Facile Synthesis of Functionalized Porous Carbon by Direct Pyrolysis of Anacardium occidentale Nut-Skin Waste and Its Utilization towards Supercapacitors
Abstract
1. Introduction
2. Experimental Section
Preparation of AO-PC Materials
3. Results and Discussion
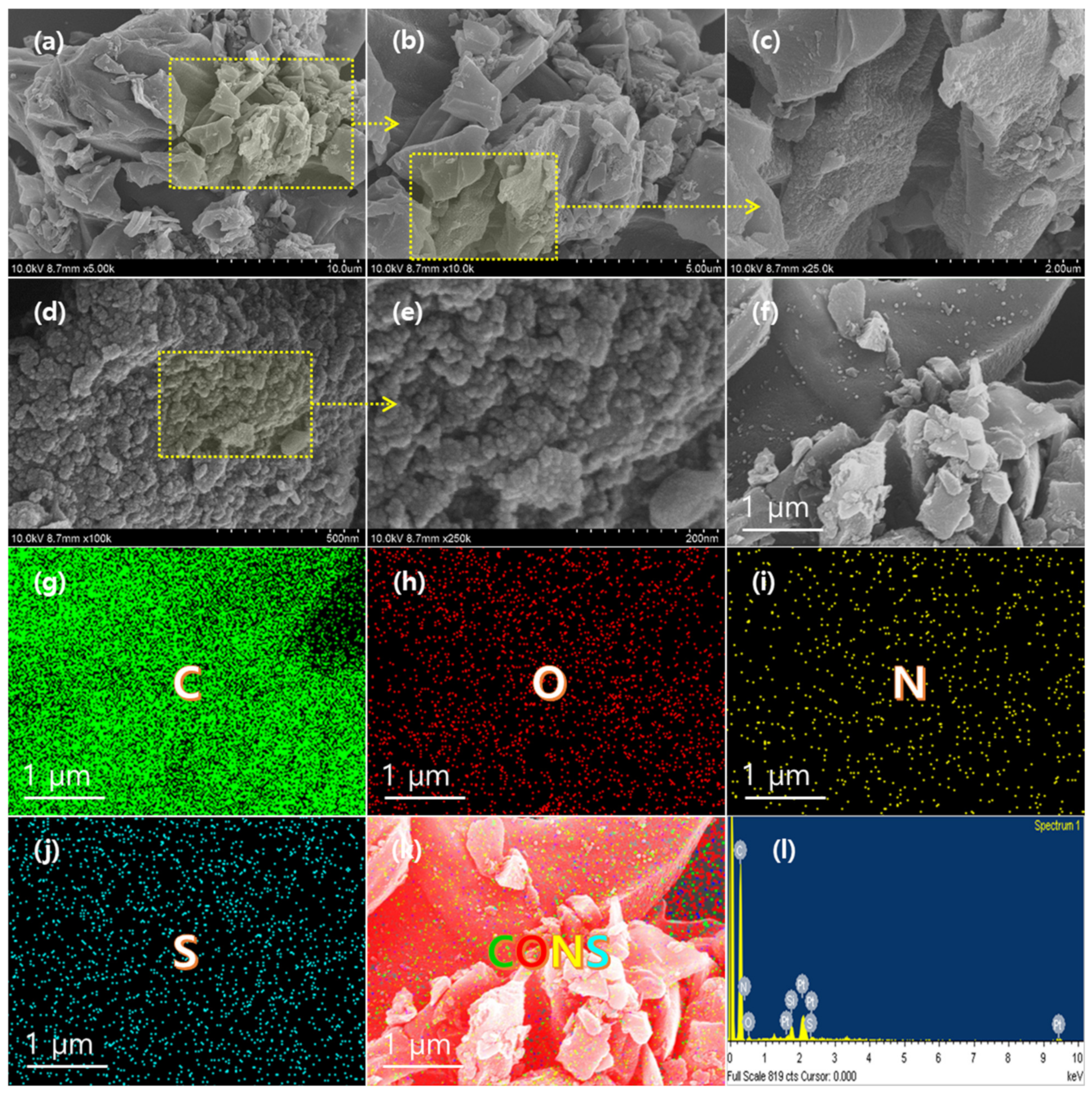
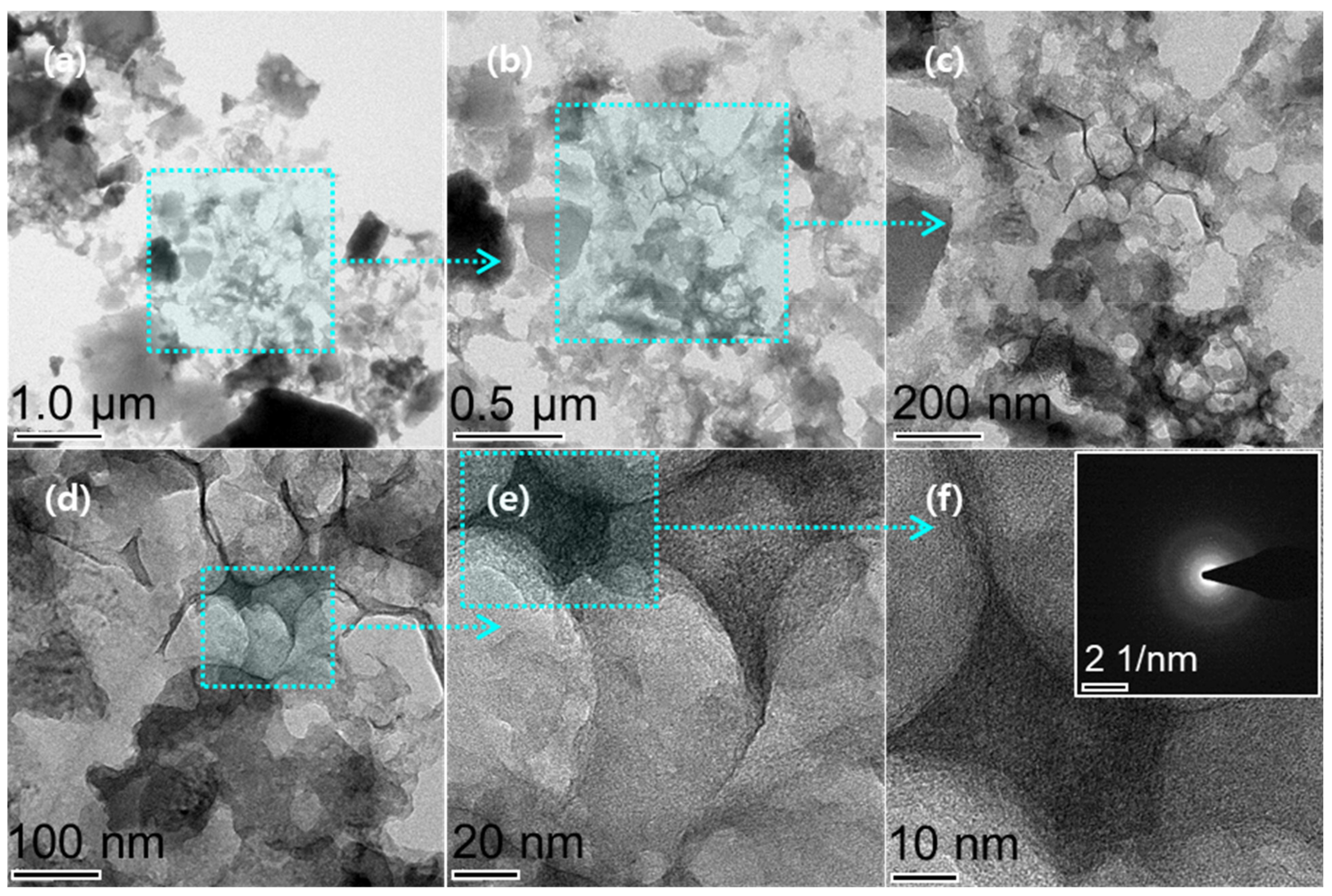
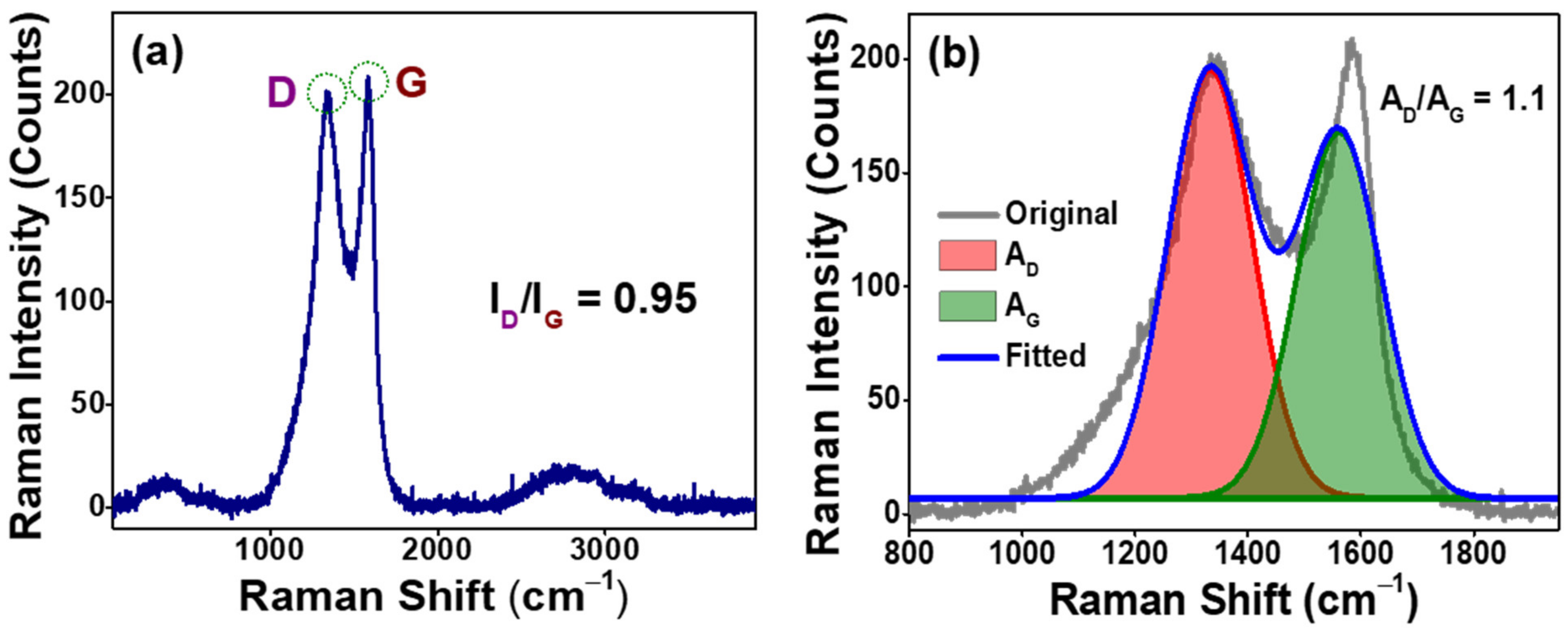
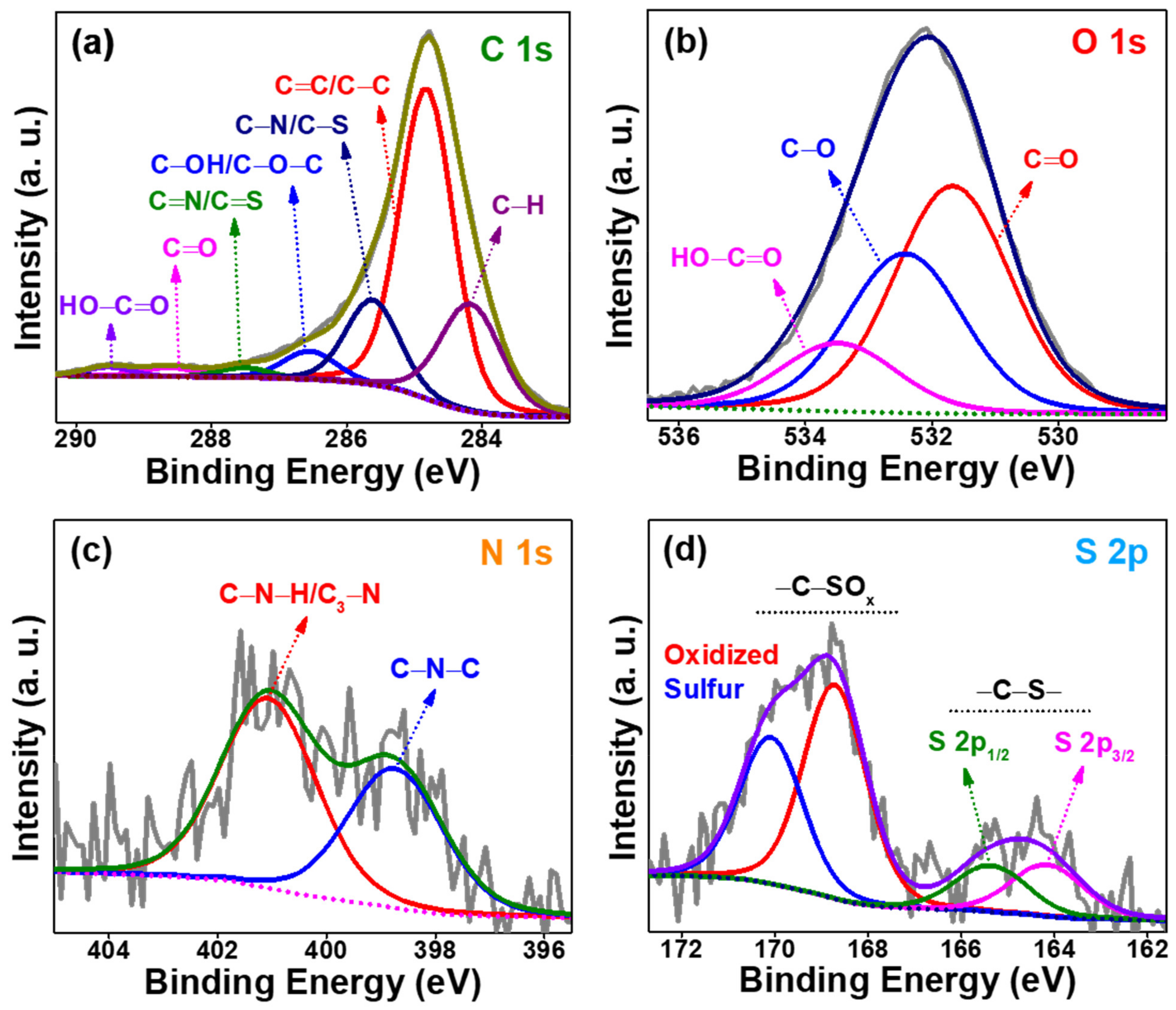
3.1. Structural Characterizations of as-Prepared AO-PC Material
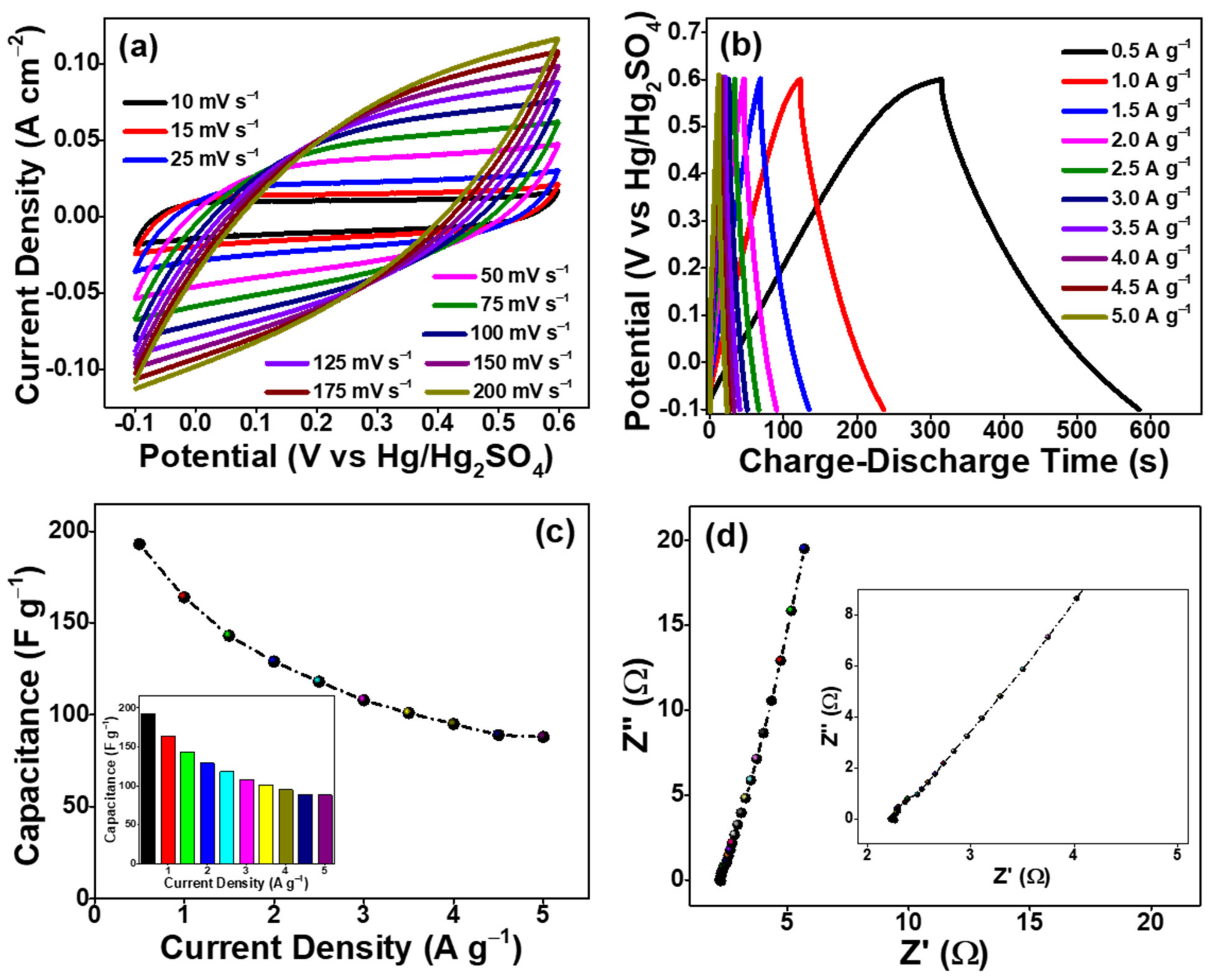
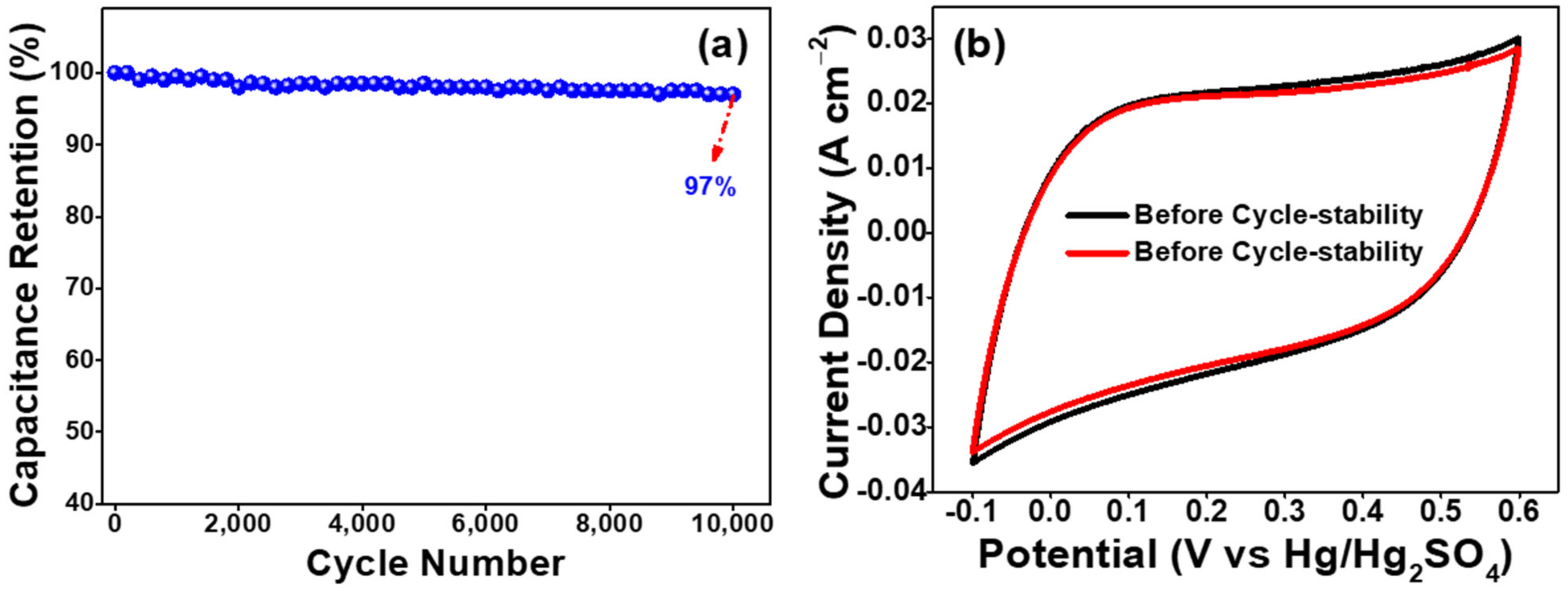
3.2. Electrochemical Studies of as-Prepared AO-PC Material
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, Y.; Liu, H.; Ke, Q.; Wang, J. Effects of nitrogen doping on supercapacitor performance of a mesoporous carbon electrode produced by a hydrothermal soft-templating process. J. Mater. Chem. A 2014, 2, 11753–11758. [Google Scholar] [CrossRef]
- Ning, K.; Zhao, G.; Liu, H.; Hu, M.; Huang, F.; Li, H.; Zhang, L.; Zhu, G.; Wang, H.; Shi, J. N and S co-doped 3D hierarchical porous carbon as high-performance electrode material for supercapacitors. Diam. Relat. Mater. 2022, 126, 109080. [Google Scholar] [CrossRef]
- Zhai, Z.; Zhang, L.; Du, T.; Ren, B.; Xu, Y.; Wang, S.; Miao, J.; Liu, Z. A review of carbon materials for supercapacitors. Mater. Des. 2022, 221, 111017. [Google Scholar] [CrossRef]
- Luo, L.; Lan, Y.; Zhang, Q.; Deng, J.; Luo, L.; Zeng, Q.; Gao, H.; Zhao, W. A review on biomass-derived activated carbon as electrode materials for energy storage supercapacitors. J. Energy Storage 2022, 55, 105839. [Google Scholar] [CrossRef]
- Sun, L.; Gong, Y.; Li, D.; Pan, C. Biomass-derived porous carbon materials: Synthesis, designing, and applications for supercapacitors. Green Chem. 2022, 24, 3864–3894. [Google Scholar] [CrossRef]
- Vinodh, R.; Gopi, C.V.V.M.; Kummara, V.G.R.; Atchudan, R.; Ahamad, T.; Sambasivam, S.; Yi, M.; Obaidat, I.M.; Kim, H.-J. A review on porous carbon electrode material derived from hypercross-linked polymers for supercapacitor applications. J. Energy Storage 2020, 32, 101831. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, G.; Li, M.-Y.; Yin, Y.-X.; Li, J.-Y.; Li, G.; Wang, W.-P.; Peng, W.; Cao, F.-F.; Guo, Y.-G. Porous carbon for high-energy density symmetrical supercapacitor and lithium-ion hybrid electrochemical capacitors. Chem. Eng. J. 2019, 375, 122020. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Li, L. Applications and challenges of porous carbon with different dimensions in supercapacitors—A mini review. Front. Energy Res. 2022, 10, 951701. [Google Scholar] [CrossRef]
