Influence of Parameters on Photodynamic Therapy of Au@TiO2–HMME Core-Shell Nanostructures
Abstract
:1. Introduction
2. Material and Methods
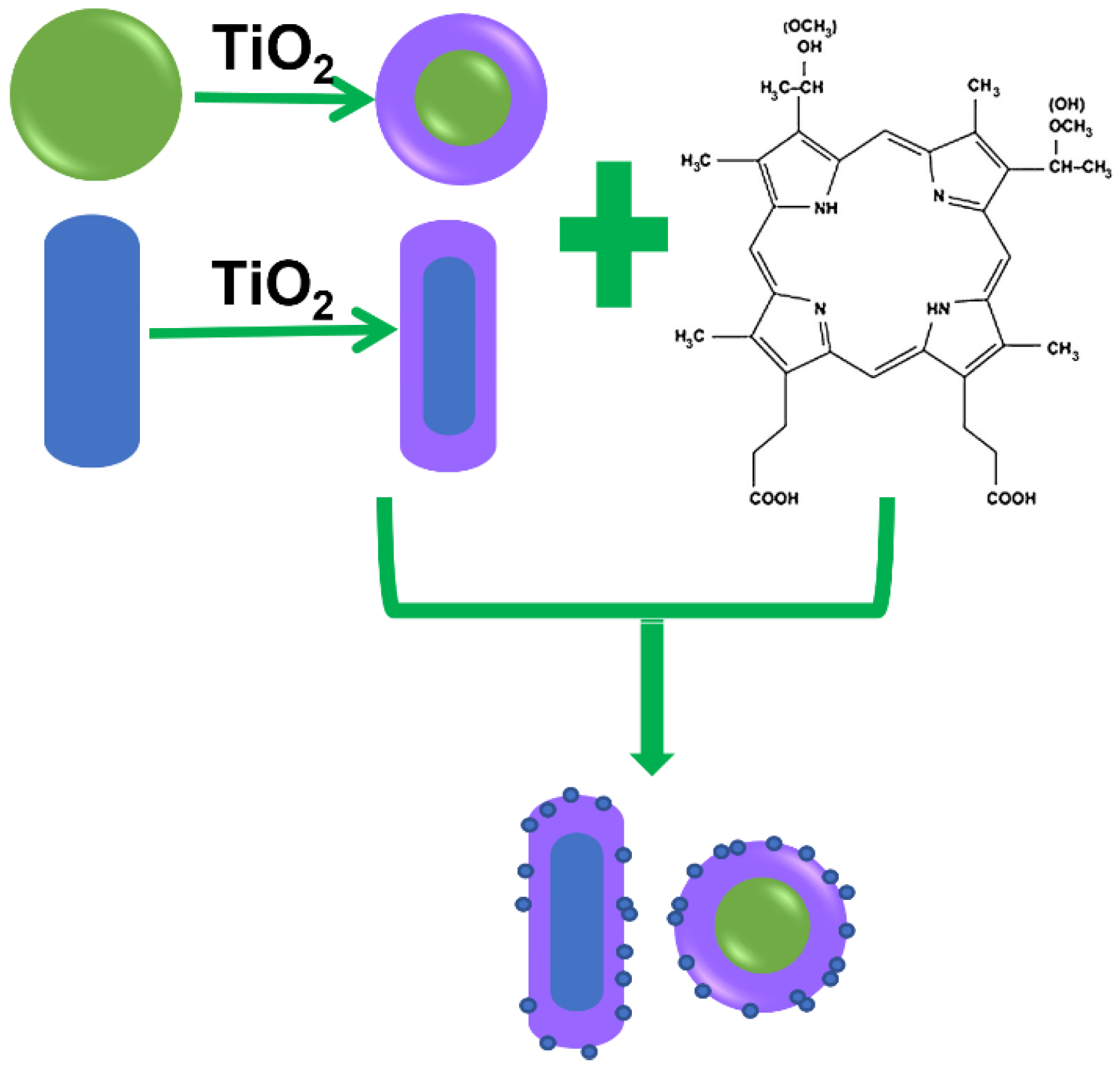
2.1. Preparation and Modification of Gold Nanoparticles
2.2. Synthesis of the Au@TiO2–HMME Conjugates
2.3. Characterization of the Au@TiO2 Core-Shell Nanostructures and Conjugates
2.4. Cell Culture
2.5. Evaluating the Intracellular Uptake of the Au@TiO2–HMME Conjugates
2.6. Evaluating the Photodynamic Effects of the Au@TiO2–HMME Conjugates on KB Cells under Different Light Sources
2.7. Statistical Analysis
3. Results and Discussion
3.1. TEM Characterization of the Samples
3.2. Absorption Spectroscopy Characterization of the Au@TiO2 Core-Shell Nanostructures and the Conjugates
3.3. Intracellular Uptake of the Au@TiO2–HMME Conjugates
3.4. Dark Cytotoxicity of the Au@TiO2–HMME Conjugate on KB Cells
3.5. Effects of Different Light Sources on PDT of Drug-Treated Cells
3.6. Effect of Different Shell Thickness of Conjugates on Cell Photodynamics
3.7. Comparison of Photodynamic Effects of Different Core-Shell Nanostructures Combined with HMME
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, C.P. Role of Radiotherapy in the Treatment of Hepatocellular Carcinoma. J. Clin. Transl. Hepatol. 2019, 7, 183–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinod, S.K.; Hau, E. Radiotherapy treatment for lung cancer: Current status and future directions. Respirology 2020, 25 (Suppl. 2), 61–71. [Google Scholar] [CrossRef] [PubMed]
- Claessens, A.K.M.; Ibragimova, K.I.E.; Geurts, S.M.E.; Bos, M.E.; Erdkamp, F.L.; Tjan-Heijnen, V.C. The role of chemotherapy in treatment of advanced breast cancer: An overview for clinical practice. Crit. Rev. Oncol. 2020, 153, 102988. [Google Scholar] [CrossRef] [PubMed]
- Farmer, Z.L.; Kim, E.S.; Carrizosa, D.R. Gene Therapy in Head and Neck Cancer. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 117. [Google Scholar] [CrossRef]
- Tang, R.; Xu, Z.G. Gene therapy: A double-edged sword with great powers. Mol. Cell. Biochem. 2020, 474, 73–81. [Google Scholar] [CrossRef]
- Dai, X.X.; Du, T.; Han, K. Engineering Nanoparticles for Optimized Photodynamic Therapy. ACS Biomater. Sci. Eng. 2019, 5, 6342–6354. [Google Scholar] [CrossRef]
- Liu, Z.; Xie, Z.; Li, W.; Wu, X.; Jiang, X.; Li, G.; Cao, L.; Zhang, D.; Wang, Q.; Xue, P.; et al. Photodynamic immunotherapy of cancers based on nanotechnology: Recent advances and future challenges. J. Nanobiotechnol. 2021, 19, 160. [Google Scholar] [CrossRef]
- Qidwai, A.; Annu; Nabi, B.; Kotta, S.; Narang, J.K.; Baboota, S.; Ali, J. Role of nanocarriers in photodynamic therapy. Photodiagn. Photodyn. Ther. 2020, 30, 101782. [Google Scholar] [CrossRef]
- Yan, J.; Gao, T.; Lu, Z.; Yin, J.; Zhang, Y.; Pei, R. Aptamer-Targeted Photodynamic Platforms for Tumor Therapy. ACS Appl. Mater. Interf. 2021, 13, 27749–27773. [Google Scholar] [CrossRef]
