Scholar] [CrossRef]
- Huang, G.; Duan, L.; Zhao, Y.; Dong, G.; Zhang, D.; Qiu, Y. Enhanced mobility of solution-processed polycrystalline zinc tin oxide thin-film transistors via direct incorporation of water into precursor solution. Appl. Phys. Lett. 2014, 105, 122105. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Chou, C.-H.; Chen, Y.-T.; Jhang, W.-C. A study of solution-processed zinc-tin-oxide semiconductors for thin-film transistors. IEEE Trans. Electron Devices 2019, 66, 2631. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Heo, J.-S.; Kim, T.-H.; Park, S.; Yoon, M.-H.; Kim, J.; Oh, M.S.; Yi, G.-R.; Noh, Y.-Y. Flexible metal-oxide devices made by room-temperature photochemical activation of sol–gel films. Nature 2012, 489, 128–132. [Google Scholar] [CrossRef]
- Jeong, J.K. Photo-bias instability of metal oxide thin film transistors for advanced active matrix displays. J. Mater. Res. 2013, 28, 2071–2084. [Google Scholar] [CrossRef]
- Xie, H.; Wu, Q.; Xu, L.; Zhang, L.; Liu, G.; Dong, C. Nitrogen-doped amorphous oxide semiconductor thin film transistors with double-stacked channel layers. Appl. Surf. Sci. 2016, 387, 237–243. [Google Scholar] [CrossRef]
- Um, J.G.; Mativenga, M.; Jang, J. Mechanism of positive bias stress-assisted recovery in amorphous-indium-gallium-zinc-oxide thin-film transistors from negative bias under illumination stress. Appl. Phys. Lett. 2013, 103, 033501. [Google Scholar] [CrossRef]
- Takagi, A.; Nomura, K.; Ohta, H.; Yanagi, H.; Kamiya, T.; Hirano, M.; Hosono, H. Carrier Transport and Electronic Structure in Amorphous Oxide Semiconductor, a-InGaZnO4. Thin Solid Film. 2005, 486, 38–41. [Google Scholar] [CrossRef]



| Ion/Off | Off-Current (A) | SS (V·dec−1) | Nit (cm−2·eV−1) | Reference | |
|---|---|---|---|---|---|
| Control group | 5.5 × 103 | ~10−7 | 8.63 | 30.10 × 1012 | This study |
| LPTA-80 | 6.7 × 106 | ~10−9 | 2.40 | 8.46 × 1012 | This study |
| LPTA-140 | 1.1 × 107 | ~10−10 | 0.73 | 2.42 × 1012 | This study |
| ZTO | 5.2 × 103 | ~10−8 | 1.52 | - | [31] |
| ZTO | 4.0 × 104 | ~10−8 | 0.58 | - | [35] |
| ZTO | 3.0 × 107 | ~10−12 | 0.39 | 1.20 × 1012 | [36] |
| INOX | 3.9 × 107 | ~10−12 | 0.45 | 1.11 × 1012 | [32] |
| μFE (cm2 V−1 S−1) | NBS | PBS | ||||
|---|---|---|---|---|---|---|
| Stress Time | Control Group | LPTA-80 | LPTA-140 | Control Group | LPTA-80 | LPTA-140 |
| 0 s | 5.1 | 3.36 | 1.99 | 3.39 | 2.89 | 2.87 |
| 10 s | 5.37 | 3.59 | 1.99 | 4.10 | 2.88 | 2.89 |
| 100 s | 5.64 | 3.91 | 2.09 | 4.35 | 3.23 | 3.00 |
| 500 s | 6.15 | 4.21 | 2.26 | 3.85 | 3.39 | 3.07 |
| 1000 s | 6.27 | 4.37 | 2.15 | 3.84 | 3.39 | 3.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Jeon, S.-H.; Park, J.; Lee, S.-H.; Jang, J.; Kang, I.M.; Kim, D.-K.; Bae, J.-H. Improvement in Switching Characteristics and Bias Stability of Solution-Processed Zinc–Tin Oxide Thin Film Transistors via Simple Low-Pressure Thermal Annealing Treatment. Nanomaterials 2023, 13, 1722. https://doi.org/10.3390/nano13111722
Feng J, Jeon S-H, Park J, Lee S-H, Jang J, Kang IM, Kim D-K, Bae J-H. Improvement in Switching Characteristics and Bias Stability of Solution-Processed Zinc–Tin Oxide Thin Film Transistors via Simple Low-Pressure Thermal Annealing Treatment. Nanomaterials. 2023; 13(11):1722. https://doi.org/10.3390/nano13111722
Chicago/Turabian StyleFeng, Junhao, Sang-Hwa Jeon, Jaehoon Park, Sin-Hyung Lee, Jaewon Jang, In Man Kang, Do-Kyung Kim, and Jin-Hyuk Bae. 2023. "Improvement in Switching Characteristics and Bias Stability of Solution-Processed Zinc–Tin Oxide Thin Film Transistors via Simple Low-Pressure Thermal Annealing Treatment" Nanomaterials 13, no. 11: 1722. https://doi.org/10.3390/nano13111722
APA StyleFeng, J., Jeon, S.-H., Park, J., Lee, S.-H., Jang, J., Kang, I. M., Kim, D.-K., & Bae, J.-H. (2023). Improvement in Switching Characteristics and Bias Stability of Solution-Processed Zinc–Tin Oxide Thin Film Transistors via Simple Low-Pressure Thermal Annealing Treatment. Nanomaterials, 13(11), 1722. https://doi.org/10.3390/nano13111722







