Electron-Level Mechanistic Insights into Ce Doping for Enhanced Efficiency Degradation of Bisphenol A under Visible Light Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Synthesis of Ce-BiOBr nanoflakes
2.3. Physicochemical Characterization
2.4. Electrochemical Characterization
2.5. Photocatalytic Degradation of BPA
2.6. Theoretical Calculation
3. Results and Discussion
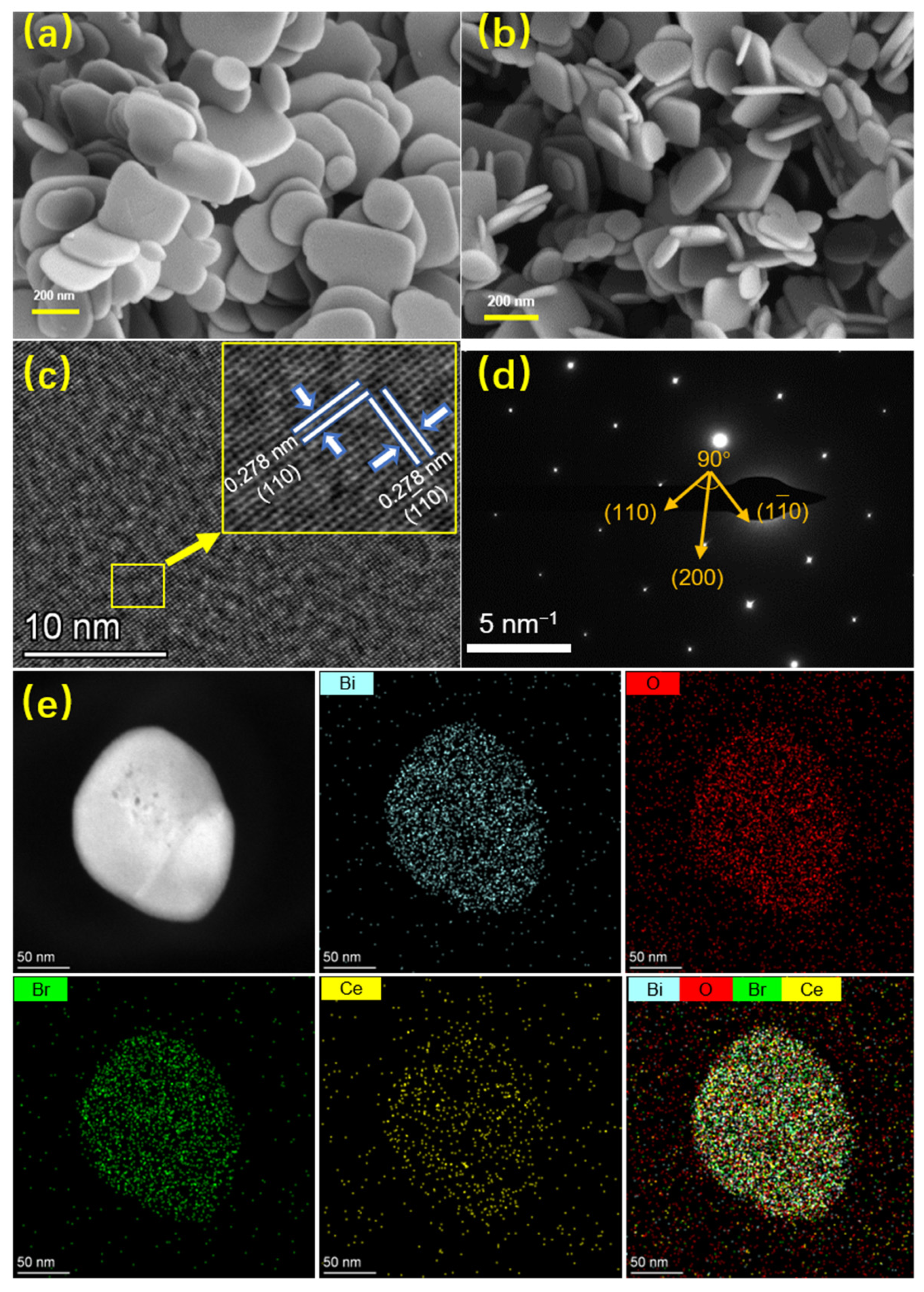
3.1. Structure and Morphology of the Catalysts
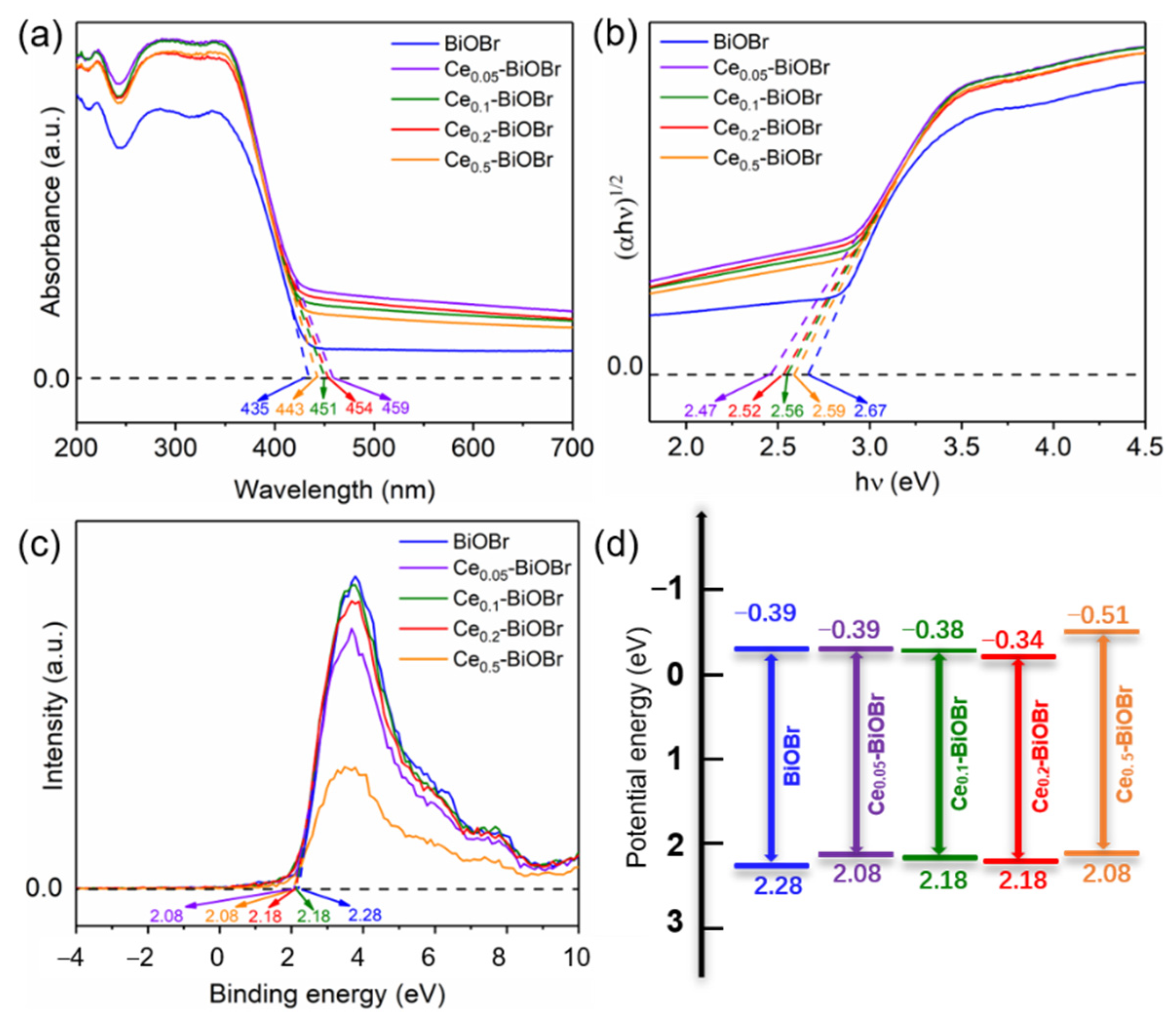
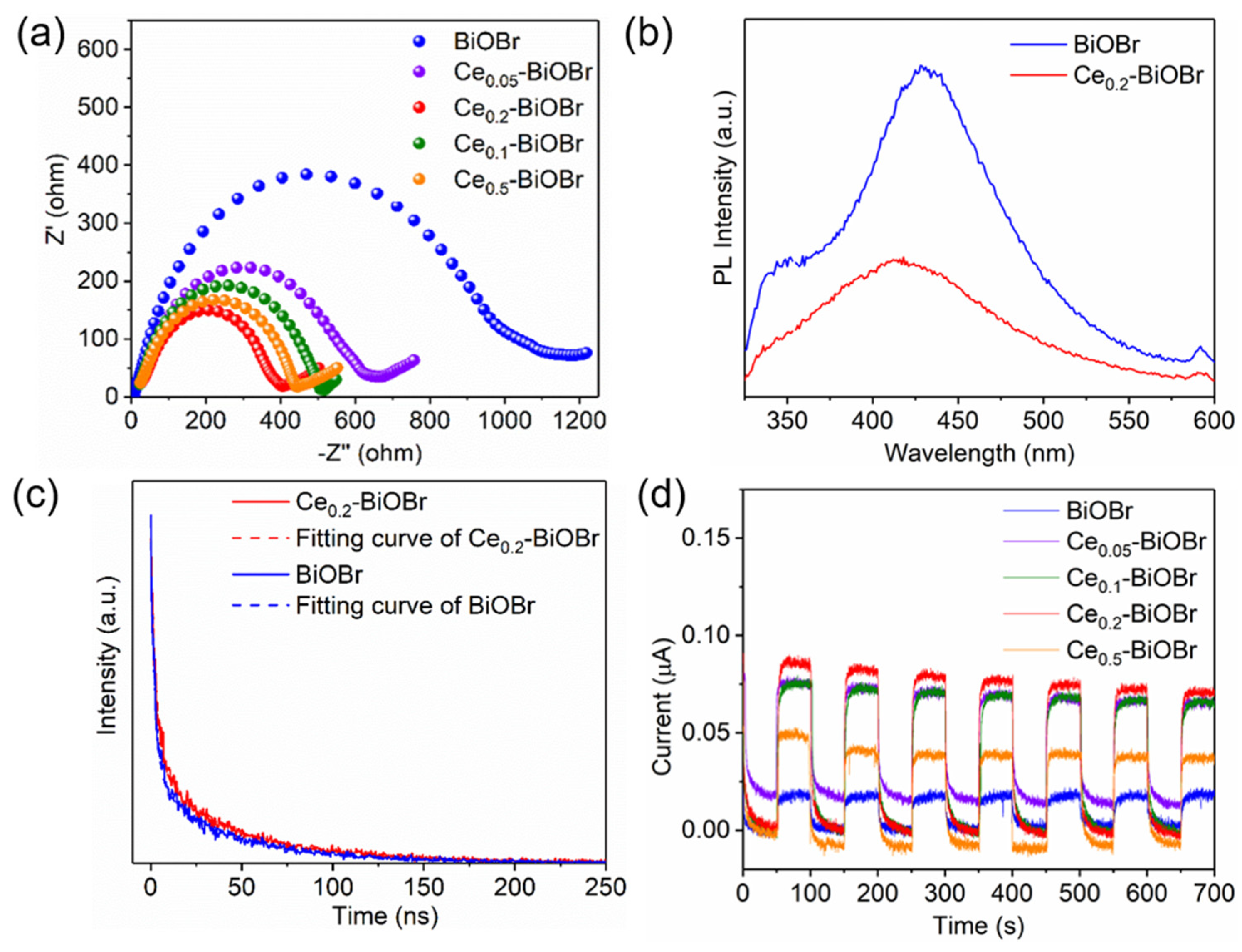
3.2. Band Structures and Photoelectrochemical Properties
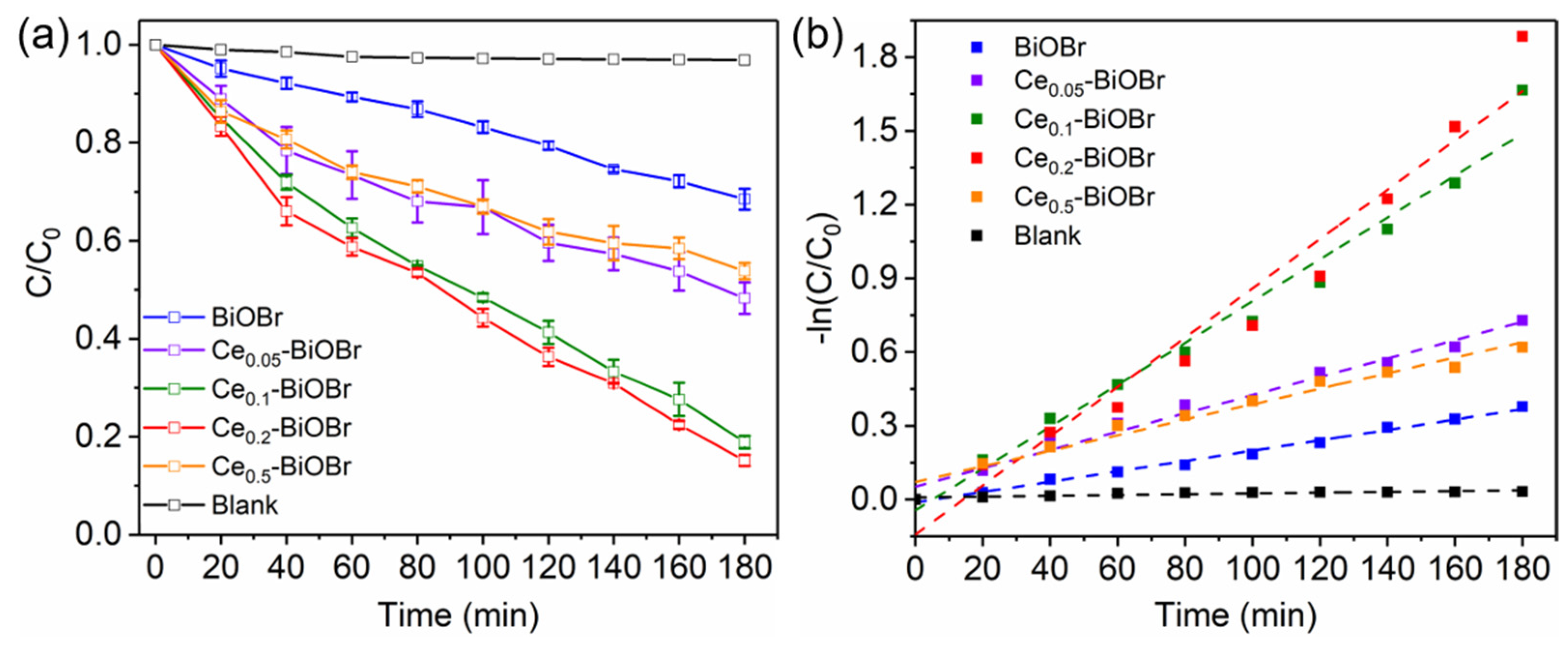
3.3. Photocatalytic Degradation of BPA
3.4. Mechanism of BPA Photocatalytic Degradation
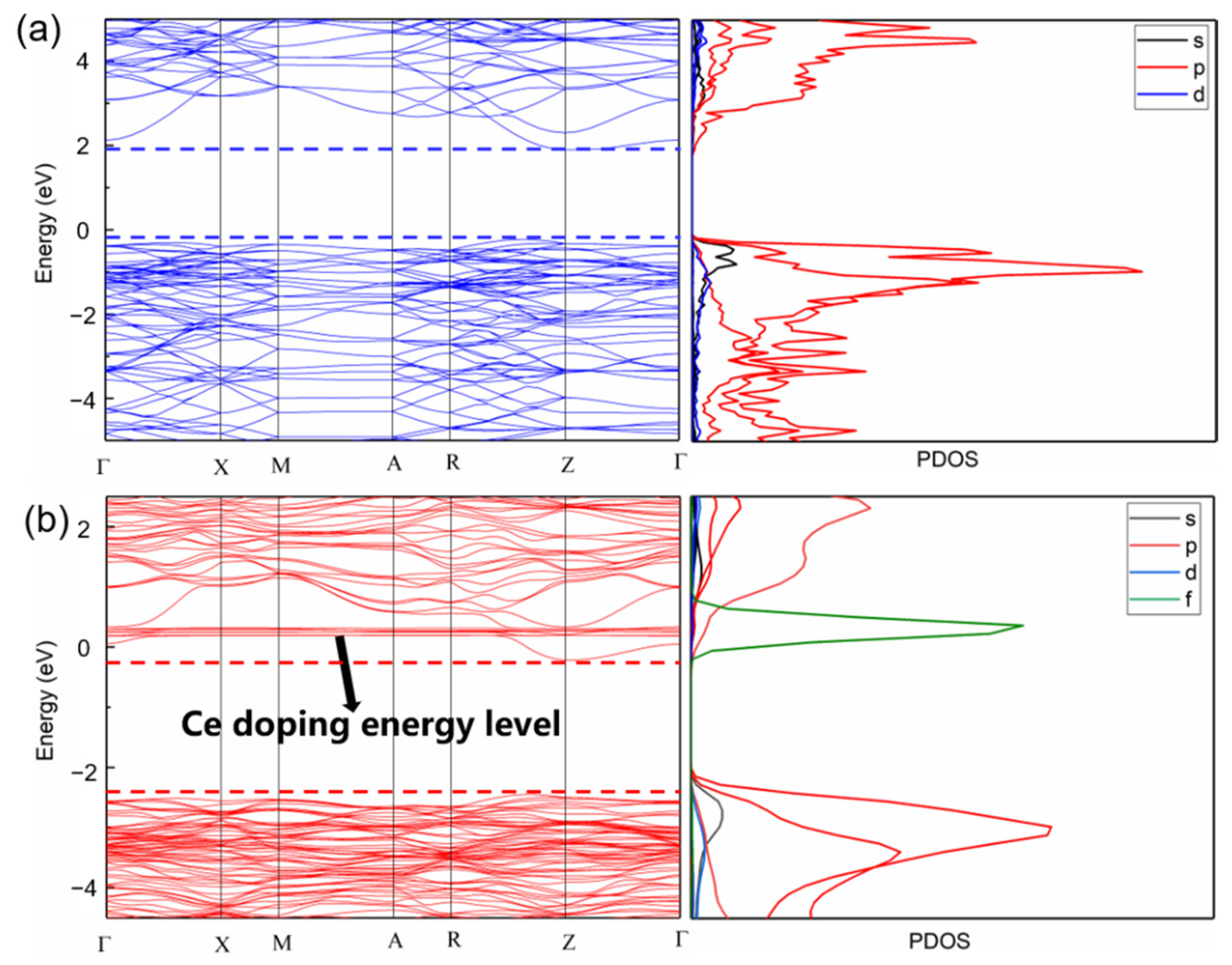
3.5. Theoretical Calculations
3.6. Reaction Model of the Photocatalytic Degradation of BPA over Ce-BiOBr Nanoflakes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shao, L.; Liu, Y.; Wang, L.; Xia, X.; Shen, X. Electronic structure tailoring of BiOBr (0 1 0) nanosheets by cobalt doping for enhanced visible-light photocatalytic activity. Appl. Surf. Sci. 2020, 502, 143895. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, F.; Xu, W.; Cao, S.; Zhu, H. Recent progress in enhancing photocatalytic efficiency of TiO2-based materials. Appl. Catal. A Gen. 2015, 495, 131–140. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chen, W.