Recent Application of Nanomaterials to Overcome Technological Challenges of Microbial Electrolysis Cells
Abstract
:1. Introduction
2. Microbial Electrolysis Cells
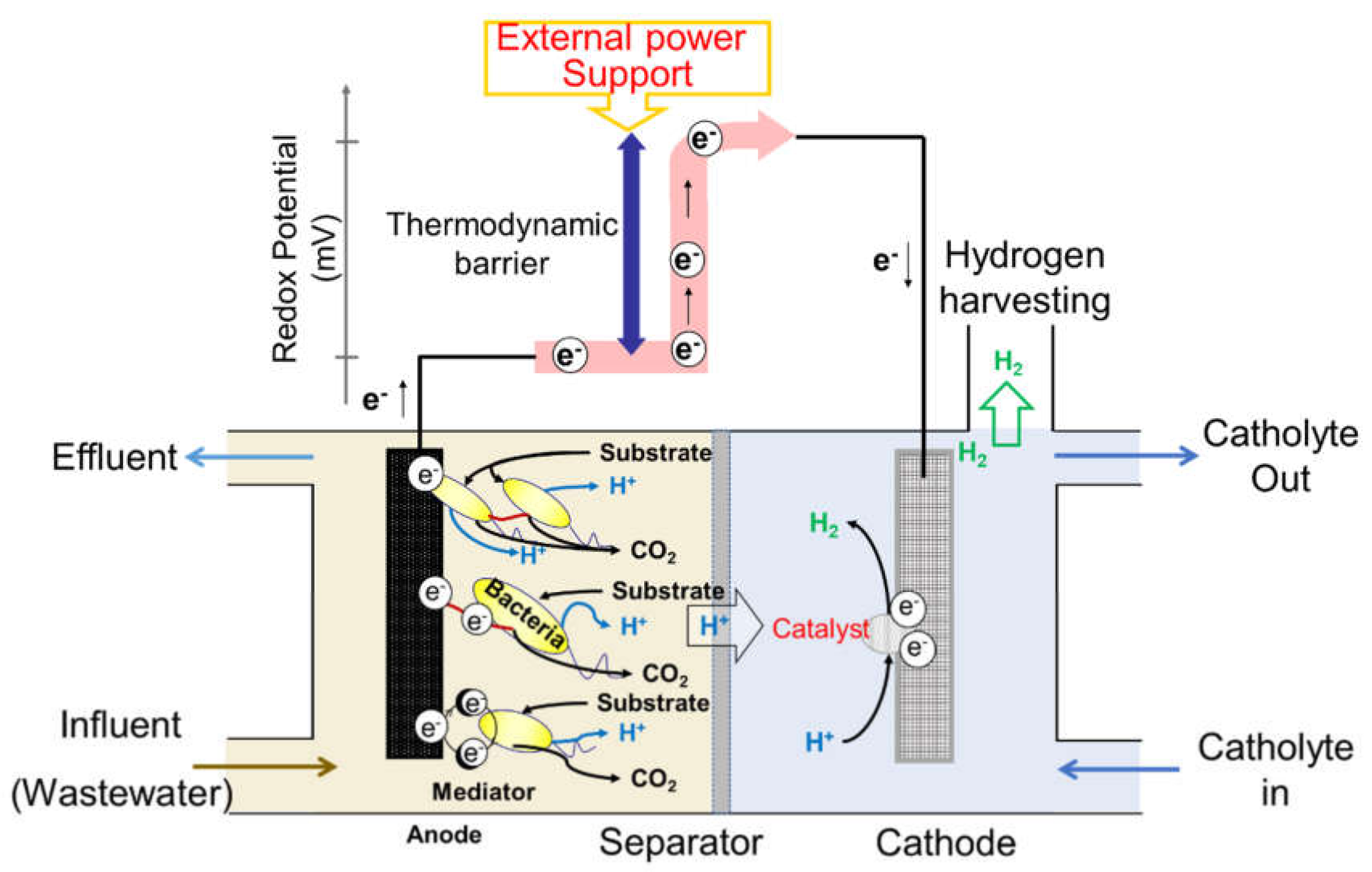
2.1. Principle of Microbial Electrolysis Cells
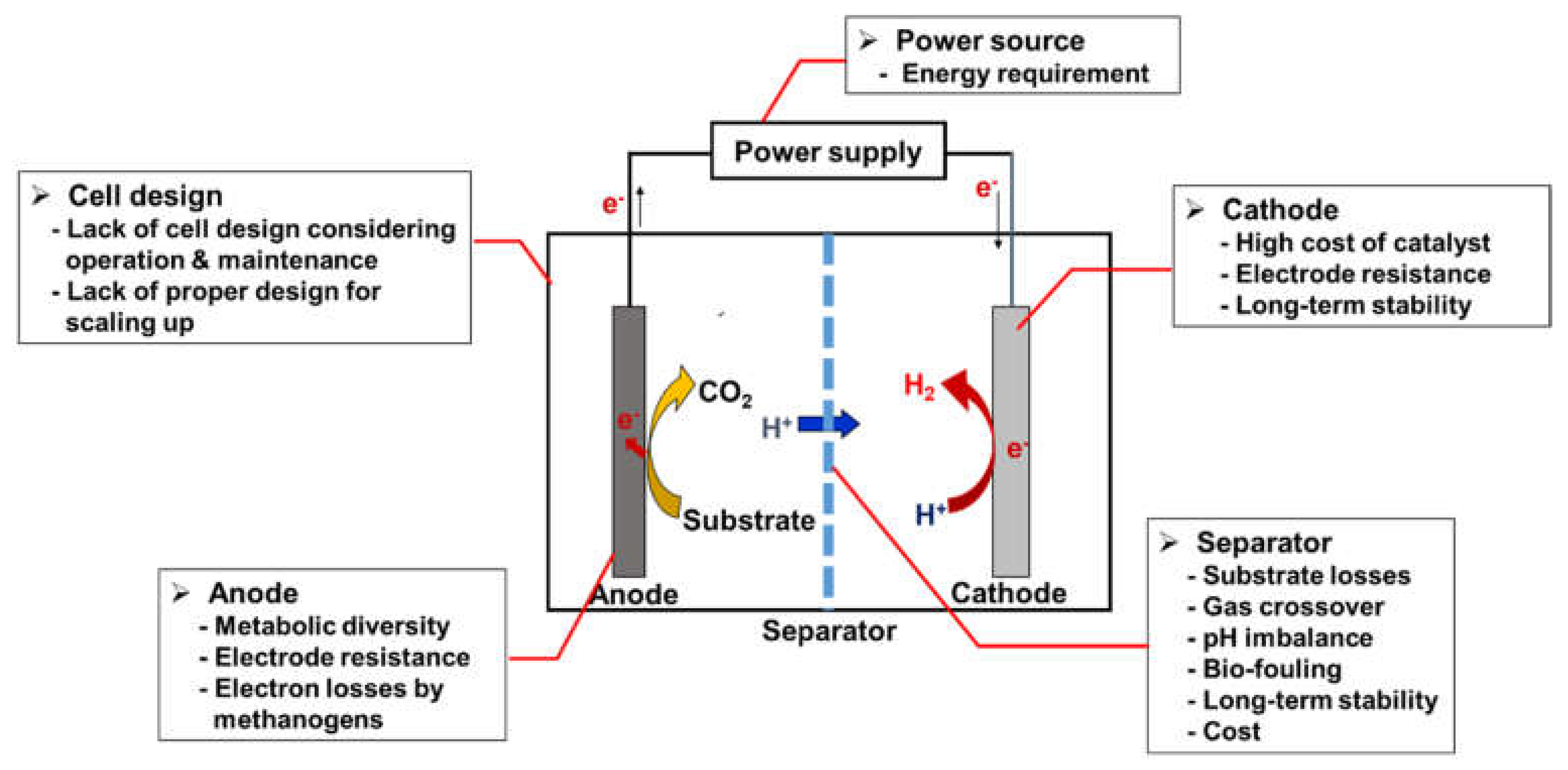
2.2. Challenges of Microbial Electrolysis Cells
3. Nanomaterials Used in Microbial Electrolysis Cells
| Application | Nanomaterial | Structure | Size (nm) | Synthesis Method | Performance | Ref. |
|---|---|---|---|---|---|---|
| Anode electrode | Au nanoparticle | 0D | 0.33 μm2 | Thermal annealing | Current density: 74.4 μA/cm2 | [26] |
| Anode electrode | Pd nanoparticle | 0D | 0.35 μm2 | Thermal annealing | Current density: 74.4 μA/cm2 | [26] |
| Photo-anode electrode | TiO2 nanotubes | 1D | Length: 4.04–4.35 μm | Anodization method | Current density: 0.371 mA/cm2 H2 production rate: 1434 mmol/m3/h | [27] |
| Photo-anode electrode | CeO2–rGO nanocomposite | 2D | - | rGO nanosheets: modified Hummer’s method and thermal reduction; CeO2–rGO nanocomposite electrode: Polymerization and carbonization | H2 production rate: 5 m3/m3/d Cathodic H2 recovery efficiency: 95% | [38] |
| Cathodic catalyst | Pt nanoparticle | 0D | 7–20 | H2 conversion efficiency: 80.6% | [39] | |
| Cathodic catalyst | Ni nanoparticle | 0D | 7–20 | - | H2 conversion efficiency: 73.0% | [39] |
| Cathodic catalyst | Pt–Ni nanoparticle (atomic ratio 1:1) | 0D | 7–20 | - | H2 conversion efficiency: 76.8% | [39] |
| Cathodic catalyst | Pt–Cu nanoparticle (atomic ratio 1:1) | 0D | 7–20 | - | H2 conversion efficiency: 72.6% | [39] |
| Cathodic catalyst | Ni nanoparticle | 0D | 30–50 | Electrodeposition | H2 conversion efficiency: 82% | [28] |
| Catalyst | Ni nanoparticle | 0D | 40 | Solution plasma | CH4 production enhancement: ~52.4% | [40] |
| Cathodic catalyst | Pd nanoparticle | 0D | 10–100 | Bioelectochemical deposition | Cathodic H2 recovery efficiency: 65.5% | [41] |
| Cathodic catalyst | Ni2P nanoparticle | 0D | 7 | Solution-phase method | Cathodic H2 recovery efficiency: 65.5% | [42] |
| Cathodic catalyst | Ni–Co–P nanoparticle | 0D | 33–35 | Electrodeposition | H2 conversion efficiency: 90.3% | [29] |
| Cathodic photocatalyst | NiFe2O4 nanoparticle | 0D | >17 | Electrodeposition/spin coating | Current density: 0.74 A/m2 H2 production rate: 288 μmol/h/g | [30] |
| Cathodic catalyst | NiMoO4 nanoparticle | 0D | <50 | Sonochemical precipitation | H2 conversion efficiency: 11.96% | [34] |
| Cathodic catalyst | NiO nanoparticle | 0D | - | Chemical precipitation | Cathodic H2 recovery efficiency: 27% | [35] |
| Cathodic catalyst | Co3O4 nanoparticle | 0D | - | Chemical precipitation | Cathodic H2 recovery efficiency: 26% | [35] |
| Cathodic catalyst | Fe3O4 nanoparticle | 0D | 12–28 | Chemical precipitation | Current density: 15.2 mA/m2 | [36] |
| Cathodic catalyst | Carbon nanoparticle | 0D | 50 | - | H2 conversion efficiency: 47% | [39] |
