Highly Active Manganese Oxide from Electrolytic Manganese Anode Slime for Efficient Removal of Antibiotics Induced by Dissociation of Peroxymonosulfate
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
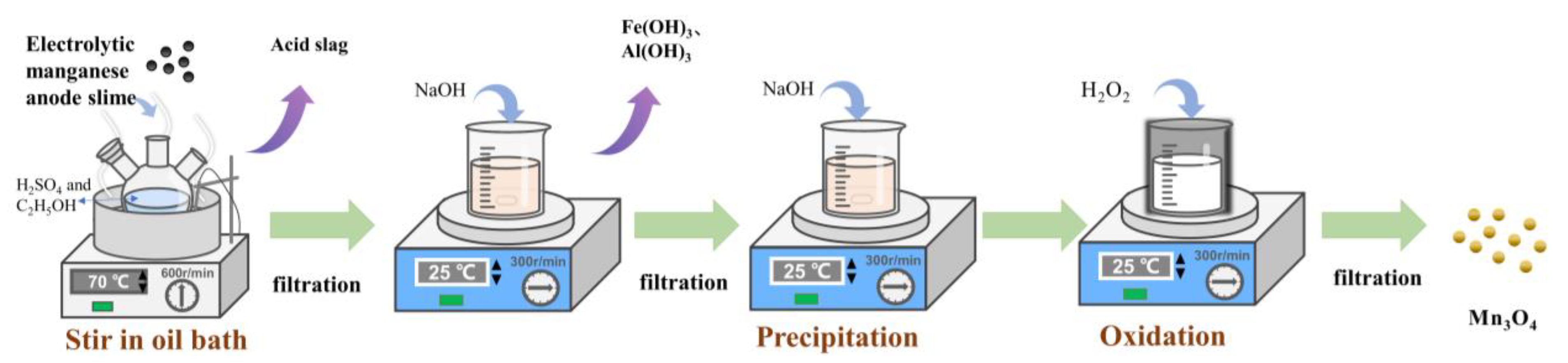
2.2. Pretreatment of Electrolytic Manganese Anode Slime
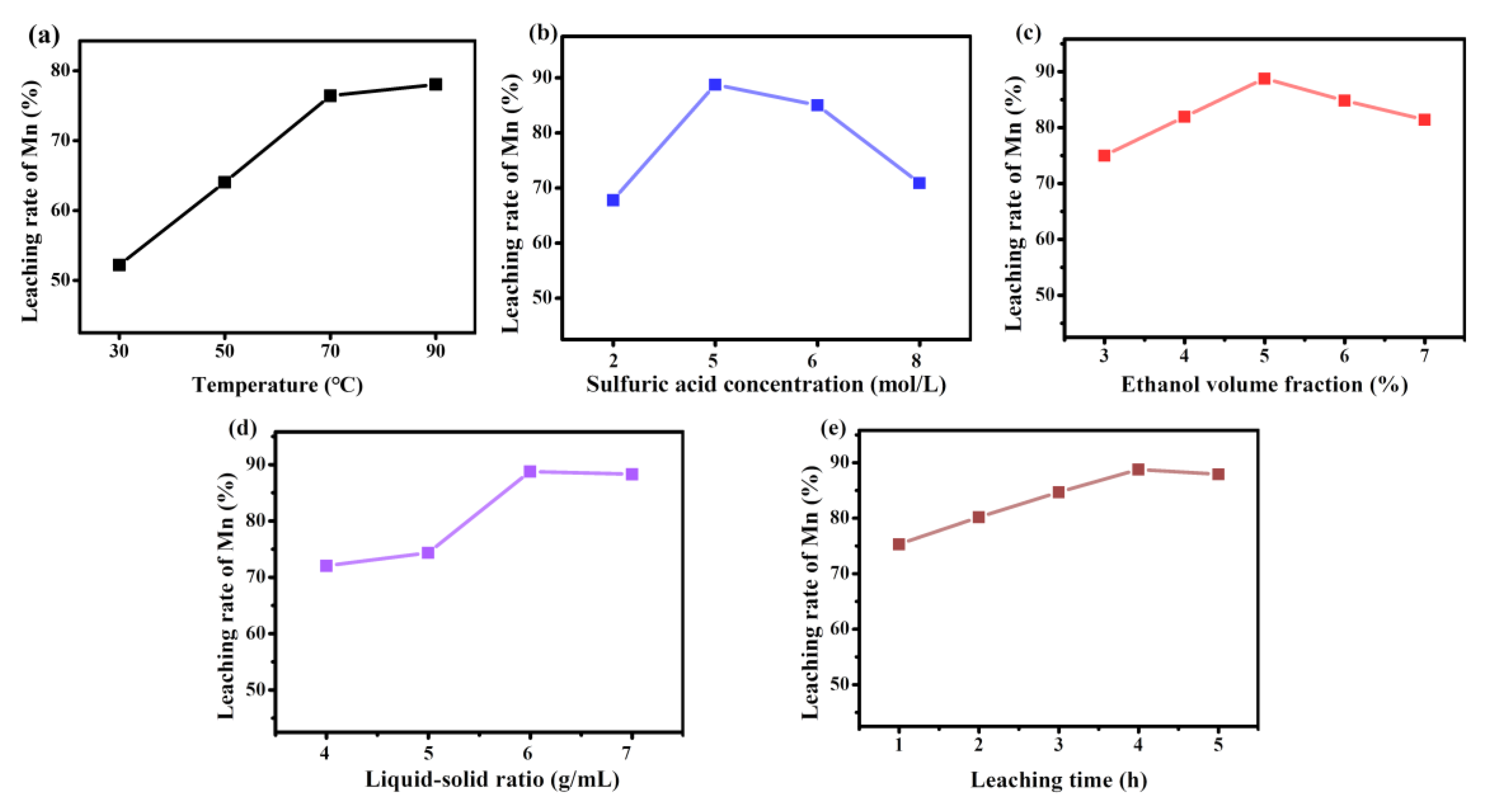
2.3. Acid Leaching
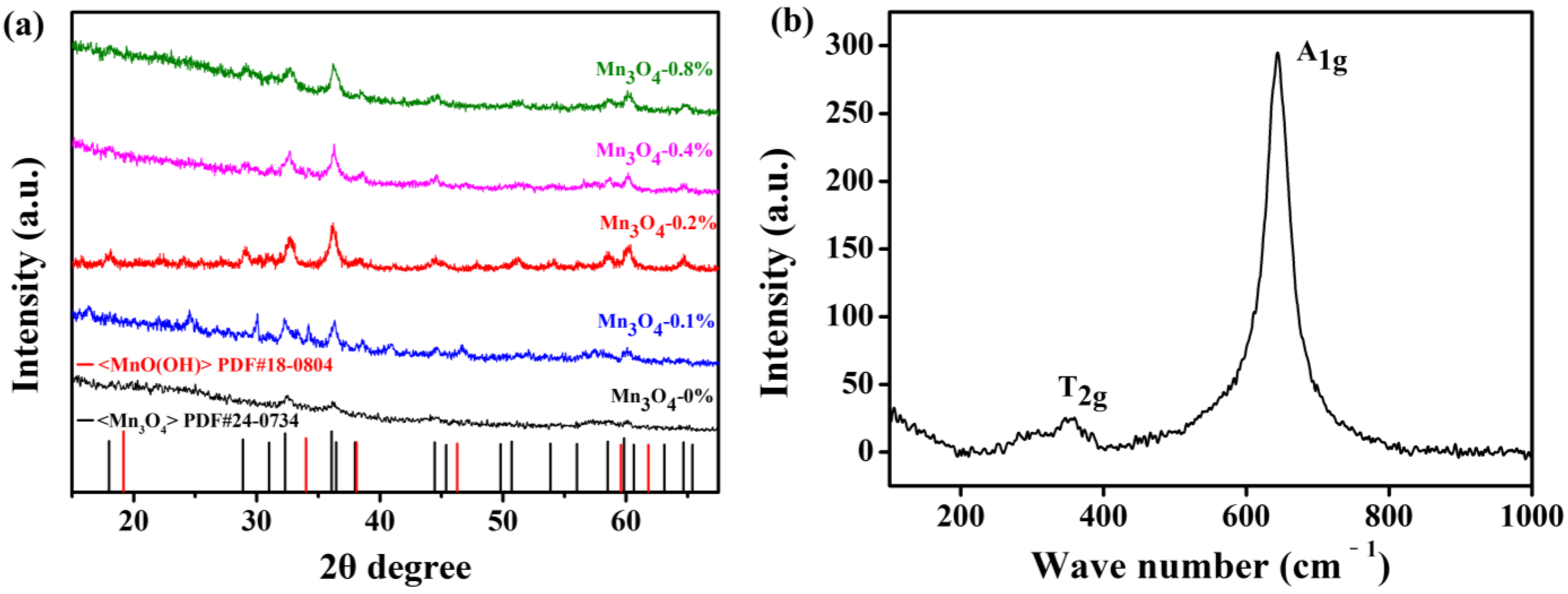
2.4. Preparation of Mn3O4
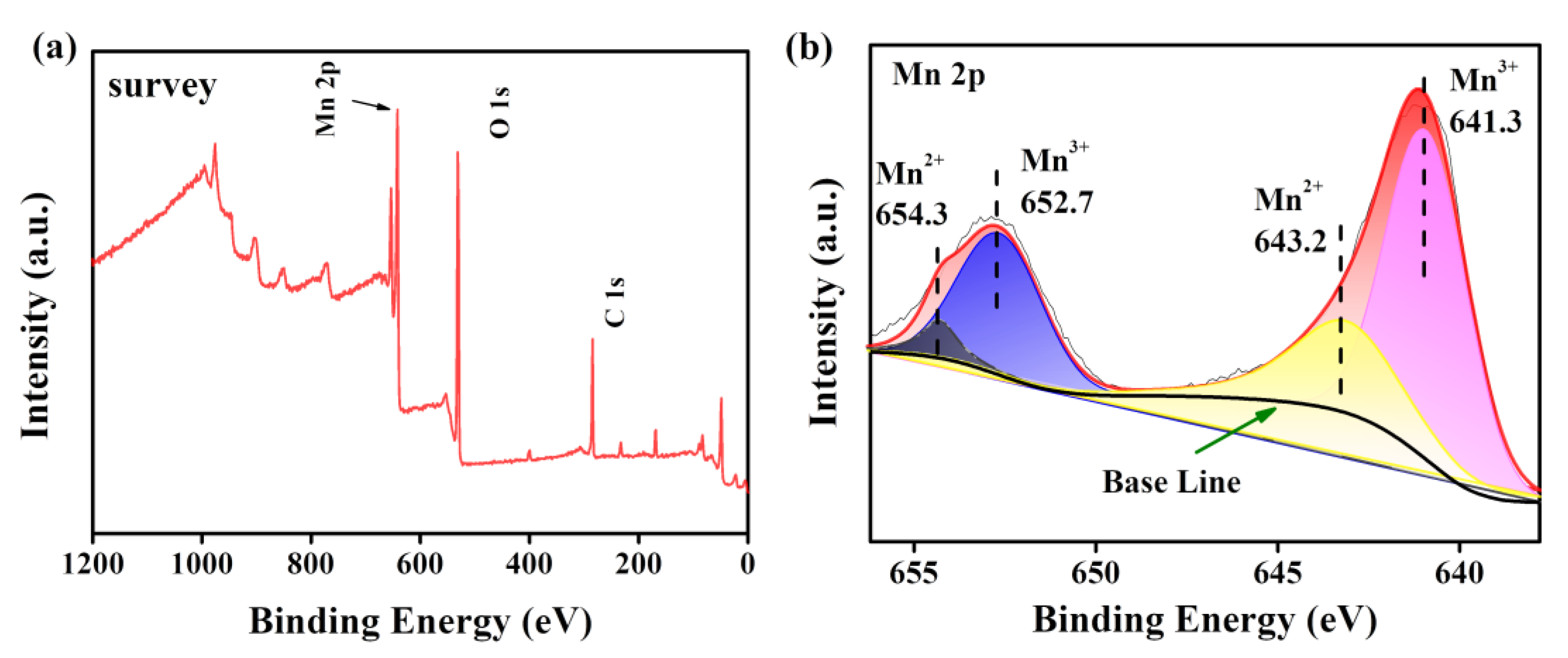
2.5. Characterizations
2.6. Activity Test
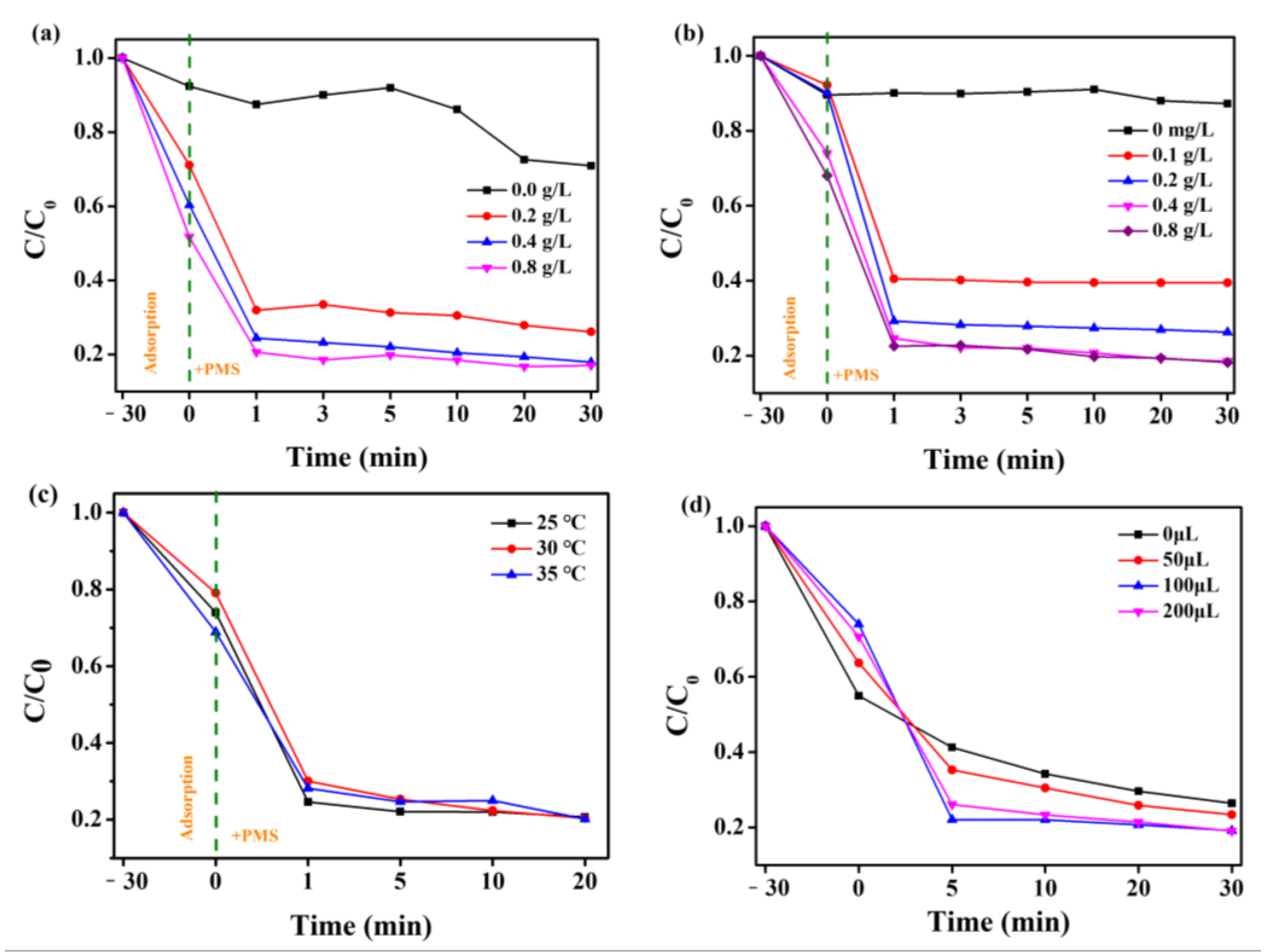
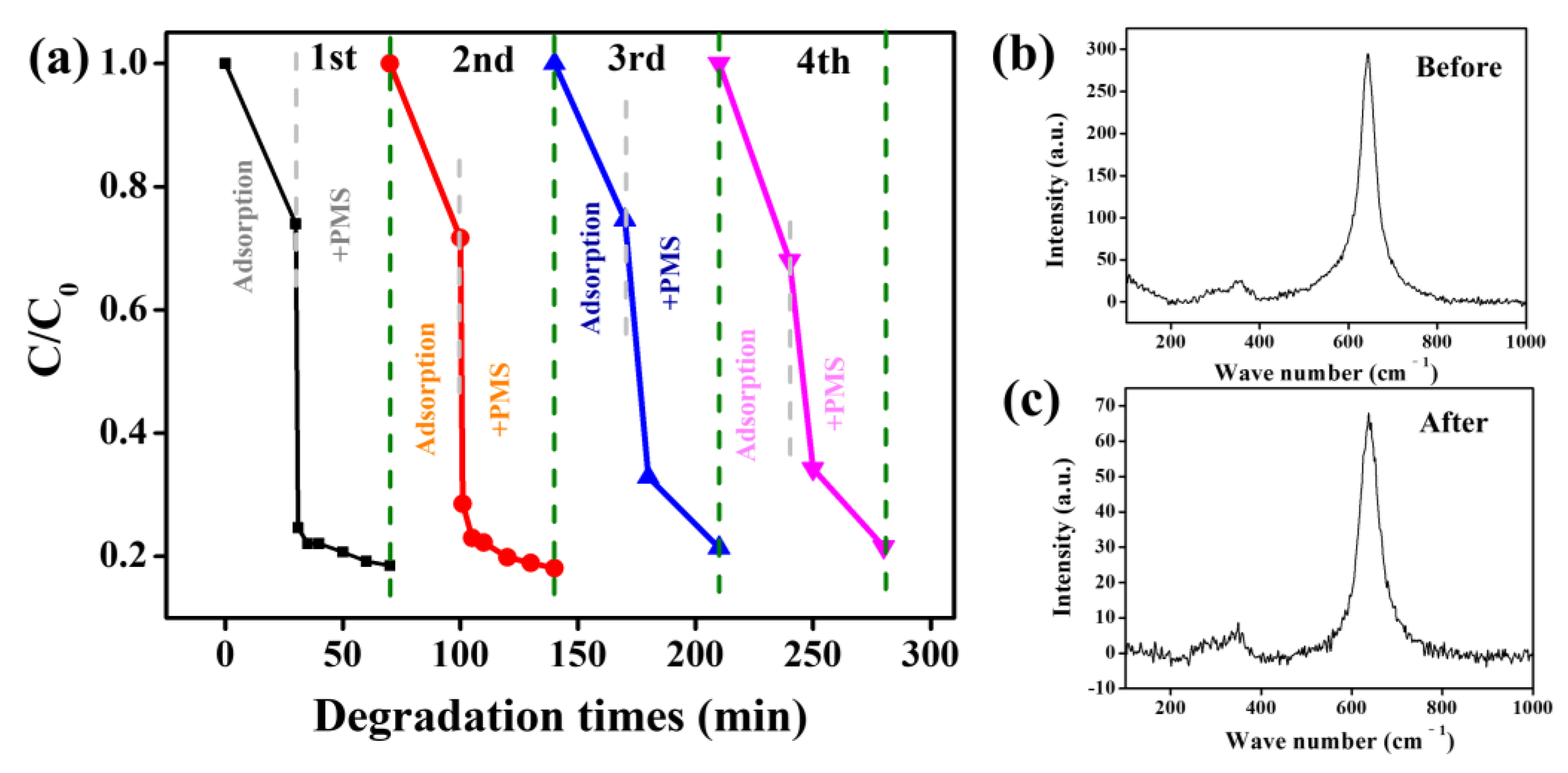
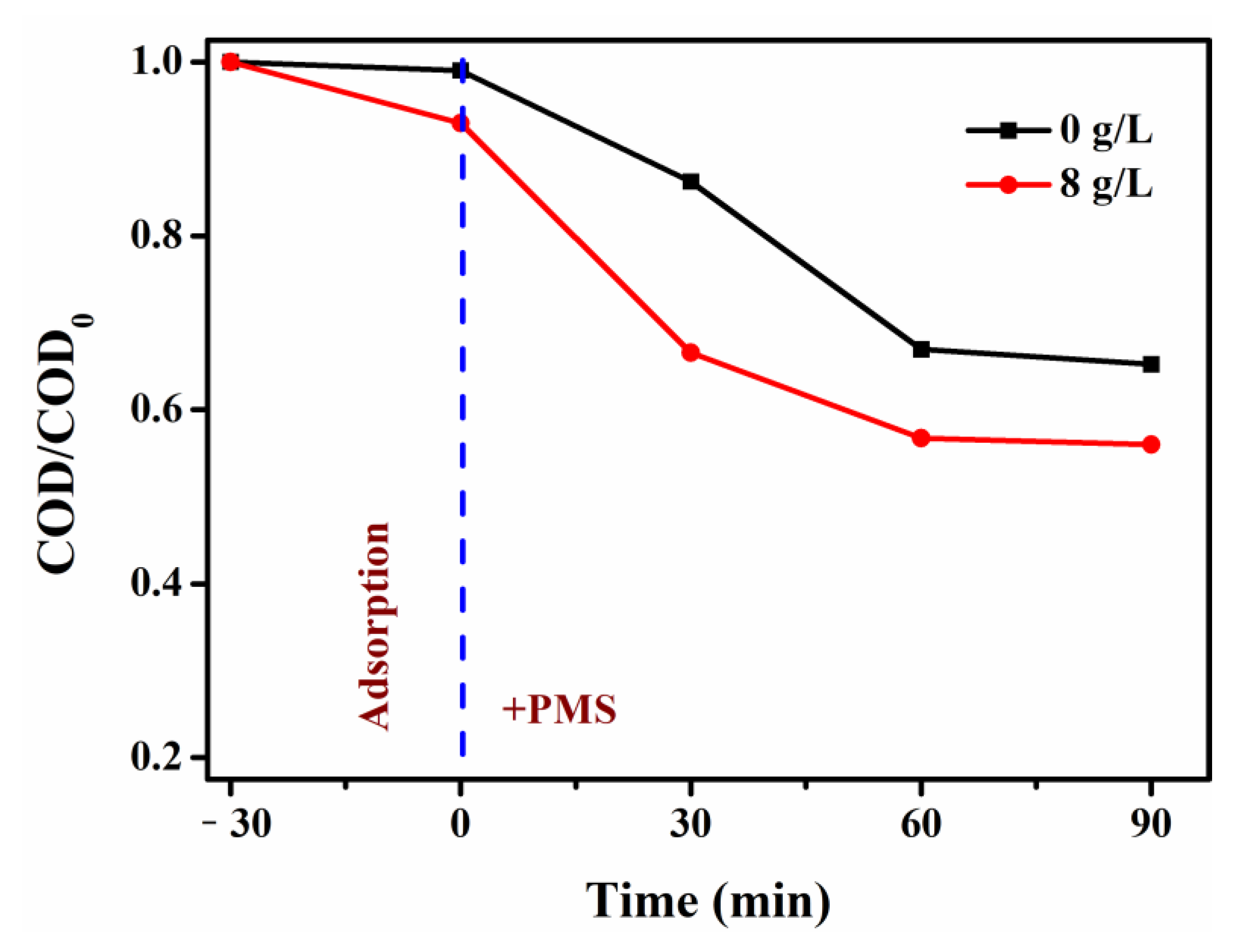
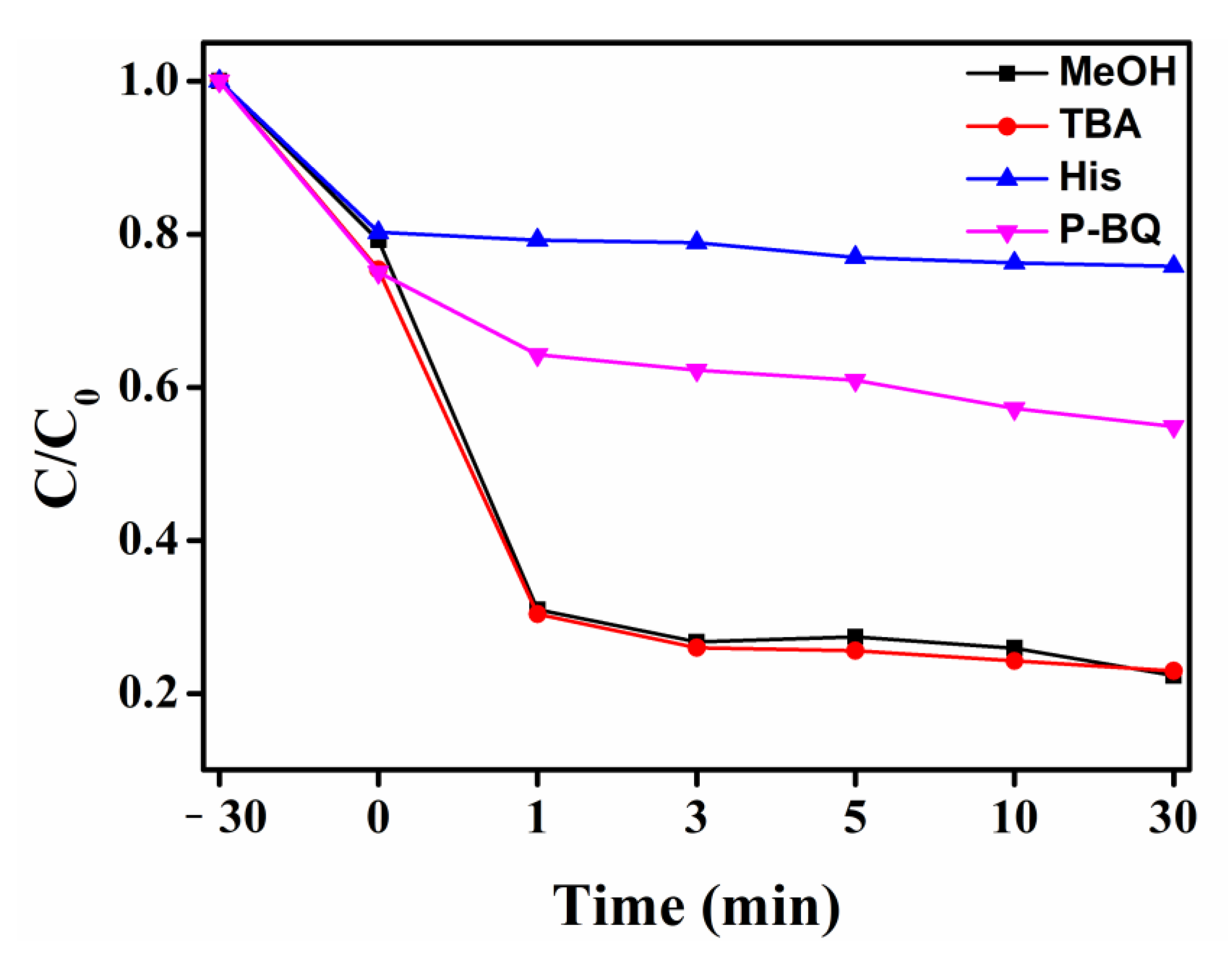
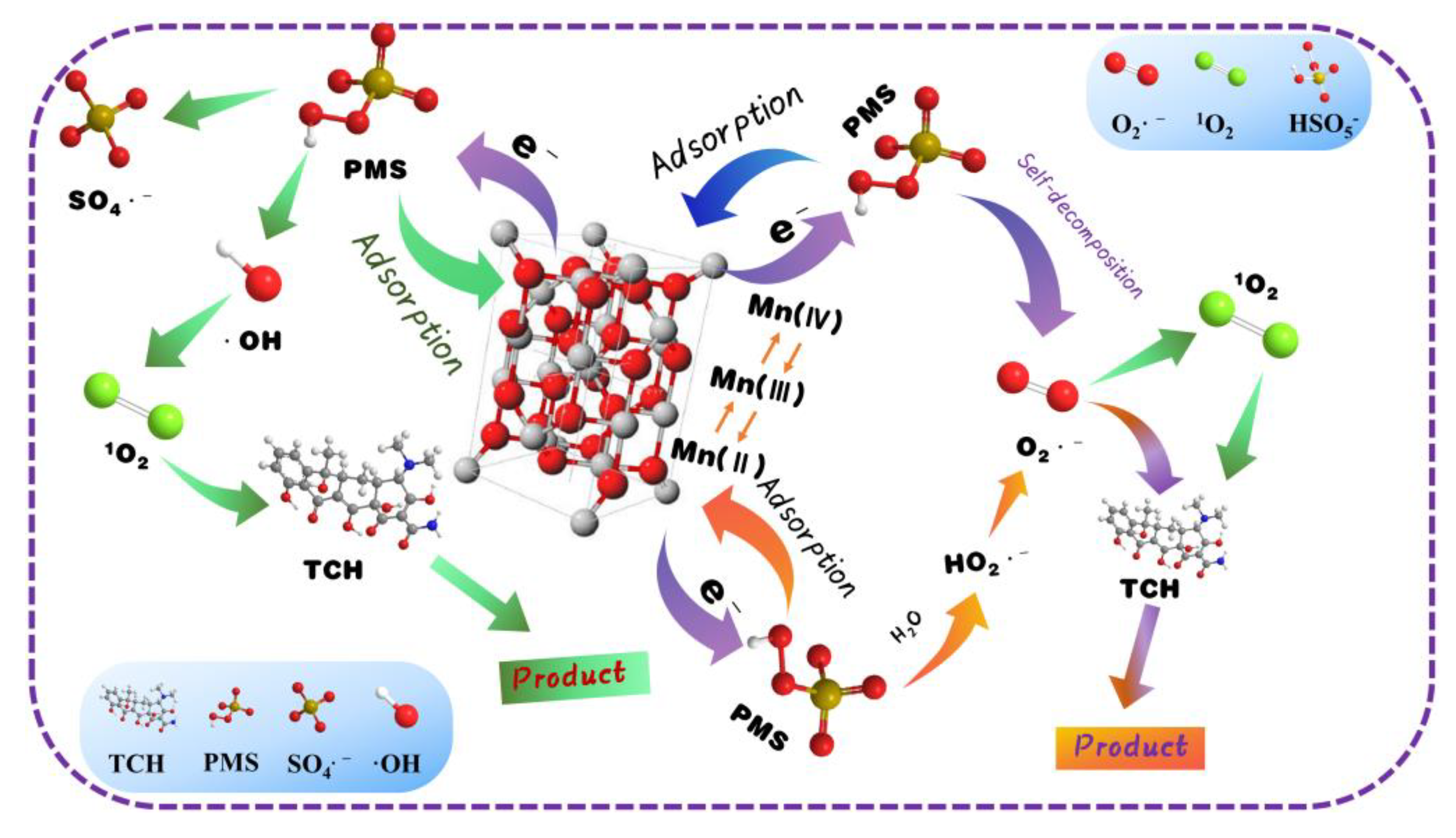
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Srivastav, M.; Gupta, M.; Agrahari, S.K.; Detwal, P. Removal of refractory organic compounds from wastewater by various advanced oxidation process—A review. Curr. Environ. Eng. 2019, 6, 8–16. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. A J. Int. Water Assoc. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, J.; Song, W.; Zhang, X.; Song, J. Decomplexation of electroplating wastewater by ozone-based advanced oxidation process. Water Sci. Technol. 2019, 79, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Lutze, H.V.; Bircher, S.; Rapp, I.; Kerlin, N.; Bakkour, R.; Geisler, M.; von Sonntag, C.; Schmidt, T.C. Degradation of chlorotriazine pesticides by sulfate radicals and the influence of organic matter. Environ. Sci. Technol. EST 2015, 49, 1673–1680. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Wang, Q.; Pan, J.; Zhang, J. Simultaneous removal of NO and SO2 using vacuum ultraviolet light (VUV)/heat/peroxymonosulfate (PMS). Chemosphere Environ. Toxicol. Risk Assess. 2018, 190, 431–441. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Lee, S.J.; Murthy, A.P.; Madhavan, J.; Choi, M.Y. Fundamental aspects and recent advances in transition metal nitrides as electrocatalysts for hydrogen evolution reaction: A review. Curr. Opin. Solid State Mater. Sci. 2020, 24, 100805. [Google Scholar] [CrossRef]