-H. Graphene family nanomaterials (GFN)-TiO2 for the photocatalytic removal of water and air pollutants: Synthesis, Characterization, and Applications. Nanomaterials 2021, 11, 3195. [Google Scholar] [CrossRef] [PubMed]
- Doustkhah, E.; Assadi, M.H.N.; Komaguchi, K.; Tsunoji, N.; Esmat, M.; Fukata, N.; Tomita, O.; Abe, R.; Ohtani, B.; Ide, Y. In situ blue titania via band shape engineering for exceptional solar H2 production in rutile TiO2. Appl. Catal. B Environ. 2021, 297, 120380. [Google Scholar] [CrossRef]
- Xiong, X.; Ding, L.; Wang, Q.; Li, Y.; Jiang, Q.; Hu, J. Synthesis and photocatalytic activity of BiOBr nanosheets with tunable exposed {0 1 0} facets. Appl. Catal. B Environ. 2016, 188, 283. [Google Scholar] [CrossRef]
- Zhang, K.L.; Liu, C.M.; Huang, F.Q.; Zheng, C.; Wang, W.D. Study of the electronic structure and photocatalytic activity of the BiOCl photocatalyst. Appl. Catal. B Environ. 2006, 68, 125. [Google Scholar] [CrossRef]
- Sheng, H.; Wang, W.; Dai, R.; Ning, J.; Zhang, L.; Wu, Q.; Zhang, F.; Yan, J.; Zhang, W. New Insights into Cd2+/Fe3+ Co-Doped BiOBr for enhancing the photocatalysis efficiency of dye decomposition under visible-light. Nanomaterials 2021, 11, 423. [Google Scholar] [CrossRef]
- Wu, D.; Yue, S.; Wang, W.; An, T.; Li, G.; Yip, H.Y.; Zhao, H.; Wong, P.K. Boron doped BiOBr nanosheets with enhanced photocatalytic inactivation of Escherichia coli. Appl. Catal. B Environ. 2016, 192, 35. [Google Scholar] [CrossRef] [Green Version]