| Cathodic photocatalyst | TiO2 nanorod | 1D | Length: 700 Diameter: 40 | Hydrothermal method | H2 production rate: 4.4 μL/h | [43] |
| Cathodic photocatalyst | MoS2 nanosheet–TiO2 nanotube composite | 1D | TiO2 nanotube diameter: about 100 | Anodization method+ bioelectrochemical deposition | H2 production rate: 0.003 m3/m3/min | [44] |
| Cathodic catalyst | Mo2N nanobelt | 1D | - | Hydrothermal synthesis + thermal annealing | H2 conversion efficiency: 74% | [45] |
| Cathodic catalyst | CoP nanoarray | 1D | - | Hydrothermal synthesis + thermal annealing | H2 conversion efficiency: 34% | [46] |
| Cathode electrode | Polyaniline/MWCNT (1) | 1D | - | MWCNT: CVD (2); polyaniline deposition: in situ chemical oxidation polymerization | Cathodic H2 recovery efficiency: 42% | [47] |
| Cathode electrode | Polyaniline/MWCNT | 1D | - | MWCNT: CVD; polyaniline deposition: in situ chemical oxidation polymerization | Cathodic H2 recovery efficiency: 56.7% | [48] |
| Cathode electrode | MoS2/CNT (3) | 1D | Outer/inner diameter: 7/3 Length: 10,000 | CNT: CVD; MoS2 deposition: Hydrothermal method | Cathodic H2 recovery efficiency: 49% | [13] |
| Cathodic catalyst | SWCNT (4) | 1D | - | CVD | H2 conversion efficiency: 38.9% | [39] |
| Cathodic photocatalyst | Polyaniline nanofibers | 1D | Thickness: 50 | Oxidizing aniline at a perchloric acid/dichloromethane interface | H2 conversion efficiency: 79.2% | [49] |
| Supporting material for membrane mechanical strength reinforcement | Polyimide nanofiber | 1D | Thickness: 200 | Electrospinning | Membrane tensile strength: >40 MPa H2 conversion efficiency: 32.4% | [37] |
| Cathodic catalyst | Graphene | 2D | - | GO nanosheets: Hummer’s method; graphene deposition: hydrothermal method | H2 production rate: 2.2 m3/m3/d | [50] |
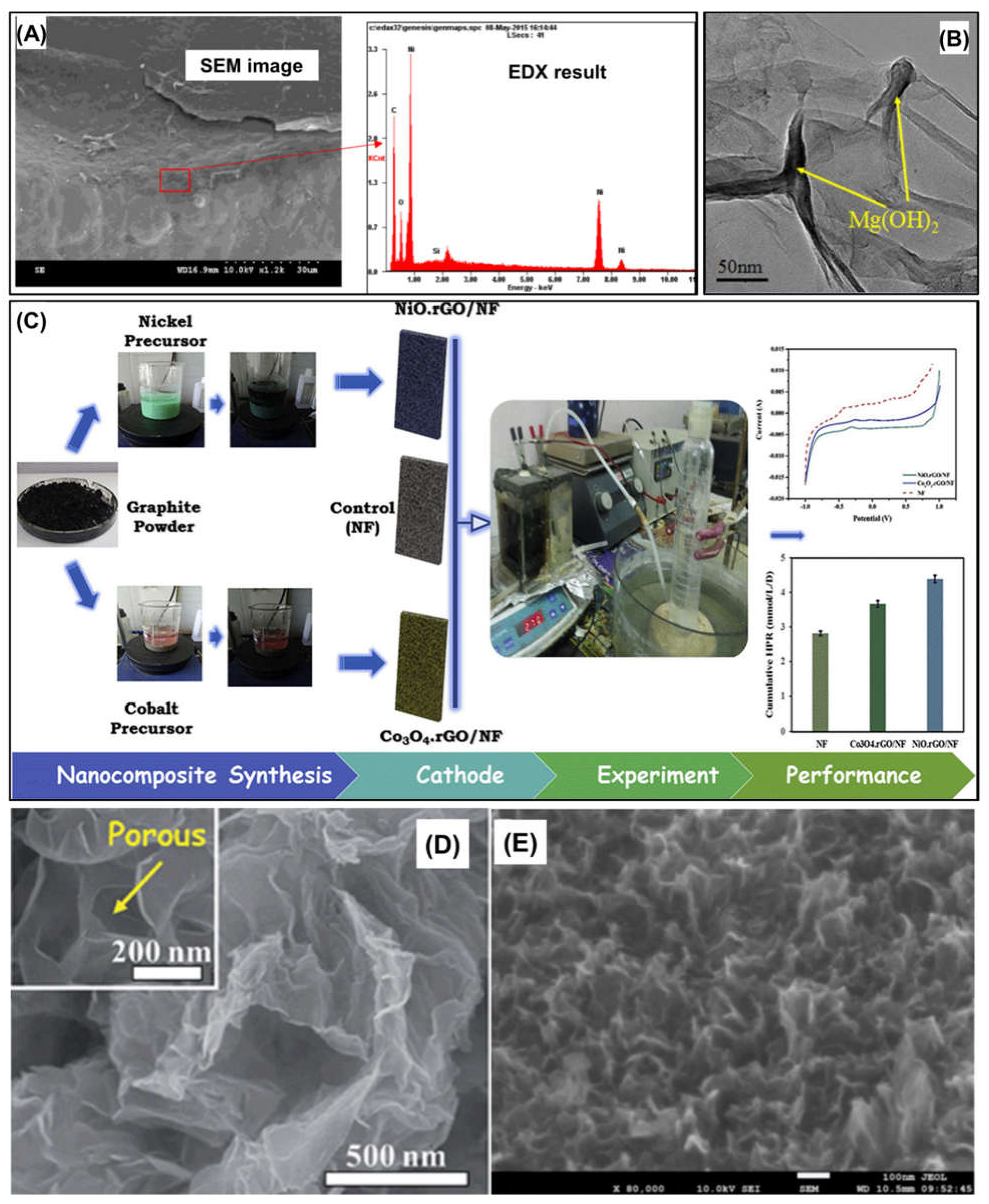
| Cathodic catalyst | Mg(OH)2/graphene nanocomposite | 2D | - | GO nanosheets: modified Hummer’s method; Mg(OH)2/graphene nanocomposites: Hydrothermal method | H2 conversion efficiency: 71% Cathodic H2 recovery efficiency: 83% H2 production rate: 0.63 m3/m3/d | [51] |
| Cathodic catalyst | NiO–rGO nanocomposte | 2D | - | GO nanosheets: modified Hummer’s method; NiO–rGO nanocomposites: chemical reduction | H2 production rate: 4.38 mmol/L/d Cathodic H2 recovery efficiency: 21.2% | [52] |
| Cathodic catalyst | NiCo2O4–rGO nanocomposite | 2D | - | GO nanosheets: modified Hummer’s method; NiO–rGO nanocomposites: chemical reduction | H2 production rate: 3.66 mmol/L/d Cathodic H2 recovery efficiency: 18.2% | [53] |
| Cathodic catalyst | MoS2 nanosheet | 2D | 150–250 | Chemical exfoliation by Li intercalation | H2 production rate: 0.133 m3/m3/d Current density: 0.6 mA/cm2 | [54] |
| Cathode electrode | MoS2/N-doped graphene nanocomposite | 2D | - | GO nanosheets: modified Hummer’s method; MoS2 nanosheet: chemical exfoliation by Li reduction; MoS2–N–GO nanocomposites: hydrothermal method | H2 production rate: 0.19 m3/m3/d | [55] |
| Cathodic catalyst | MoS2–GO (5) nanocomposite | 2D | - | Solvothermal method | H2 production rate: 0.183 m3/m3/d | [56] |