- Jyothibasu, J.P.; Kuo, D.-W.; Lee, R.-H. Flexible and freestanding electrodes based on polypyrrole/carbon nanotube/cellulose composites for supercapacitor application. Cellulose 2019, 26, 4495–4513. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Hou, H.; Xu, W.; Duan, G.; He, S.; Liu, K.; Jiang, S. Recent progress in carbon-based materials for supercapacitor electrodes: A review. J. Mater. Sci. 2021, 56, 173–200. [Google Scholar] [CrossRef]
- Lu, W.; Hao, L.; Wang, Y. Highly active N, S Co-Doped Ultramicroporous Carbon for High-Performance Supercapacitor Electrodes. Micromachines 2022, 13, 905. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chang, Y.; Song, H.; Hou, W.; Zhang, Y.; Zhao, Y.; Xiao, Y.; Zhu, S.; Han, G. Polymerization-Pyrolysis Assisted Construction of Multiscale Porous Carbon for High-Performance Supercapacitors. ECS J. Solid State Sci. Technol. 2022, 11, 081007. [Google Scholar] [CrossRef]
- Zhang, X.; Han, R.; Liu, Y.; Li, H.; Shi, W.; Yan, X.; Zhao, X.; Li, Y.; Liu, B. Porous and graphitic structure optimization of biomass-based carbon materials from 0D to 3D for supercapacitors: A review. Chem. Eng. J. 2023, 460, 141607. [Google Scholar] [CrossRef]
- Jalalah, M.; Han, H.; Mahadani, M.; Nayak, A.K.; Harraz, F.A. Novel interconnected hierarchical porous carbon derived from biomass for enhanced supercapacitor application. J. Electroanal. Chem. 2023, 935, 117355. [Google Scholar] [CrossRef]
- Zekenova, A.; Nazhipkyzy, M.; Li, W.; Kalybayeva, A.; Zhumanova, G.; Zubova, O. Advances of Biowaste-Derived Porous Carbon and Carbon–Manganese Dioxide Composite in Supercapacitors: A Review. Inorganics 2022, 10, 160. [Google Scholar]
- Wu, S.; Wei, D.; Yin, Y.; Li, Q.; Wang, H.; Chen, W.; Jiang, Y.; Tao, X. N, S co-doped activated carbon with porous architecture derived from partial poly (2, 2′-dithiodianiline) for supercapacitors. J. Energy Storage 2021, 33, 102043. [Google Scholar] [CrossRef]
- Jin, T.; Su, J.; Luo, Q.; Zhu, W.; Lai, H.; Huang, D.; Wang, C. Preparation of N,P Co-doped Porous Carbon Derived from Daylily for Supercapacitor Applications. ACS Omega 2022, 7, 37564–37571. [Google Scholar] [CrossRef]
