Synthesis of Polyaniline Supported CdS/CdS-ZnS/CdS-TiO2 Nanocomposite for Efficient Photocatalytic Applications
Abstract
1. Introduction
2. Methodology
2.1. Materials
2.2. Synthesis of PANI/CdS-PANI/CdS-ZnS-PANI/CdS-TiO2-PANI Nano Composites
2.3. Preparation of Polyaniline (PANI)
2.4. Preparation of CdS-PANI Nanocomposite (PC)
2.5. Preparation of CdS-ZnS-PANI Nanocomposite (CZP)
2.6. Preparation of CdS-TiO2-PANI Nanocomposite (CTP)
2.7. Characterization Techniques
2.8. Photocatalytic Experiment
3. Results and Discussion:
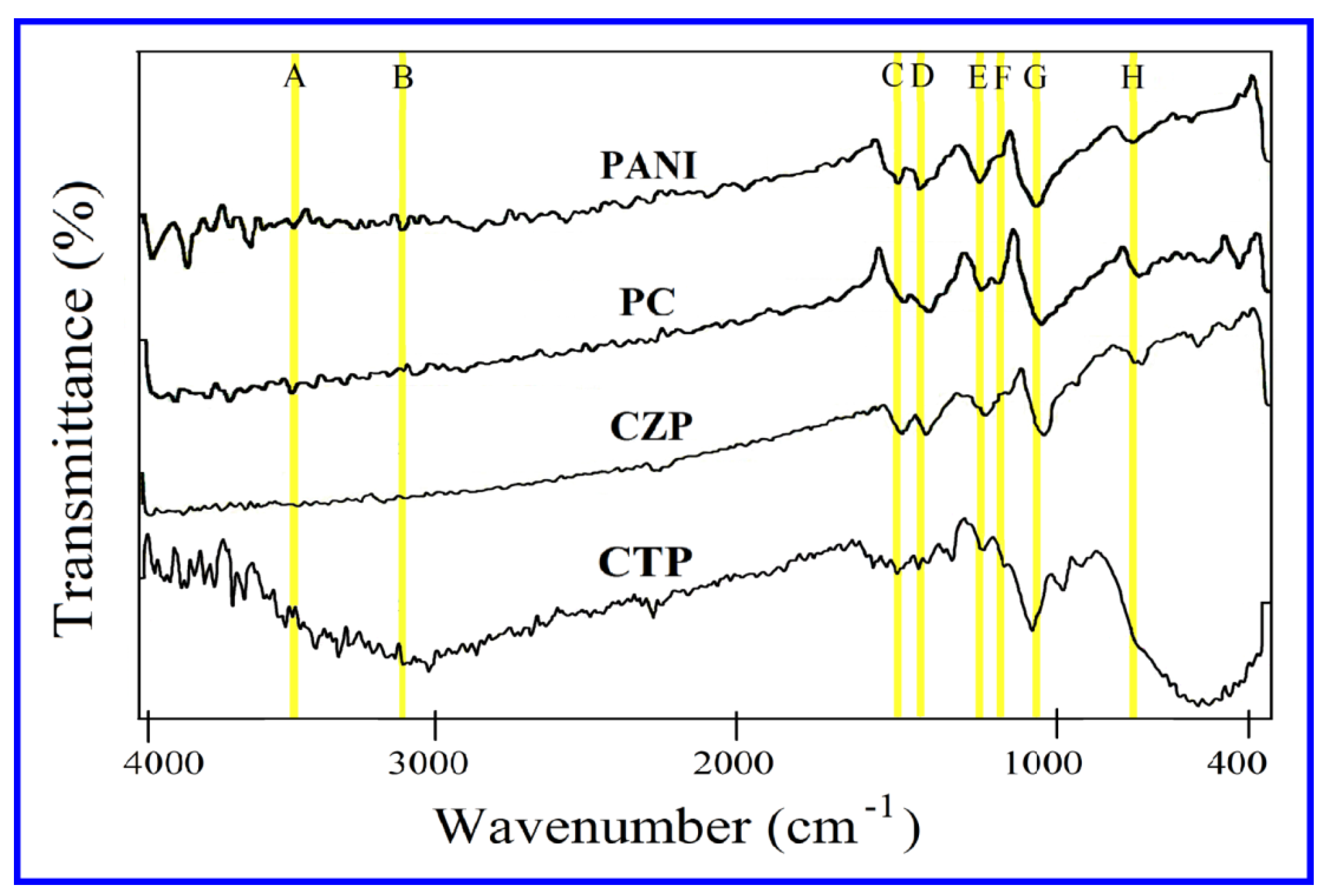
3.1. Fourier Transform Infrared (FTIR) Spectroscopy
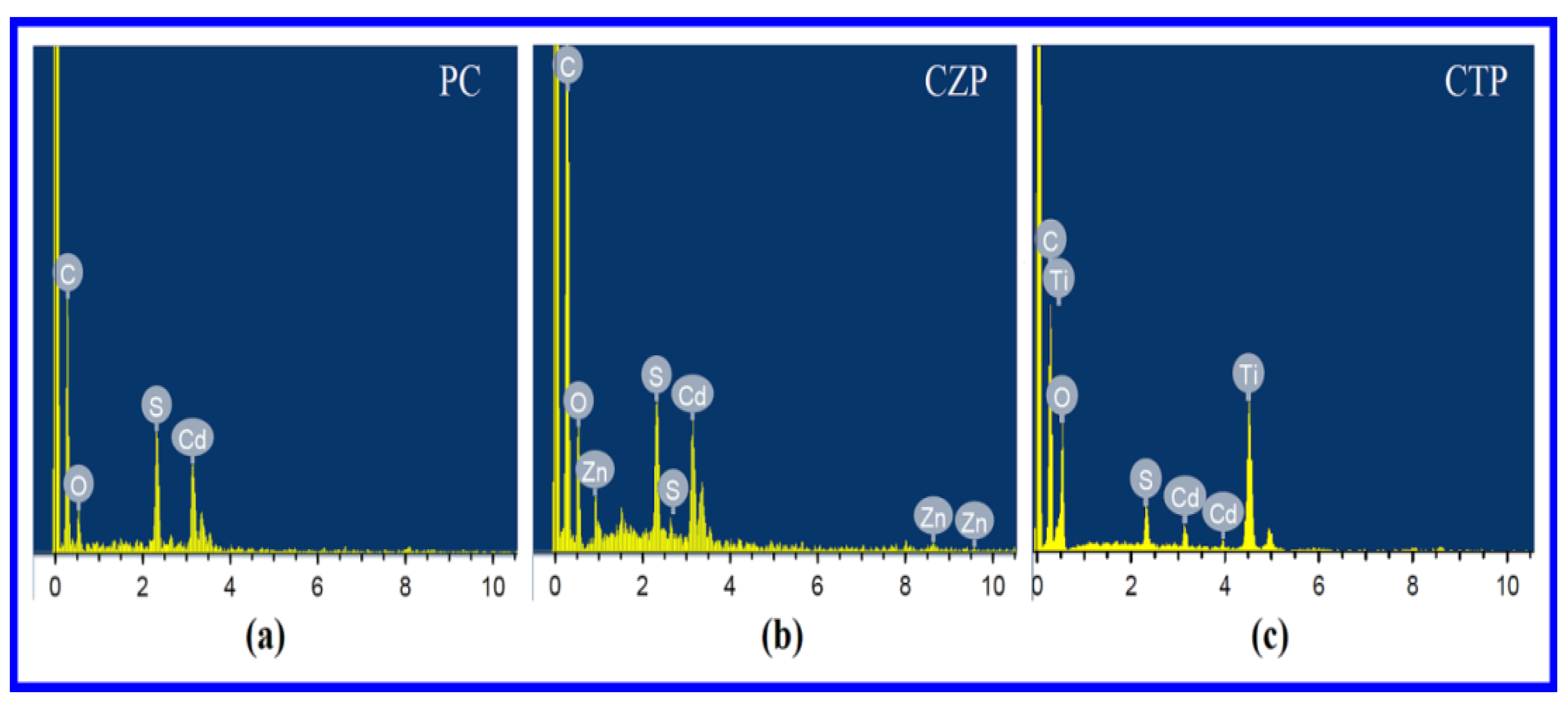
3.2. Energy Dispersive X-ray Spectroscopy (EDS)
3.3. Scanning Electron Microscopy (SEM)
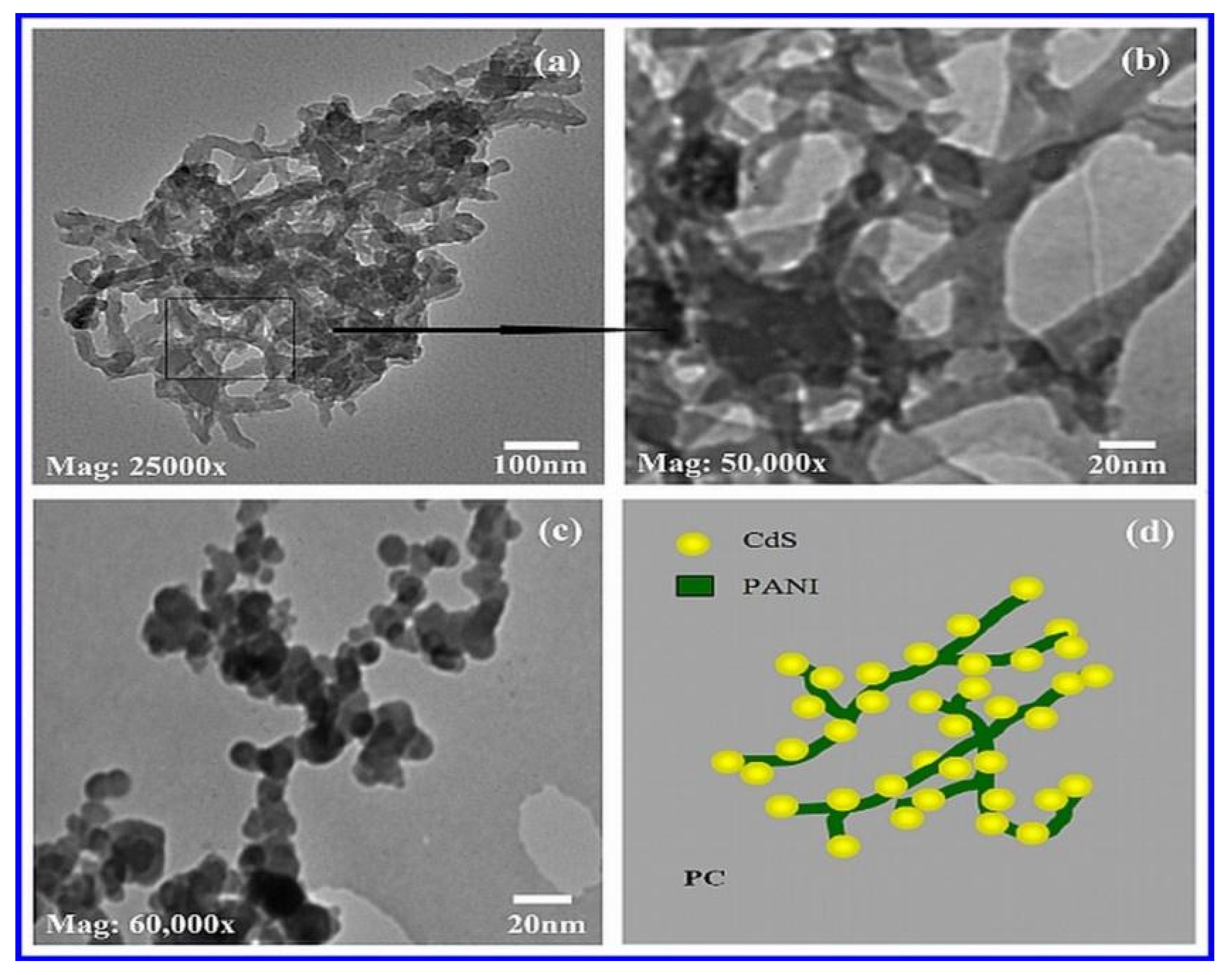
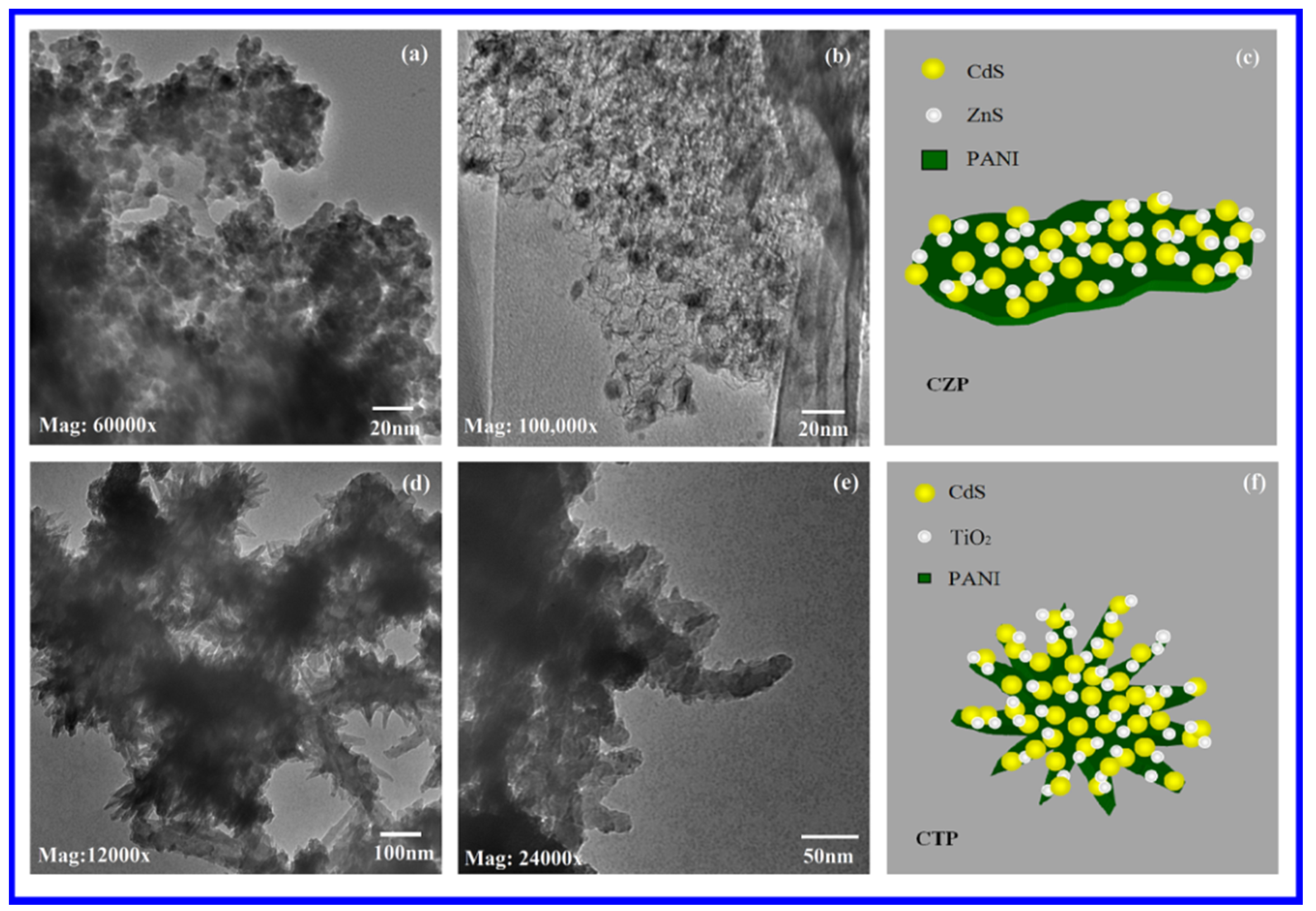
3.4. Transmission Electron Microscopy
3.5. X-ray Diffraction (XRD) Spectroscopy
3.6. Thermal Analysis
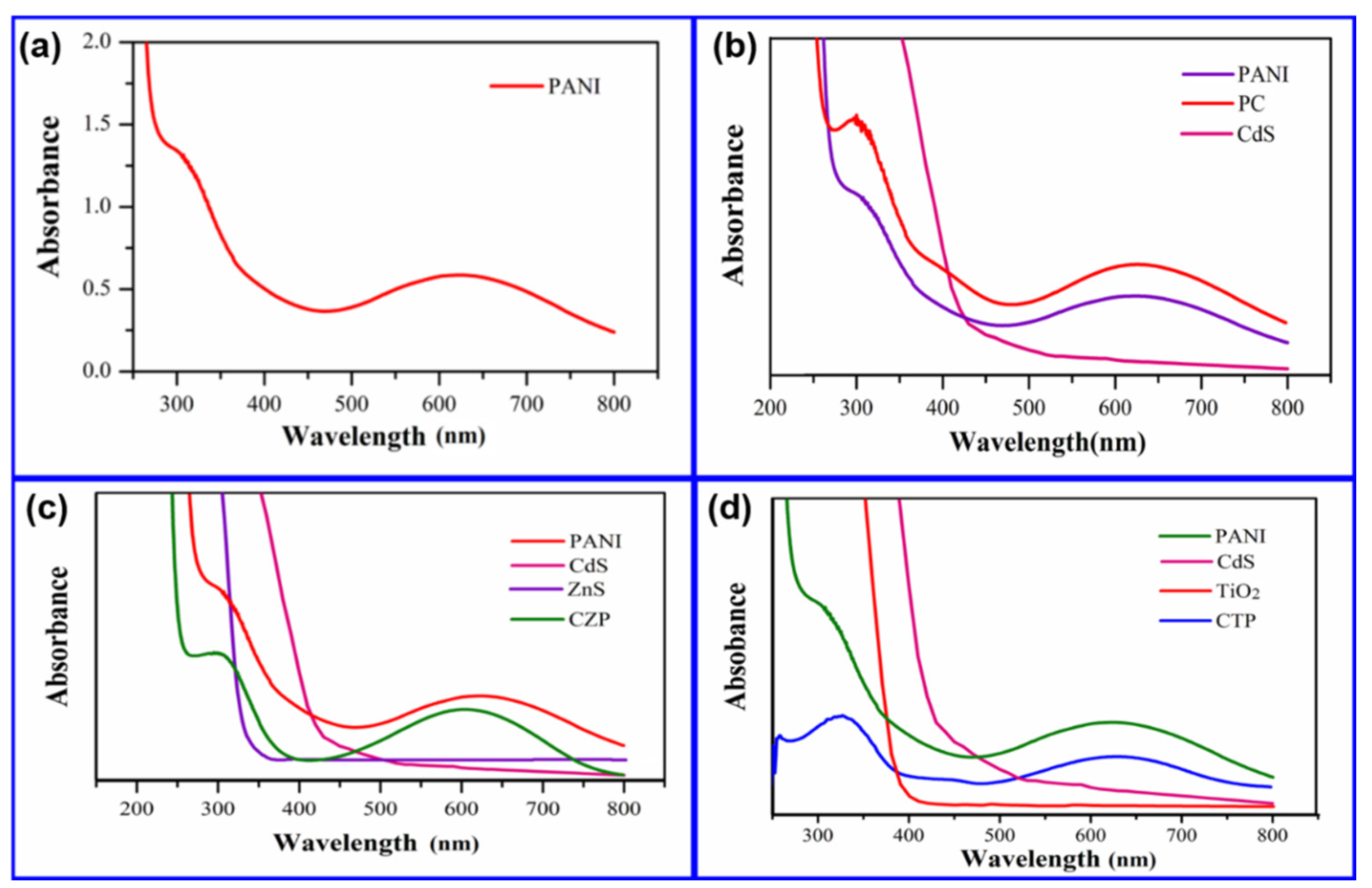
3.7. Optical Analysis
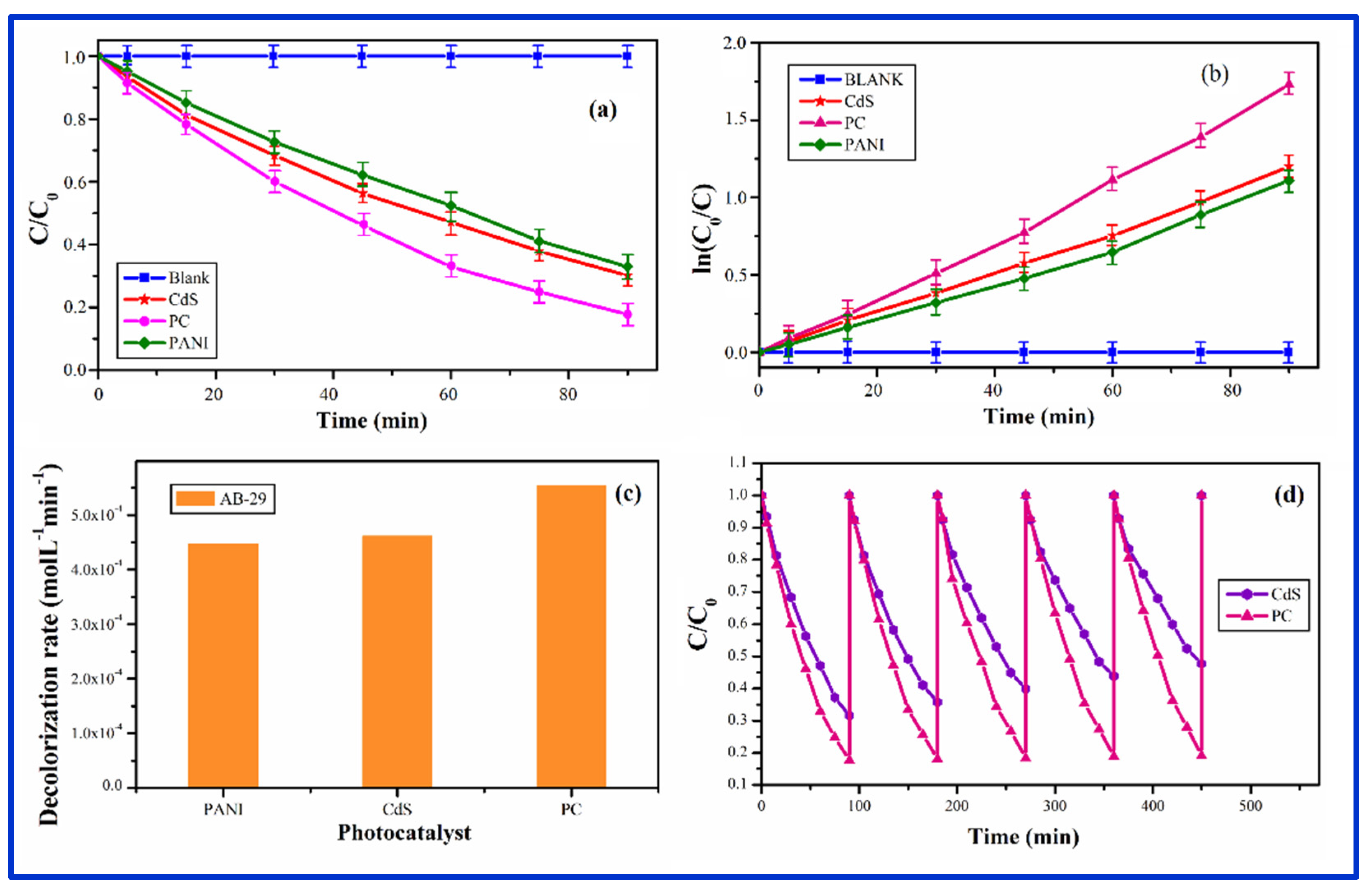
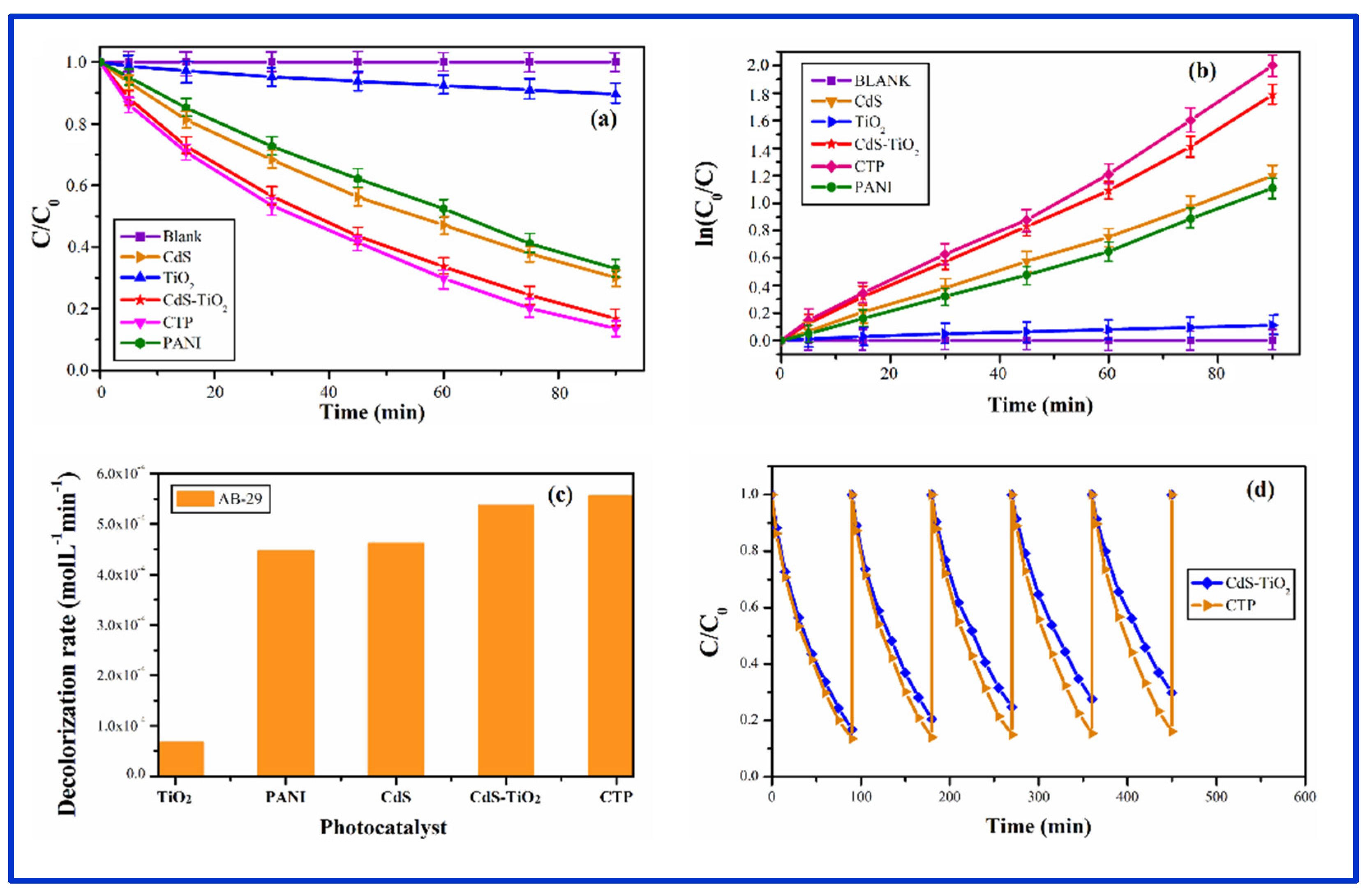
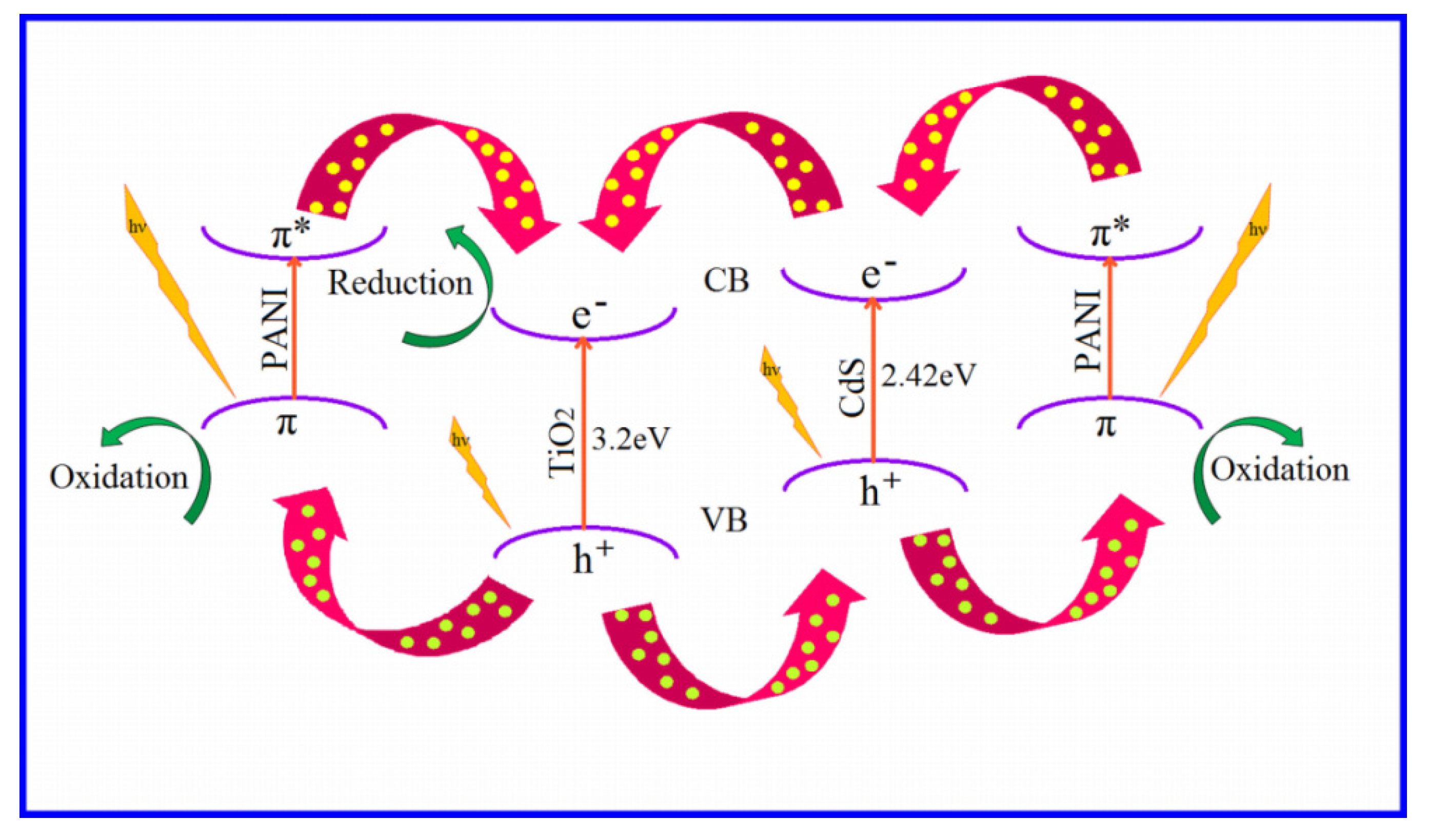
3.8. Photocatalytic Activity:
3.8.1. Photocatalysis by PC
3.8.2. Photocatalysis by CZP
3.8.3. Photocatalysis by CTP
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tuzen, M.; Sarı, A.; Saleh, T.A. Response surface optimization, kinetic and thermodynamic studies for effective removal of rhodamine B by magnetic AC/CeO2 nanocomposite. J. Environ. Manag. 2018, 206, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Xu, F.; Chen, M.; Xu, Z.; Zhu, Z. Adsorption behavior of methylene blue on carbon nanotubes. Bioresour. Technol. 2010, 101, 3040–3046. [Google Scholar] [CrossRef] [PubMed]
- Koysuren, O.; Koysuren, H.N. Photocatalytic activity of polyaniline/Fe-doped TiO2 composites by in situ polymerization method. J. Macromol. Sci. Part A 2019, 56, 267–276. [Google Scholar] [CrossRef]
- Preeti, S.; Abdullah, M.M.; Ikram, S. Role of Nanomaterials and their Applications as Photo-catalyst and Senors: A Review. Nano Res. Appl. 2016, 2, 1. [Google Scholar]
- Singh, P.; MAbdullah, M.; Sagadevan, S.; Kaur, C.; Ikram, S. Highly sensitive ethanol sensor based on TiO2 nanoparticles and its photocatalyst activity. Opt.-Int. J. Light Electron Opt. 2019, 182, 512–518. [Google Scholar] [CrossRef]
- Material Safety Data. Available online: https://biotech.bio5.org/sites/default/files/msds.Acid_Blue_29.pdf (accessed on 26 October 2009).
- Saleh, T.A.; Tuzen, M.; Sarı, A. Polyamide magnetic palygorskite for the simultaneous removal of Hg(II) and methyl mercury; with factorial design analysis. J. Environ. Manag. 2018, 211, 323–333. [Google Scholar] [CrossRef]