- Di, J.; Chen, C.; Zhu, C.; Song, P.; Xiong, J.; Ji, M.; Zhou, J.; Fu, Q.; Xu, M.; Hao, W.; et al. Bismuth vacancy-tuned bismuth oxybromide ultrathin nanosheets toward photocatalytic CO2 reduction. Acs Appl. Mater. Interfaces 2019, 11, 30786. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Zeng, Q.; Zhu, G.C. Novel S-doped BiOBr nanosheets for the enhanced photocatalytic degradation of bisphenol A under visible light irradiation. Chemosphere 2021, 268, 128854. [Google Scholar] [CrossRef]
- Cheng, H.; Huang, B.; Dai, Y. Engineering BiOX (X = Cl, Br, I) nanostructures for highly efficient photocatalytic applications. Nanoscale 2014, 6, 2009. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; He, J.; Zhu, Y.M.; Zhang, H.; Yang, C.; Wang, K.; Wu, S.-C.; Chueh, L.; Jiang, W. Hierarchical Bi-doped BiOBr microspheres assembled from nanosheets with (001) facet exposed via crystal facet engineering toward highly efficient visible light photocatalysis. Appl. Surf. Sci. 2020, 514, 145927. [Google Scholar] [CrossRef]
- Faisal, M.; Ismail, A.A.; Ibrahim, A.A.; Bouzid, H.; Al-Sayari, S.A. Highly efficient photocatalyst based on Ce doped ZnO nanorods: Controllable synthesis and enhanced photocatalytic activity. Chem. Eng. J. 2013, 229, 225. [Google Scholar] [CrossRef]