- Rustamaji, H.; Prakoso, T.; Devianto, H.; Widiatmoko, P.; Kurnia, K.A. Facile synthesis of N, S-modified activated carbon from biomass residue for promising supercapacitor electrode applications. Bioresour. Technol. Rep. 2023, 21, 101301. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, H.; Sun, S.; Lu, G.; Liu, H.; Huang, M.; Shi, J.; Liu, W.; Li, H. Sustainable nitrogen-doped carbon electrodes for use in high-performance supercapacitors and Li-ion capacitors. Sustain. Energy Fuels 2020, 4, 1789–1800. [Google Scholar] [CrossRef]
- Li, J.; Gao, Y.; Han, K.; Qi, J.; Li, M.; Teng, Z. High performance hierarchical porous carbon derived from distinctive plant tissue for supercapacitor. Sci. Rep. 2019, 9, 17270. [Google Scholar] [CrossRef]
- Yang, H.; Ye, S.; Zhou, J.; Liang, T. Biomass-Derived Porous Carbon Materials for Supercapacitor. Front. Chem. 2019, 7, 274. [Google Scholar] [CrossRef]
- Ge, L.; Wu, Y.; Wang, F.; Huang, Y. Algae-Derived Nitrogen Self-Doped Porous Carbon Materials with High Supercapacitor Performances. Energy Fuels 2021, 35, 15118–15125. [Google Scholar] [CrossRef]
- Liu, S.; Xu, Y.; Wu, J.; Huang, J. Celery-derived porous carbon materials for superior performance supercapacitors. Nanoscale Adv. 2021, 3, 5363–5372. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lin, Q.; Wang, Z.; Du, A.; Luo, H.; Liu, Y.-Q. Hierarchical porous carbon with high specific surface area and superb capacitance made from palm shells for supercapacitors. Diam. Relat. Mater. 2023, 135, 109852. [Google Scholar] [CrossRef]
- Manasa, P.; Sambasivam, S.; Ran, F. Recent progress on biomass waste derived activated carbon electrode materials for supercapacitors applications—A review. J. Energy Storage 2022, 54, 105290. [Google Scholar] [CrossRef]
- Hepsiba, P.; Rajkumar, S.; Elanthamilan, E.; Wang, S.-F.; Princy Merlin, J. Biomass-derived porous activated carbon from anacardium occidentale shell as electrode material for supercapacitors. New J. Chem. 2022, 46, 8863–8873. [Google Scholar] [CrossRef]
- Le, P.-A.; Nguyen, V.-T.; Sahoo, S.K.; Tseng, T.Y.; Wei, K.-H. Porous carbon materials derived from areca palm leaves for high performance symmetrical solid-state supercapacitors. J. Mater. Sci. 2020, 55, 10751–10764. [Google Scholar] [CrossRef]
