Exploring the Influence of Synthesis Parameters on the Optical Properties for Various CeO2 NPs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of CeO2 Nanoparticles
2.2.1. Synthesis of CeO2 Nanoparticles V1, V2 and V3
2.2.2. Synthesis of CeO2 NPs by Version 2 (V4)
2.2.3. Synthesis of CeO2 NPs by Version 3 (V5, V6)
2.3. Preparation of Co-Doped CeO2 Nanoparticles (V2-Co and V5-Co)
2.4. Characterization
3. Results and Discussion
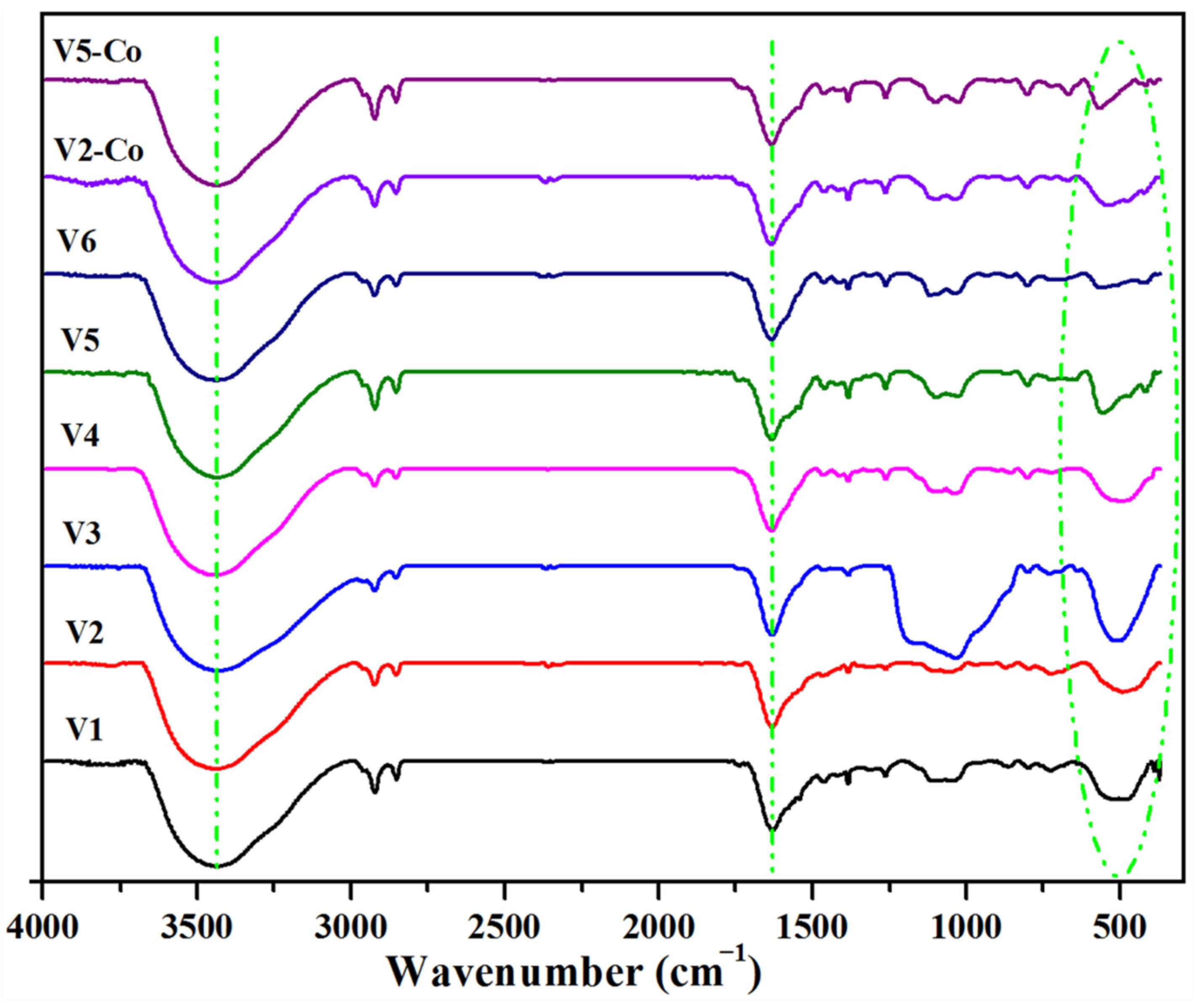
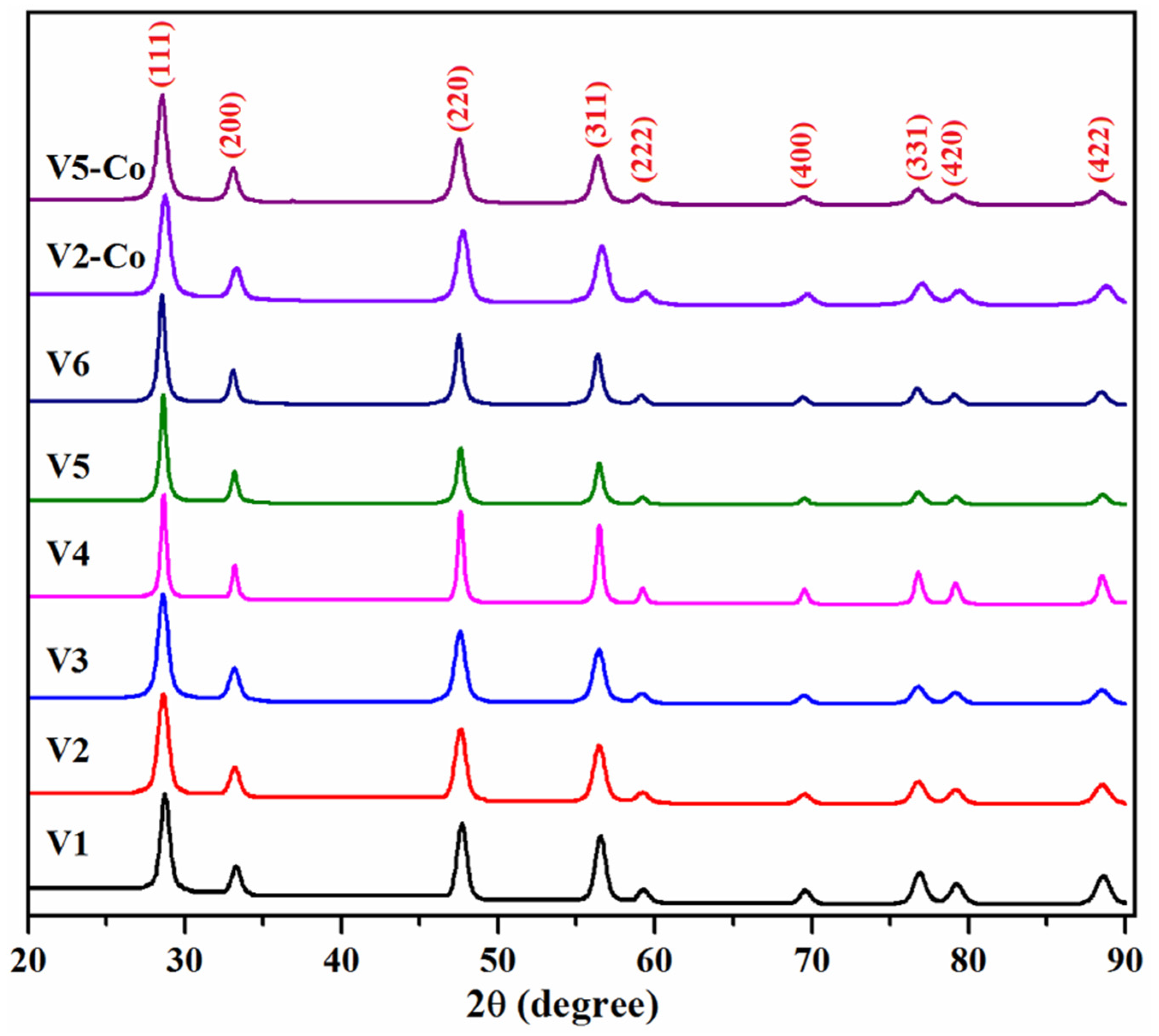
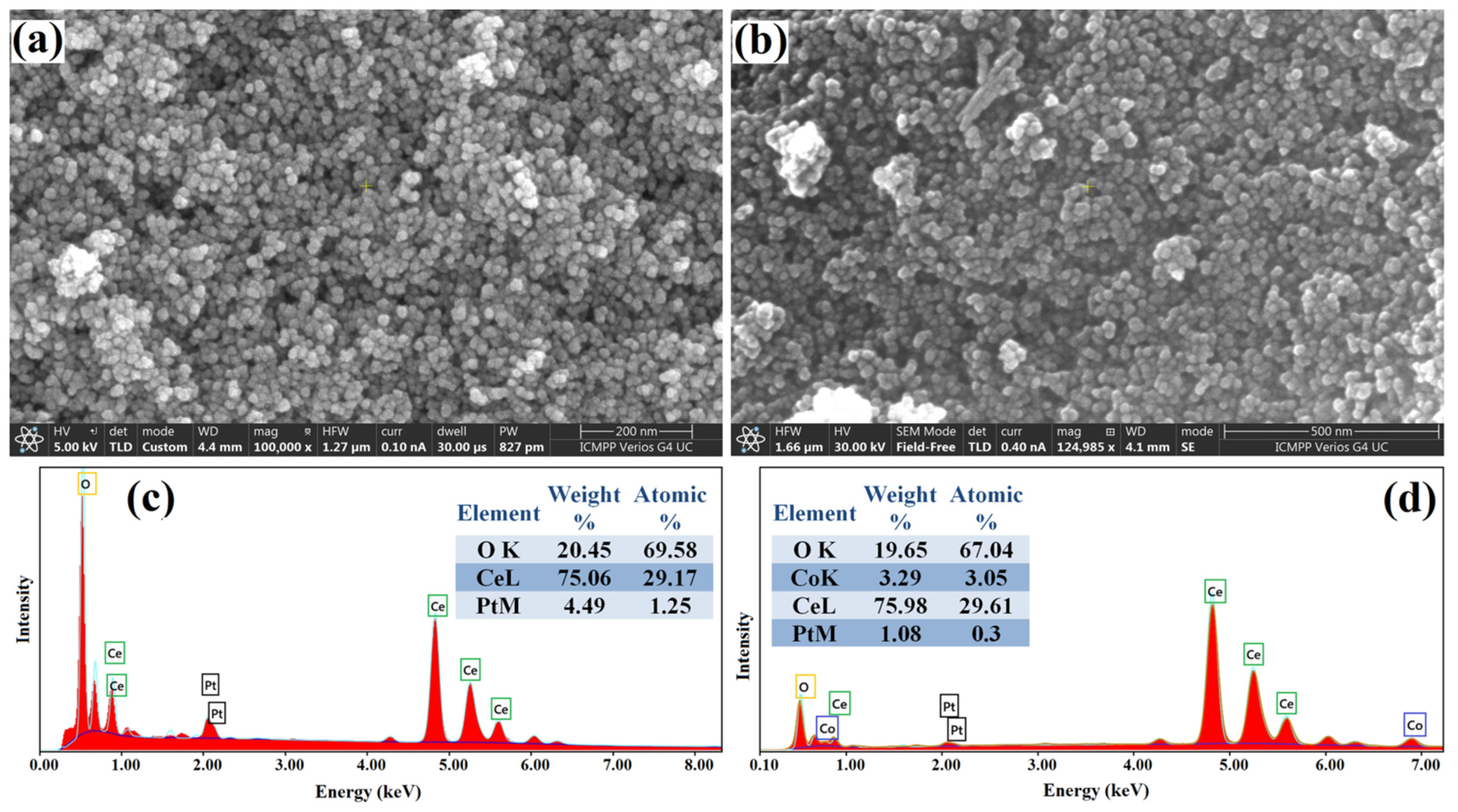
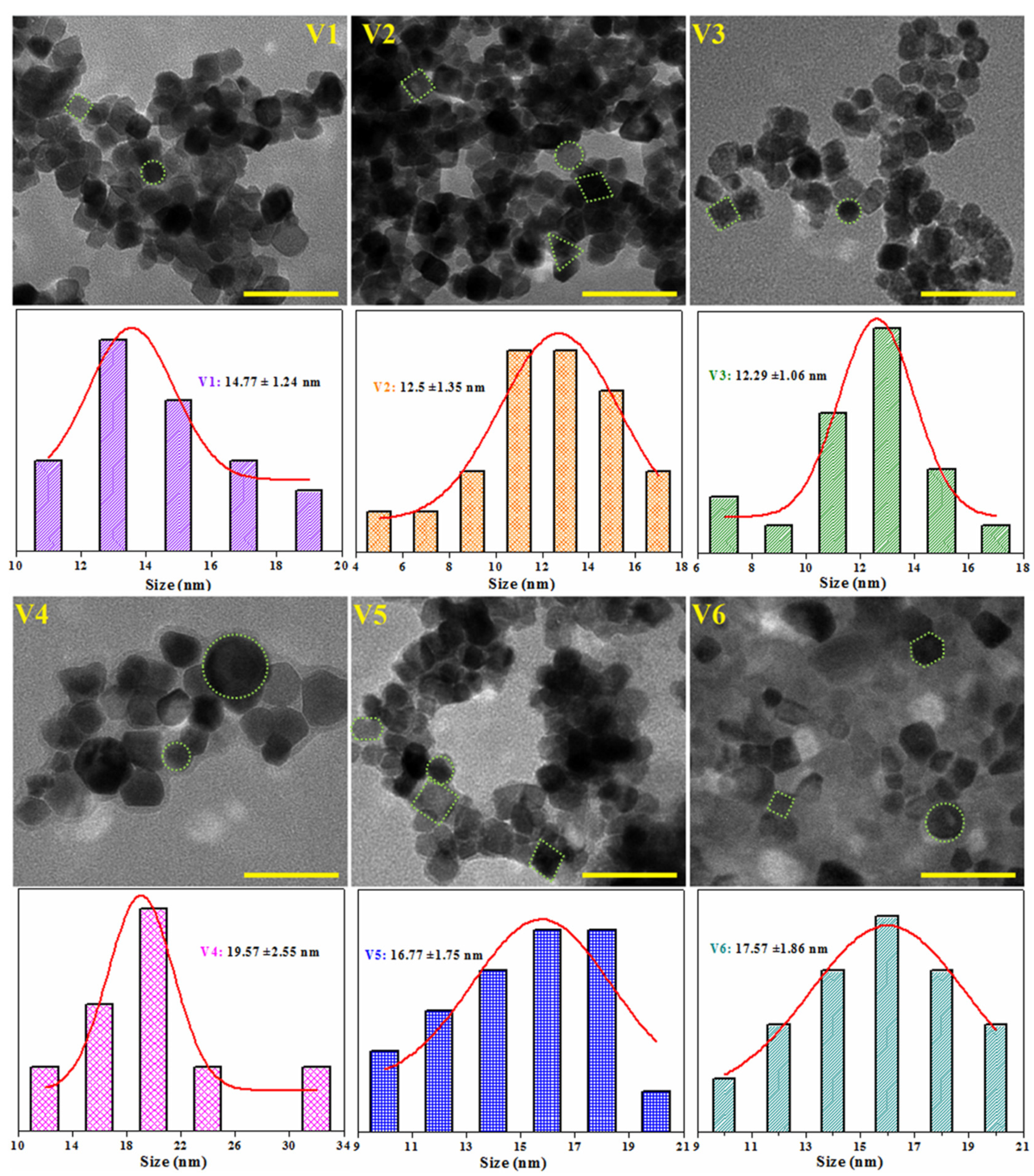
3.1. Structural and Morphological Characterization
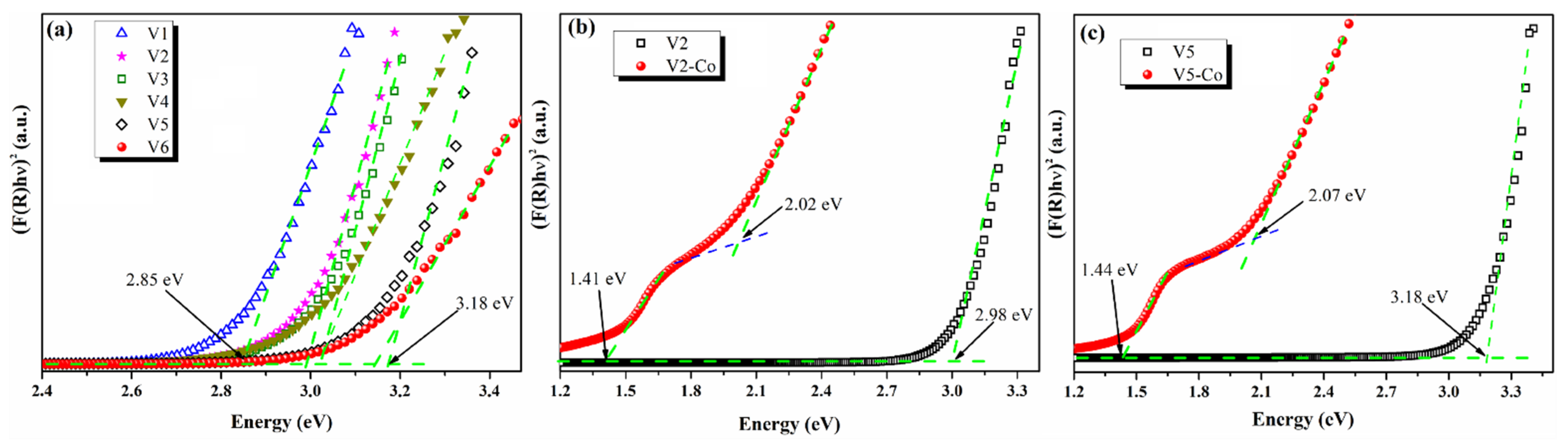
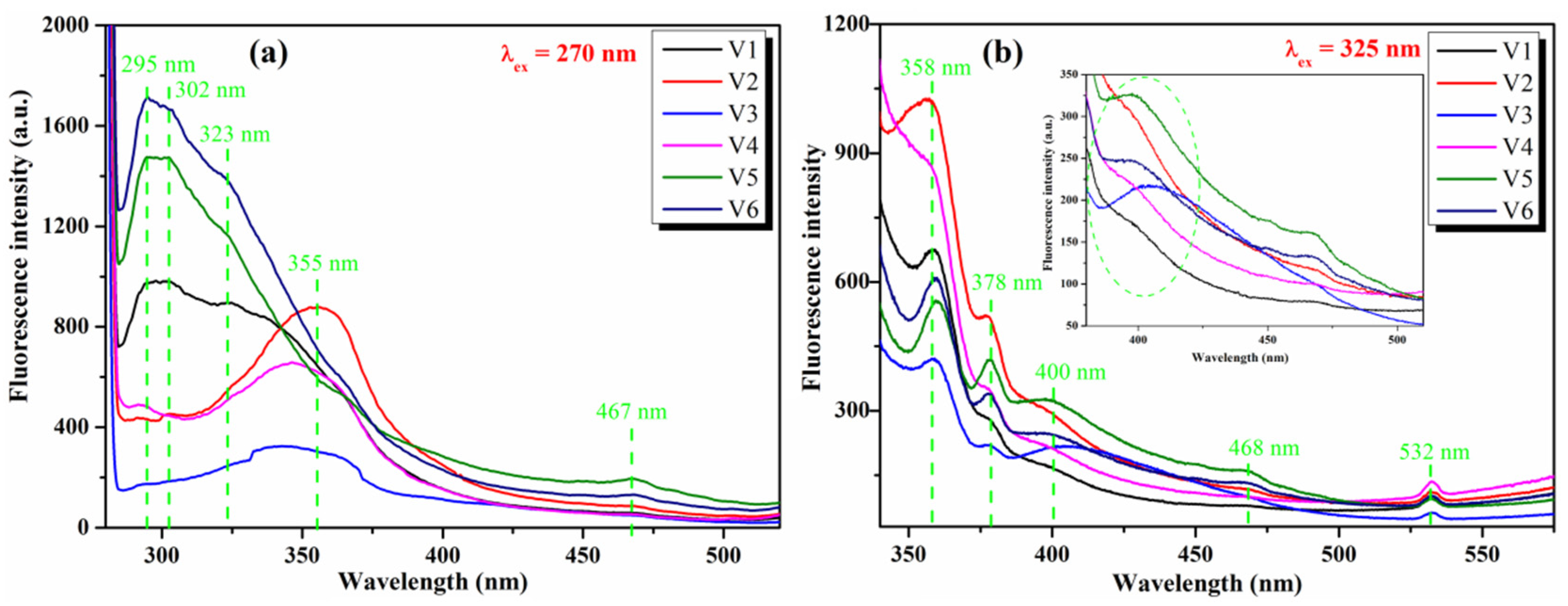
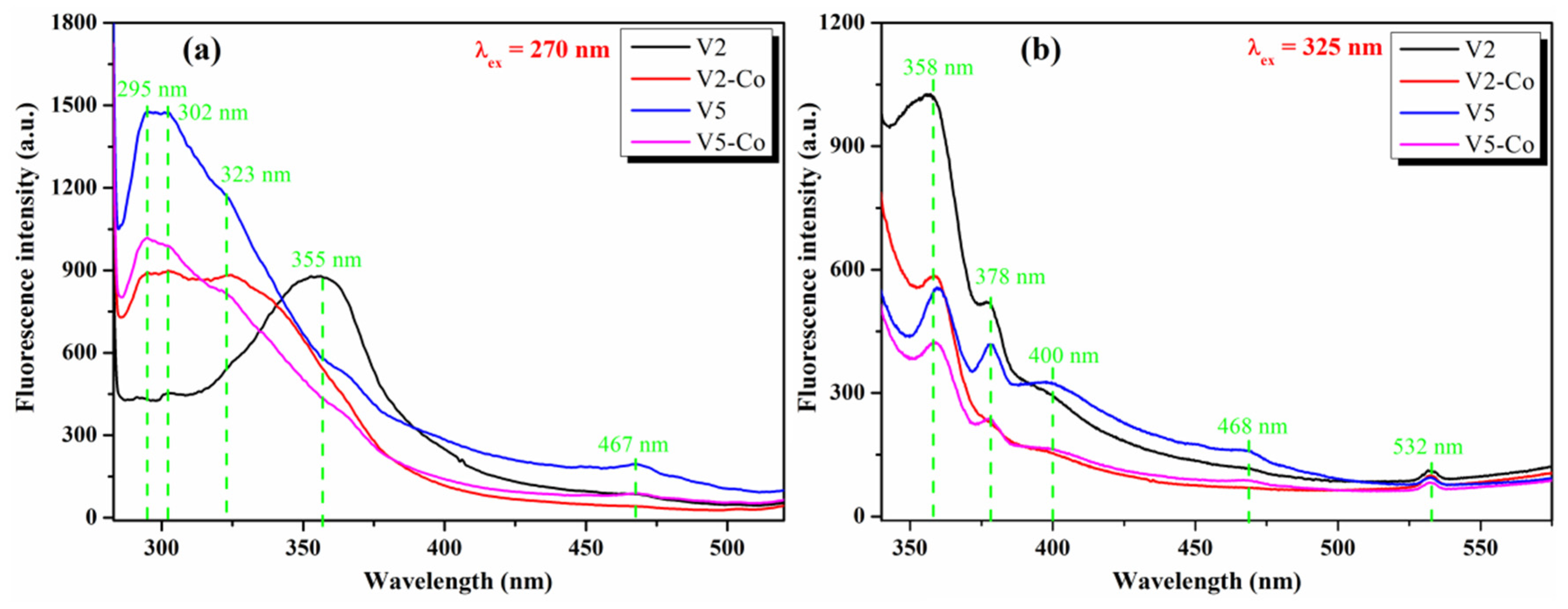
3.2. Optical Study of Pristine and Co-Doped CeO2 NPs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amoresi, R.A.C.; Oliveira, R.C.; Marana, N.L.; De Almeida, P.B.; Prata, P.S.; Zaghete, M.A.; Longo, E.; Sambrano, J.R.; Simões, A.Z. CeO2 nanoparticle morphologies and their corresponding crystalline planes for the photocatalytic degradation of organic pollutants. ACS Appl. Nano Mater. 2019, 2, 6513–6526. [Google Scholar] [CrossRef]
- Trovarelli, A.; Llorca, J. Ceria catalysts at nanoscale: How do crystal shapes shape catalysis? ACS Catal. 2017, 7, 4716–4735. [Google Scholar] [CrossRef]
- Mullins, D.R.; Albrecht, P.M.; Calaza, F. Variations in reactivity on different crystallographic orientations of cerium oxide. Top. Catal. 2013, 56, 1345–1362. [Google Scholar] [CrossRef]
- Ma, Y.; Gao, W.; Zhang, Z.; Zhang, S.; Tian, Z.; Liu, Y.; Ho, J.C.; Qu, Y. Regulating the surface of nanoceria and its applications in heterogeneous catalysis. Surf. Sci. Rep. 2018, 73, 1–36. [Google Scholar] [CrossRef]
- Álvarez-Asencio, R.; Corkery, R.W.; Ahniyaz, A. Solventless synthesis of cerium oxide nanoparticles and their application in UV protective clear coatings. RSC Adv. 2020, 10, 14818–14825. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chang, K.H.; Chiang, H.Y.; Shih, S.J. Preparation of a porous ceria coating for a resistive oxygen sensor. Sens. Actuators B Chem. 2014, 204, 31–41. [Google Scholar] [CrossRef]