- Shen, H.; Bu, J.; Wang, W.; Wu, C.; Cao, Y.; Zhang, B.; Zhang, Q.; Zhang, H. Insight into Ce doping induced oxygen vacancies over Ce-doped MnO2 Catalysts for Imine Synthesis. Chin. J. Chem. 2020, 38, 1353. [Google Scholar] [CrossRef]
- Ahmed, A.; Siddique, M.N.; Ali, T.; Tripathi, P. Defect assisted improved room temperature ferromagnetism in Ce doped SnO2 nanoparticles. Appl. Surf. Sci. 2019, 483, 463. [Google Scholar] [CrossRef]
- Hu, M.; Yan, A.; Wang, X.; Huang, F.; Cui, Q.; Li, F.; Huang, J. Hydrothermal method to prepare Ce-doped BiOBr nanoplates with enhanced carrier transfer and photocatalytic activity. Mater. Res. Bull. 2019, 116, 89. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab-Initio molecular-dynamics simulation of the liquid-metal amorphous-semiconductor transition in germanium. Phy. Rev. B 1994, 49, 14251. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. J. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Hammer, B.; Hansen, L.B.; Norskov, J.K. Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals. Phys. Rev. B 1999, 59, 7413. [Google Scholar] [CrossRef] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Liu, H.; Du, C.; Li, M.; Zhang, S.; Bai, H.; Yang, L.; Zhang, S. One-pot hydrothermal synthesis of SnO2/BiOBr heterojunction photocatalysts for the efficient degradation of organic pollutants under visible light. ACS Appl. Mater. Interfaces 2018, 10, 28686. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yong, D.; Chen, S.; Jiang, S.; Zhang, X.; Shao, W.; Yan, W.; Pan, B.; Xie, Y. Oxygen-vacancy-mediated exciton dissociation in BiOBr for boosting charge-carrier-involved molecular oxygen activation. J. Am. Chem. Soc. 2018, 140, 1760. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yuan, C.; Chen, R.; Wang, J.; Dong, F.; Li, J.; Sun, Y. Mechanisms of interfacial charge transfer and photocatalytic NO oxidation on BiOBr/SnO2 p-n heterojunctions. ACS Appl. Mater. Interfaces 2020, 12, 43741. [Google Scholar] [CrossRef]
- Yang, J.; Liang, Y.; Li, K.; Yang, G.; Yin, S. One-step low-temperature synthesis of 0D CeO2 quantum dots/2D BiOX (X = Cl, Br) nanoplates heterojunctions for highly boosting photo-oxidation and reduction ability. Appl. Catal. B Environ. 2019, 250, 17. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Wong, H.; Yu, D.Q.; Kakushima, K.; Iwai, H. X-ray photoelectron spectroscopy study of high-k CeO2/La2O3 stacked dielectrics. AIP Adv. 2014, 4, 117117. [Google Scholar] [CrossRef] [Green Version]
- Keerthana, S.; Yuvakkumar, R.; Ravi, G.; Hong, S.; Al-Sehemi, A.G.; Velauthapillai, D. Fabrication of Ce doped TiO2 for efficient organic pollutants removal from wastewater. Chemosphere 2022, 293, 133540. [Google Scholar] [CrossRef]