- Saleh, T.A.; Adio, S.O.; Asif, M.; Dafalla, H. Statistical analysis of phenols adsorption on diethylenetriamine-modified activated carbon. J. Clean. Prod. 2018, 182, 960–968. [Google Scholar] [CrossRef]
- Xiong, Y.; Tong, Q.; Shan, W.; Xing, Z.; Wang, Y.; Wen, S.; Lou, Z. Arsenic transformation and adsorption by iron hydroxide/manganese dioxide doped straw activated carbon. Appl. Surf. Sci. 2017, 416, 618–627. [Google Scholar] [CrossRef]
- Li, Y.; Cui, W.; Liu, L.; Zong, R.; Yao, W.; Liang, Y.; Zhu, Y. Removal of Cr(VI) by 3D TiO2 -graphene hydrogel via adsorption enriched with photocatalytic reduction. Appl. Catal. B Environ. 2016, 199, 412–423. [Google Scholar] [CrossRef]
- Saleh, T.; Sarı, A.; Tuzen, M. Effective adsorption of antimony(III) from aqueous solutions by polyamide-graphene composite as a novel adsorbent. Chem. Eng. J. 2017, 307, 230–238. [Google Scholar] [CrossRef]
- Saleh, T.A. Simultaneous adsorptive desulfurization of diesel fuel over bimetallic nanoparticles loaded on activated carbon. J. Clean. Prod. 2018, 172, 2123–2132. [Google Scholar] [CrossRef]
- Kumar, R.; Oves, M.; Almeelbi, T.; Al-Makishah, N.H.; Barakat, M. Hybrid chitosan/polyaniline-polypyrrole biomaterial for enhanced adsorption and antimicrobial activity. J. Colloid Interface Sci. 2017, 490, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Chotsuwan, C.; Asawapirom, U.; Shimoi, Y.; Akiyama, H.; Ngamaroonchote, A.; Jiemsakul, T.; Jiramitmongkon, K. Investigation of the electrochromic properties of tri-block polyaniline-polythiophene-polyaniline under visible light. Synth. Met. 2017, 226, 80–88. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Z.; Wen, H.; Cai, Z.; He, C.; Wang, Z.; Yan, W. Microwave assisted modification of activated carbons by organic acid ammoniums activation for enhanced adsorption of acid red 18. Powder Technol. 2018, 323, 230–237. [Google Scholar] [CrossRef]
- Zhu, K.; Duan, Y.; Wang, F.; Gao, P.; Jia, H.; Ma, C.; Wang, C. Silane-modified halloysite/Fe3 O4 nanocomposites: Simultaneous removal of Cr(VI) and Sb(V) and positive effects of Cr(VI) on Sb(V) adsorption. Chem. Eng. J. 2017, 311, 236–246. [Google Scholar] [CrossRef]
- Chen, J.; Feng, J.; Yan, W. Influence of metal oxides on the adsorption characteristics of PPy/metal oxides for methylene blue. J. Colloid Interface Sci. 2016, 475, 26–35. [Google Scholar] [CrossRef]
- Chang, C.-J.; Chao, P.-Y. Efficient photocatalytic hydrogen production by doped ZnS grown on Ni foam as porous immobilized photocatalysts. Int. J. Hydrogen Energy 2018, 44, 20805–20814. [Google Scholar] [CrossRef]
- Zangeneh, H.; Zinatizadeh, A.; Habibi, M.; Akia, M.; Isa, M.H. Photocatalytic oxidation of organic dyes and pollutants in wastewater using different modified titanium dioxides: A comparative review. J. Ind. Eng. Chem. 2014, 26, 1–36. [Google Scholar] [CrossRef]
