Monolithic MXene Aerogels Encapsulated Phase Change Composites with Superior Photothermal Conversion and Storage Capability
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Ti3C2Tx MXene Nanoflake Dispersions and Aerogels
2.3. Preparation of PEG@MXene Composites
2.4. Characterization
3. Results and Discussion
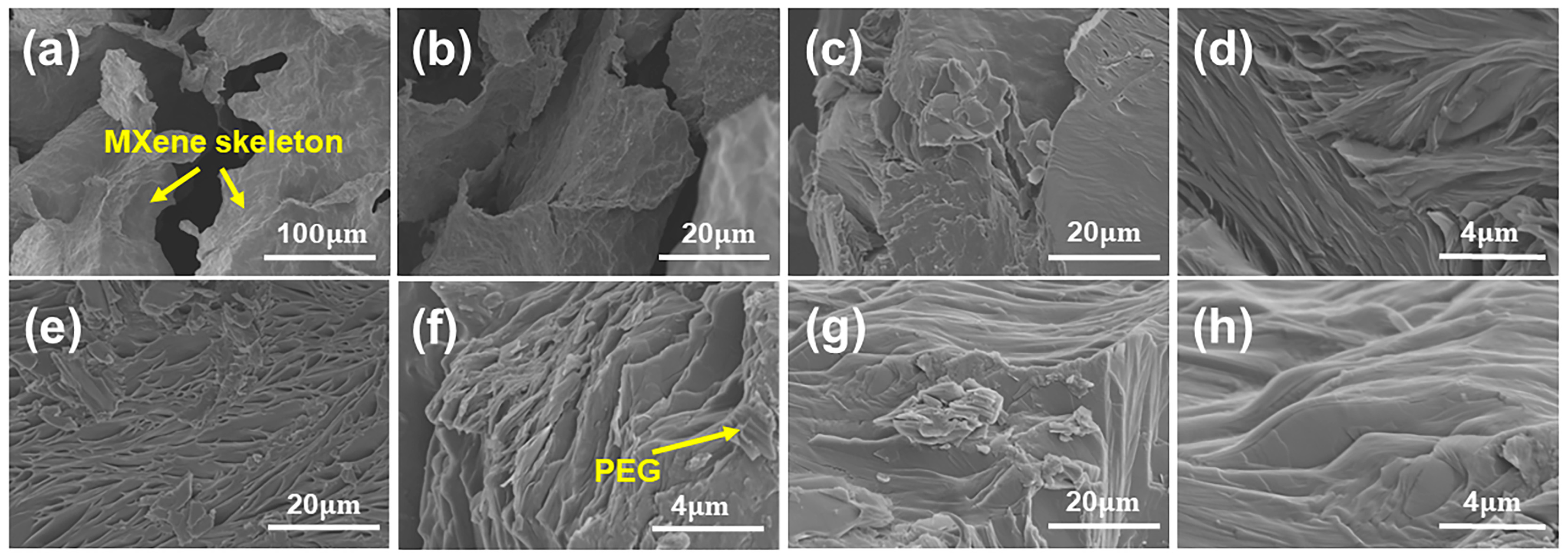
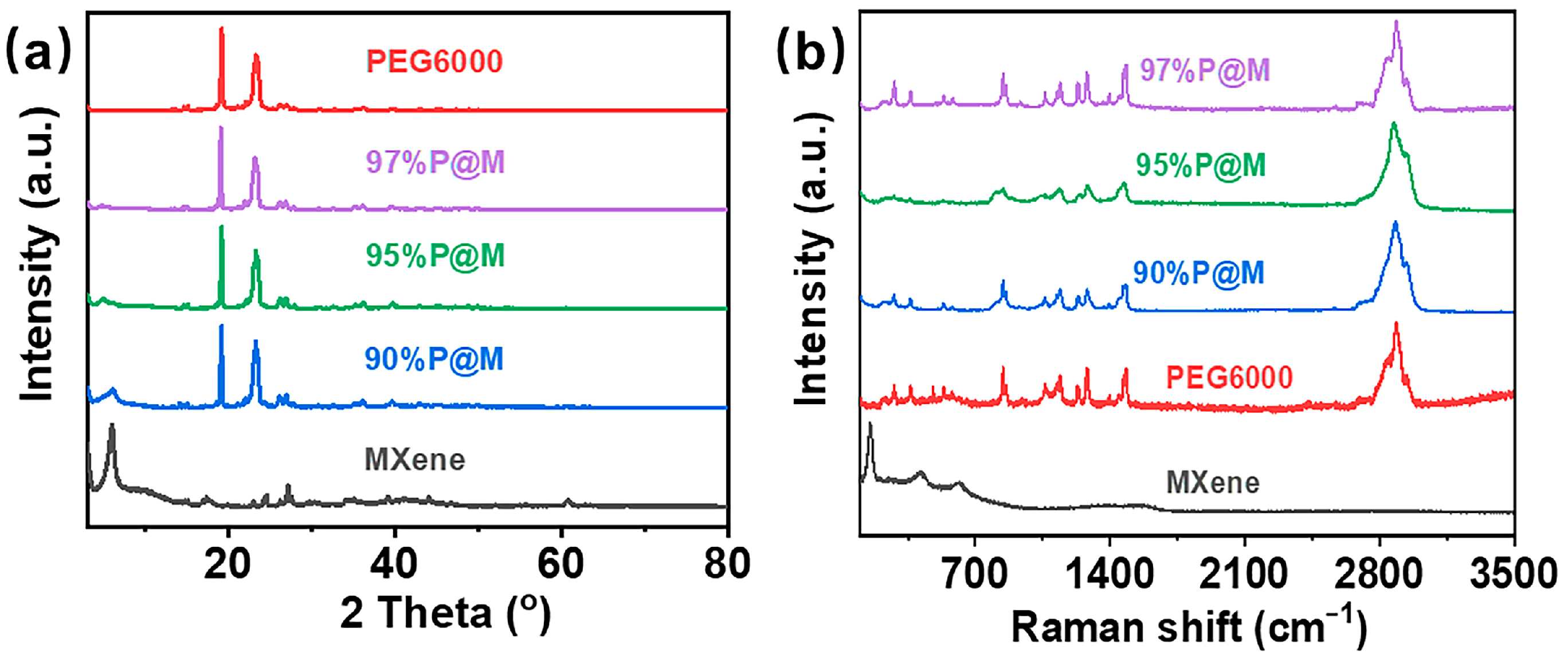
3.1. Morphology and Structures of MXene and PEG@MXene PCMs
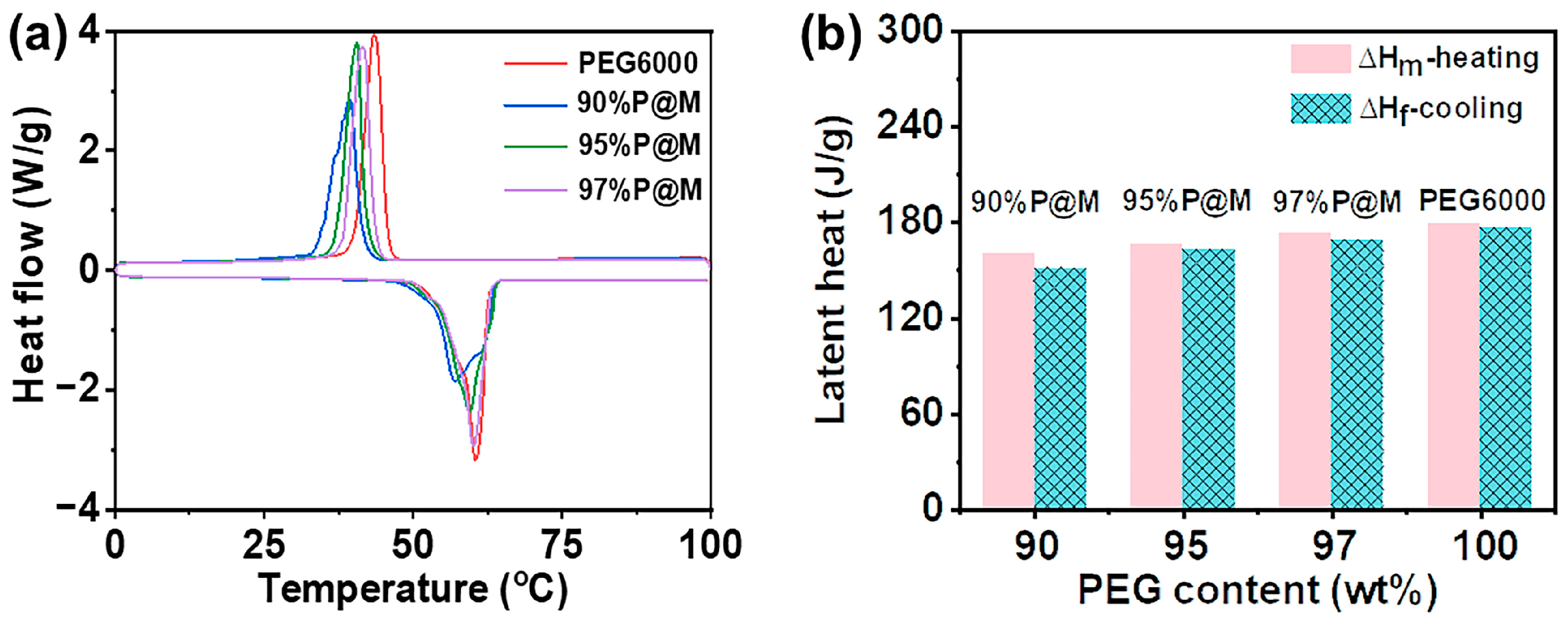
3.2. Phase Change Properties of xP@M PCMs
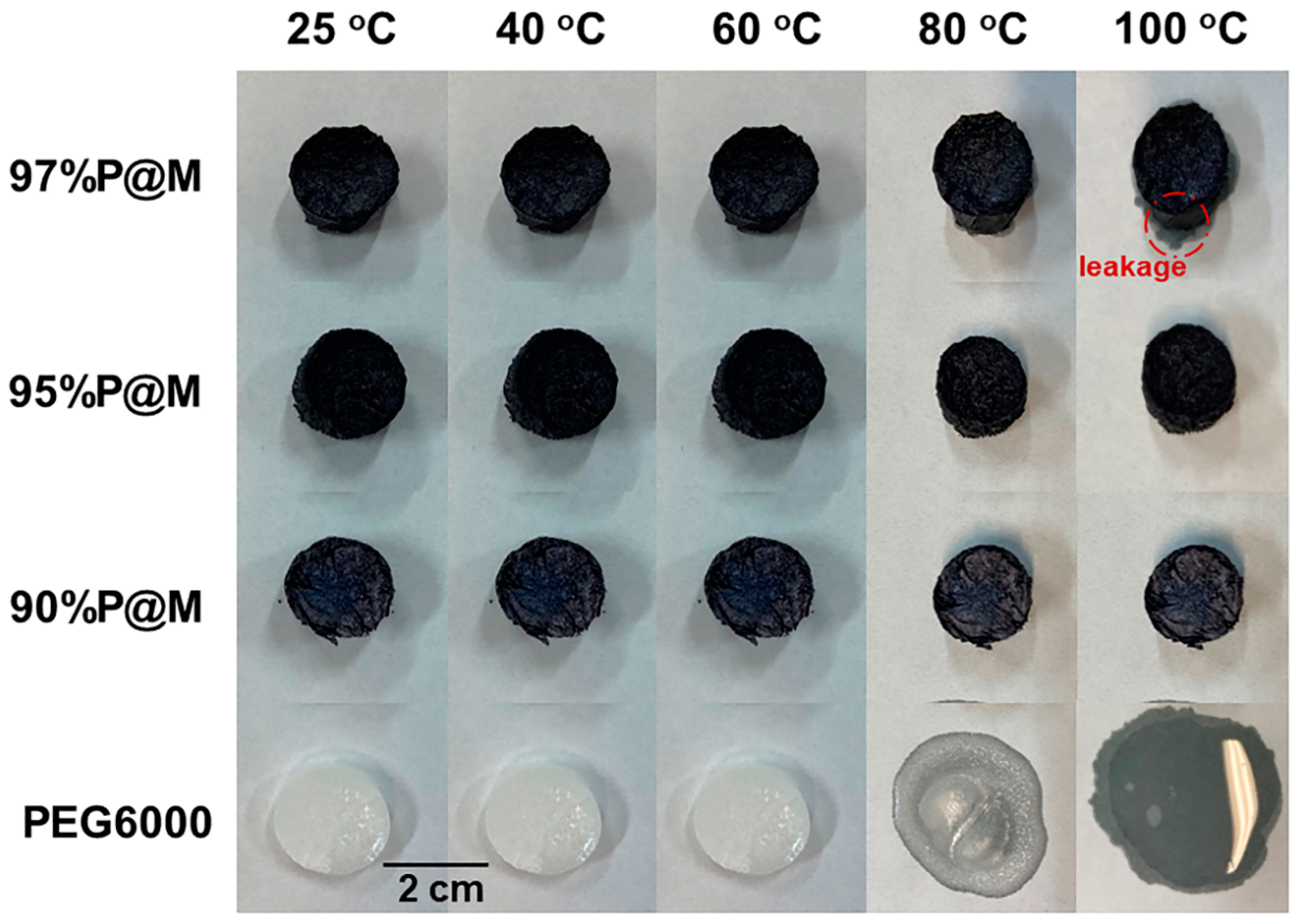
3.3. Thermal and Cyclic Stability of PEG6000 and xP@M Composites
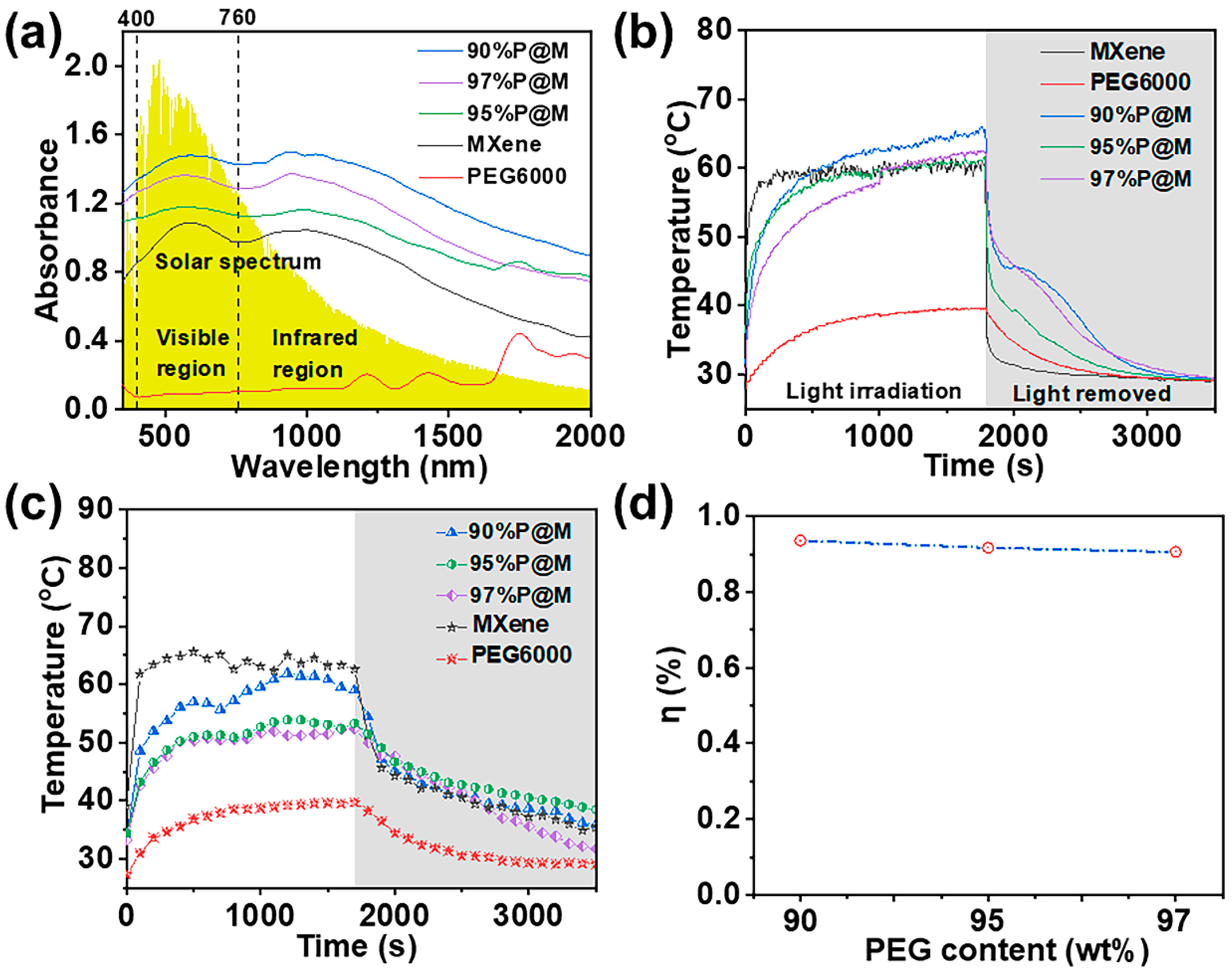
3.4. Photo-Thermal Conversion and Storage of xP@M
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carrillo, A.J.; González-Aguilar, J.; Romero, M.; Coronado, J.M. Solar Energy on Demand: A Review on High Temperature Thermochemical Heat Storage Systems and Materials. Chem. Rev. 2019, 119, 4777–4816. [Google Scholar] [CrossRef] [PubMed]
- Shchukina, E.M.; Graham, M.; Zheng, Z.; Shchukin, D.G. Nanoencapsulation of phase change materials for advanced thermal energy storage systems. Chem. Soc. Rev. 2018, 47, 4156–4175. [Google Scholar] [CrossRef] [PubMed]
- Jebasingh, B.E.; Arasu, A.V. A comprehensive review on latent heat and thermal conductivity of nanoparticle dispersed phase change material for low-temperature applications. Energy Storage Mater. 2020, 24, 52–74. [Google Scholar] [CrossRef]
- Zhao, S.; Li, J.; Song, S. Preparation of porous titanium dioxide foam impregnated with polyethylene glycol as shape-stable composite phase change materials. J. Energy Storage 2022, 51, 104416. [Google Scholar] [CrossRef]
- Li, T.; Pan, H.; Xu, L.; Ni, K.; Shen, Y.; Li, K. Shape-stabilized phase change material with high phase change enthalpy made of PEG compounded with lignin-based carbon. Int. J. Biol. Macromol. 2022, 213, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Lu, X.; Wu, H.; Hu, X.; Li, X.; Liu, S.; Qu, J.-P. Constructing heat conduction path and flexible support skeleton for PEG-based phase change composites through salt template method. Compos. Sci. Technol. 2022, 226, 109532. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett. 2007, 8, 902–907. [Google Scholar] [CrossRef]