- Xu, H.; Shan, C.; Wu, X.; Sun, M.; Huang, B.; Tang, Y.; Yan, C. Fabrication of layered double hydroxide microcapsules mediated by cerium dop-ing in metal–organic frameworks for boosting water splitting. Energy Environ. Sci. 2020, 13, 2949–2956. [Google Scholar] [CrossRef]
- Si, Y.H.; Xia, Y.; Shang, S.K.; Xiong, X.B.; Zeng, X.R.; Zhou, J.; Li, Y.Y. Enhanced visible light driven photocatalytic behavior of BiFeO3/reduced graphene oxide composites. Nanomaterials 2018, 8, 526. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Ma, D.; Zhou, Z.; Xu, C.; Cao, C.; Zhao, P.; Huang, Q. Efficient photocatalytic degradation of herbicide glyphosate in water by magnetically separable and recyclable BiOBr/Fe3O4 nanocomposites under visible light irradiation. Chem. Eng. J. 2019, 368, 212. [Google Scholar] [CrossRef]
- El-Sheikh, S.M.; Khedr, T.M.; Zhang, G.; Vogiazi, V.; Ismail, A.A.; O’Shea, K.; Dionysiou, D.D. Tailored synthesis of anatase–brookite heterojunction photocatalysts for degradation of cylindrospermopsin under UV–Vis light. Chem. Eng. J. 2017, 310, 428. [Google Scholar] [CrossRef] [Green Version]
- Cui, Z.; Yang, H.; Zhao, X. Enhanced photocatalytic performance of g-C3N4/Bi4Ti3O12 heterojunction nanocomposites. Mater. Sci. Eng. B 2018, 229, 160. [Google Scholar] [CrossRef]