- Paz, Y. Application of TiO2 photocatalysis for air treatment: Patents’ overview. Appl. Catal. B 2010, 99, 448–460. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Qiu, X.; Song, L.; Meyer, H.M., III; Paranthaman, M.P.; Eres, G.; Zhang, Z.; Gu, B. Comparing Cr, and N only doping with (Cr, N)-codoping for enhancing visible light reactivity of TiO2. Appl. Catal. B 2011, 110, 148–153. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.-H.; Chang, C.-J. S-doped ZnO nanorods on stainless-steel wire mesh as immobilized hierarchical photocatalysts for photocatalytic H 2 production. Int. J. Hydrogen Energy 2014, 39, 16524–16533. [Google Scholar] [CrossRef]
- Chang, C.-J.; Wei, Y.-H.; Huang, K.-P. Photocatalytic hydrogen production by flower-like graphene supported ZnS composite photocatalysts. Int. J. Hydrogen Energy 2017, 42, 23578–23586. [Google Scholar] [CrossRef]
- Ponzio, E.A.; Benedetti, T.M.; Torresi, R.M. Electrochemical and morphological stabilization of V2O5 nanofibers by the addition of polyaniline. Electrochim. Acta 2007, 52, 4419–4427. [Google Scholar] [CrossRef]
- Malta, M.; Torresi, R.M. Electrochemical and kinetic studies of lithium intercalation in composite nanofibers of vanadium oxide/polyaniline. Electrochim. Acta 2005, 50, 5009–5014. [Google Scholar] [CrossRef]
- Karim, M.R.; Yeum, J.H.; Lee, M.S.; Lim, K.T. Preparation of conducting polyaniline/TiO2 composite submicron-rods by the gamma-radiolysis oxidative polymerization method. React. Funct. Polym. 2008, 68, 1371–1376. [Google Scholar] [CrossRef]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Zhu, S.; Xu, T.; Fu, H.; Zhao, J.; Zhu, Y. Synergetic Effect of Bi2WO6 Photocatalyst with C60 and Enhanced Photoactivity Under Visible Irradiation. Environ. Sci. Technol. 2007, 41, 6234–6239. [Google Scholar] [CrossRef]
- Lee, S.L.; Chang, C.-J. Recent Developments about Conductive Polymer Based Composite Photocatalysts. Polymers 2019, 11, 206. [Google Scholar] [CrossRef]
- Ma, J.; Dai, J.; Duan, Y.; Zhang, J.; Qiang, L.; Xue, J. Fabrication of PANI-TiO2/rGO hybrid composites for enhanced photocatalysis of pollutant removal and hydrogen production. Renew. Energy 2020, 156, 1008–1018. [Google Scholar] [CrossRef]
- Wang, N.; Chen, J.; Wang, J.; Feng, J.; Yan, W. Removal of methylene blue by Polyaniline/TiO2 hydrate: Adsorption kinetic, isotherm and mechanism studies. Powder Technol. 2019, 347, 93–102. [Google Scholar] [CrossRef]
- Shang, M.; Wang, W.; Sun, S.; Ren, J.; Zhou, L.; Zhang, L. Efficient Visible Light-Induced Photocatalytic Degradation of Contaminant by Spindle-Like PANI/BiVO4. J. Phys. Chem. C 2009, 113, 20228–20233. [Google Scholar] [CrossRef]