| Cathodic electrode | MoS2–Cu–rGO (6) nanocomposite | 2D | GO nanosheets: modified Hummer’s method; MoS2–Cu–rGO nanocomposites: Hydrothermal method | H2 production rate: 0.449 m3/m3/d | [57] | |
| Cathodic catalyst | MoSx nanoparticle | 3D | - | Electrodeposition method | Cathodic H2 recovery efficiency: 98% | [31] |
| Cathodic catalyst | Y Zeolites–NiO nanocomposite | 3D | - | Y zeolites: Hydrothermal process; Y Zeolite–NiO nanocomposites: incipient wetness impregnation | H2 production rate: 0.83 m3/m3/d | [58] |
| Cathodic catalyst | NiO/MoO2/MoO3/C | 3D | Electrodeposition method | Current density: 37.5 A/m2 | [33] | |
| Cathodic catalyst | CoNi/CoFe2O4 composite | 3D | - | CoFe2O4: Hydrothermal method and calcination; CoNi/CoFe2O4 composite: Unpolar pulse electrodeposition | H2 production rate: 1.25 m3/m3/d | [33] |
| Cathodic catalyst | Activated carbon + Ni210 powder | 3D | 0.5–1 μm | - | H2 production rate: 0.28 m3/m3/d Cathodic H2 recovery efficiency: 98% | [59] |
| Antibiofouling membrane | Ag nanoparticle | 3D | - | Chemical reduction | Biofouling reduction: 80.74% H2 conversion efficiency: 61.82% | [60] |
4. Nanomaterials Used in Microbial Electrolysis Cells
4.1. Anode Electrodes
4.2. Cathode Electrodes and Catalysts
4.2.1. Metal-Based Nanomaterials
Metal Nanoparticles
Metal Compound Nanoparticles
Transition Metal Nanobelt and Nanotube
4.2.2. Carbon-Based Nanomaterials
Carbon Nanoparticles
Carbon Nanotubes
Graphene Derivatives
Other Carbon-Based Nanomaterials
4.2.3. Polymer Nanofiber
4.3. Membrane Modification
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lan, L.; Wood, K.L.; Yuen, C. A holistic design approach for residential net-zero energy buildings: A case study in Singapore. Sustain. Cities Soc. 2019, 50, 101672. [Google Scholar] [CrossRef]
- Buck, H.J. Ending Fossil Fuels: Why Net Zero Is Not Enough; Verso Books: London, UK, 2021. [Google Scholar]
- Howarth, R.W.; Jacobson, M.Z. How green is blue hydrogen? Energy Sci. Eng. 2021, 9, 1676–1687. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Kadier, A.; Kalil, M.S.; Abdeshahian, P.; Chandrasekhar, K.; Mohamed, A.; Azman, N.F.; Logroño, W.; Simayi, Y.; Hamid, A.A. Recent advances and emerging challenges in microbial electrolysis cells (MECs) for microbial production of hydrogen and value-added chemicals. Renew. Sustain. Energy Rev. 2016, 61, 501–525. [Google Scholar] [CrossRef]
- Kadier, A.; Simayi, Y.; Abdeshahian, P.; Azman, N.F.; Chandrasekhar, K.; Kalil, M.S. A comprehensive review of microbial electrolysis cells (MEC) reactor designs and configurations for sustainable hydrogen gas production. Alex. Eng. J. 2016, 55, 427–443. [Google Scholar] [CrossRef] [Green Version]
- Rousseau, R.; Etcheverry, L.; Roubaud, E.; Basséguy, R.; Délia, M.-L.; Bergel, A. Microbial electrolysis cell (MEC): Strengths, weaknesses and research needs from electrochemical engineering standpoint. Appl. Energy 2020, 257, 113938. [Google Scholar] [CrossRef]
