The Nanostructure of Polymer-Active Principle Microparticles Produced by Supercritical CO2 Assisted Processing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microparticles Preparation Procedure and SAA Plant Description
2.2. Characterization Methods of the Microparticles
3. Results and Discussion
3.1. Dextran-Fe3O4 Composite Microparticles: Model System
3.2. PVP-Curcumin Microparticles
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Grigorov, P.I.; Glasser, B.J.; Muzzio, F.J. Improving dissolution kinetics of pharmaceuticals by fluidized bed impregnation of active pharmaceutical ingredients. AIChE J. 2016, 62, 4201–4214. [Google Scholar] [CrossRef]
- Zheng, K.; Lin, Z.; Capece, M.; Kunnath, K.; Chen, L.; Davé, R.N. Effect of particle size and polymer loading on dissolution behavior of amorphous griseofulvin powder. J. Pharm. Sci. 2019, 108, 234–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rascioni, R.; Censi, R.; Malaj, L.; Martino, P. Effect of particle size reduction and crystalline form on dissolution behaviour of nimesulide. J. Therm. Anal. Calorim. 2016, 123, 2213–2223. [Google Scholar] [CrossRef]
- Yan, A.; Xue, Y.X.C.; Cui, Z.; Zhang, Z.; Wang, S. Size-dependent dissolution kinetics of CaCO nanoparticles in theory and experiment. J. Mater. Sci. 2017, 52, 4412–4420. [Google Scholar] [CrossRef]