- Liu, X.-X.; Zhang, L.; Li, Y.-B.; Bian, L.-J.; Huo, Y.-Q.; Su, Z. Electrosynthesis of Polyaniline/SiO2 Composite at high pH in the Absence of Extra Supporting Electrolyte. Polym. Bull. 2006, 57, 825–832. [Google Scholar] [CrossRef]
- Manjunath, S.; Koppalkar, A.K.; Prasad, M.V.N.A. Dielectric Spectroscopy of Polyaniline/Stanic Oxide (PANI/SnO2) Composites. Ferroelectrics 2008, 366, 22–28. [Google Scholar] [CrossRef]
- Singh, N.; Kulkarni, M.V.; Lonkar, S.P.; Viswanath, A.K.; Khanna, P.K. CdS/Polyaniline Nanocomposites: Synthesis and Characterization. Synth. React. Inorganic, Met. Nano-Metal Chem. 2007, 37, 153–159. [Google Scholar] [CrossRef]
- Li, G.; Zhang, C.; Peng, H.; Chen, K. One-Dimensional V2O5@Polyaniline Core/Shell Nanobelts Synthesized by an In Situ Polymerization Method. Macromol. Rapid Commun. 2009, 30, 1841–1845. [Google Scholar] [CrossRef]
- Zhao, M.; Wu, X.; Cai, C. Polyaniline Nanofibers: Synthesis, Characterization, and Application to Direct Electron Transfer of Glucose Oxidase. J. Phys. Chem. C 2009, 113, 4987–4996. [Google Scholar] [CrossRef]
- Khairy, M.; Kamar, E.M.; Yehia, M.; Masoud, E.M. High Removal Efficiency of Methyl Orange Dye by Pure and (Cu, N) Doped TiO2/Polyaniline Nanocomposites. Biointerface Res. Appl. Chem. 2021, 12, 893–909. [Google Scholar] [CrossRef]
- Qutub, N.; Singh, P.; Sabir, S.; Sagadevan, S.; Oh, W.C. Enhanced photocatalytic degradation of Acid Blue dye using CdS/TiO2 nanocomposite. Sci. Rep. 2022, 12, 5759. [Google Scholar] [CrossRef]
- Borah, J.P.; Barman, J.; Sarma, K.C. Structural and Optical Properties of ZnS Nanoparticles. Chalcogenide Lett. 2008, 5, 201–208. [Google Scholar]
- Peng, X.; Schlamp, M.C.; Kadavanich, A.V.; Alivisatos, A.P. Epitaxial Growth of Highly Luminescent CdSe/CdS Core/Shell Nanocrystals with Photostability and Electronic Accessibility. J. Am. Chem. Soc. 1997, 119, 7019–7029. [Google Scholar] [CrossRef]
- Mirov, S.B.; Fedorov, V.; Graham, K.; Moskalev, I.S.; Badikov, V.V.; Panyutin, V. Erbium fiber laser–pumped continuous-wave microchip Cr2+:ZnS and Cr2+:ZnSe lasers. Opt. Lett. 2002, 27, 909–911. [Google Scholar] [CrossRef] [PubMed]
- Behboudnia, M.; Habibi-Yangjeh, A.; Jafari-Tarzanag, Y.; Khodayari, A. Facile and Room Temperature Preparation and Characterization of PbS Nanoparticles in Aqueous [EMIM][EtSO4] Ionic Liquid Using Ultrasonic Irradiation. Bull. Korean Chem. Soc. 2008, 29, 53–56. [Google Scholar]
- Kim, E.; Kim, D.; Ahn, B. Synthesis of Mesoporous TiO2and Its Application to Photocatalytic Activation of Methylene Blue and E. coli. Bull. Korean Chem. Soc. 2009, 30, 193–196. [Google Scholar] [CrossRef]