- Wang, X.; Huang, Z.; Jin, H.; Wang, S.; Zhang, Q.; Xiao, H.; Hu, X.; Li, J.; Sheng, S. Cistanches herba residues derived nitrogen and oxygen self-doped porous carbon for high performance supercapacitor. J. Alloys Compd. 2023, 956, 170264. [Google Scholar] [CrossRef]
- Zhang, Y.; Yue, P.; Zhang, C.; Wang, Y.; Wu, X. Facile synthesis of three-dimensional interconnected porous carbon for high performance supercapacitor. Diam. Relat. Mater. 2023, 136, 109941. [Google Scholar] [CrossRef]
- Taslim, R.; Refanza, R.; Hamdy, M.I.; Apriwandi, A.; Taer, E. One-step strategy of 3D hierarchical porous carbon with self-heteroatom-doped derived bread waste for high-performance supercapacitor. J. Anal. Appl. Pyrolysis 2023, 171, 105956. [Google Scholar] [CrossRef]
- Yan, Y.; Manickam, S.; Lester, E.; Wu, T.; Pang, C.H. Synthesis of graphene oxide and graphene quantum dots from miscanthus via ultrasound-assisted mechano-chemical cracking method. Ultrason. Sonochem. 2021, 73, 105519. [Google Scholar] [CrossRef] [PubMed]
- Atchudan, R.; Jebakumar Immanuel Edison, T.N.; Perumal, S.; RanjithKumar, D.; Lee, Y.R. Direct growth of iron oxide nanoparticles filled multi-walled carbon nanotube via chemical vapour deposition method as high-performance supercapacitors. Int. J. Hydrogen Energy 2019, 44, 2349–2360. [Google Scholar] [CrossRef]
- Jänes, A.; Kurig, H.; Lust, E. Characterisation of activated nanoporous carbon for supercapacitor electrode materials. Carbon 2007, 45, 1226–1233. [Google Scholar] [CrossRef]
- Gupta, G.K.; Sagar, P.; Pandey, S.K.; Srivastava, M.; Singh, A.K.; Singh, J.; Srivastava, A.; Srivastava, S.K.; Srivastava, A. In Situ Fabrication of Activated Carbon from a Bio-Waste Desmostachya bipinnata for the Improved Supercapacitor Performance. Nanoscale Res. Lett. 2021, 16, 85. [Google Scholar] [CrossRef] [PubMed]
- Perumal, S.; Atchudan, R.; Cheong, I.W. Poly[2-(methacryloyloxy)ethyl phosphorylcholine]-Stabilized graphene-iron oxide composites for water splitting. Int. J. Hydrogen Energy 2021, 46, 10850–10861. [Google Scholar] [CrossRef]
- Maiaugree, W.; Lowpa, S.; Towannang, M.; Rutphonsan, P.; Tangtrakarn, A.; Pimanpang, S.; Maiaugree, P.; Ratchapolthavisin, N.; Sang-aroon, W.; Jarernboon, W.; et al. A dye sensitized solar cell using natural counter electrode and natural dye derived from mangosteen peel waste. Sci. Rep. 2015, 5, 15230. [Google Scholar] [CrossRef] [PubMed]