- Šimková, K.; Joost, B.; Imanidis, G. Production of fast-dissolving low-density powders for improved lung deposition by spray drying of a nanosuspension. Eur. J. Pharm. Biopharm. 2020, 146, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Hodnett, B.K.; Hudson, S.; Davern, P. Modification of the zeta potential of montmorillonite to achieve high active pharmaceutical ingredient nanoparticle loading and stabilization with optimum dissolution properties. Colloids Surf. B 2020, 193, 111120. [Google Scholar] [CrossRef] [PubMed]
- Carlan, I.C.; Estevinho, B.N.; Rocha, F. Production of vitamin B1 microparticles by a spray drying process using different biopolymers as wall materials. Can. J. Chem. Eng. 2020, 98, 1682–1695. [Google Scholar] [CrossRef]
- Pérez-Landa, I.D.; Bonilla-Landa, I.; Monribot-Villanueva, J.L.; Ramírez-Vázquez, M.; Lasa, R.; Ramos-Torres, W.; Olivares-Romero, J.L.; Barrera-Méndez, F. Photoprotection and release study of spinosad biopolymeric microparticles obtained by spray drying. Powder Technol. 2021, 377, 514–522. [Google Scholar] [CrossRef]
- Tavares, L.; Barbosa Barros, H.L.; Pacheco Vaghetti, J.C.; Zapata Noreña, C.P. Microencapsulation of garlic extract by complex coacervation using whey protein isolate/chitosan and gum arabic/chitosan as wall materials: Influence of anionic biopolymers on the physicochemical and structural properties of microparticles. Food Bioprocess Technol. 2019, 12, 2093–2106. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, H. Alginate/pectin aerogel microspheres for controlled release of proanthocyanidins. Int. J. Biol. Macromol. 2019, 136, 936–943. [Google Scholar] [CrossRef]
- Schroeder da Silva, T.; Kasper Silva, D.A.; Nogueira, A.L. Metformin hydrochloride sustained release biopolymeric system composed by PLLA-CMC microparticles. J. Appl. Polym. Sci. 2021, 138, 50806. [Google Scholar] [CrossRef]