- Huang, X.; Wang, G.; Yang, M.; Guo, W.; Gao, H. Synthesis of polyaniline-modified Fe3O4/SiO2/TiO2 composite microspheres and their photocatalytic application. Mater. Lett. 2011, 65, 2887–2890. [Google Scholar] [CrossRef]
- Wang, N.; Li, J.; Lv, W.; Feng, J.; Yan, W. Synthesis of polyaniline/TiO2 composite with excellent adsorption performance on acid red G. RSC Adv. 2015, 5, 21132–21141. [Google Scholar] [CrossRef]
- Qutub, N.; Pirzada, B.M.; Umar, K.; Sabir, S. Synthesis of CdS nanoparticles using different sulfide ion precursors: Formation mechanism and photocatalytic degradation of Acid Blue-29. J. Environ. Chem. Eng. 2016, 4, 808–817. [Google Scholar] [CrossRef]
- Lu, X.; Yu, Y.; Chen, L.; Mao, H.; Zhang, W.; Wei, Y. Preparation and characterization of polyaniline microwires containing CdS nanoparticles. Chem. Commun. 2004, 13, 1522–1523. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, C.; Li, J. Photocatalysis and wave-absorbing properties of polyaniline/TiO2 microbelts composite by in situ polymerization method. Appl. Surf. Sci. 2010, 257, 944–948. [Google Scholar] [CrossRef]
- Savitha, K.; Prabu, H.G. One-pot synthesis of PANI–TiO2 (anatase) hybrid of low electrical resistance using TiCl4 as precursor. Mater. Chem. Phys. 2011, 130, 275–279. [Google Scholar] [CrossRef]
- Nyamukamba, P.; Moloto, M.J.; Mungondori, H. Visible Light-Active CdS/TiO2 Hybrid Nanoparticles Immobilized on Polyacrylonitrile Membranes for the Photodegradation of Dyes in Water. J. Nanotechnol. 2019, 2019, 5135618. [Google Scholar] [CrossRef]
- Qutub, N.; Pirzada, B.M.; Umar, K.; Mehraj, O.; Muneer, M.; Sabir, S. Synthesis, characterization and visible-light driven photocatalysis by differently structured CdS/ZnS sandwich and core–shell nanocomposites. Phys. E Low-Dimens. Syst. Nanostructures 2015, 74, 74–86. [Google Scholar] [CrossRef]
- He, K.; Li, M.; Guo, L. Preparation and photocatalytic activity of PANI-CdS composites for hydrogen evolution. Int. J. Hydrogen Energy Energy 2012, 37, 755–759. [Google Scholar] [CrossRef]
- Nur, H.; Misnon, I.I.; Wei, L.K. Stannic Oxide-Titanium Dioxide Coupled Semiconductor Photocatalyst Loaded with Polyaniline for Enhanced Photocatalytic Oxidation of 1-Octene. Int. J. Photoenergy 2007, 2007, 098548. [Google Scholar] [CrossRef]
- Du, X.-S.; Zhou, C.-F.; Mai, Y.-W. Facile Synthesis of Hierarchical Polyaniline Nanostructures with Dendritic Nanofibers as Scaffolds. J. Phys. Chem. C 2008, 112, 19836–19840. [Google Scholar] [CrossRef]
- Mallick, K.; Witcomb, M.J.; Scurrell, M.; Strydom, A. In-situ chemical synthesis route for a fiber shaped gold-polyaniline nanocomposite. Gold Bull. 2008, 41, 246–250. [Google Scholar] [CrossRef][Green Version]