- Zhang, W.; Ahmed, A.; Cong, H.; Wang, S.; Shen, Y.; Yu, B. Application of multifunctional BODIPY in photodynamic therapy. Dyes Pigments 2021, 185, 108937. [Google Scholar] [CrossRef]
- Sharman, W.M.; Allen, C.M.; van Lier, J.E. Photodynamic therapeutics: Basic principles and clinical applications. Drug Discov. Today 1999, 4, 507–517. [Google Scholar] [CrossRef]
- Mfouo-Tynga, I.S.; Dias, L.D.; Inada, N.M.; Kurachi, C. Features of third generation photosensitizers used in anticancer photodynamic therapy: Review. Photodiagn. Photodyn. Ther. 2021, 34, 102091. [Google Scholar] [CrossRef] [PubMed]
- Gai, S.L.; Yang, G.X.; Yang, P.P.; He, F.; Lin, J.; Jin, D.; Xing, B. Recent advances in functional nanomaterials for light-triggered cancer therapy. Nano Today 2018, 19, 146–187. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z. Photodynamic therapy in China: Over 25 years of unique clinical experience Part two-Clinical experience. Photodiagn. Photodyn. Ther. 2006, 3, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Xu, Q.; Liu, F.; Zhou, P.; Gu, Y.; Zeng, J.; An, J.; Dai, W.; Li, X. Hematoporphyrin monomethyl ether photodynamic damage on HeLa cells by means of reactive oxygen species production and cytosolic free calcium concentration elevation. Cancer Lett. 2004, 216, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.C.; Glazner, G.F.; Duffy, M.; Scherrer, L.; Pendyala, S.; Li, B.; Wang, X.; Wang, H.; Huang, Z. Optical properties of hematoporphyrin monomethyl ether (HMME), a PDT photosensitizer. Photodiagn. Photodyn. Ther. 2012, 9, 232–242. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, X.; Chen, H.; Yang, Y.; Lin, H.; Guo, X. Clinical study on clinical operation and post-treatment reactions of HMME-PDT in treatment of PWS. Photodiagn. Photodyn. Ther. 2017, 20, 253–256. [Google Scholar] [CrossRef]
- Xin, J.; Wang, S.; Wang, B.; Wang, J.; Wang, J.; Zhang, L.; Xin, B.; Shen, L.; Zhang, Z.; Yao, C. AIPcS(4)-PDT for gastric cancer therapy using gold nanorod, cationic liposome, and Pluronic (R) F127 nanomicellar drug carriers. Int. J. Nanomed. 2018, 13, 2017–2036. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Jiang, N.; Fu, B.; Huang, F.; Liu, J. Self-assembling peptide-based nanodrug delivery systems. Biomater. Sci. 2019, 7, 4888–4911. [Google Scholar] [CrossRef]
- Derycke, A.S.L.; de Witte, P.A.M. Liposomes for photodynamic therapy. Adv. Drug Deliv. Rev. 2004, 56, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.Z.; Cheng, L.; Dong, Z.L.; Tao, D.; Barnhart, T.E.; Cai, W.; Chen, M.; Liu, Z. Theranostic Liposomes with HypoxiaActivated Prodrug to Effectively Destruct Hypoxic Tumors Post-Photodynamic Therapy. ACS Nano 2017, 11, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.P.; Lin, S.Y.; McDonagh, A.; Feng, Z.; Lin, S.; McDonagh, A.; Yu, C. Natural Hydrogels Applied in Photodynamic Therapy. Curr. Med. Chem. 2020, 27, 2681–2703. [Google Scholar] [CrossRef] [PubMed]
- Duse, L.; Baghdan, E.; Pinnapireddy, S.R.; Engelhardt, K.H.; Jedelská, J.; Schaefer, J.; Quendt, P.; Bakowsky, U. Preparation and Characterization of Curcumin Loaded Chitosan Nanoparticles for Photodynamic Therapy. Phys. Status Solidi A 2018, 215, 1700709. [Google Scholar] [CrossRef]