- Han, F.Y.; Thurecht, K.J.; Whittaker, A.K.; Smith, M.T. Bioerodable PLGA-based microparticles for producing sustained-release drug formulations and strategies for improving drug loading. Front. Pharmacol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosnea, L.A.; Moschakis, T.; Nigam, P.S.; Biliaderis, C.G. Growth adaptation of probiotics in biopolymer-based coacervate structures to enhance cell viability. LWT 2017, 77, 282–289. [Google Scholar] [CrossRef]
- Rios-Mera, J.D.; Saldaña, E.; Ramírez, Y.; Auquiñivín, E.A.; Alvim, I.D.; Contreras-Castillo, C.J. Encapsulation optimization and pH- and temperature-stability of the complex coacervation between soy protein isolate and inulin entrapping fish oil. LWT 2019, 116, 108555. [Google Scholar] [CrossRef]
- Córdova Aguilar, K.; Tello, F.; Bierhalz, A.C.K.; Garnica Romo, M.G.; Martínez Flores, H.E.; Grosso, C.R.F. Protein adsorption onto alginate-pectin microparticles and films produced by ionic gelation. J. Food Eng. 2015, 154, 17–24. [Google Scholar] [CrossRef]
- Jurić, S.; Jurić, M.; Režek Jambrak, A.; Vinceković, M. Tailoring alginate/chitosan microparticles loaded with chemical and biological agents for agricultural application and production of value-added foods. Appl. Sci. 2021, 11, 4061. [Google Scholar] [CrossRef]
- Zhao, T.; Qiu, D. One-pot synthesis of highly folded microparticles by suspension polymerization. Langmuir 2011, 27, 12771–12774. [Google Scholar] [CrossRef]