- Dutta, K.; De, S. Optical and electrical characterizations of self-assembled CdS nanorods—Polyaniline composites. J. Appl. Phys. 2007, 101, 093711. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Sudha, J.D.; Reena, V.L. Nanostructured Polyaniline-Polytitanate-Clay Composite for Photocatalytic Applications: Preparation and Properties. RSC Adv. 2012, 2, 6228–6236. [Google Scholar] [CrossRef]
- Gu, L.; Wang, J.; Qi, R.; Wang, X.; Xu, P.; Han, X. A novel incorporating style of polyaniline/TiO2 composites as effective visible photocatalysts. J. Mol. Catal. A Chem. 2012, 357, 19–25. [Google Scholar] [CrossRef]
- Lu, X.; Gao, H.; Chen, J.; Chao, D.; Zhang, W.; Wei, Y. Poly(acrylic acid)-guided synthesis of helical polyaniline/CdS composite microwires. Nanotechnology 2005, 16, 113–117. [Google Scholar] [CrossRef]
- Khiew, P.; Huang, N.M.; Radiman, S.; Ahmad, S. Synthesis and characterization of conducting polyaniline-coated cadmium sulphide nanocomposites in reverse microemulsion. Mater. Lett. 2004, 58, 516–521. [Google Scholar] [CrossRef]
- Ummartyotin, S.; Bunnak, N.; Juntaro, J.; Sain, M.; Manuspiya, H. Synthesis and Luminescence Properties of ZnS and Metal (Mn, Cu)-doped-ZnS Ceramic Powder. Solid State Sci. 2012, 14, 299–304. [Google Scholar] [CrossRef]
- Devi, B.S.R.; Raveendran, R.; Vaidyan, A.V. Synthesis and characterization of Mn2+-doped ZnS nanoparticles. Pramana 2007, 68, 679–687. [Google Scholar] [CrossRef]
- Niziol, J.; Sniechowski, M.; Podraza-Guba, A.; Pielichowski, J. Alternative Oxidizers in Polyaniline Synthesis. Polym. Bull. 2011, 66, 761–770. [Google Scholar] [CrossRef]
- Girginer, B.; Galli, G.; Chiellini, E.; Bicak, N. Preparation of stable CdS nanoparticles in aqueous medium and their hydrogen generation efficiencies in photolysis of water. Int. J. Hydrogen Energy 2008, 34, 1176–1184. [Google Scholar] [CrossRef]
- Uwe, H.; Neil, G. The Scherrer Equation Versus the ‘Debye-Scherrer Equation’. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar]
- Scherrer, P. Determination of the Size and Internal Structure of Colloidal Particles using X-rays. Göttinger Nachr. Math. Phys. 1918, 2, 98–100. [Google Scholar]
- Chen, X.; Xu, H.; Xu, N.; Zhao, F.; Lin, W.; Lin, G.; Fu, Y.; Huang, Z.; Wang, H.; Wu, M. Kinetically Controlled Synthesis of Wurtzite ZnS Nanorods through Mild Thermolysis of a Covalent Organic−Inorganic Network. Inorg. Chem. 2003, 42, 3100–3106. [Google Scholar] [CrossRef]
- Li, Q.; Wang, C. Fabrication of wurtzite ZnS nanobelts via simple thermal evaporation. Appl. Phys. Lett. 2003, 83, 359–361. [Google Scholar] [CrossRef]
- Oh, M.; Kim, S. Synthesis and electrochemical analysis of polyaniline/TiO2 composites prepared with various molar ratios between aniline monomer and para-toluenesulfonic acid. Electrochimica Acta 2012, 78, 279–285. [Google Scholar] [CrossRef]