- Al-Hazmi, G.H.; Saad, H.A.; Refat, M.S.; Adam, A.M.A. Fe3O4-Carbon-Based Composite Derived from the Charge-Transfer Reaction Using Waste Tea Leaves as the Carbon Precursor for Enhanced Removing of Azocarmine G2, Methyl Violet 2B, Eosin B, and Toluidine Blue from Aqueous Solution. Crystals 2022, 12, 1355. [Google Scholar] [CrossRef]
- Kristianto, H.; Arie, A.A.; Susanti, R.F.; Halim, M.; Lee, J.K. The effect of activated carbon support surface modification on characteristics of carbon nanospheres prepared by deposition precipitation of Fe-catalyst. IOP Conf. Ser. Mater. Sci. Eng. 2016, 162, 012034. [Google Scholar] [CrossRef]
- Chu, M.; Li, M.; Han, Z.; Cao, J.; Li, R.; Cheng, Z. Novel biomass-derived smoke-like carbon as a supercapacitor electrode material. R. Soc. Open Sci. 2019, 6, 190132. [Google Scholar] [CrossRef]
- Elanthamilan, E.; Sathiyan, A.; Rajkumar, S.; Sheryl, E.J.; Merlin, J.P. Polyaniline based charcoal/Ni nanocomposite material for high performance supercapacitors. Sustain. Energy Fuels 2018, 2, 811–819. [Google Scholar] [CrossRef]
- Karnan, M.; Subramani, K.; Sudhan, N.; Ilayaraja, N.; Sathish, M. Aloe vera Derived Activated High-Surface-Area Carbon for Flexible and High-Energy Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 35191–35202. [Google Scholar] [CrossRef]
- Mopoung, S.; Dejang, N. Activated carbon preparation from eucalyptus wood chips using continuous carbonization–steam activation process in a batch intermittent rotary kiln. Sci. Rep. 2021, 11, 13948. [Google Scholar] [CrossRef]
- Atchudan, R.; Perumal, S.; Edison, T.N.J.I.; Albasher, G.; Sundramoorthy, A.K.; Vinodh, R.; Lee, Y.R. Lotus-biowaste derived sulfur/nitrogen-codoped porous carbon as an eco-friendly electrocatalyst for clean energy harvesting. Environ. Res. 2022, 214, 113910. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, Y.; Wang, P.; Yang, Y.; Wang, Y.; Xu, J.; Wang, Y.; Yu, W.W. Synthesis of Nitrogen and Sulfur Co-doped Carbon Dots from Garlic for Selective Detection of Fe3+. Nanoscale Res. Lett. 2016, 11, 110. [Google Scholar] [CrossRef]