- Li, P.; Chen, X.; Li, Y.; Schwank, J.W. A review on oxygen storage capacity of CeO2 -based materials: Influence factors, measurement techniques, and applications in reactions related to catalytic automotive emissions control. Catal. Today 2019, 327, 90–115. [Google Scholar] [CrossRef]
- Khan, A.J.; Hanif, M.; Javed, M.S.; Hussain, S.; Zhong, W.; Saleem, M.; Liu, Z. Energy storage properties of hydrothermally processed, nanostructured, porous CeO2 nanoparticles. J. Electroanal. Chem. 2020, 865, 114158. [Google Scholar] [CrossRef]
- Divya, T.; Anjali, C.; Sunajadevi, K.R.; Anas, K.; Renuka, N.K. Influence of hydrothermal synthesis conditions on lattice defects in cerium oxide. J. Solid State Chem. 2021, 300, 122253. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, Q.; Yuan, S.; Zhang, M.; Ohno, T. Morphology control and characterization of broom-like porous CeO2. Chem. Eng. J. 2015, 260, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, N.S.; Angélica, R.S.; Marques, V.B.; De Lima, C.C.O.; Silva, M.S. Cassava-starch-assisted sol-gel synthesis of CeO2 nanoparticles. Mater. Lett. 2016, 165, 139–142. [Google Scholar] [CrossRef]
- Tamizhdurai, P.; Sakthinathan, S.; Chen, S.M.; Shanthi, K.; Sivasanker, S.; Sangeetha, P. Environmentally friendly synthesis of CeO2 nanoparticles for the catalytic oxidation of benzyl alcohol to benzaldehyde and selective detection of nitrite. Sci. Rep. 2017, 7, 46372. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Sahu, K.; Bisht, A.; Singhal, R.; Mohapatra, S. Template-free and surfactant-free synthesis of CeO2nanodiscs with enhanced photocatalytic activity. Appl. Surf. Sci. 2020, 503, 144102. [Google Scholar] [CrossRef]
- Ko, H.H.; Yang, G.; Cheng, H.Z.; Wang, M.C.; Zhao, X. Growth and optical properties of cerium dioxide nanocrystallites prepared by coprecipitation routes. Ceram. Int. 2014, 40, 4055–4064. [Google Scholar] [CrossRef]
- Pang, J.H.; Liu, Y.; Li, J.; Yang, X.J. Solvothermal synthesis of nano-CeO2 aggregates and its application as a high-efficient arsenic adsorbent. Rare Met. 2019, 38, 73–80. [Google Scholar] [CrossRef]
- Shlapa, Y.; Sarnatskaya, V.; Timashkov, I.; Yushko, L.; Antal, I.; Gerashchenko, B.; Nychyporenko, I.; Belous, A.; Nikolaev, V.; Timko, M. Synthesis of CeO2 nanoparticles by precipitation in reversal microemulsions and their physical-chemical and biological properties. Appl. Phys. A Mater. Sci. Process. 2019, 125, 412. [Google Scholar] [CrossRef]