- Stanisz, M.; Klapiszewski, Ł.; Jesionowski, T. Recent advances in the fabrication and application of biopolymer-based micro- and nanostructures: A comprehensive review. Chem. Eng. J. 2020, 397, 125409. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Veloso, C.M. Microencapsulation of natural dyes with biopolymers for application in food: A review. Food Hydrocolloids 2021, 112, 106374. [Google Scholar] [CrossRef]
- Cortés-Morales, E.A.; Mendez-Montealvo, G.; Velazquez, G. Interactions of the molecular assembly of polysaccharide-protein systems as encapsulation materials. A review. Adv. Colloid Interface Sci. 2021, 295, 102398. [Google Scholar] [CrossRef]
- Aquino, R.P.; Auriemma, G.; Mencherini, T.; Russo, P.; Porta, A.; Adami, R.; Liparoti, S.; Della Porta, G.; Reverchon, E.; Del Gaudio, P. Design and production of gentamicin/dextrans microparticles by supercritical assisted atomisation for the treatment of wound bacterial infections. Int. J. Pharm. 2013, 440, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Adami, R.; Reverchon, E. Composite polymer-Fe3O4 microparticles for biomedical applications, produced by Supercritical Assisted Atomization. Powder Technol. 2012, 218, 102–108. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I.; Della Porta, G. Tailoring of nano- and micro-particles of some superconductor precursors by supercritical antisolvent precipitation. J. Supercrit. Fluids 2002, 23, 81–87. [Google Scholar] [CrossRef]