- Aydin, M.I.; Karaca, A.E.; Qureshy, A.M.M.I.; Dincer, I. A comparative review on clean hydrogen production from wastewaters. J. Environ. Manag. 2021, 279, 111793. [Google Scholar] [CrossRef]
- Muddasar, M.; Liaquat, R.; Aslam, A.; Ur Rahman, M.Z.; Abdullah, A.; Khoja, A.H.; Latif, K.; Bahadar, A. Performance efficiency comparison of microbial electrolysis cells for sustainable production of biohydrogen—A comprehensive review. Int. J. Energy Res. 2022, 46, 5625–5645. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Park, S.-G.; Pandit, S.; Yang, E.; Ali Abdelkareem, M.; Jang, J.-K.; Chae, K.-J. Scalability of microbial electrochemical technologies: Applications and challenges. Bioresour. Technol. 2022, 345, 126498. [Google Scholar] [CrossRef]
- Leicester, D.; Amezaga, J.; Heidrich, E. Is bioelectrochemical energy production from wastewater a reality? Identifying and standardising the progress made in scaling up microbial electrolysis cells. Renew. Sustain. Energy Rev. 2020, 133, 110279. [Google Scholar] [CrossRef]
- Olabi, A.; Wilberforce, T.; Sayed, E.T.; Elsaid, K.; Rezk, H.; Abdelkareem, M.A. Recent progress of graphene based nanomaterials in bioelectrochemical systems. Sci. Total Environ. 2020, 749, 141225. [Google Scholar] [CrossRef]
- Yuan, H.; Li, J.; Yuan, C.; He, Z. Facile Synthesis of MoS2@CNT as an Effective Catalyst for Hydrogen Production in Microbial Electrolysis Cells. ChemElectroChem 2014, 1, 1828–1833. [Google Scholar] [CrossRef]
- Kim, I.-S.; Chae, K.-J.; Choi, M.-J.; Verstraete, W. Microbial fuel cells: Recent advances, bacterial communities and application beyond electricity generation. Environ. Eng. Res. 2008, 13, 51–65. [Google Scholar] [CrossRef]
- Gil-Carrera, L.; Escapa, A.; Mehta, P.; Santoyo, G.; Guiot, S.; Morán, A.; Tartakovsky, B. Microbial electrolysis cell scale-up for combined wastewater treatment and hydrogen production. Bioresour. Technol. 2013, 130, 584–591. [Google Scholar] [CrossRef]
- Cotterill, S.; Dolfing, J.; Jones, C.; Curtis, T.; Heidrich, E. Low Temperature Domestic Wastewater Treatment in a Microbial Electrolysis Cell with 1 m2 Anodes: Towards System Scale-Up. Fuel Cells 2017, 17, 584–592. [Google Scholar] [CrossRef] [Green Version]
- Escapa, A.; San-Martín, M.; Mateos, R.; Morán, A. Scaling-up of membraneless microbial electrolysis cells (MECs) for domestic wastewater treatment: Bottlenecks and limitations. Bioresour. Technol. 2015, 180, 72–78. [Google Scholar] [CrossRef]
- Baeza, J.A.; Martínez-Miró, À.; Guerrero, J.; Ruiz, Y.; Guisasola, A. Bioelectrochemical hydrogen production from urban wastewater on a pilot scale. J. Power Sources 2017, 356, 500–509. [Google Scholar] [CrossRef]
- Shirpay, A. Effects of electrode size on the power generation of the microbial fuel cell by Saccharomyces cerevisiae. Ionics 2021, 27, 3967–3973. [Google Scholar] [CrossRef]
- An, J.; Kim, B.; Jang, J.K.; Lee, H.-S.; Chang, I.S. New architecture for modulization of membraneless and single-chambered microbial fuel cell using a bipolar plate-electrode assembly (BEA). Biosens. Bioelectron. 2014, 59, 28–34. [Google Scholar] [CrossRef]