- He, J.; Zhou, L.; Liu, J.; Yang, L.; Zou, L.; Xiang, J.; Dong, S.; Yang, X. Modulation of surface structure and catalytic properties of cerium oxide nanoparticles by thermal and microwave synthesis techniques. Appl. Surf. Sci. 2017, 402, 469–477. [Google Scholar] [CrossRef]
- Mishra, S.; Soren, S.; Debnath, A.K.; Aswal, D.K.; Das, N.; Parhi, P. Rapid microwave—Hydrothermal synthesis of CeO2 nanoparticles for simultaneous adsorption/photodegradation of organic dyes under visible light. Optik 2018, 169, 125–136. [Google Scholar] [CrossRef]
- Polychronopoulou, K.; Zedan, A.F.; Katsiotis, M.S.; Baker, M.A.; AlKhoori, A.A.; AlQaradawi, S.Y.; Hinder, S.J.; AlHassan, S. Rapid microwave assisted sol-gel synthesis of CeO2 and CexSm1-xO2 nanoparticle catalysts for CO oxidation. J. Mol. Catal. 2017, 428, 41–55. [Google Scholar] [CrossRef]
- Qiao, Z.A.; Wu, Z.; Dai, S. Shape-controlled ceria-based nanostructures for catalysis applications. ChemSusChem 2013, 6, 1821–1833. [Google Scholar] [CrossRef]
- Kurian, M. Cerium oxide based materials for water treatment-A review. J. Environ. Chem. Eng. 2020, 8, 104439. [Google Scholar] [CrossRef]
- Nyoka, M.; Choonara, Y.E.; Kumar, P.; Kondiah, P.P.D.; Pillay, V. Synthesis of cerium oxide nanoparticles using various methods: Implications for biomedical applications. Nanomaterials 2020, 10, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvache-Muñoz, J.; Prado, F.A.; Rodríguez-Páez, J.E. Cerium oxide nanoparticles: Synthesis, characterization and tentative mechanism of particle formation. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 146–159. [Google Scholar] [CrossRef]
- Krishnan, A.; Sreeremya, T.S.; Ghosh, S. Size-tunable hydrophilic cerium oxide nanoparticles as a “turn-on” fluorescence sensor for the rapid detection of ultralow concentrations of Vitamin C. RSC Adv. 2016, 6, 53550–53559. [Google Scholar] [CrossRef]
- Dos Santos, A.P.B.; Dantas, T.C.M.; Costa, J.A.P.; Souza, L.D.; Soares, J.M.; Caldeira, V.P.S.; Araújo, A.S.; Santos, A.G.D. Formation of CeO2 nanotubes through different conditions of hydrothermal synthesis. Surf. Interfaces 2020, 21, 100746. [Google Scholar] [CrossRef]