- Wei, M.; Gao, L.; Li, J.; Fang, J.; Cai, W.; Li, X.; Xu, A. Activation of peroxymonosulfate by graphitic carbon nitride loaded on activated carbon for organic pollutants degradation. J. Hazard. Mater. 2016, 316, 60–68. [Google Scholar] [CrossRef]
- Wang, J.; Guo, H.; Liu, Y.; Li, W.; Yang, B. Peroxymonosulfate activation by porous BiFeO3 for the degradation of bisphenol AF: Non-radical and radical mechanism. Appl. Surf. Sci. 2020, 507, 145097. [Google Scholar] [CrossRef]
- Xu, L.; Wang, X.; Sun, Y.; Gong, H.; Guo, M.; Zhang, X.; Meng, L.; Gan, L. Mechanistic study on the combination of ultrasound and peroxymonosulfate for the decomposition of endocrine disrupting compounds. Ultrason. Sonochemistry 2020, 60, 104749. [Google Scholar] [CrossRef]
- Zhu, S.; Xiao, P.; Wang, X.; Liu, Y.; Yi, X.; Zhou, H. Efficient peroxymonosulfate (PMS) activation by visible-light-driven formation of polymorphic amorphous manganese oxides. J. Hazard. Mater. 2022, 427, 127938. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, T.; Zhu, Y.; Liang, X.; Zhao, Z.; Wang, D.; Li, J.; Gao, Y.; Hu, C. Efficient light-free activation of peroxymonosulfate by carbon ring conjugated carbon nitride for elimination of organic pollutants. Chem. Eng. J. 2021, 420, 129671. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Wang, J. Fenton/Fenton-like processes with in-situ production of hydrogen peroxide/hydroxyl radical for degradation of emerging contaminants: Advances and prospects. J. Hazard. Mater. 2021, 404, 124191. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Chen, Z.; Zheng, Z.; Xu, H.; Wang, H.; Hu, K.; Yan, K. Remarkably enhanced sulfate radical-based photo-Fenton-like degradation of levofloxacin using the reduced mesoporous MnO@MnOx microspheres. Chem. Eng. J. 2020, 379, 122340. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, Q.; Yang, X.; Ferronato, C.; Chovelon, J.M.; Sleiman, M.; Richard, C. Sulfate radical mediated degradation of 5-halogenosalicylic acids: Phenoxyl radical transformation pathways. Chem. Eng. J. 2020, 394, 124839. [Google Scholar] [CrossRef]
- Sun, P.; Liu, H.; Feng, M.; Guo, L.; Zhai, Z.; Fang, Y.; Zhang, X.; Sharma, V.K. Nitrogen-sulfur co-doped industrial graphene as an efficient peroxymonosulfate activator: Singlet oxygen-dominated catalytic degradation of organic contaminants. Appl. Catal. B Environ. 2019, 251, 335–345. [Google Scholar] [CrossRef]
- Chen, Z.; Bi, S.; Zhao, G.; Chen, Y.; Hu, Y. Enhanced degradation of triclosan by cobalt manganese spinel-type oxide activated peroxymonosulfate oxidation process via sulfate radicals and singlet oxygen: Mechanisms and intermediates identification. Sci. Total Environ. 2020, 711, 134715. [Google Scholar] [CrossRef]
- Li, D.; Zhang, S.; Li, S.; Tang, J.; Hua, T.; Li, F. Mechanism of the application of single-atom catalyst-activated PMS/PDS to the degradation of organic pollutants in water environment: A review. J. Clean. Prod. 2023, 397, 136468. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, X.; Yang, F. Research progress in heterogeneous catalytic activation of persulfate catalysts for wastewater treatment. Shandong Chem. Ind. 2021, 50, 70–71, 74. [Google Scholar]
- Liu, Q.; Chen, L. Research progress on activation generation of sulfate radical. Appl. Chem. Ind. 2021, 50, 3135–3139. [Google Scholar]
- Lee, S.J.; Theerthagiri, J.; Nithyadharseni, P.; Arunachalam, P.; Balaji, D.; Kumar, A.M.; Madhavan, J.; Mittal, V.; Choi, M.Y. Heteroatom-doped graphene-based materials for sustainable energy applications: A review. Renew. Sustain. Energy Rev. 2021, 143, 110849. [Google Scholar] [CrossRef]