- Jin, W.; Jiang, L.; Han, L.; Huang, H.; Zhang, J.; Guo, M.; Gu, Y.; Zhi, F.; Chen, Z.; Yang, G. Investigation of thermal conductivity enhancement of water-based graphene and graphene/MXene nanofluids. J. Mol. Liq. 2022, 367, 120455. [Google Scholar] [CrossRef]
- Li, R.; Zhang, L.; Shi, L.; Wang, P. MXene Ti3C2: An Effective 2D Light-to-Heat Conversion Material. ACS Nano 2017, 11, 3752–3759. [Google Scholar] [CrossRef]
- Huang, X.; Chen, X.; Li, A.; Atinafu, D.; Gao, H.; Dong, W.; Wang, G. Shape-stabilized phase change materials based on porous supports for thermal energy storage applications. Chem. Eng. J. 2019, 356, 641–661. [Google Scholar] [CrossRef]
- Chen, X.; Tang, Z.; Liu, P.; Gao, H.; Chang, Y.; Wang, G. Smart Utilization of Multifunctional Metal Oxides in Phase Change Materials. Matter 2020, 3, 708–741. [Google Scholar] [CrossRef]
- Fan, X.; Liu, L.; Jin, X.; Wang, W.; Zhang, S.; Tang, B. MXene Ti3C2Tx for phase change composite with superior photothermal storage capability. J. Mater. Chem. A 2019, 7, 14319–14327. [Google Scholar] [CrossRef]
- Mo, Z.; Mo, P.; Yi, M.; Hu, Z.; Tan, G.; Selim, M.S.; Chen, Y.; Chen, X.; Hao, Z.; Wei, X. Ti3C2Tx@Polyvinyl alcohol foam-Supported Phase Change Materials with Simultaneous Enhanced Thermal Conductivity and Solar-Thermal Conversion Performance. Sol. Energy Mater. Sol. Cells 2021, 219, 110813. [Google Scholar] [CrossRef]
- Sheng, X.; Dong, D.; Lu, X.; Zhang, L.; Chen, Y. MXene-wrapped bio-based pomelo peel foam/polyethylene glycol composite phase change material with enhanced light-to-thermal conversion efficiency, thermal energy storage capability and thermal conductivity. Compos. Part A Appl. Sci. Manuf. 2020, 138, 106067. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, S.; Li, X.; Hu, X.; Wu, H.; Lu, X.; Qu, J. Biomass porous potatoes/MXene encapsulated PEG-based PCMs with improved photo-to-thermal conversion capability. Sol. Energy Mater. Sol. Cells 2022, 237, 111559. [Google Scholar] [CrossRef]