- MohdFadzil, N.A.; Ab Rahim, M.H.; PragasManiam, G. Brief review of ceria and modified ceria: Synthesis and application. Mater. Res. Express 2018, 5, 085019. [Google Scholar] [CrossRef] [Green Version]
- Mal, D.; Balouch, A.; Sirajuddin; Abdullah; Mahar, A.M.; Pato, A.H.; Kumar, S.; Lal, S.; Kumar, A. Synthesis and catalytic practicality of CeO2 nanoparticle: An excellent heterogenous candidate for 4-nitrophenol reduction. Appl. Nanosci. 2020, 10, 3443–3455. [Google Scholar] [CrossRef]
- Li, H.; Wang, G.; Zhang, F.; Cai, Y.; Wang, Y.; Djerdj, I. Surfactant-assisted synthesis of CeO2 nanoparticles and their application in wastewater treatment. RSC Adv. 2012, 2, 12413–12423. [Google Scholar] [CrossRef]
- Eka Putri, G.; Rilda, Y.; Syukri, S.; Labanni, A.; Arief, S. Highly antimicrobial activity of cerium oxide nanoparticles synthesized using Moringa oleifera leaf extract by a rapid green precipitation method. J. Mater. Res. Technol. 2021, 15, 2355–2364. [Google Scholar] [CrossRef]
- Kusmierek, E. A CeO2 semiconductor as a photocatalytic and photoelectrocatalytic material for the remediation of pollutants in industrial wastewater: A review. Catalysts 2020, 10, 1435. [Google Scholar] [CrossRef]
- Choudhury, B.; Chetri, P.; Choudhury, A. Annealing temperature and oxygen-vacancy-dependent variation of lattice strain, band gap and luminescence properties of CeO2 nanoparticles. J. Exp. Nanosci. 2015, 10, 103–114. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Li, H.; Chen, L. Nanostructured ceria-based materials: Synthesis, properties, and applications. Energy Environ. Sci. 2012, 5, 8475–8505. [Google Scholar] [CrossRef]
- Tatar, B.; Sam, E.D.; Kutlu, K.; Ürgen, M. Synthesis and optical properties of CeO2 nanocrystalline films grown by pulsed electron beam deposition. J. Mater. Sci. 2008, 43, 5102–5108. [Google Scholar] [CrossRef]
- Ortega, P.P.; Hangai, B.; Moreno, H.; Rocha, L.S.R.; Ramírez, M.A.; Ponce, M.A.; Longo, E.; Simões, A.Z. Tuning structural, optical, and gas sensing properties of ceria-based materials by rare-earth doping. J. Alloys Compd. 2021, 888, 161517. [Google Scholar] [CrossRef]
- Qi, Y.; Ye, J.; Zhang, S.; Tian, Q.; Xu, N.; Tian, P.; Ning, G. Controllable synthesis of transition metal ion-doped CeO2 micro/nanostructures for improving photocatalytic performance. J. Alloys Compd. 2019, 782, 780–788. [Google Scholar] [CrossRef]