- Tarver, J.; Yoo, J.E.; Dennes, T.J.; Schwartz, J.; Loo, Y.-L. Polymer Acid Doped Polyaniline Is Electrochemically Stable beyond pH 9. Chem. Mater. 2008, 21, 280–286. [Google Scholar] [CrossRef]
- Fan, J.; Ji, X.; Zhang, W.; Yan, Y. Preparation and Photoelectronic Properties of Q-CdS/Polyaniline (PANI) Nanocomposites. CJI 2004, 6, 45–50. [Google Scholar]
- Singh, V.; Sharma, P.; Chauhan, P. Synthesis of CdS nanoparticles with enhanced optical properties. Mater. Charact. 2011, 62, 43–52. [Google Scholar] [CrossRef]
- Heera, T.; Cindrella, L. PbS/CoS–Pani Composite Semiconductor Films. Mater. Sci. Semicond. Processing 2011, 14, 151–156. [Google Scholar] [CrossRef]
- Rathore, K.S.; Patidar, D.; Janu, Y.; Saxena, N.S.; Sharma, K.; Sharma, T.P. Structural and Optical Characterization of Chemically Synthesized ZnS Nanoparticles. Chalcogenide Lett. 2008, 5, 105–110. [Google Scholar]
- Zhang, S.; Chen, Q.; Jing, D.; Wang, Y.; Guo, L. Visible photoactivity and antiphotocorrosion performance of PdS–CdS photocatalysts modified by polyaniline. Int. J. Hydrogen Energy 2012, 37, 791–796. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, Y. Significant Visible Photoactivity and Antiphotocorrosion Performance of CdS Photocatalysts after Monolayer Polyaniline Hybridization. J. Phys. Chem. C 2010, 114, 5822–5826. [Google Scholar] [CrossRef]
- Deshpande, A.; Shah, P.; Gholap, R.; Gupta, N.M. Interfacial and physico-chemical properties of polymer-supported CdS⋅ZnS nanocomposites and their role in the visible-light mediated photocatalytic splitting of water. J. Colloid Interface Sci. 2009, 333, 263–268. [Google Scholar] [CrossRef]
- Wu, L.; Yu, J.; Fu, X. Characterization and photocatalytic mechanism of nanosized CdS coupled TiO2 nanocrystals under visible light irradiation. J. Mol. Catal. A Chem. 2006, 244, 25–32. [Google Scholar] [CrossRef]
- Nasuha, N.; Hameed, B.H.; Okoye, P.U. Dark-Fenton oxidative degradation of methylene blue and acid blue 29 dyes using sulfuric acid-activated slag of the steel-making process. J. Environ. Chem. Eng. 2021, 9, 10483. [Google Scholar] [CrossRef]
- Nasuha, N.; Ismail, S.; Hameed, B.H. Activated electric arc furnace slag as an effective and reusable Fenton-like catalyst for the photodegradation of methylene blue and acid blue 29. J. Environ. Manag. 2017, 196, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Khanday, W.A.; Asif, M.; Hameed, B. Cross-linked beads of activated oil palm ash zeolite/chitosan composite as a bio-adsorbent for the removal of methylene blue and acid blue 29 dyes. Int. J. Biol. Macromol. 2017, 95, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, M.K.; Sayoo, L. Removal of Acid Blue 29 in aqueous solution by Fenton and Fenton-like processes. Desalination Water Treat. 2014, 52, 3411–3420. [Google Scholar] [CrossRef]