- Wang, H.; Deng, Y.; Liu, Y.; Wu, F.; Wang, W.; Jin, H.; Zheng, J.; Lei, J. In situ preparation of light-driven cellulose-Mxene aerogels based composite phase change materials with simultaneously enhanced light-to-heat conversion, heat transfer and heat storage. Compos. Part A Appl. Sci. Manuf. 2022, 155, 106853. [Google Scholar] [CrossRef]
- Lin, P.; Xie, J.; He, Y.; Lu, X.; Li, W.; Fang, J.; Yan, S.; Zhang, L.; Sheng, X.; Chen, Y. MXene aerogel-based phase change materials toward solar energy conversion. Sol. Energy Mater. Sol. Cells 2020, 206, 110229. [Google Scholar] [CrossRef]
- Lu, X.; Huang, H.; Zhang, X.; Lin, P.; Huang, J.; Sheng, X.; Zhang, L.; Qu, J.-P. Novel light-driven and electro-driven polyethylene glycol/two-dimensional MXene form-stable phase change material with enhanced thermal conductivity and electrical conductivity for thermal energy storage. Compos. Part B Eng. 2019, 177, 107372. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, P.; Song, L.; Li, J.; Ji, B.; Li, J.; Chen, L. Polyethylene glycol supported by phosphorylated polyvinyl alcohol/graphene aerogel as a high thermal stability phase change material. Compos. Part B Eng. 2019, 179, 107545. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, Y.; Wang, F.; Deng, H.; Song, Y.; Li, C.; Ling, Z. Water permeability in MXene membranes: Process matters. Chin. Chem. Lett. 2020, 31, 1665–1669. [Google Scholar] [CrossRef]
- Sun, K.; Kou, Y.; Zhang, Y.; Liu, T.; Shi, Q. Photo-triggered Hierarchical Porous Carbon-Based Composite Phase-Change Materials with Superior Thermal Energy Conversion Capacity. ACS Sustain. Chem. Eng. 2020, 8, 3445–3453. [Google Scholar] [CrossRef]
- Deng, Y.; Shang, T.; Wu, Z.; Tao, Y.; Luo, C.; Liang, J.; Han, D.; Lyu, R.; Qi, C.; Lv, W.; et al. Fast Gelation of Ti 3 C 2 T x MXene Initiated by Metal Ions. Adv. Mater. 2019, 31, e1902432. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Liu, H.; Wang, J.; Yang, M.; Zhao, J.; Zhang, L.; Song, Y.; Ling, Z. Vermiculite aerogels assembled from nanosheets via metal ion induced fast gelation. Appl. Clay Sci. 2022, 218, 106431. [Google Scholar] [CrossRef]