- Prosapio, V.; De Marco, I.; Scognamiglio, M.; Reverchon, E. Folic acid–PVP nanostructured composite microparticles by supercritical antisolvent precipitation. Chem. Eng. J. 2015, 277, 286–294. [Google Scholar] [CrossRef]
- Franco, P.; Reverchon, E.; De Marco, I. Zein/diclofenac sodium coprecipitation at micrometric and nanometric range by supercritical antisolvent processing. J. CO2 Utiliz. 2018, 27, 366–373. [Google Scholar] [CrossRef]
- Della Porta, G.; Reverchon, E. Nanostructured microspheres produced by supercritical fluid extraction of emulsions. Biotechnol. Bioeng. 2008, 100, 1020–1033. [Google Scholar] [CrossRef]
- Gimenez-Rota, C.; Palazzo, I.; Scognamiglio, M.; Mainar, A.; Reverchon, E.; Della Porta, G. β-Carotene, α-tocoferol and rosmarinic acid encapsulated within PLA/PLGA microcarriers by supercritical emulsion extraction: Encapsulation efficiency, drugs shelf-life and antioxidant activity. J. Supercrit. Fluids 2019, 146, 199–207. [Google Scholar] [CrossRef]
- Guastaferro, M.; Baldino, L.; Cardea, S.; Reverchon, E. Supercritical assisted electrospray/spinning to produce PVP+quercetin microparticles and microfibers. J. Taiwan Inst. Chem. Eng. 2020, 117, 278–286. [Google Scholar] [CrossRef]
- Guastaferro, M.; Cardea, S.; Baldino, L.; Reverchon, E. Cellulose acetate nanocarrier production by supercritical assisted electrospray. Chem. Eng. Trans. 2021, 87, 391–396. [Google Scholar] [CrossRef]
- Cooper, A.I. Recent developments in materials synthesis and processing using supercritical CO2. Adv. Mater. 2001, 13, 1111–1114. [Google Scholar] [CrossRef]