- Makihara, K.; Onoda, J.; Miyakawa, T. Low energy dissipation electric circuit for energy harvesting. Smart Mater. Struct. 2006, 15, 1493. [Google Scholar] [CrossRef]
- Yang, E.; Mohamed, H.O.; Park, S.-G.; Obaid, M.; Al-Qaradawi, S.Y.; Castaño, P.; Chon, K.; Chae, K.-J. A review on self-sustainable microbial electrolysis cells for electro-biohydrogen production via coupling with carbon-neutral renewable energy technologies. Bioresour. Technol. 2021, 320, 124363. [Google Scholar] [CrossRef] [PubMed]
- Sleutels, T.H.; Hamelers, H.V.; Rozendal, R.A.; Buisman, C.J. Ion transport resistance in microbial electrolysis cells with anion and cation exchange membranes. Int. J. Hydrogen Energy 2009, 34, 3612–3620. [Google Scholar] [CrossRef]
- Kim, B.; Chang, I.S.; Dinsdale, R.M.; Guwy, A.J. Accurate measurement of internal resistance in microbial fuel cells by improved scanning electrochemical impedance spectroscopy. Electrochim. Acta 2021, 366, 137388. [Google Scholar] [CrossRef]
- Frattini, D.; Karunakaran, G.; Cho, E.-B.; Kwon, Y. Sustainable Syntheses and Sources of Nanomaterials for Microbial Fuel/Electrolysis Cell Applications: An Overview of Recent Progress. Processes 2021, 9, 1221. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, S.; Schaller, R.; Jiao, J.; Chaplen, F.; Liu, H. Nanoparticle decorated anodes for enhanced current generation in microbial electrochemical cells. Biosens. Bioelectron. 2011, 26, 1908–1912. [Google Scholar] [CrossRef]
- Kim, K.N.; Lee, S.H.; Kim, H.; Park, Y.H.; In, S.-I. Improved Microbial Electrolysis Cell Hydrogen Production by Hybridization with a TiO2 Nanotube Array Photoanode. Energies 2018, 11, 3184. [Google Scholar] [CrossRef] [Green Version]
- Hrapovic, S.; Manuel, M.-F.; Luong, J.; Guiot, S.; Tartakovsky, B. Electrodeposition of nickel particles on a gas diffusion cathode for hydrogen production in a microbial electrolysis cell. Int. J. Hydrogen Energy 2010, 35, 7313–7320. [Google Scholar] [CrossRef] [Green Version]
- Chaurasia, A.K.; Goyal, H.; Mondal, P. Hydrogen gas production with Ni, Ni–Co and Ni–Co–P electrodeposits as potential cathode catalyst by microbial electrolysis cells. Int. J. Hydrogen Energy 2020, 45, 18250–18265. [Google Scholar] [CrossRef]
- Tahir, M.B. Microbial photoelectrochemical cell for improved hydrogen evolution using nickel ferrite incorporated WO3 under visible light irradiation. Int. J. Hydrogen Energy 2019, 44, 17316–17322. [Google Scholar] [CrossRef]
- Kokko, M.; Bayerköhler, F.; Erben, J.; Zengerle, R.; Kurz, P.; Kerzenmacher, S. Molybdenum sulphides on carbon supports as electrocatalysts for hydrogen evolution in acidic industrial wastewater. Appl. Energy 2017, 190, 1221–1233. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Wu, A.; Li, J. Preparation of Carbon-Based Composite NiO/MoO2/MoO3/C by Electrodeposition and Its Application in Microbial Electrolysis Cells. Int. J. Electrochem. Sci 2019, 14, 9231–9238. [Google Scholar] [CrossRef]