- Shokoohi, R.; Godini, K.; Latifi, Z. Catalytic oxidation of reactive blue 222 dye using peroxymonosulfate activated by Mn3O4, Parameter optimization using response surface methodology. Inorg. Chem. Commun. 2023, 149, 12. [Google Scholar] [CrossRef]
- Muhammad, S.; Nugraha, M.W.; Saputra, E.; Arahman, N. Mn3O4 catalysts for advanced oxidation of phenolic contaminants in aqueous solutions. Water 2022, 14, 2124. [Google Scholar] [CrossRef]
- Liu, P.C.; Zheng, K.; Su, X.-D.; Huang, F.; Li, J.-R.; Chen, X.-H.; Dong, X.-W. Present situation and prospect of the comprehensive utilization of electrolytic manganese slag resources. Liaoning Chem. Ind. 2022, 51, 235–238. [Google Scholar]
- Kai, Z.; Fanghai, L.; Junqi, L.; Xiongwen, D.; Xiangdong, S. Present situation and prospect of the comprehensive utilization of electrolytic manganese slag resources. Chem. Eng. Des. Commun. 2020, 46, 138–139+158. [Google Scholar]
- Zhao, J.; Cai, L.; Shu, J.; Cao, J.; Yang, Y.; Chen, M. Hydrometallurgy leaching of manganese from electrolytic manganese anode slime using hydrogen peroxide as reducing agent. Chin. J. Eng. 2023, 45, 206–213. [Google Scholar]
- Yang, Y.; Shu, J.; Su, P.; Wu, H.; Zhang, L.; Liu, R.; Liu, Z.; Chen, M.; Chen, F.; Ming, X. Enhanced removal of Pb from electrolytic manganese anode slime and preparation of chemical MnO2. Environ. Technol. 2022. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, C.; Wang, J.; Wang, H.; Chen, Z.; Wang, S.; Chen, Z. Recycling application of modified waste electrolytic manganese anode slag as efficient catalyst for PMS activation. Sci. Total Environ. 2021, 762, 143120. [Google Scholar] [CrossRef]
- Li, N.; Tian, Y.; Zhao, J.; Zhang, J.; Zhang, J.; Zuo, W.; Ding, Y. Efficient removal of chromium from water by Mn3O4@ZnO/Mn3O4 composite under simulated sunlight irradiation: Synergy of photocatalytic reduction and adsorption. Appl. Catal. B Environ. 2017, 214, 126–136. [Google Scholar] [CrossRef]