- Nunes, A.V.M.; Duarte, C.M.M. Dense CO2 as a solute, co-solute or co-solvent in particle formation processes: A review. Materials 2011, 4, 2017–2041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, B.; Ryan, K.M.; Padrela, L. From batch to continuous—New opportunities for supercritical CO2 technology in pharmaceutical manufacturing. Eur. J. Pharm. Sci. 2019, 137, 104971. [Google Scholar] [CrossRef] [PubMed]
- Manjare, S.D.; Dhingra, K. Supercritical fluids in separation and purification: A review. Mater. Sci. Energy Technol. 2019, 2, 463–484. [Google Scholar] [CrossRef]
- Adami, R.; Di Capua, A.; Reverchon, E. Supercritical Assisted Atomization for the production of curcumin-biopolymer microspheres. Powder Technol. 2017, 305, 455–461. [Google Scholar] [CrossRef]
- Di Capua, A.; Adami, R.; Cosenza, E.; Jalaber, V.; Crampon, C.; Badens, E.; Reverchon, E. β-Carotene/PVP microspheres produced by Supercritical Assisted Atomization. Powder Technol. 2019, 346, 228–236. [Google Scholar] [CrossRef]
- Schwendner, J.F.; Konnerth, C.; Romeis, S.; Schmidt, J.; Peukert, W. Formation of drug-loaded nanoemulsions in stirred media mills. Adv. Powder Technol. 2019, 30, 1584–1591. [Google Scholar] [CrossRef]
- Li, W.; Chen, S.; Zhang, L.; Zhang, Y.; Yang, X.; Xie, B.; Guo, J.; He, Y.; Wang, C. Inhalable functional mixed-polymer microspheres to enhance doxorubicin release behavior for lung cancer treatment. Colloids Surf. B 2020, 196, 111350. [Google Scholar] [CrossRef]
- Weiss, A.-V.; Koch, M.; Schneider, M. Combining cryo-TEM and energy-filtered TEM for imaging organic core-shell nanoparticles and defining the polymer distribution. Int. J. Pharm. 2019, 570, 118650. [Google Scholar] [CrossRef]