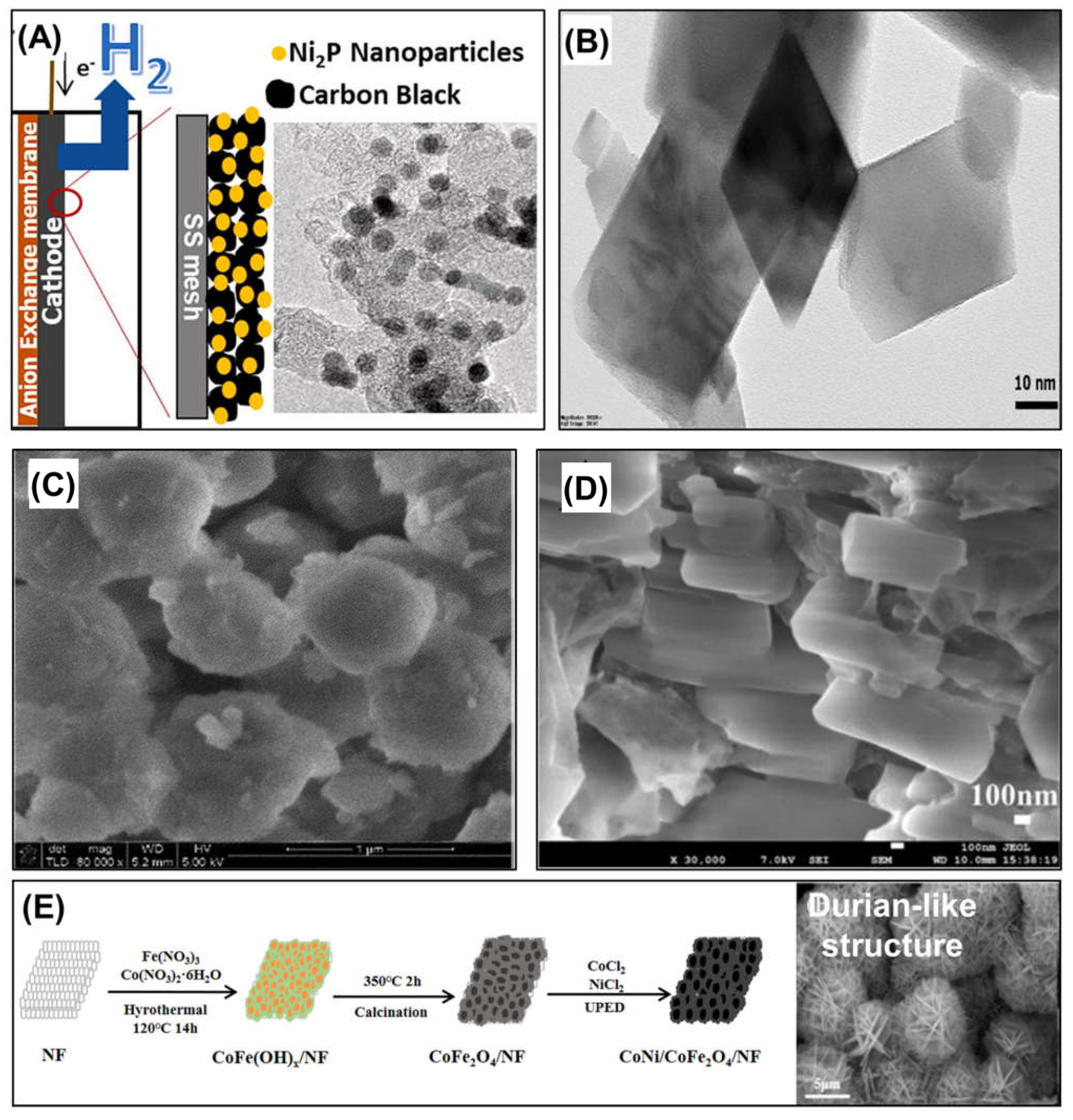
- Fang, X.; Cheng, Y.; Li, S.; Dai, H.; Zhao, Y.; Du, X.; Ma, X.; Hao, X. Fabrication of durian-like CoNi/CoFe2O4 composite electrocatalysts on nickel foam for hydrogen evolution in a microbial electrolysis cell. Int. J. Energy Res. 2022, 46, 340–350. [Google Scholar] [CrossRef]
- Jayabalan, T.; Matheswaran, M.; Radhakrishnan, T.K.; Naina Mohamed, S. Influence of Nickel molybdate nanocatalyst for enhancing biohydrogen production in microbial electrolysis cell utilizing sugar industrial effluent. Bioresour. Technol. 2021, 320, 124284. [Google Scholar] [CrossRef]
- Jayabalan, T.; Naina Mohamed, S.; Matheswaran, M.; Radhakrishnan, T.K.; Pugazhendhi, A.; Alagarsamy, A. Enhanced biohydrogen production from sugar industry effluent using nickel oxide and cobalt oxide as cathode nanocatalysts in microbial electrolysis cell. Int. J. Energy Res. 2021, 45, 17431–17439. [Google Scholar] [CrossRef]
- Rani, G.; Krishna, K.; Yogalakshmi, K. Enhancing the electrochemical performance of Fe3O4 nanoparticles layered carbon electrodes in Microbial Electrolysis Cell. Int. J. Energy Res. 2021, 9, 106326. [Google Scholar] [CrossRef]
- Park, S.-G.; Chae, K.-J.; Lee, M. A sulfonated poly(arylene ether sulfone)/polyimide nanofiber composite proton exchange membrane for microbial electrolysis cell application under the coexistence of diverse competitive cations and protons. J. Membr. Sci. 2017, 540, 165–173. [Google Scholar] [CrossRef]
- Pophali, A.; Singh, S.; Verma, N. Simultaneous hydrogen generation and COD reduction in a photoanode-based microbial electrolysis cell. Int. J. Hydrogen Energy 2020, 45, 25985–25995. [Google Scholar] [CrossRef]
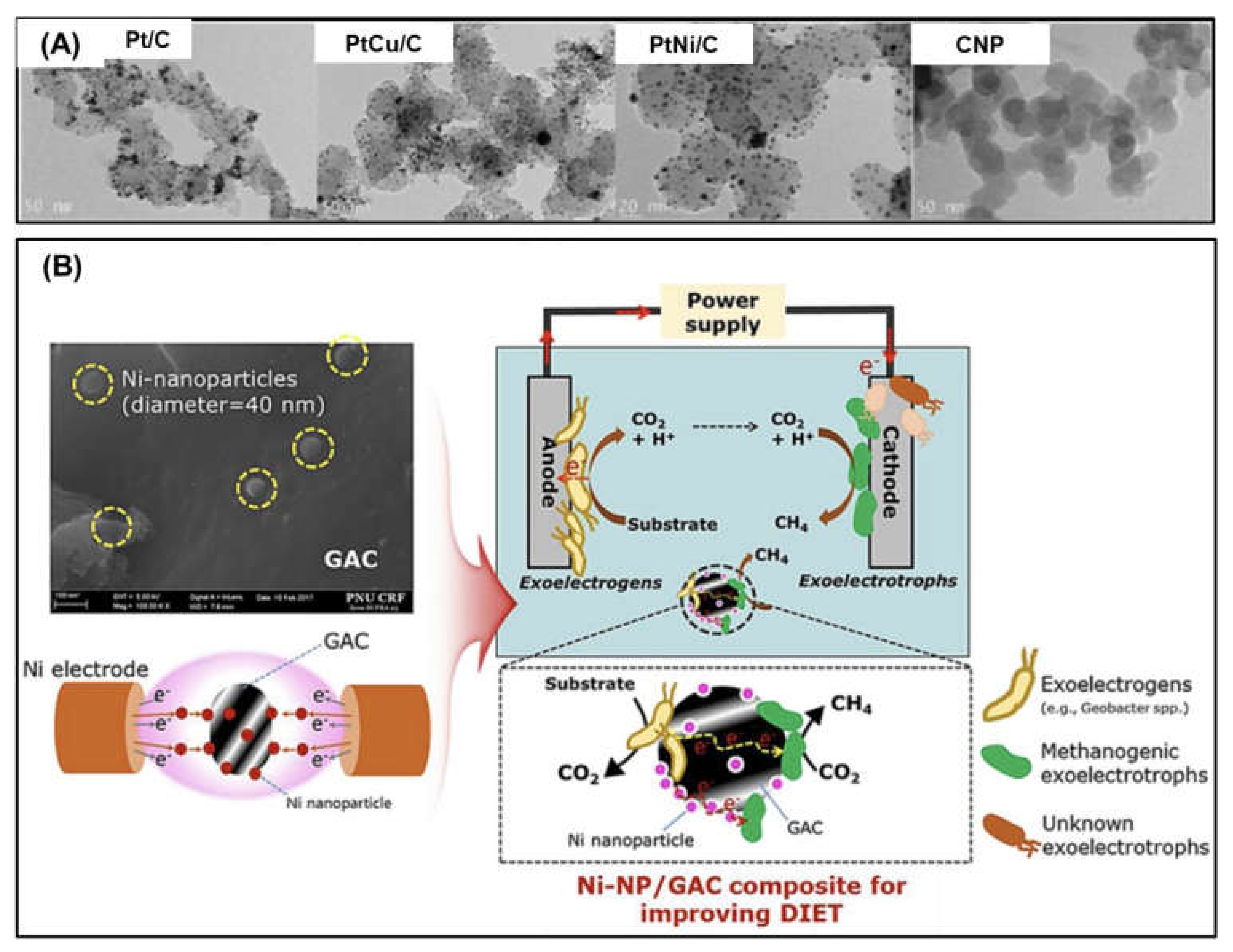
- Choi, M.-J.; Yang, E.; Yu, H.-W.; Kim, I.S.; Oh, S.-E.; Chae, K.-J. Transition metal/carbon nanoparticle composite catalysts as platinum substitutes for bioelectrochemical hydrogen production using microbial electrolysis cells. Int. J. Hydrogen Energy 2019, 44, 2258–2265. [Google Scholar] [CrossRef]
- Kim, K.-R.; Kang, J.; Chae, K.-J. Improvement in methanogenesis by incorporating transition metal nanoparticles and granular activated carbon composites in microbial electrolysis cells. Int. J. Hydrogen Energy 2017, 42, 27623–27629. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, B.; He, Z. Bioelectrochemical deposition of palladium nanoparticles as catalysts by Shewanella oneidensis MR-1 towards enhanced hydrogen production in microbial electrolysis cells. Electrochim. Acta 2019, 318, 794–800. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Habas, S.E.; Schaidle, J.A.; Logan, B.E. Application of phase-pure nickel phosphide nanoparticles as cathode catalysts for hydrogen production in microbial electrolysis cells. Bioresour. Technol. 2019, 293, 122067. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.-Y.; Liu, J.-S.; Liu, Y.; Wang, Y.-H. Hydrogen production on TiO2 nanorod arrays cathode coupling with bio-anode with additional electricity generation. J. Power Sources 2013, 238, 345–349. [Google Scholar] [CrossRef]