- Sun, J.Y.; Kormakov, S.; Liu, Y.; Huang, Y.; Wu, D.; Yang, Z. Recent Progress in Metal-Based Nanoparticles Mediated Photodynamic Therapy. Molecules 2018, 23, 1704. [Google Scholar] [CrossRef] [Green Version]
- Dhanalekshmi, K.I.; Magesan, P.; Sangeetha, K.; Zhang, X.; Jayamoorthy, K.; Srinivasan, N. Preparation and characterization of core-shell type Ag@SiO2 nanoparticles for photodynamic cancer therapy. Photodiagn. Photodyn. Ther. 2019, 28, 324–329. [Google Scholar] [CrossRef]
- Lan, G.X.; Ni, K.Y.; Lin, W.B. Nanoscale metal-organic frameworks for phototherapy of cancer. Coord. Chem. Rev. 2019, 379, 65–81. [Google Scholar] [CrossRef]
- Park, J.; Jiang, Q.; Feng, D.W.; Zhou, H.-C. Controlled Generation of Singlet Oxygen in Living Cells with Tunable Ratios of the Photochromic Switch in Metal-Organic Frameworks. Angew. Chem.-Int. Ed. 2016, 55, 7188–7193. [Google Scholar] [CrossRef]
- Fan, H.Y.; Yu, X.H.; Wang, K.; Yin, Y.-J.; Tang, Y.-J.; Liang, X.-H. Graphene quantum dots (GQDs)-based nanomaterials for improving photodynamic therapy in cancer treatment. Eur. J. Med. Chem. 2019, 182, 111620. [Google Scholar] [CrossRef]
- Liu, Y.; Lee, S.H.; Lee, W.K.; Yoon, I. Ionic Liquid-dependent Gold Nanoparticles of Purpurin-18 for Cellular Imaging and Photodynamic Therapy In Vitro. Bull. Korean Chem. Soc. 2020, 41, 230–233. [Google Scholar] [CrossRef]
- Kang, M.S.; Lee, S.Y.; Kim, K.S.; Han, D.-W. State of the Art Biocompatible Gold Nanoparticles for Cancer Theragnosis. Pharmaceutics 2020, 12, 701. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.L.; Yi, Y.H.; Wu, G.Q.; Liu, W. Gold nanotriangles: Green synthesis and PDT & PTT effect. Mater. Lett. 2017, 187, 148–150. [Google Scholar] [CrossRef]
- Pacioni, N.L.; Gonzalez-Bejar, M.; Alarcon, E.; McGilvray, K.L.; Scaiano, J.C. Surface Plasmons Control the Dynamics of Excited Triplet States in the Presence of Gold Nanoparticles. J. Am. Chem. Soc. 2010, 132, 6298. [Google Scholar] [CrossRef] [PubMed]
- Hah, H.J.; Kim, G.; Orringer, D.A.; Sagher, O.; Philbert, M.A.; Kopelman, R.; Lee, Y.-E.K. Methylene Blue-Conjugated Hydrogel Nanoparticles and Tumor-Cell Targeted Photodynamic Therapy. Macromol. Biosci. 2011, 11, 90–99. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Zhou, C.Y.; Ma, Z.B.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Burschka, J.; Pellet, N.; Moon, S.J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Gratzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316. [Google Scholar] [CrossRef]
- Kafshgari, M.H.; Goldmann, W.H. Insights into Theranostic Properties of Titanium Dioxide for Nanomedicine. Nanomicro Lett. 2020, 12, 22. [Google Scholar] [CrossRef] [Green Version]
- Yadav, H.M.; Thorat, N.D.; Yallapu, M.M.; Tofail, S.A.M.; Kim, J.-S. Functional TiO2 nanocoral architecture for light-activated cancer chemotherapy. J. Mater. Chem. B 2017, 5, 1461–1470. [Google Scholar] [CrossRef]