- Lei, W.; Mochalin, V.N.; Liu, D.; Qin, S.; Gogotsi, Y.; Chen, Y. Boron nitride colloidal solutions, ultralight aerogels and freestanding membranes through one-step exfoliation and functionalization. Nat. Commun. 2015, 6, 8849. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, Z.; Eloi, J.; Titirici, M.; Eichhorn, S.J. Ice-Templated, Sustainable Carbon Aerogels with Hierarchically Tailored Channels for Sodium- and Potassium-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2110862. [Google Scholar] [CrossRef]
- Sarycheva, A.; Gogotsi, Y. Raman Spectroscopy Analysis of the Structure and Surface Chemistry of Ti3C2Tx MXene. Chem. Mater. 2020, 32, 3480–3488. [Google Scholar] [CrossRef]
- Li, Y.; Sun, K.; Kou, Y.; Liu, H.; Wang, L.; Yin, N.; Dong, H.; Shi, Q. One-step synthesis of graphene-based composite phase change materials with high solar-thermal conversion efficiency. Chem. Eng. J. 2022, 429, 132439. [Google Scholar] [CrossRef]
- Kashyap, S.; Kabra, S.; Kandasubramanian, B. Graphene aerogel-based phase changing composites for thermal energy storage systems. J. Mater. Sci. 2020, 55, 4127–4156. [Google Scholar] [CrossRef]
- Cao, Q.; He, F.; Li, Y.; He, Z.; Fan, J.; Wang, R.; Hu, W.; Zhang, K.; Yang, W. Graphene-carbon nanotube hybrid aerogel/polyethylene glycol phase change composite for thermal management. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 656–662. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Qiu, J.; Jin, X.; Umair, M.M.; Lu, R.; Zhang, S.; Tang, B. Ag-graphene/PEG composite phase change materials for enhancing solar-thermal energy conversion and storage capacity. Appl. Energy 2019, 237, 83–90. [Google Scholar] [CrossRef]
- Song, S.; Qiu, F.; Zhu, W.; Guo, Y.; Zhang, Y.; Ju, Y.; Feng, R.; Liu, Y.; Chen, Z.; Zhou, J.; et al. Polyethylene glycol/halloysite@Ag nanocomposite PCM for thermal energy storage: Simultaneously high latent heat and enhanced thermal conductivity. Sol. Energy Mater. Sol. Cells 2019, 193, 237–245. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, H.; Tang, B.; Xu, S.; Shufen, Z. Novel light–driven CF/PEG/SiO2 composite phase change materials with high thermal conductivity. Sol. Energy Mater. Sol. Cells 2018, 174, 538–544. [Google Scholar] [CrossRef]