- Zeng, L.; Li, X.; Fan, S.; Zhang, M.; Yin, Z.; Tadé, M.; Liu, S. Photo-driven bioelectrochemical photocathode with polydopamine-coated TiO2 nanotubes for self-sustaining MoS2 synthesis to facilitate hydrogen evolution. J. Power Sources 2019, 413, 310–317. [Google Scholar] [CrossRef]
- Lu, S.; Lu, B.; Tan, G.; Moe, W.; Xu, W.; Wang, Y.; Xing, D.; Zhu, X. Mo2N nanobelt cathodes for efficient hydrogen production in microbial electrolysis cells with shaped biofilm microbiome. Biosens. Bioelectron. 2020, 167, 112491. [Google Scholar] [CrossRef]
- Liang, D.; Zhang, L.; He, W.; Li, C.; Liu, J.; Liu, S.; Lee, H.-S.; Feng, Y. Efficient hydrogen recovery with CoP-NF as cathode in microbial electrolysis cells. Appl. Energy 2020, 264, 114700. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Y.; Chen, L.; Li, P.; Zhu, S.; Shen, S. Microbial electrolysis cells with polyaniline/multi-walled carbon nanotube-modified biocathodes. Energy 2015, 88, 377–384. [Google Scholar] [CrossRef]
- Yang, Q.; Jiang, Y.; Xu, Y.; Qiu, Y.; Chen, Y.; Zhu, S.; Shen, S. Hydrogen production with polyaniline/multi-walled carbon nanotube cathode catalysts in microbial electrolysis cells. J. Chem. Technol. Biotechnol. 2015, 90, 1263–1269. [Google Scholar] [CrossRef]
- Jeon, Y.; Kim, S. Persistent Hydrogen Production by the Photo-Assisted Microbial Electrolysis Cell Using a p-Type Polyaniline Nanofiber Cathode. ChemSusChem 2016, 9, 3276–3279. [Google Scholar] [CrossRef]
- Cai, W.; Liu, W.; Han, J.; Wang, A. Enhanced hydrogen production in microbial electrolysis cell with 3D self-assembly nickel foam-graphene cathode. Biosens. Bioelectron. 2016, 80, 118–122. [Google Scholar] [CrossRef] [Green Version]
- Hongyan, D.; Yang, H.; Liu, X.; Jian, X.; Liang, Z. Electrochemical evaluation of nano-Mg(OH)2/graphene as a catalyst for hydrogen evolution in microbial electrolysis cell. Fuel 2016, 174, 251–256. [Google Scholar]
- Jayabalan, T.; Matheswaran, M.; Preethi, V.; Naina Mohamed, S. Enhancing biohydrogen production from sugar industry wastewater using metal oxide/graphene nanocomposite catalysts in microbial electrolysis cell. Int. J. Hydrogen Energy 2020, 45, 7647–7655. [Google Scholar] [CrossRef]
- Jayabalan, T.; Manickam, M.; Naina Mohamed, S. NiCo2O4-graphene nanocomposites in sugar industry wastewater fed microbial electrolysis cell for enhanced biohydrogen production. Renew. Energy 2020, 154, 1144–1152. [Google Scholar] [CrossRef]
- Rozenfeld, S.; Teller, H.; Schechter, M.; Farber, R.; Krichevski, O.; Schechter, A.; Cahan, R. Exfoliated molybdenum di-sulfide (MoS2) electrode for hydrogen production in microbial electrolysis cell. Bioelectrochemistry 2018, 123, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zhang, B.; Wen, Z.; Cui, S.; Guo, X.; He, Z.; Chen, J. A 3D hybrid of layered MoS2/nitrogen-doped graphene nanosheet aerogels: An effective catalyst for hydrogen evolution in microbial electrolysis cells. J. Mater. Chem. A 2014, 2, 13795–13800. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Tu, L.; Qin, S.; Yu, Z.; Yan, Y.; Xu, Y.; Song, H.; Lin, H.; Chen, Y.; Wang, S. Dye wastewater treatment and hydrogen production in microbial electrolysis cells using MoS2-graphene oxide cathode: Effects of dye concentration, co-substrate and buffer solution. Process Biochem. 2021, 102, 51–58. [Google Scholar] [CrossRef]