- Khan, S.U.M.; Al-Shahry, M.; Ingler, W.B. Efficient photochemical water splitting by a chemically modified n-TiO2. Science 2002, 297, 2243–2245. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Fu, K.; Su, Z.Q. Fabrication of 3D MoS2-TiO2@PAN electro-spun membrane for efficient and recyclable photocatalytic degradation of organic dyes. Mater. Sci. Eng. B Adv. Funct. Solid-State Mater. 2021, 269, 115179. [Google Scholar] [CrossRef]
- Morales-Garcia, A.; Escatllar, A.M.; Illas, F.; Bromley, S.T. Understanding the interplay between size, morphology and energy gap in photoactive TiO2 nanoparticles. Nanoscale 2019, 11, 9032–9041. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.X.; Li, N.; Astruc, D. State of the art in gold nanoparticle synthesis. Coord. Chem. Rev. 2013, 257, 638–665. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Fang, C.H.; Jia, H.L.; Chang, S.; Ruan, Q.; Wang, P.; Chen, T.; Wang, J. (Gold core)/(titania shell) nanostructures for plasmon-enhanced photon harvesting and generation of reactive oxygen species. Energy Environ. Sci. 2014, 7, 3431–3438. [Google Scholar] [CrossRef]
- Huang, X.H.; Neretina, S. El-Sayed, M.A. Gold Nanorods: From Synthesis and Properties to Biological and Biomedical Applications. Adv. Mater. 2009, 21, 4880–4910. [Google Scholar] [CrossRef]
- Liao, H.W.; Hafner, J.H. Gold nanorod bioconjugates. Chem. Mater. 2005, 17, 4636–4641. [Google Scholar] [CrossRef]
- Tsung, C.K.; Kou, X.S.; Shi, Q.H.; Zhang, J.; Yeung, M.H.; Wang, J.; Stucky, G.D. Selective shortening of single-crystalline gold nanorods by mild oxidation. J. Am. Chem. Soc. 2006, 128, 5352–5353. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Wang, S.J.; Xu, H.; Wang, B.; Yao, C. Role of 5-aminolevulinic acid-conjugated gold nanoparticles for photodynamic therapy of cancer. J. Biomed. Opt. 2015, 20, 51043. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C.W. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Chan, W.C.W. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007, 7, 1542–1550. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Zhang, L.; Zhang, Z.; Wang, S.; Yao, C. Influence of Parameters on Photodynamic Therapy of Au@TiO2–HMME Core-Shell Nanostructures. Nanomaterials 2022, 12, 1358. https://doi.org/10.3390/nano12081358
Wang P, Zhang L, Zhang Z, Wang S, Yao C. Influence of Parameters on Photodynamic Therapy of Au@TiO2–HMME Core-Shell Nanostructures. Nanomaterials. 2022; 12(8):1358. https://doi.org/10.3390/nano12081358
Chicago/Turabian StyleWang, Ping, Luwei Zhang, Zhenxi Zhang, Sijia Wang, and Cuiping Yao. 2022. "Influence of Parameters on Photodynamic Therapy of Au@TiO2–HMME Core-Shell Nanostructures" Nanomaterials 12, no. 8: 1358. https://doi.org/10.3390/nano12081358
APA StyleWang, P., Zhang, L., Zhang, Z., Wang, S., & Yao, C. (2022). Influence of Parameters on Photodynamic Therapy of Au@TiO2–HMME Core-Shell Nanostructures. Nanomaterials, 12(8), 1358. https://doi.org/10.3390/nano12081358