- Qian, T.; Li, J.; Feng, W.; Nian, H. Single-walled carbon nanotube for shape stabilization and enhanced phase change heat transfer of polyethylene glycol phase change material. Energy Convers. Manag. 2017, 143, 96–108. [Google Scholar] [CrossRef]
- Zhang, X.; Qiao, J.; Zhang, W.; Cheng, F.; Yin, Z.; Huang, Z.; Min, X. Thermal behavior of composite phase change materials based on polyethylene glycol and expanded vermiculite with modified porous carbon layer. J. Mater. Sci. 2018, 53, 13067–13080. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, Y.; Zhuo, H.; Liu, L.; Jing, S.; Zhong, L.; Peng, X.; Sun, R.-C. Compressible, Elastic, and Pressure-Sensitive Carbon Aerogels Derived from 2D Titanium Carbide Nanosheets and Bacterial Cellulose for Wearable Sensors. Chem. Mater. 2019, 31, 3301–3312. [Google Scholar] [CrossRef]
- Sarı, A.; Bicer, A.; Al-Sulaiman, F.; Karaipekli, A.; Tyagi, V. Diatomite/CNTs/PEG composite PCMs with shape-stabilized and improved thermal conductivity: Preparation and thermal energy storage properties. Energy Build. 2018, 164, 166–175. [Google Scholar] [CrossRef]
- Ji, R.; Wei, S.; Xia, Y.; Huang, C.; Huang, Y.; Zhang, H.; Xu, F.; Sun, L.; Lin, X. Enhanced thermal performance of form-stable composite phase-change materials supported by novel porous carbon spheres for thermal energy storage. J. Energy Storage 2020, 27, 101134. [Google Scholar] [CrossRef]
- Chen, J.L.; Mo, Z.J.; Chen, Y.Z.; Mo, P.J.; Hu, Z.Y.; Chen, X.; Yu, J.; Hao, Z.F.; Zeng, X.L.; Sun, R.; et al. Highly Stable MXene-Based Phase Change Composites with Enhanced Thermal Conductivity and Photothermal Storage Capability. ACS Appl. Energy Mater. 2022, 5, 11669–11683. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, X.; Sheng, D.; Liu, X.; Yang, Y. Light-driven PEG/Ti3C2Tx form-stable phase change film for energy storage. Sol. Energy Mater. Sol. Cells 2022, 248, 112021. [Google Scholar] [CrossRef]