- Ma, R.; Zhang, S.; Wen, T.; Gu, P.; Li, L.; Zhao, G.; Niu, F.; Huang, Q.; Tang, Z.; Wang, X. A critical review on visible-light-response CeO2-based photocatalysts with enhanced photooxidation of organic pollutants. Catal. Today 2019, 335, 20–30. [Google Scholar] [CrossRef]
- Gnanam, S.; Rajendran, V. Facile sol-gel preparation of Cd-doped cerium oxide (CeO2) nanoparticles and their photocatalytic activities. J. Alloys Compd. 2018, 735, 1854–1862. [Google Scholar] [CrossRef]
- Podasca, V.-E.; Damaceanu, M.-D. Photopolymerized films with ZnO and doped ZnO particles used as efficient photocatalysts in malachite green dye decomposition. Appl. Sci. 2020, 10, 1954. [Google Scholar] [CrossRef] [Green Version]
- Dong, B.; Li, L.; Dong, Z.; Xu, R.; Wu, Y. Fabrication of CeO2 nanorods for enhanced solar photocatalysts. Int. J. Hydrogen Energy 2018, 43, 5275–5282. [Google Scholar] [CrossRef]
- Li, S.; Wang, N.; Yue, Y.; Wang, G.; Zu, Z.; Zhang, Y. Copper doped ceria porous nanostructures towards a highly efficient bifunctional catalyst for carbon monoxide and nitric oxide elimination. Chem. Sci. 2015, 6, 2495–2500. [Google Scholar] [CrossRef] [Green Version]
- Akbari, A.; Khammar, M.; Taherzadeh, D.; Rajabian, A.; Khorsand Zak, A.; Darroudi, M. Zinc-doped cerium oxide nanoparticles: Sol-gel synthesis, characterization, and investigation of their in vitro cytotoxicity effects. J. Mol. Struct. 2017, 1149, 771–776. [Google Scholar] [CrossRef]
- Suresh, R.; Ponnuswamy, V.; Mariappan, R. Effect of annealing temperature on the microstructural, optical and electrical properties of CeO2 nanoparticles by chemical precipitation method. Appl. Surf. Sci. 2013, 273, 457–464. [Google Scholar] [CrossRef]
- Ortega, P.P.; Rocha, L.S.R.; Cortés, J.A.; Ramirez, M.A.; Buono, C.; Ponce, M.A.; Simões, A.Z. Towards carbon monoxide sensors based on europium doped cerium dioxide. Appl. Surf. Sci. 2019, 464, 692–699. [Google Scholar] [CrossRef]
- Bell, A.T. The impact of nanoscience on heterogeneous catalysis. Science 2003, 299, 1688–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, M.; Wang, W.; Sun, S.; Zhou, L.; Zhang, L. Bi2WO6 nanocrystals with high photocatalytic activities under visible light. J. Phys. Chem. C 2008, 112, 10407–10411. [Google Scholar] [CrossRef]