- Xu, Y.; Ai, J.; Zhang, H. The mechanism of degradation of bisphenol A using the magnetically separable CuFe2O4/peroxymonosulfate heterogeneous oxidation process. J. Hazard. Mater. 2016, 309, 87–96. [Google Scholar] [CrossRef]
- Yang, L.; Cheng, S.; Ji, X.; Jiang, Y.; Zhou, J.; Liu, M. Investigations into the origin of pseudocapacitive behavior of Mn3O4 electrodes using in operando Raman spectroscopy. J. Mater. Chem. A 2015, 3, 7338–7344. [Google Scholar] [CrossRef]
- Rani, B.J.; Ravi, G.; Yuvakkumar, R.; Hong, S.I.; Velauthapillai, D.; Thambidurai, M.; Dang, C.; Saravanakumar, B. Neutral and alkaline chemical environment dependent synthesis of Mn3O4 for oxygen evolution reaction (OER). Mater. Chem. Phys. 2020, 247, 122864. [Google Scholar] [CrossRef]
- Subramani, K.; Jeyakumar, D.; Sathish, M. Manganese hexacyanoferrate derived Mn3O4 nanocubes-reduced graphene oxide nanocomposites and their charge storage characteristics in supercapacitors. Phys. Chem. Chem. Phys. 2014, 16, 4952–4961. [Google Scholar] [CrossRef] [PubMed]
- Thareja, S.; Kumar, A. One-Pot In Situ Synthesis of Mn3O4/N-rGO nanohybrids for the fabrication of high cell voltage aqueous symmetric supercapacitors: An analysis of redox activity of Mn3O4 toward stabilizing the high potential window in salt-in-water and water- in-salt electrolytes. Energy Fuels 2022, 36, 15177–15187. [Google Scholar]
- Shokoohi, R.; Khazaei, M.; Godini, K.; Azarian, G.; Latifi, Z.; Javadimanesh, L.; Nasab, H.Z. Degradation and mineralization of methylene blue dye by peroxymonosulfate/Mn3O4 nanoparticles using central composite design: Kinetic study. Inorg. Chem. Commun. 2021, 127, 1–10. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.; Li, Y.; Fan, X. Simple and rapid preparation of nano-Mn3O4 and its Fenton-like catalytic oxidation of methylene Blue. Min. Metall. Eng. 2020, 40, 153–155+160. [Google Scholar]