- Cao, Y.; Li, W.; Huang, D.; Zhang, J.; Lin, P.; Zhang, L.; Sheng, X.; Chen, Y.; Lu, X. One-step construction of novel phase change composites supported by a biomass/MXene gel network for efficient thermal energy storage. Sol. Energy Mater. Sol. Cells 2022, 241, 111729. [Google Scholar] [CrossRef]
- Quan, B.; Wang, J.; Li, Y.; Sui, M.; Xie, H.; Liu, Z.; Wu, H.; Lu, X.; Tong, Y. Cellulose nanofibrous/MXene aerogel encapsulated phase change composites with excellent thermal energy conversion and storage capacity. Energy 2023, 262, 125505. [Google Scholar] [CrossRef]
- Gong, S.; Ding, Y.; Li, X.; Liu, S.; Wu, H.; Lu, X.; Qu, J. Novel flexible polyurethane/MXene composites with sensitive solar thermal energy storage behavior. Compos. Part A Appl. Sci. Manuf. 2021, 149, 106505. [Google Scholar] [CrossRef]
- Du, Y.; Huang, H.; Hu, X.; Liu, S.; Sheng, X.; Li, X.; Lu, X.; Qu, J. Melamine foam/polyethylene glycol composite phase change material synergistically modified by polydopamine/MXene with enhanced solar-to-thermal conversion. Renew. Energy 2021, 171, 1–10. [Google Scholar] [CrossRef]
- Shao, Y.-W.; Hu, W.-W.; Gao, M.-H.; Xiao, Y.-Y.; Huang, T.; Zhang, N.; Yang, J.-H.; Qi, X.-D.; Wang, Y. Flexible MXene-coated melamine foam based phase change material composites for integrated solar-thermal energy conversion/storage, shape memory and thermal therapy functions. Compos. Part A Appl. Sci. Manuf. 2021, 143, 106291. [Google Scholar] [CrossRef]
- Hu, W.-W.; Shi, X.-Y.; Gao, M.-H.; Huang, C.-H.; Huang, T.; Zhang, N.; Yang, J.-H.; Qi, X.-D.; Wang, Y. Light-actuated shape memory and self-healing phase change composites supported by MXene/waterborne polyurethane aerogel for superior solar-thermal energy storage. Compos. Commun. 2021, 28, 100980. [Google Scholar] [CrossRef]
- Cao, Y.; Weng, M.; Mahmoud, M.H.H.; Elnaggar, A.Y.; Zhang, L.; El Azab, I.H.; Chen, Y.; Huang, M.; Huang, J.; Sheng, X. Flame-retardant and leakage-proof phase change composites based on MXene/polyimide aerogels toward solar thermal energy harvesting. Adv. Compos. Hybrid Mater. 2022, 5, 1253–1267. [Google Scholar] [CrossRef]