- Ranjith, K.S.; Saravanan, P.; Chen, S.H.; Dong, C.L.; Chen, C.L.; Chen, S.Y.; Asokan, K.; Kumar, R.T.R. Enhanced room-temperature ferromagnetism on Co-doped CeO2 nanoparticles: Mechanism, electronic and optical properties. J. Phys. Chem. C 2014, 118, 27039–27047. [Google Scholar] [CrossRef]
- Hancock, M.L.; Yokel, R.A.; Beck, M.J.; Calahan, J.L.; Jarrells, T.W.; Munson, E.J.; Olaniyan, G.A.; Grulke, E.A. The characterization of purified citrate-coated cerium oxide nanoparticles prepared via hydrothermal synthesis. Appl. Surf. Sci. 2021, 535, 147681. [Google Scholar] [CrossRef]
- Melinte, V.; Chibac-Scutaru, A.L.; Culica, M.E.; Coseri, S. Mineralization versus photoreduction of 4-nitrophenol under the influence of surface functionalized CeO2 nanoparticles, hosted by versatile cellulose supports. Appl. Surf. Sci. 2021, 565, 150494. [Google Scholar] [CrossRef]
- Choudhury, B.; Chetri, P.; Choudhury, A. Oxygen defects and formation of Ce3+ affecting the photocatalytic performance of CeO2 nanoparticles. RSC Adv. 2014, 4, 4663–4671. [Google Scholar] [CrossRef]
- Soni, S.; Vats, V.S.; Kumar, S.; Dalela, B.; Mishra, M.; Meena, R.S.; Gupta, G.; Alvi, P.A.; Dalela, S. Structural, optical and magnetic properties of Fe-doped CeO2 samples probed using X-ray photoelectron spectroscopy. J. Mater. Sci. Mater. Electron. 2018, 29, 10141–10153. [Google Scholar] [CrossRef]
- Goharshadi, E.K.; Samiee, S.; Nancarrow, P. Fabrication of cerium oxide nanoparticles: Characterization and optical properties. J. Colloid Interface Sci. 2011, 356, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.E.; Khan, M.M.; Cho, M.H. Ce3+-ion, surface oxygen vacancy, and visible light-induced photocatalytic dye degradation and photocapacitive performance of CeO2-graphene nanostructures. Sci. Rep. 2017, 7, 5928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Meng, F.; Li, K.; Lu, F. Characterization and optical properties of pole-like nano-CeO2 synthesized by a facile hydrothermal method. Appl. Surf. Sci. 2013, 286, 269–274. [Google Scholar] [CrossRef]
- Kubelka, P. New contributions to the optics of intensely light-scattering materials. Part II: Nonhomogeneous layers. J. Opt. Soc. Am. 1954, 44, 330–335. [Google Scholar] [CrossRef]