- Prieto, C.; Evtoski, Z.; Pardo-Figuerez, M.; Hrakovsky, J.; Lagaron, J.M. Nanostructured valsartan microparticles with enhanced bioavailability produced by high-throughput electrohydrodynamic room-temperature atomization. Mol. Pharmaceutics 2021, 18, 2947–2958. [Google Scholar] [CrossRef]
- Falsafi, S.R.; Rostamabadi, H.; Assadpour, E.; Jafari, S.M. Morphology and microstructural analysis of bioactive-loaded micro/nanocarriers via microscopy techniques; CLSM/SEM/TEM/AFM. Adv. Colloid Interface Sci. 2020, 280, 102166. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, S.; Wang, W. A dynamic laser light-scattering study of chitosan in aqueous solution. Biopolymers 1995, 35, 385–392. [Google Scholar] [CrossRef]
- Lyutova, E.M.; Kasakov, A.S.; Gurvits, B.Y. Effects of arginine on kinetics of protein aggregation studied by dynamic laser light scattering and tubidimetry techniques. Biotechnol. Prog. 2007, 23, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Mutalik, S.; Suthar, N.A.; Managuli, R.S.; Shetty, P.K.; Avadhani, K.; Kalthur, G.; Kulkarni, R.V.; Thomas, R. Development and performance evaluation of novel nanoparticles of a grafted copolymer loaded with curcumin. Int. J. Biol. Macromol. 2016, 86, 709–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, G.H.; Li, J.; Cho, J.H.; Kim, J.T.; Park, H.J. Enhancement of curcumin solubility by phase change from crystalline to amorphous in Cur-TPGS nanosuspension. J. Food Sci. 2016, 81, 494–501. [Google Scholar] [CrossRef] [PubMed]