- Dai, H.; Yang, H.; Liang, Z. Electrochemical Evaluation of MoS2-Cu-RGO as a Catalyst for Hydrogen Evolution in Microbial Electrolysis Cell. Int. J. Electrochem. Sci. 2021, 16, 210458. [Google Scholar] [CrossRef]
- Wang, G.; Bo, Q.; Yang, D.; Li, Y.; Li, Y.; Ge, C. Cathodic hydrogen recovery using Y zeolites loaded nickel(II) Oxide instead of Pt/C in microbial electrolysis cell. Energy Sources Part A Recovery Util. Environ. Eff. 2019. published online. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Logan, B.E. Nickel powder blended activated carbon cathodes for hydrogen production in microbial electrolysis cells. Int. J. Hydrog. Energy 2019, 44, 13169–13174. [Google Scholar] [CrossRef]
- Park, S.-G.; Rajesh, P.; Hwang, M.-H.; Chu, K.H.; Cho, S.; Chae, K.-J. Long-term effects of anti-biofouling proton exchange membrane using silver nanoparticles and polydopamine on the performance of microbial electrolysis cells. Int. J. Hydrogen Energy 2021, 46, 11345–11356. [Google Scholar] [CrossRef]
- Zhou, K.L.; Wang, Z.; Han, C.B.; Ke, X.; Wang, C.; Jin, Y.; Zhang, Q.; Liu, J.; Wang, H.; Yan, H. Platinum single-atom catalyst coupled with transition metal/metal oxide heterostructure for accelerating alkaline hydrogen evolution reaction. Nat. Commun. 2021, 12, 3783. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Huang, Q.; Feng, Y.; Zhu, S.; Shen, S. Hydrogen production with carbon nanotubes based cathode catalysts in microbial electrolysis cells. J. Chem. Technol. Biotechnol. 2012, 87, 1150–1156. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.; Yang, E.; Kim, B.; Obaid, M.; Jang, J.K.; Chae, K.-J. Recent Application of Nanomaterials to Overcome Technological Challenges of Microbial Electrolysis Cells. Nanomaterials 2022, 12, 1316. https://doi.org/10.3390/nano12081316
Kim B, Yang E, Kim B, Obaid M, Jang JK, Chae K-J. Recent Application of Nanomaterials to Overcome Technological Challenges of Microbial Electrolysis Cells. Nanomaterials. 2022; 12(8):1316. https://doi.org/10.3390/nano12081316
Chicago/Turabian StyleKim, Byeongcheol, Euntae Yang, Bongkyu Kim, M. Obaid, Jae Kyung Jang, and Kyu-Jung Chae. 2022. "Recent Application of Nanomaterials to Overcome Technological Challenges of Microbial Electrolysis Cells" Nanomaterials 12, no. 8: 1316. https://doi.org/10.3390/nano12081316
APA StyleKim, B., Yang, E., Kim, B., Obaid, M., Jang, J. K., & Chae, K.-J. (2022). Recent Application of Nanomaterials to Overcome Technological Challenges of Microbial Electrolysis Cells. Nanomaterials, 12(8), 1316. https://doi.org/10.3390/nano12081316