- Lu, X.; Zheng, D.; Zhang, P.; Liang, C.; Liu, P.; Tong, Y. Facile synthesis of free-standing CeO2 nanorods for photoelectrochemical applications. Chem. Commun. 2010, 46, 7721–7723. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Ce3+ and oxygen vacancy mediated tuning of structural and optical properties of CeO2 nanoparticles. Mater. Chem. Phys. 2012, 131, 666–671. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Kalathil, S.; Lee, J.; Cho, M.H. Band gap engineering of CeO2 nanostructure by electrochemically active biofilm for visible light applications. RSC Adv. 2014, 4, 16782–16791. [Google Scholar] [CrossRef]
- Pastor-Pérez, L.; Ramos-Fernández, E.V.; Sepúlveda-Escribano, A. Effect of the CeO2 synthesis method on the behaviour of Pt/CeO2 catalysis for the water-gas shift reaction. Int. J. Hydrogen Energy 2019, 44, 21837–21846. [Google Scholar] [CrossRef]
- Lykaki, M.; Stefa, S.; Carabineiro, S.A.C.; Soria, M.A.; Madeira, L.M.; Konsolakis, M. Shape effects of ceria nanoparticles on the water-gas shift performance of CuOx/CeO2 catalysts. Catalysts 2021, 11, 753. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV-Vis spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [Green Version]
- Yue, L.; Zhang, X.M. Structural characterization and photocatalytic behaviors of doped CeO2 nanoparticles. J. Alloys Compd. 2009, 475, 702–705. [Google Scholar] [CrossRef]
- Saranya, J.; Ranjith, K.S.; Saravanan, P.; Mangalaraj, D.; Rajendra Kumar, R.T. Cobalt-doped cerium oxide nanoparticles: Enhanced photocatalytic activity under UV and visible light irradiation. Mater. Sci. Semicond. Process. 2014, 26, 218–224. [Google Scholar] [CrossRef]
- Zimou, J.; Nouneh, K.; Hsissou, R.; El-habib, A.; El Gana, L.; Talbi, A.; Beraich, M.; Lotfi, N.; Addou, M. Structural, morphological, optical, and electrochemical properties of Co-doped CeO2 thin films. Mater. Sci. Semicond. Process. 2021, 135, 106049. [Google Scholar] [CrossRef]
- Wang, Y.; Ge, H.X.; Chen, Y.P.; Meng, X.Y.; Ghanbaja, J.; Horwat, D.; Pierson, J.F. Wurtzite CoO: A direct band gap oxide suitable for a photovoltaic absorber. Chem. Commun. 2018, 54, 13949–13952. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, M.M.; Kalathil, S.; Nisar, A.; Lee, J.; Cho, M.H. Oxygen vacancy induced band gap narrowing of ZnO nanostructures by an electrochemically active biofilm. Nanoscale 2013, 5, 9238–9246. [Google Scholar] [CrossRef]