- Zhao, Z.; Zhao, J.; Yang, C. Efficient removal of ciprofloxacin by peroxymonosulfate/Mn3O4-MnO2 catalytic oxidation system. Chem. Eng. J. 2017, 327, 481–489. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, C.; Wang, Y.; Jia, F.; Song, S. Construction of 3D-sized Mn (II)-doped MoS2@activated alumina beads as PMS activator for tetracycline degradation under light irradiation. Chem. Phys. Lett. 2022, 806, 139996. [Google Scholar] [CrossRef]
- Wang, Z.; Han, Y.; Fan, W.; Wang, Y.; Huang, L. Shell-core MnO2/Carbon@Carbon nanotubes synthesized by a facile one-pot method for peroxymonosulfate oxidation of tetracycline. Sep. Purif. Technol. 2022, 278, 119558. [Google Scholar] [CrossRef]
- Liang, F.; Liu, Z.; Jiang, X.; Li, J.; Xiao, K.; Xu, W.; Chen, X.; Liang, J.; Lin, Z.; Li, M.; et al. NaOH-modified biochar supported Fe/Mn bimetallic composites as efficient peroxymonosulfate activator for enhance tetracycline removal. Chem. Eng. J. 2023, 454, 139949. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, X.; Yan, Y.; Sun, C.; Wu, H.; He, J.; Wang, D. Heterogeneous activation of peroxymonosulfate by different ferromanganese oxides for tetracycline degradation: Structure dependence and catalytic mechanism. Chem. Eng. J. 2018, 348, 263–270. [Google Scholar] [CrossRef]
- Xu, R.; Xiong, J.; Liu, D.; Wang, Y.; Ming, Y.A. Inverse micelle fabrication of ordered mesoporous manganese oxide and degradation of tetracycline hydrochloride. J. Colloid Interface Sci. 2022, 625, 397–404. [Google Scholar] [CrossRef]
- Liang, C.J.; Su, H.W. Identification of sulfate and hydroxyl radicals in thermally activated persulfate. Ind. Eng. Chem. Res. 2009, 48, 5558–5562. [Google Scholar] [CrossRef]
- Chen, C.; Ma, T.; Shang, Y.; Gao, B.; Jin, B.; Dan, H.; Li, Q.; Yue, Q.; Li, Y.; Wang, Y.; et al. In-situ pyrolysis of Enteromorpha as carbocatalyst for catalytic removal of organic contaminants: Considering the intrinsic N/Fe in Enteromorpha and non-radical reaction. Appl. Catal. B Environ. 2019, 250, 382–395. [Google Scholar] [CrossRef]