- Shang, L.; Brandholt, S.; Stockmar, F.; Trouillet, V.; Bruns, M.; Nienhaus, G.U. Effect of protein adsorption on the fluorescence of ultrasmall gold nanoclusters. Small 2012, 8, 661. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; McLeod, J.A.; Yang, Y.; Wang, Y.; Wu, Z.; Bai, S.; Yuan, Z.; Song, T.; Wang, Y.; Si, J.; et al. Iodomethane-mediated organometal halide perovskite with record photoluminescence lifetime. ACS Appl. Mater. Interfaces 2016, 8, 23181. [Google Scholar] [CrossRef]
- Zhang, T.; Shao, X.; Zhang, D.; Pu, X.; Tang, Y.; Yin, J.; Ge, B.; Li, W. Synthesis of direct z-scheme g-C3N4/Ag2VO2PO4 photocatalysts with enhanced visible light photocatalytic activity. Sep. Purif. Technol. 2018, 195, 332. [Google Scholar] [CrossRef]
- Masoumi, Z.; Tayebi, M.; Kolaei, M.; Tayyebi, A.; Ryu, H.; Jang, J.I.; Lee, B.K. Simultaneous enhancement of charge separation and hole transportation in a W: Alpha-Fe2O3/MoS2 Photoanode: A Collaborative Approach of MoS2 as a Heterojunction and W as a Metal Dopant. ACS Appl. Mater. Interfaces 2021, 13, 33. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Zhang, Y.-J.; Wang, W.-K.; Pei, D.-N.; Huang, G.; Chen, J.-J.; Zhang, X.; Yu, H.-Q. Enhanced photocatalytic degradation of bisphenol A by Co-doped BiOCl nanosheets under visible light irradiation. Appl. Catal. B Environ. 2018, 221, 320. [Google Scholar] [CrossRef]
- Wei, F.; Wang, H.; Ran, W.; Liu, T.; Liu, X. Preparation of S–N co-doped CoFe2O4@rGO@TiO2 nanoparticles and their superior UV-Vis light photocatalytic activities. RSC Adv. 2019, 9, 6152. [Google Scholar] [CrossRef] [Green Version]