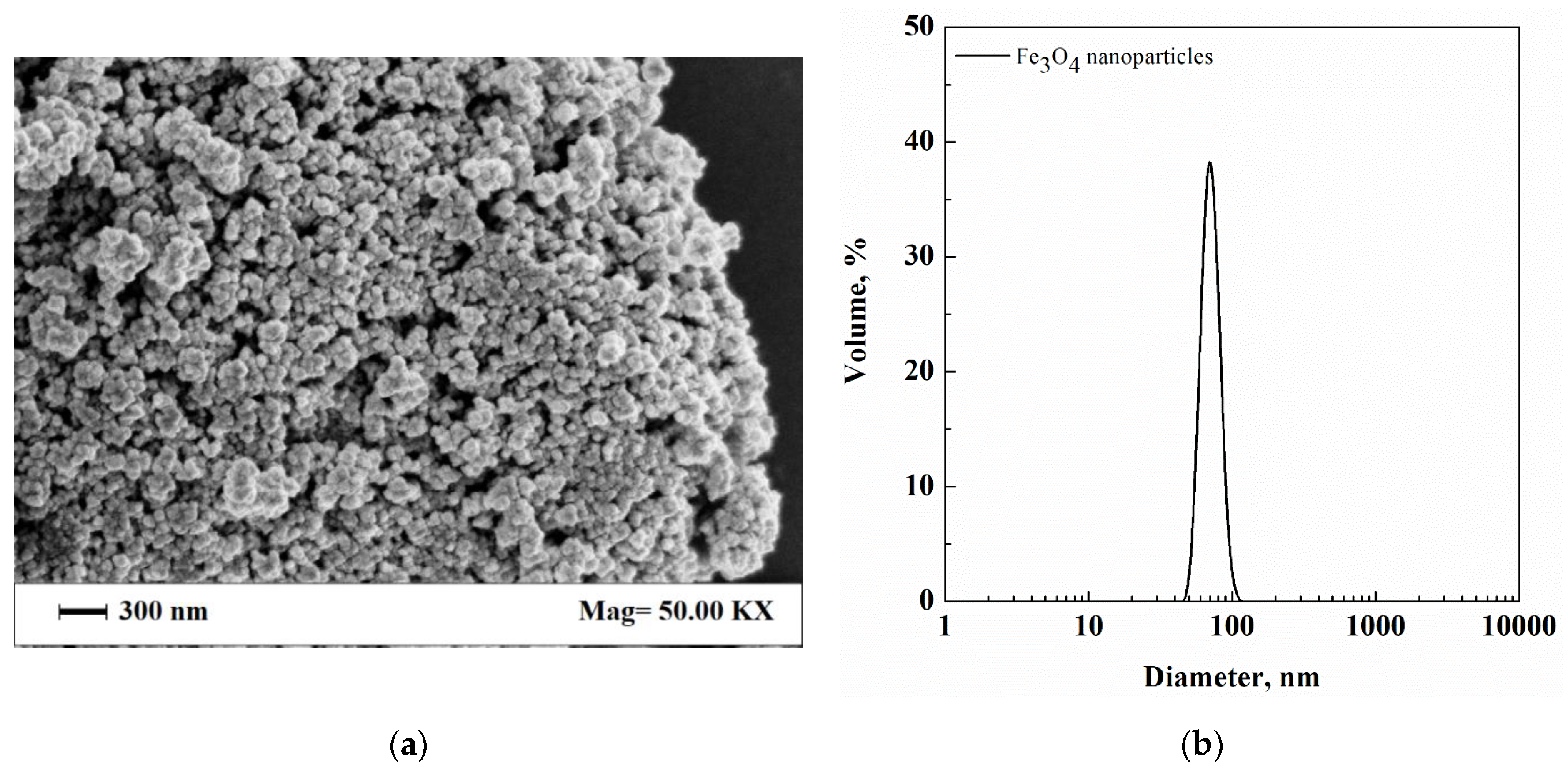
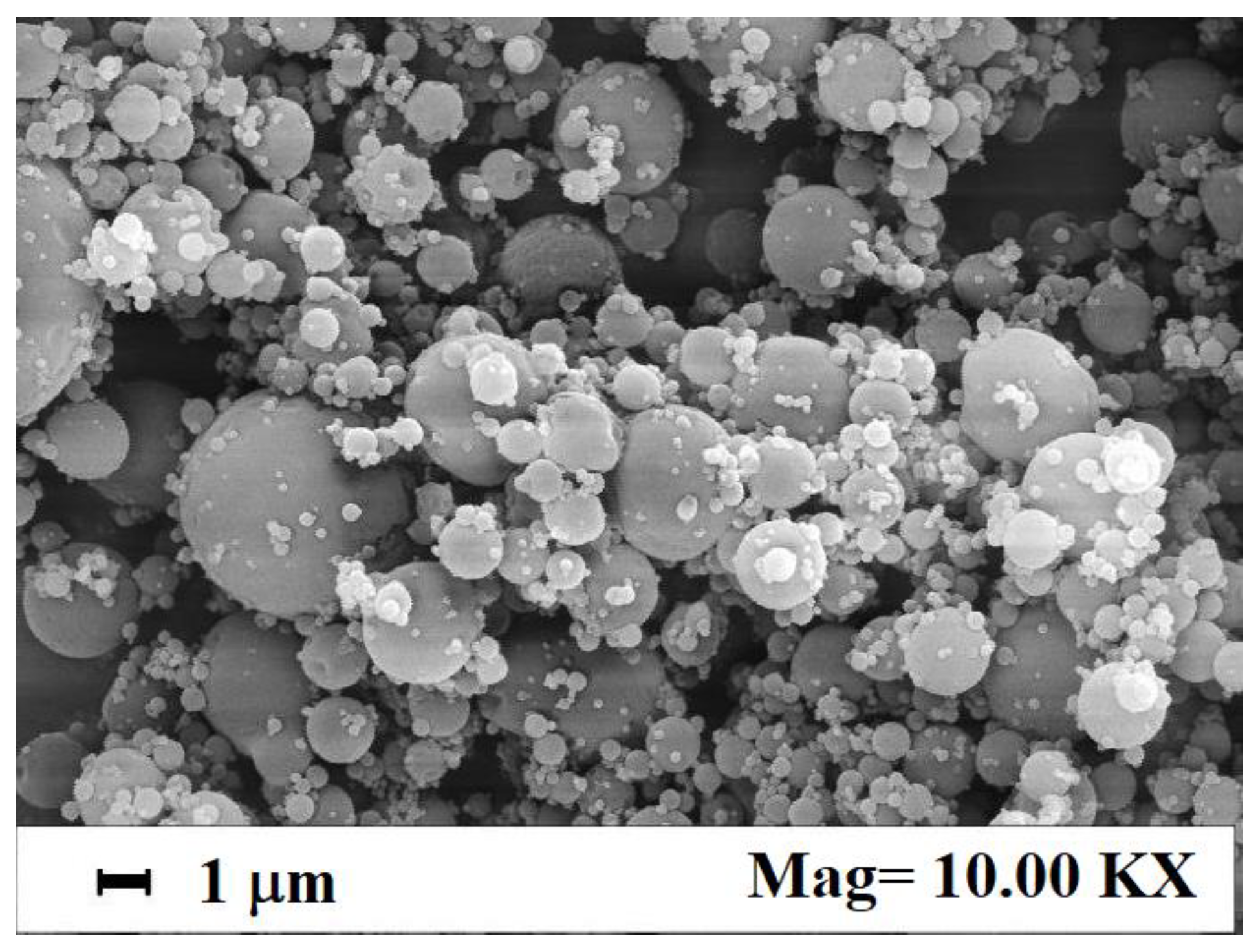
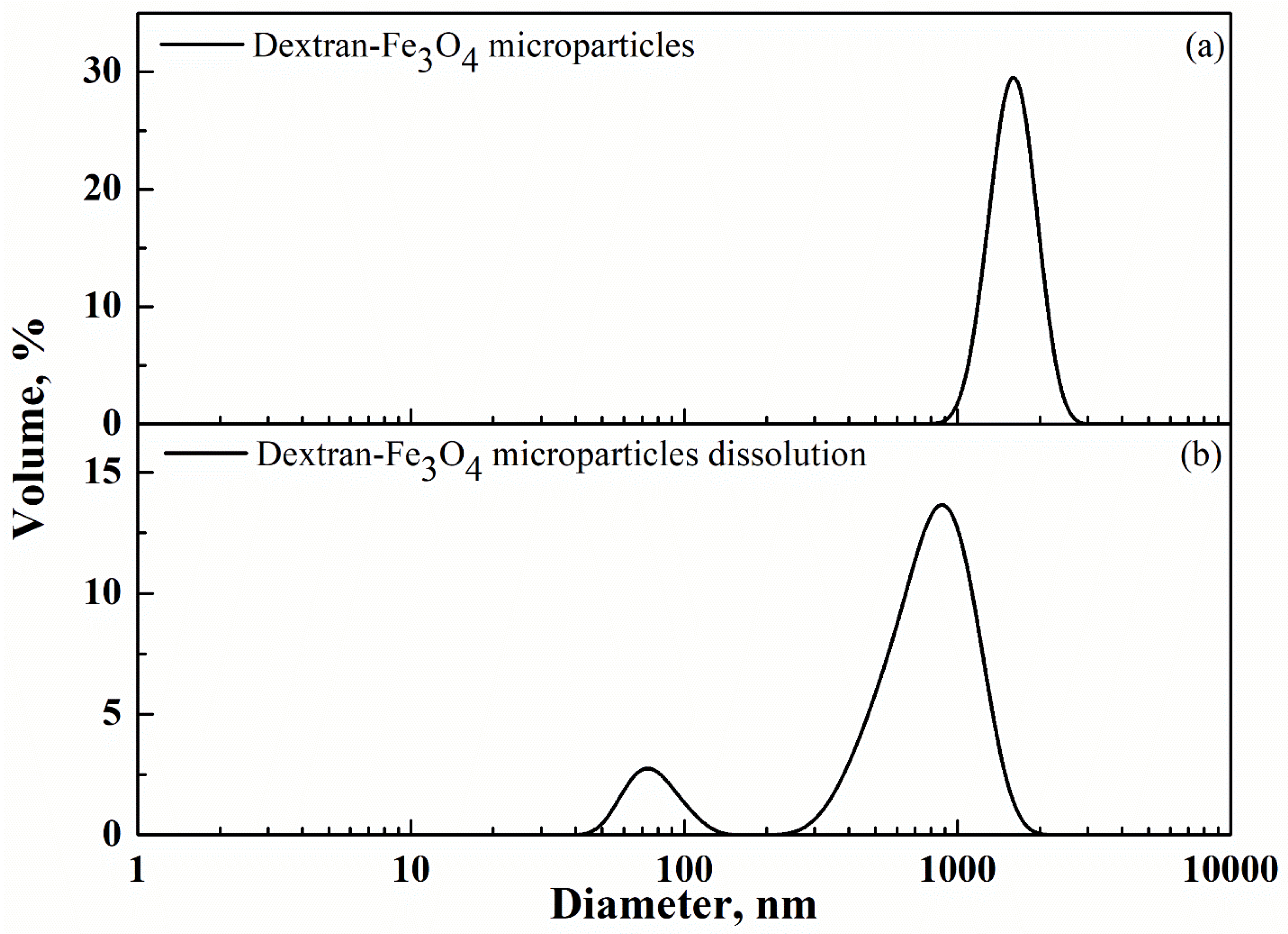


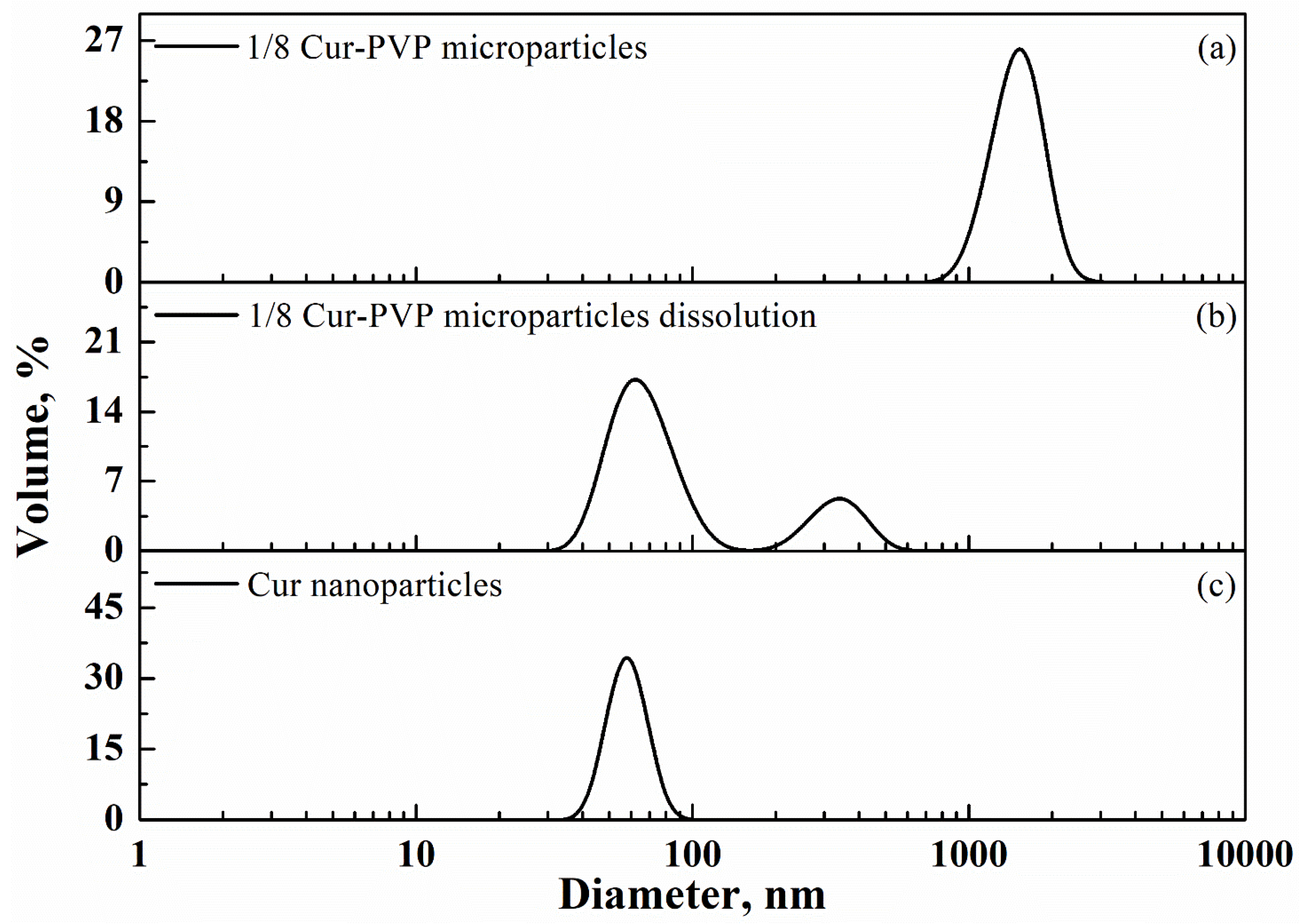

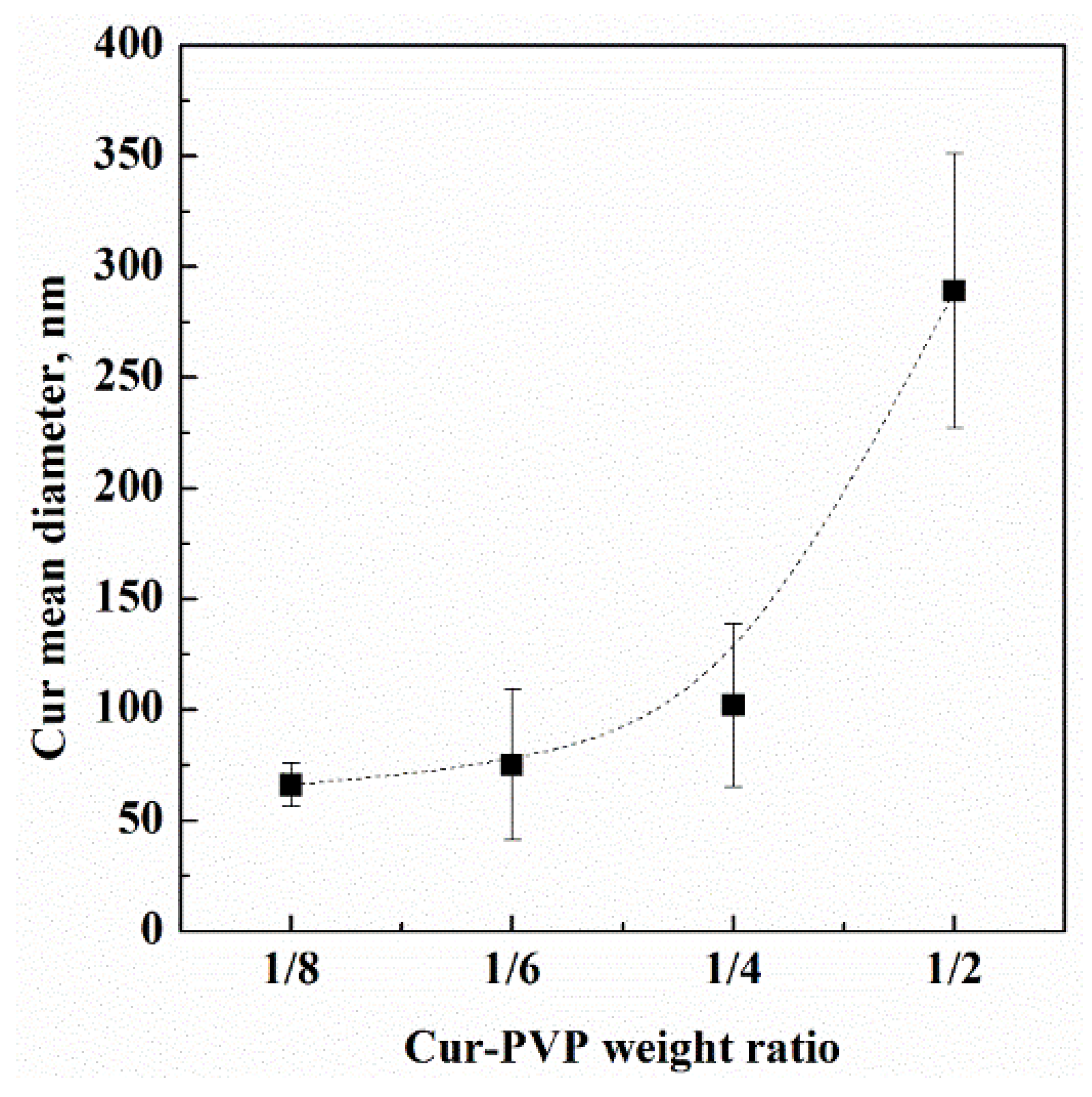
| Cur-PVP Ratio by Weight | Microparticles Mean Diameter ± Standard Deviation, nm | Cur Nanoparticles Mean Diameter ± Standard Deviation, nm |
|---|---|---|
| 1/2 | 2530 ± 440 | 289 ± 62 |
| 1/4 | 2180 ± 580 | 102 ± 37 |
| 1/6 | 1790 ± 270 | 75 ± 34 |
| 1/8 | 1520 ± 300 | 66 ± 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reverchon, E.; Scognamiglio, M.; Baldino, L. The Nanostructure of Polymer-Active Principle Microparticles Produced by Supercritical CO2 Assisted Processing. Nanomaterials 2022, 12, 1401. https://doi.org/10.3390/nano12091401
Reverchon E, Scognamiglio M, Baldino L. The Nanostructure of Polymer-Active Principle Microparticles Produced by Supercritical CO2 Assisted Processing. Nanomaterials. 2022; 12(9):1401. https://doi.org/10.3390/nano12091401
Chicago/Turabian StyleReverchon, Ernesto, Mariarosa Scognamiglio, and Lucia Baldino. 2022. "The Nanostructure of Polymer-Active Principle Microparticles Produced by Supercritical CO2 Assisted Processing" Nanomaterials 12, no. 9: 1401. https://doi.org/10.3390/nano12091401
APA StyleReverchon, E., Scognamiglio, M., & Baldino, L. (2022). The Nanostructure of Polymer-Active Principle Microparticles Produced by Supercritical CO2 Assisted Processing. Nanomaterials, 12(9), 1401. https://doi.org/10.3390/nano12091401