- Du, X.; Qiu, J.; Deng, S.; Du, Z.; Cheng, X.; Wang, H. Ti3C2Tx@PDA-Integrated Polyurethane Phase Change Composites with Superior Solar-Thermal Conversion Efficiency and Improved Thermal Conductivity. ACS Sustain. Chem. Eng. 2020, 8, 5799–5806. [Google Scholar] [CrossRef]
- Tang, L.; Zhao, X.; Feng, C.; Bai, L.; Yang, J.; Bao, R.; Liu, Z.; Yang, M.; Yang, W. Bacterial cellulose/MXene hybrid aerogels for photodriven shape-stabilized composite phase change materials. Sol. Energy Mater. Sol. Cells 2019, 203, 110174. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, T.; Wang, J.; Lv, J.; Zheng, Y.; Zhang, Y.; Wang, Y. Novel properties of stearic acid/MXene—Graphene oxide shape—Stabilized phase change material: Ascended phase transition temperature and hierarchical transition. Sol. Energy Mater. Sol. Cells 2022, 247, 111948. [Google Scholar] [CrossRef]
- Wang, X.; Yu, W.; Wang, L.; Xie, H. Vertical orientation graphene/MXene hybrid phase change materials with anisotropic properties, high enthalpy, and photothermal conversion. Sci. China Technol. Sci. 2022, 65, 882–892. [Google Scholar] [CrossRef]
- Ye, X.; Ma, Y.; Tian, Z.; Sun, H.; Zhu, Z.; Li, J.; Liang, W.; Li, A. Shape-stable MXene/sodium alginate/carbon nanotubes hybrid phase change material composites for efficient solar energy conversion and storage. Compos. Sci. Technol. 2022, 230, 109794. [Google Scholar] [CrossRef]
- Du, X.; Wang, J.; Jin, L.; Deng, S.; Dong, Y.; Lin, S. Dopamine-Decorated Ti(3)C(2)T(x) MXene/Cellulose Nanofiber Aerogels Supported Form-Stable Phase Change Composites with Superior Solar-Thermal Conversion Efficiency and Extremely High Thermal Storage Density. ACS Appl Mater Interfaces 2022, 14, 15225–15234. [Google Scholar] [CrossRef] [PubMed]
- Aslfattahi, N.; Saidur, R.; Arifutzzaman, A.; Sadri, R.; Bimbo, N.; Sabri, M.F.M.; Maughan, P.A.; Bouscarrat, L.; Dawson, R.J.; Said, S.M.; et al. Experimental investigation of energy storage properties and thermal conductivity of a novel organic phase change material/MXene as A new class of nanocomposites. J. Energy Storage 2020, 27, 101115. [Google Scholar] [CrossRef]