- Turczyn, R.; Krukiewicz, K.; Katunin, A.; Sroka, J.; Sul, P. Fabrication and application of electrically conducting composites for electromagnetic interference shielding of remotely piloted aircraft systems. Compos. Struct. 2020, 232, 111498. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Parveen, A.S.; Lee, Y.R. Electrocatalytic and energy storage performance of bio-derived sulphur-nitrogen-doped carbon. J. Electroanal. Chem. 2019, 833, 357–369. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Q.; Li, M.; Yan, J.; Zhang, Y.; Liu, J.; Wu, Y. Nitrogen, sulfur-codoped micro–mesoporous carbon derived from boat-fruited sterculia seed for robust lithium–sulfur batteries. RSC Adv. 2019, 9, 15715–15726. [Google Scholar] [CrossRef]
- Tao, Y.; Wu, D.; Wang, T.; Ren, P.; Jia, D. A universal method of preparing high yield and N riched porous carbon by carbonizing coal tar pitch in air for supercapacitor. J. Power Sources 2023, 573, 233114. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Ramalingam, S.; Aldawood, S.; Devarajan, N.; Lee, W.; Lee, Y.R. Silver nanoparticles loaded graphene-poly-vinylpyrrolidone composites as an effective recyclable antimicrobial agent. Environ. Res. 2023, 216, 114706. [Google Scholar] [CrossRef]
- Sun, Y.; Xue, S.; Sun, J.; Li, X.; Ou, Y.; Zhu, B.; Demir, M. Silk-derived nitrogen-doped porous carbon electrodes with enhanced ionic conductivity for high-performance supercapacitors. J. Colloid Interface Sci. 2023, 645, 297–305. [Google Scholar] [CrossRef]
- Wu, P.; Sun, M.-H.; Yu, Y.; Peng, Z.; Bulbula, S.T.; Li, Y.; Chen, L.-H.; Su, B.-L. Physical and chemical dual-confinement of polysulfides within hierarchically meso-microporous nitrogen-doped carbon nanocages for advanced Li–S batteries. RSC Adv. 2017, 7, 42627–42633. [Google Scholar] [CrossRef]
- Chen, H.; Yu, F.; Wang, G.; Chen, L.; Dai, B.; Peng, S. Nitrogen and Sulfur Self-Doped Activated Carbon Directly Derived from Elm Flower for High-Performance Supercapacitors. ACS Omega 2018, 3, 4724–4732. [Google Scholar] [CrossRef]
- Atchudan, R.; Perumal, S.; Jebakumar Immanuel Edison, T.N.; Aldawood, S.; Vinodh, R.; Sundramoorthy, A.K.; Ghodake, G.; Lee, Y.R. Facile synthesis of novel molybdenum disulfide decorated banana peel porous carbon electrode for hydrogen evolution reaction. Chemosphere 2022, 307, 135712. [Google Scholar] [CrossRef]
- Guo, Z.; Feng, X.; Li, X.; Zhang, X.; Peng, X.; Song, H.; Fu, J.; Ding, K.; Huang, X.; Gao, B. Nitrogen Doped Carbon Nanosheets Encapsulated in situ Generated Sulfur Enable High Capacity and Superior Rate Cathode for Li-S Batteries. Front. Chem. 2018, 6. [Google Scholar] [CrossRef]