- Cheng, X.; Guo, H.; Zhang, Y.; Wu, X.; Liu, Y. Non-photochemical production of singlet oxygen via activation of persulfate by carbon nanotubes. Water Res. 2017, 113, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Mao, Q.; Zhou, Y.; Wei, J.; Liu, X.; Yang, J.; Luo, L.; Zhang, J.; Chen, H.; Chen, H.; et al. Metal-free carbon materials-catalyzed sulfate radical-based advanced oxidation processes: A review on heterogeneous catalysts and applications. Chemosphere 2017, 189, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Gong, M.; Wang, W.; Mu, Y.; Hu, Z.H. α-MnO2/Palygorskite composite as an effective catalyst for heterogeneous activation of peroxymonosulfate (PMS) for the degradation of Rhodamine B. Sep. Purif. Technol. 2020, 230, 115877. [Google Scholar] [CrossRef]
- Nie, M.; Zhang, W.; Yan, C.; Xu, W.; Wu, L.; Ye, Y.; Hu, Y.; Dong, W. Enhanced removal of organic contaminants in water by the combination of peroxymonosulfate and carbonate. Sci. Total Environ. 2019, 647, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, D.; Fan, S.; Yang, T.; Zhou, Q. Facile template synthesis of dumbbell-like Mn2O3 with oxygen vacancies for efficient degradation of organic pollutants by activating peroxymonosulfate. Catal. Sci. Technol. 2020, 10, 864–875. [Google Scholar] [CrossRef]
- Lin, H.; Li, S.; Deng, B.; Tan, W.; Li, R.; Xu, Y.; Zhang, H. Degradation of bisphenol A by activating peroxymonosulfate with Mn0.6Zn0.4Fe2O4 fabricated from spent Zn-Mn alkaline batteries. Chem. Eng. J. 2019, 364, 541–551. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, H.; Zhang, Y.; Tang, W.; Cheng, X.; Li, W. Heterogeneous activation of peroxymonosulfate by sillenite Bi25FeO40: Singlet oxygen generation and degradation for aquatic levofloxacin. Chem. Eng. J. 2018, 343, 128–137. [Google Scholar] [CrossRef]

| Sample | Pollutants | Operation Condition | Activities | Fourth Cycle | Ref. |