- Salem, I.A.; El-Ghamry, H.A.; El-Ghobashy, M.A. Catalytic decolorization of Acid blue 29 dye by H2O2 and a heterogeneous catalyst. Beni-Suef Univ. J. Basic Appl. Sci. 2014, 3, 186–192. [Google Scholar] [CrossRef]
- Chen, C.; Chaudhary, A.; Grimes, S. The effect of sodium hydroxide in photolytic and photocatalytic degradation of Acid Blue 29 and Ethyl Violet. Dye. Pigment. 2007, 73, 55–58. [Google Scholar] [CrossRef]





| S.No. | Synthesized Material or Medium | Rate of Degradation (%) | Decolorization Rate (molL−1min−1) | References |
|---|---|---|---|---|
| 1. | Sulfuric acid-activated slug | 95% | [81] | |
| 2. | Activated electric arc furnace slag | 98% | [82] | |
| 3. | Zeolite/chitosan composite | The Langmuir monolayer adsorption capacities of the Z-AC/C composites are 212.76, 238.09, and 270.27 mg/g for AB29 at 30 °C, 40 14 °C, and 50 °C, respectively | [83] | |
| 4. | In the presence and absence of air by Fenton and Fenton-like processes using hydrogen peroxide (HP) and sodium persulfate (SPS), respectively, as oxidants | 95.8% at pH 3 in 180 min. | [84] | |
| 5. | Montmorillonite K10-Cu(II)ethylenediamine (MMTK10-Cu(en)2) | 83.27% | [85] | |
| 6. | In the presence/absence of sodium hydroxide (NaOH) | 98% (in absence of NaOH)100% (in presence of NaOH) | [86] | |
| 7. | Polyaniline (PANI) with CdS (PC) PANI with CdS-ZnS (CZP) PANI with CdS-TiO2 (CTP) | 82.2 89.8 89.8 | 5.54 × 10−4 5.84 × 10−4 5.56 × 10−4 | Present Study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qutub, N.; Singh, P.; Sabir, S.; Umar, K.; Sagadevan, S.; Oh, W.-C. Synthesis of Polyaniline Supported CdS/CdS-ZnS/CdS-TiO2 Nanocomposite for Efficient Photocatalytic Applications. Nanomaterials 2022, 12, 1355. https://doi.org/10.3390/nano12081355
Qutub N, Singh P, Sabir S, Umar K, Sagadevan S, Oh W-C. Synthesis of Polyaniline Supported CdS/CdS-ZnS/CdS-TiO2 Nanocomposite for Efficient Photocatalytic Applications. Nanomaterials. 2022; 12(8):1355. https://doi.org/10.3390/nano12081355
Chicago/Turabian StyleQutub, Nida, Preeti Singh, Suhail Sabir, Khalid Umar, Suresh Sagadevan, and Won-Chun Oh. 2022. "Synthesis of Polyaniline Supported CdS/CdS-ZnS/CdS-TiO2 Nanocomposite for Efficient Photocatalytic Applications" Nanomaterials 12, no. 8: 1355. https://doi.org/10.3390/nano12081355
APA StyleQutub, N., Singh, P., Sabir, S., Umar, K., Sagadevan, S., & Oh, W.-C. (2022). Synthesis of Polyaniline Supported CdS/CdS-ZnS/CdS-TiO2 Nanocomposite for Efficient Photocatalytic Applications. Nanomaterials, 12(8), 1355. https://doi.org/10.3390/nano12081355