| Sample | B1 (a.u.) | B2 (a.u.) | ||
|---|---|---|---|---|
| BiOBr | 562.68 | 3.79 | 199.95 | 47.85 |
| Ce0.2-BiOBr | 560.90 | 4.68 | 228.19 | 52.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Q.; Wang, C.-Y.; Xu, B.-X.; Han, J.; Fang, X.; Zhu, G. Electron-Level Mechanistic Insights into Ce Doping for Enhanced Efficiency Degradation of Bisphenol A under Visible Light Irradiation. Nanomaterials 2022, 12, 1382. https://doi.org/10.3390/nano12081382
Zeng Q, Wang C-Y, Xu B-X, Han J, Fang X, Zhu G. Electron-Level Mechanistic Insights into Ce Doping for Enhanced Efficiency Degradation of Bisphenol A under Visible Light Irradiation. Nanomaterials. 2022; 12(8):1382. https://doi.org/10.3390/nano12081382
Chicago/Turabian StyleZeng, Qi, Chu-Ya Wang, Bo-Xing Xu, Jianyu Han, Xin Fang, and Guangcan Zhu. 2022. "Electron-Level Mechanistic Insights into Ce Doping for Enhanced Efficiency Degradation of Bisphenol A under Visible Light Irradiation" Nanomaterials 12, no. 8: 1382. https://doi.org/10.3390/nano12081382
APA StyleZeng, Q., Wang, C.-Y., Xu, B.-X., Han, J., Fang, X., & Zhu, G. (2022). Electron-Level Mechanistic Insights into Ce Doping for Enhanced Efficiency Degradation of Bisphenol A under Visible Light Irradiation. Nanomaterials, 12(8), 1382. https://doi.org/10.3390/nano12081382