- Shan, Y.-Q.; Xu, Z.-X.; Duan, P.-G.; Fan, H.-L.; Hu, X.; Luque, R. Nitrogen- and Sulfur-Doped Carbon Obtained from Direct Hydrothermal Carbonization of Cellulose and Ammonium Sulfate for Supercapacitor Applications. ACS Sustain. Chem. Eng. 2020, 8, 15809–15814. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Xia, W.; Gong, J.; Jia, S.; Zhang, J. Nitrogen, Phosphorus and Sulfur Co-Doped Pyrolyzed Bacterial Cellulose Nanofibers for Supercapacitors. Nanomaterials 2020, 10, 1912. [Google Scholar] [CrossRef]
- Pedro Aguiar dos Santos, J.; Cesar Rufino, F.; Yutaka Ota, J.I.; Fernandes, R.C.; Vicentini, R.; Pagan, C.J.B.; Morais Da Silva, L.; Zanin, H. Best practices for electrochemical characterization of supercapacitors. J. Energy Chem. 2023, 80, 265–283. [Google Scholar] [CrossRef]
- Stoller, M.D.; Ruoff, R.S. Best practice methods for determining an electrode material’s performance for ultracapacitors. Energy Environ. Sci. 2010, 3, 1294–1301. [Google Scholar] [CrossRef]
- Lu, K.-L.; Wei, X.-Y.; Mao, F.-Z.; Zhu, Z.-J.; Li, Z.; Yin, F.; Li, J.-H.; Zong, Z.-M. Biomass-derived nitrogen and sulfur co-doped sheet-like porous carbon for high-performance supercapacitors. New J. Chem. 2023, 47, 1247–1255. [Google Scholar] [CrossRef]
- Li, Y.; Wang, G.; Wei, T.; Fan, Z.; Yan, P. Nitrogen and sulfur co-doped porous carbon nanosheets derived from willow catkin for supercapacitors. Nano Energy 2016, 19, 165–175. [Google Scholar] [CrossRef]
- Raji, A.; Thomas Nesakumar, J.I.E.; Mani, S.; Perumal, S.; Rajangam, V.; Thirunavukkarasu, S.; Lee, Y.R. Biowaste-originated heteroatom-doped porous carbonaceous material for electrochemical energy storage application. J. Ind. Eng. Chem. 2021, 98, 308–317. [Google Scholar] [CrossRef]
- Perumal, S.; Chandra Kishore, S.; Atchudan, R.; Sundramoorthy, A.K.; Alagan, M.; Lee, Y.R. Sustainable Synthesis of N/S-Doped Porous Carbon from Waste-Biomass as Electroactive Material for Energy Harvesting. Catalysts 2022, 12, 436. [Google Scholar] [CrossRef]
- Atchudan, R.; Jebakumar Immanuel Edison, T.N.; Perumal, S.; Vinodh, R.; Babu, R.S.; Sundramoorthy, A.K.; Renita, A.A.; Lee, Y.R. Facile synthesis of nitrogen-doped porous carbon materials using waste biomass for energy storage applications. Chemosphere 2022, 289, 133225. [Google Scholar] [CrossRef]
- Jain, A.; Ghosh, M.; Krajewski, M.; Kurungot, S.; Michalska, M. Biomass-derived activated carbon material from native European deciduous trees as an inexpensive and sustainable energy material for supercapacitor application. J. Energy Storage 2021, 34, 102178. [Google Scholar] [CrossRef]