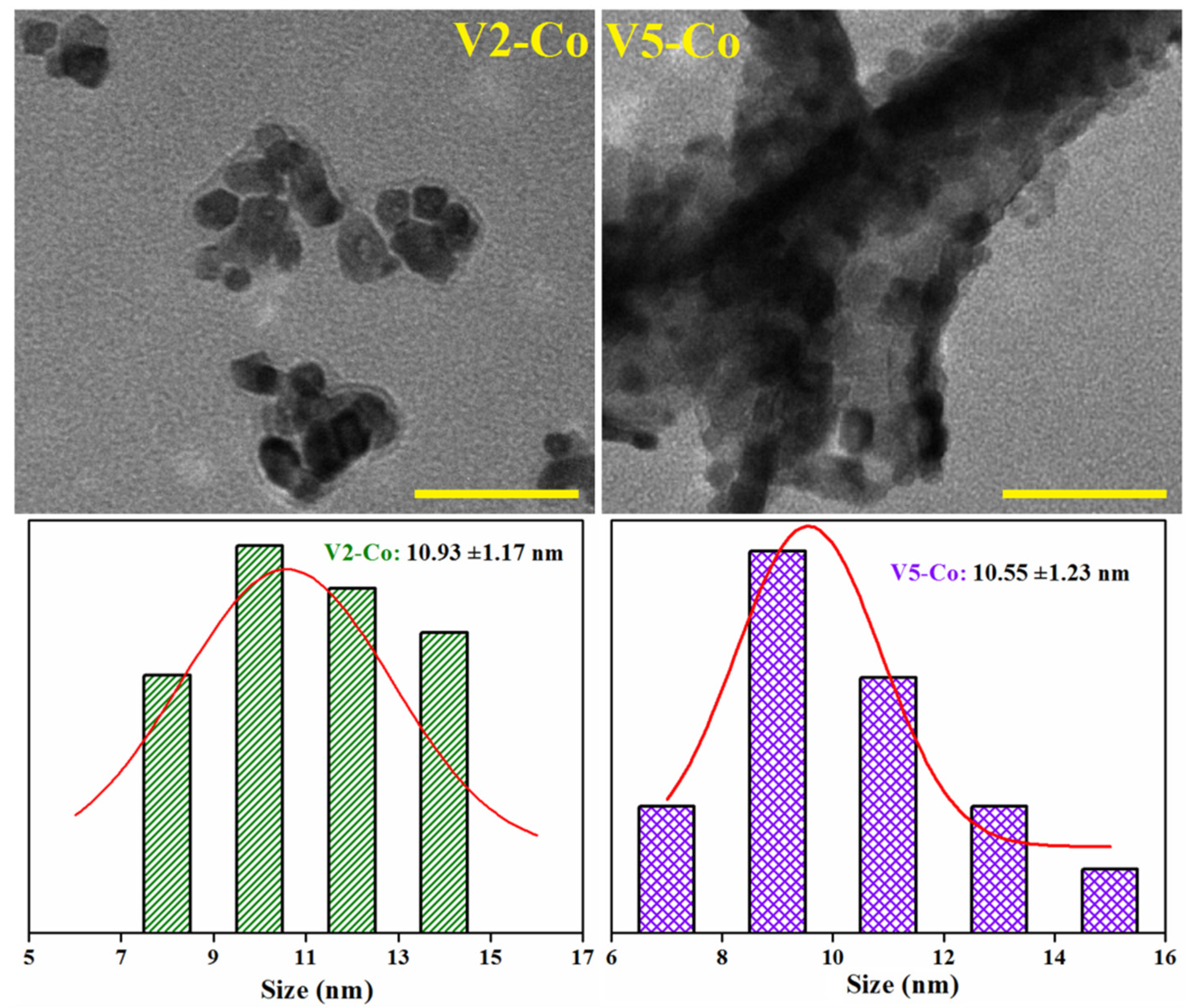
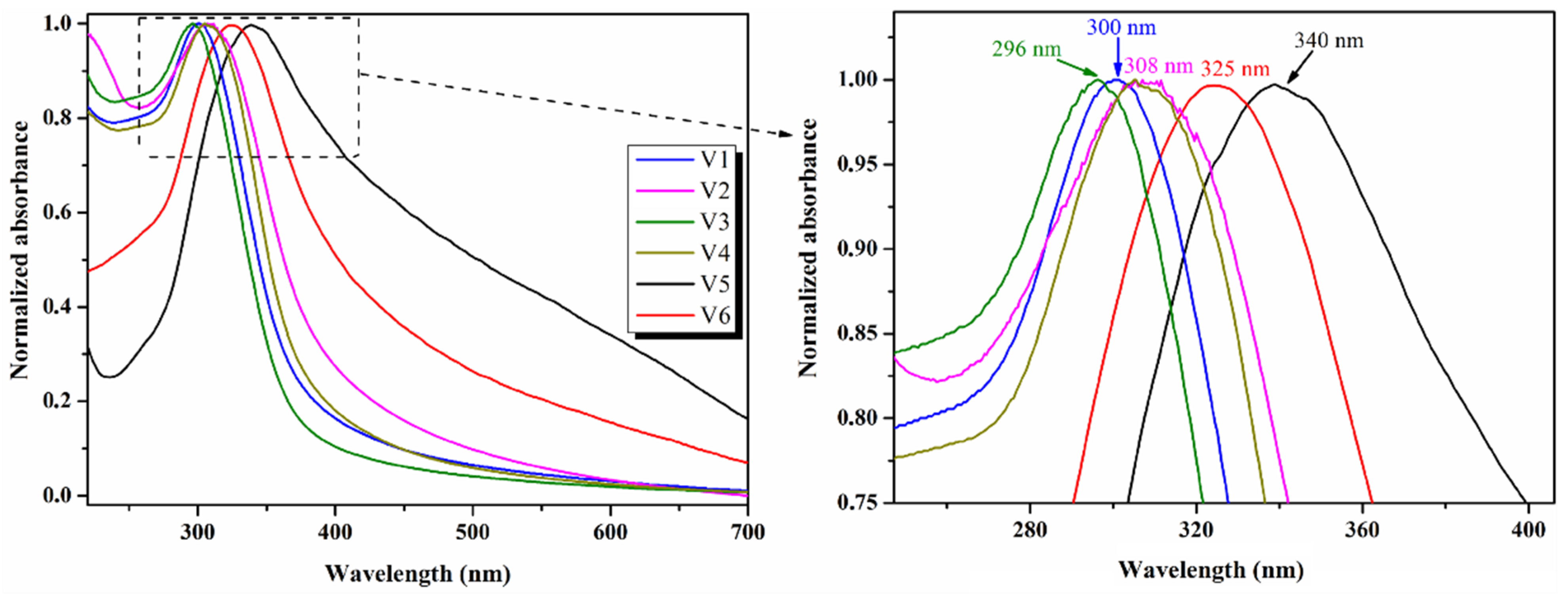
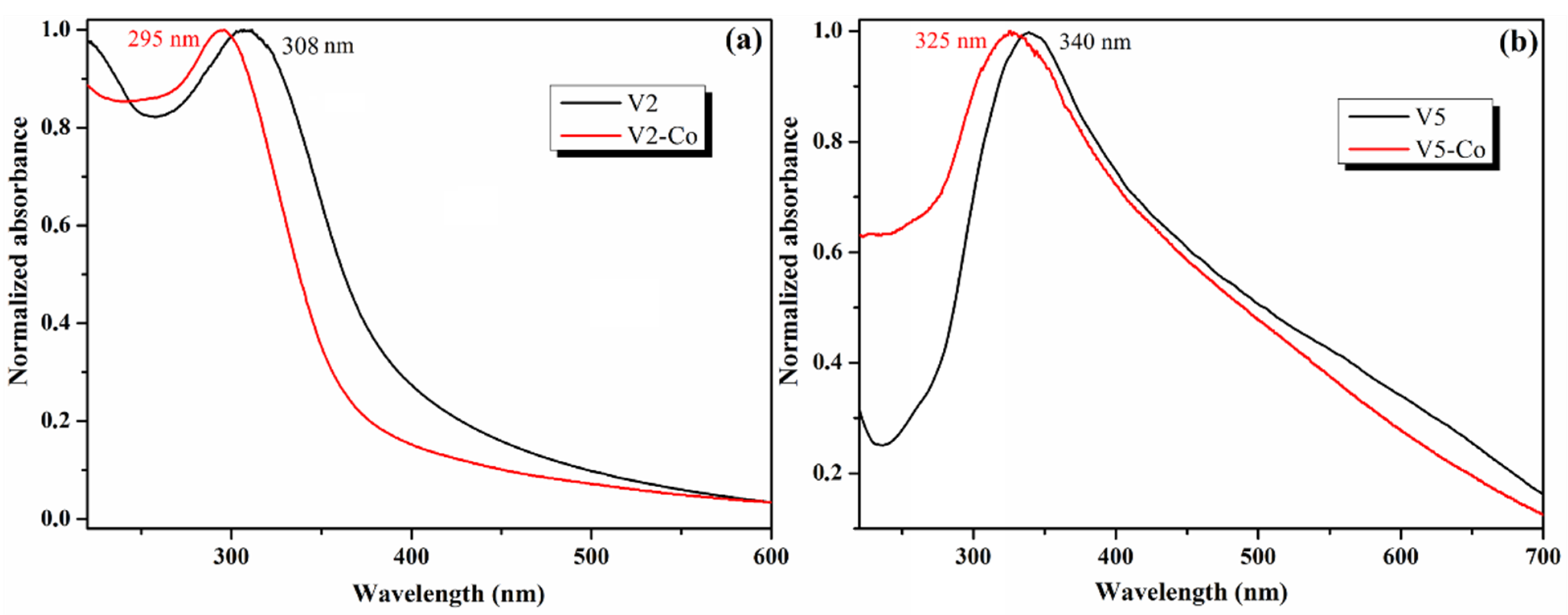
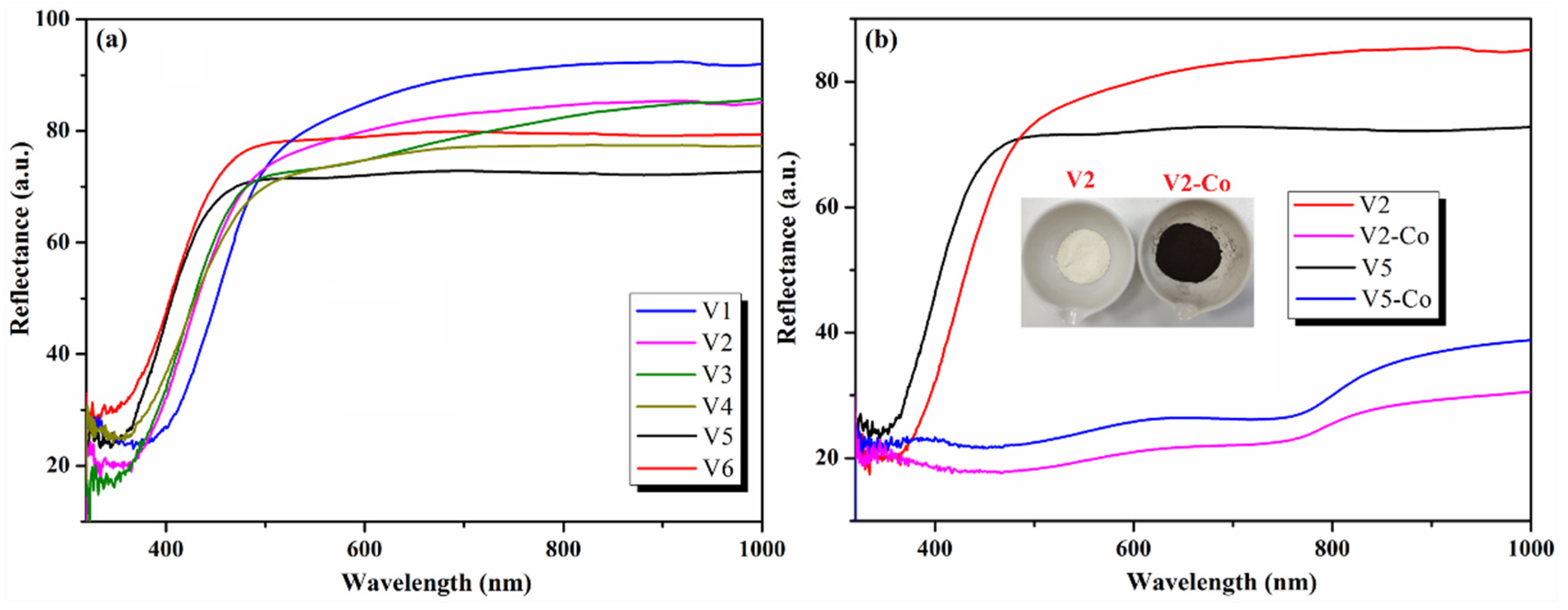
| Sample | Precursor | Co-Reagent | pH | Time (h) | |
|---|---|---|---|---|---|
| 1 | V1 | Ce(NO3)3·6H2O | NH4OH | 8 | 3 |
| 2 | V2 | Ce(NO3)3·6H2O | NH4OH | 10 | 3 |
| 3 | V3 | Ce(NO3)3·6H2O | NH4OH | 10 | 72 |
| 4 | V4 | Ce(NO3)3·6H2O | NaOH | 12 | 3 |
| 5 | V5 | Ce(NO3)3·6H2O | terephthalic acid | 4 | 3 |
| 6 | V6 | Ce(NO3)3·6H2O | terephthalic acid | 4 | 72 |
| 7 | V2-Co | Ce(NO3)3·6H2O/Co(NO3)2·6H2O | NH4OH | 10 | 3 |
| 8 | V5-Co | Ce(NO3)3·6H2O/Co(NO3)2·6H2O | terephthalic acid | 4 | 3 |
| Sample | V1 | V2 | V3 | V4 | V5 | V6 | V2-Co | V5-Co |
|---|---|---|---|---|---|---|---|---|
| dXRD (nm) | 12.70 | 11.24 | 11.55 | 21.65 | 19.21 | 17.64 | 11.60 | 12.60 |
| dTEM (nm) | 14.77 | 12.50 | 12.29 | 19.57 | 16.77 | 17.57 | 10.93 | 10.55 |
| λabs (nm) | 300 | 308 | 296 | 308 | 340 | 325 | 295 | 325 |
| Eg (eV) | 2.85 | 2.98 | 3.02 | 3.02 | 3.18 | 3.14 | 2.02 | 2.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chibac-Scutaru, A.L.; Podasca, V.; Dascalu, I.A.; Melinte, V. Exploring the Influence of Synthesis Parameters on the Optical Properties for Various CeO2 NPs. Nanomaterials 2022, 12, 1402. https://doi.org/10.3390/nano12091402
Chibac-Scutaru AL, Podasca V, Dascalu IA, Melinte V. Exploring the Influence of Synthesis Parameters on the Optical Properties for Various CeO2 NPs. Nanomaterials. 2022; 12(9):1402. https://doi.org/10.3390/nano12091402
Chicago/Turabian StyleChibac-Scutaru, Andreea L., Viorica Podasca, Ioan A. Dascalu, and Violeta Melinte. 2022. "Exploring the Influence of Synthesis Parameters on the Optical Properties for Various CeO2 NPs" Nanomaterials 12, no. 9: 1402. https://doi.org/10.3390/nano12091402
APA StyleChibac-Scutaru, A. L., Podasca, V., Dascalu, I. A., & Melinte, V. (2022). Exploring the Influence of Synthesis Parameters on the Optical Properties for Various CeO2 NPs. Nanomaterials, 12(9), 1402. https://doi.org/10.3390/nano12091402