- Zheng, J.; Deng, Y.; Liu, Y.; Wu, F.; Wang, W.; Wang, H.; Sun, S.; Lu, J. Paraffin/polyvinyl alcohol/MXene flexible phase change composite films for thermal management applications. Chem. Eng. J. 2023, 453, 139727. [Google Scholar] [CrossRef]


| Composition of the Composite PCMs | Content of PEG | Retention of (R) | References and Year |
|---|---|---|---|
| xP@M | 97.0% | 96.5% | This work |
| Graphene aerogel/PEG2000 | 97.7% | 91.0% | 2020 [28] |
| CNT/graphene/PEG2000 | 98.8% | 96.5% | 2020 [29] |
| Ti3C2Tx@PVA/PEG2000 | 92.3% | 90.6% | 2021 [13] |
| Ag/GNS/PEG6000 | 92.0% | 89.6% | 2019 [30] |
| HNT/Ag/PEG1000 | 45.0% | 43.8% | 2019 [31] |
| Ti3C2/PEG6000 | 80.0% | 72.6% | 2019 [12] |
| CF/SiO2/PEG6000 | 80.0% | 67.3% | 2018 [32] |
| SWCNTs/PEG6000 | 98.0% | 97.8% | 2017 [33] |
| Vermiculite/PEG4000 | 56.0% | 54.2% | 2018 [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, F.; Shi, C.; Dong, H.; Song, Y.; Zhao, J.; Ling, Z. Monolithic MXene Aerogels Encapsulated Phase Change Composites with Superior Photothermal Conversion and Storage Capability. Nanomaterials 2023, 13, 1661. https://doi.org/10.3390/nano13101661
Wang Y, Wang F, Shi C, Dong H, Song Y, Zhao J, Ling Z. Monolithic MXene Aerogels Encapsulated Phase Change Composites with Superior Photothermal Conversion and Storage Capability. Nanomaterials. 2023; 13(10):1661. https://doi.org/10.3390/nano13101661
Chicago/Turabian StyleWang, Yan, Fuqiang Wang, Changrui Shi, Hongsheng Dong, Yongchen Song, Jiafei Zhao, and Zheng Ling. 2023. "Monolithic MXene Aerogels Encapsulated Phase Change Composites with Superior Photothermal Conversion and Storage Capability" Nanomaterials 13, no. 10: 1661. https://doi.org/10.3390/nano13101661
APA StyleWang, Y., Wang, F., Shi, C., Dong, H., Song, Y., Zhao, J., & Ling, Z. (2023). Monolithic MXene Aerogels Encapsulated Phase Change Composites with Superior Photothermal Conversion and Storage Capability. Nanomaterials, 13(10), 1661. https://doi.org/10.3390/nano13101661