| Source (Biomass) | Preparation Method | Electrolytes | SC * (F g−1)/ CD * (A g−1) | Cycle/ Retention (%) | Reference |
|---|---|---|---|---|---|
| Succulent leaves | Carbonization | 1 M H2SO4 | 128.4/1.0 | - | [2] |
| Ginger straw | Annealing | EMIM-BF4 | 122/0.5 | 10,000/82.7 | [19] |
| Garlic sprout | Carbonization | 6 M KOH | 568/0.1 | 5000/97 | [20] |
| A. turbinata | Carbonization | 1 M H2SO4 | 142/0.5 | 10,000/95 | [62] |
| Areca palm | Carbonization | PVA–Li2SO4 | 132/0.5 | 5000/92 | [27] |
| Kusha grass | Carbonization | 6 M KOH | 218/0.7 | 5000/88 | [34] |
| Lotus fruit | Carbonization | 1 M H2SO4 | 160/0.5 | 10,000/95 | [63] |
| Hornbeam | Pyrolysis | 1 M H2SO4 | 74/0.25 | 3500/99 | [64] |
| Banana Peel | Carbonization | 1 M H2SO4 | 137/0.5 | 10,000/94 | [61] |
| AO Nut Skin | Pyrolysis | 1 M H2SO4 | 193/0.5 | 10,000/97 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atchudan, R.; Perumal, S.; Sundramoorthy, A.K.; Manoj, D.; Kumar, R.S.; Almansour, A.I.; Lee, Y.R. Facile Synthesis of Functionalized Porous Carbon by Direct Pyrolysis of Anacardium occidentale Nut-Skin Waste and Its Utilization towards Supercapacitors. Nanomaterials 2023, 13, 1654. https://doi.org/10.3390/nano13101654
Atchudan R, Perumal S, Sundramoorthy AK, Manoj D, Kumar RS, Almansour AI, Lee YR. Facile Synthesis of Functionalized Porous Carbon by Direct Pyrolysis of Anacardium occidentale Nut-Skin Waste and Its Utilization towards Supercapacitors. Nanomaterials. 2023; 13(10):1654. https://doi.org/10.3390/nano13101654
Chicago/Turabian StyleAtchudan, Raji, Suguna Perumal, Ashok K. Sundramoorthy, Devaraj Manoj, Raju Suresh Kumar, Abdulrahman I. Almansour, and Yong Rok Lee. 2023. "Facile Synthesis of Functionalized Porous Carbon by Direct Pyrolysis of Anacardium occidentale Nut-Skin Waste and Its Utilization towards Supercapacitors" Nanomaterials 13, no. 10: 1654. https://doi.org/10.3390/nano13101654
APA StyleAtchudan, R., Perumal, S., Sundramoorthy, A. K., Manoj, D., Kumar, R. S., Almansour, A. I., & Lee, Y. R. (2023). Facile Synthesis of Functionalized Porous Carbon by Direct Pyrolysis of Anacardium occidentale Nut-Skin Waste and Its Utilization towards Supercapacitors. Nanomaterials, 13(10), 1654. https://doi.org/10.3390/nano13101654