|---|---|---|---|---|---|
| Mn-MoS2@AABs | TCH | Pollutant dosage 20 ppm. Catalyst dosage 2.3 g/L. PMS dosage 0.4 ppm. | 80% (60 min) | 40% | [38] |
| MnO2/C@CNT | TCH | Pollutant dosage 10 ppm. Catalyst dosage 0.1 g/L. PMS dosage 1.84 ppm. | 85% (10 min) | 78.8% | [39] |
| Fe-Mn/AW-BC | TCH | Pollutant dosage 20 ppm. Catalyst dosage 0.5 g/L. PMS dosage 0.5 ppm. | 97.9% (60 min) | 77.8% | [40] |
| FMO-46 (FeMnO3) | TCH | Pollutant dosage 50 ppm. Catalyst dosage 0.4 g/L. PMS dosage 0.8 ppm. | 79.8% (80 min) | / | [41] |
| MnOx | TCH | Pollutant dosage 20 ppm. Catalyst dosage 0.2 g/L. PMS dosage 0.4 ppm. | 87.89% (180 min) | / | [42] |
| Mn3O4 | TCH | Pollutant dosage 50 ppm. Catalyst dosage 0.4 g/L. PMS dosage 0.4 ppm. | 82.1% (30 min) | 78.5% | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Xiong, R.; Peng, S.; Xu, D.; Ke, J. Highly Active Manganese Oxide from Electrolytic Manganese Anode Slime for Efficient Removal of Antibiotics Induced by Dissociation of Peroxymonosulfate. Nanomaterials 2023, 13, 1600. https://doi.org/10.3390/nano13101600
Zhang H, Xiong R, Peng S, Xu D, Ke J. Highly Active Manganese Oxide from Electrolytic Manganese Anode Slime for Efficient Removal of Antibiotics Induced by Dissociation of Peroxymonosulfate. Nanomaterials. 2023; 13(10):1600. https://doi.org/10.3390/nano13101600
Chicago/Turabian StyleZhang, He, Ruixue Xiong, Shijie Peng, Desheng Xu, and Jun Ke. 2023. "Highly Active Manganese Oxide from Electrolytic Manganese Anode Slime for Efficient Removal of Antibiotics Induced by Dissociation of Peroxymonosulfate" Nanomaterials 13, no. 10: 1600. https://doi.org/10.3390/nano13101600
APA StyleZhang, H., Xiong, R., Peng, S., Xu, D., & Ke, J. (2023). Highly Active Manganese Oxide from Electrolytic Manganese Anode Slime for Efficient Removal of Antibiotics Induced by Dissociation of Peroxymonosulfate. Nanomaterials, 13(10), 1600. https://doi.org/10.3390/nano13101600





