Piezoelectric Effect Enhanced Photocatalytic Activity of Pt/Bi3.4Gd0.6Ti3O12 Plasmonic Photocatalysis
Abstract
:1. Introduction
2. Experimental Method
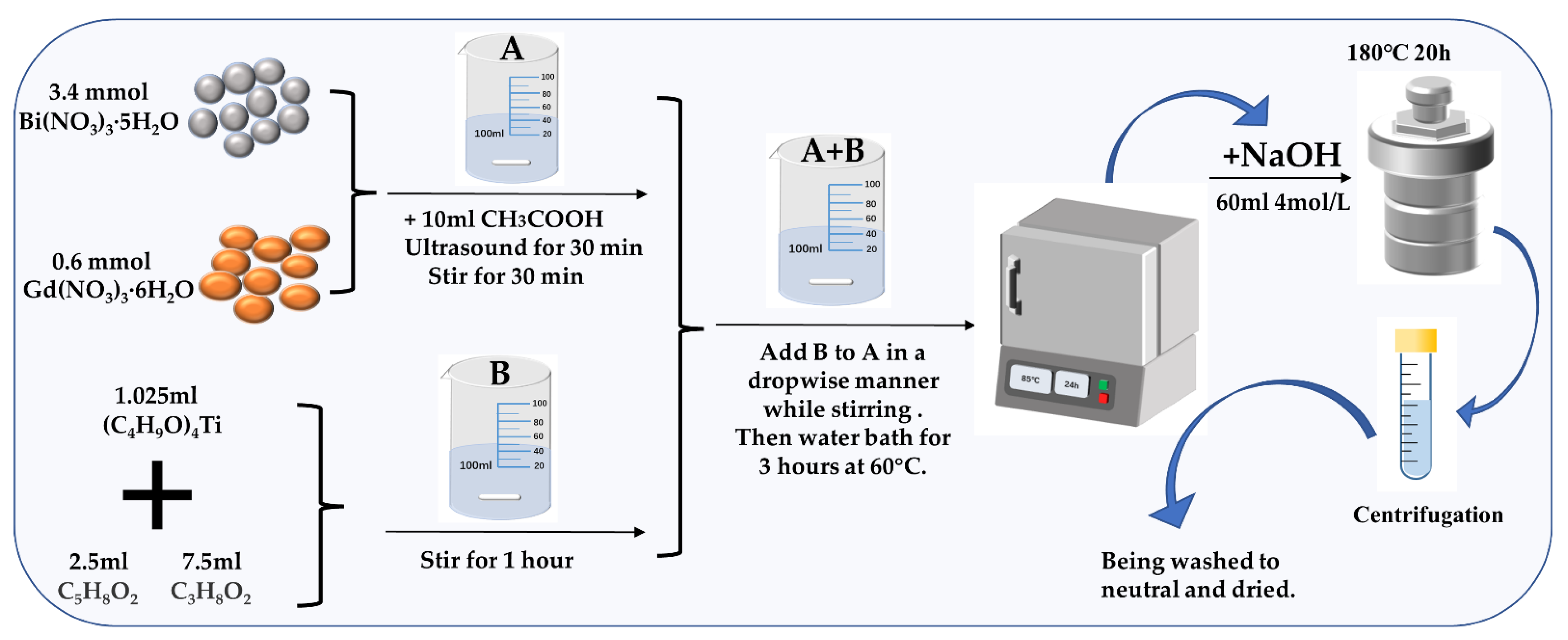
2.1. Synthesis of BGTO and Pt/BGTO Samples
2.2. Samples Characterization
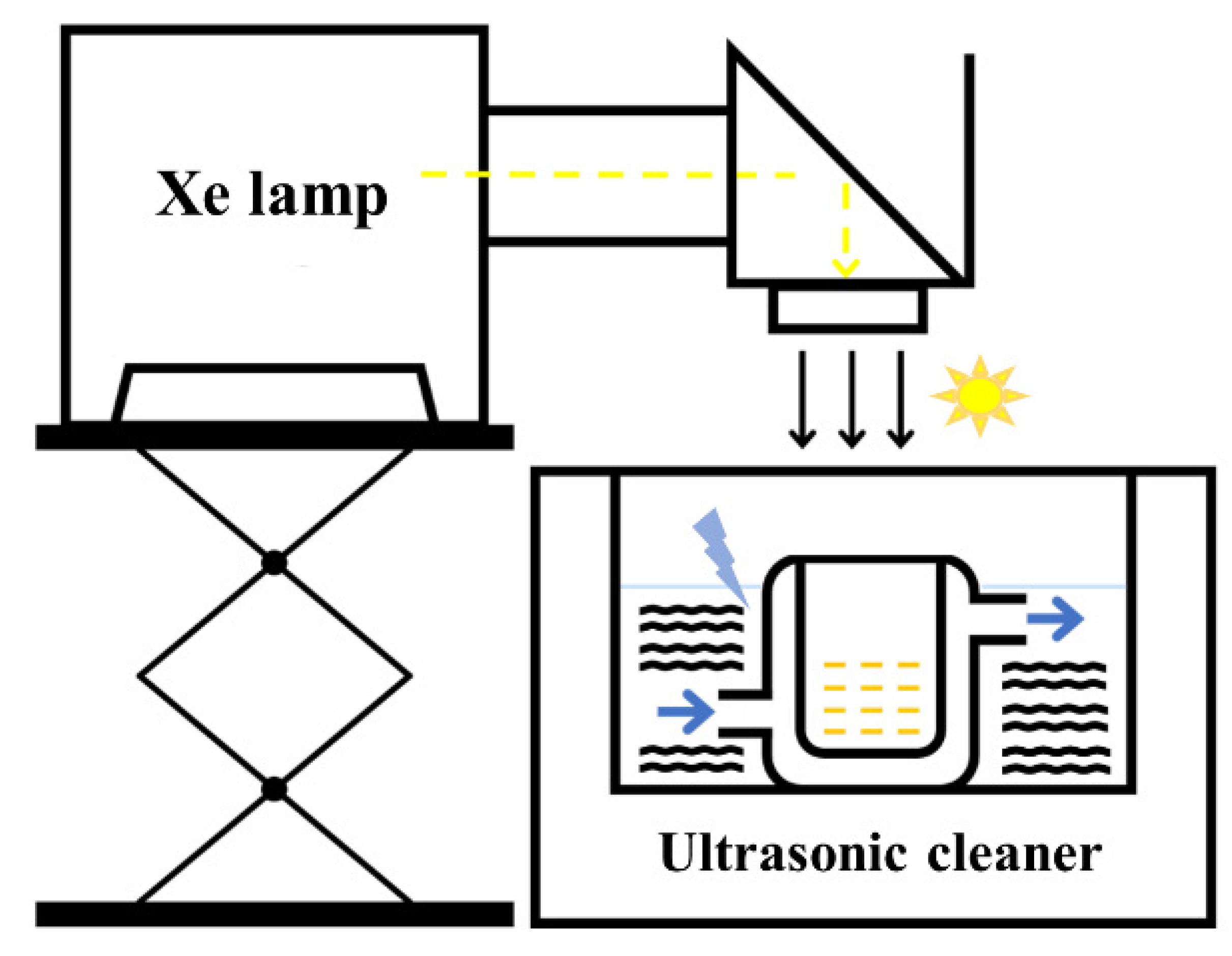
2.3. Catalytic Activity Assessment
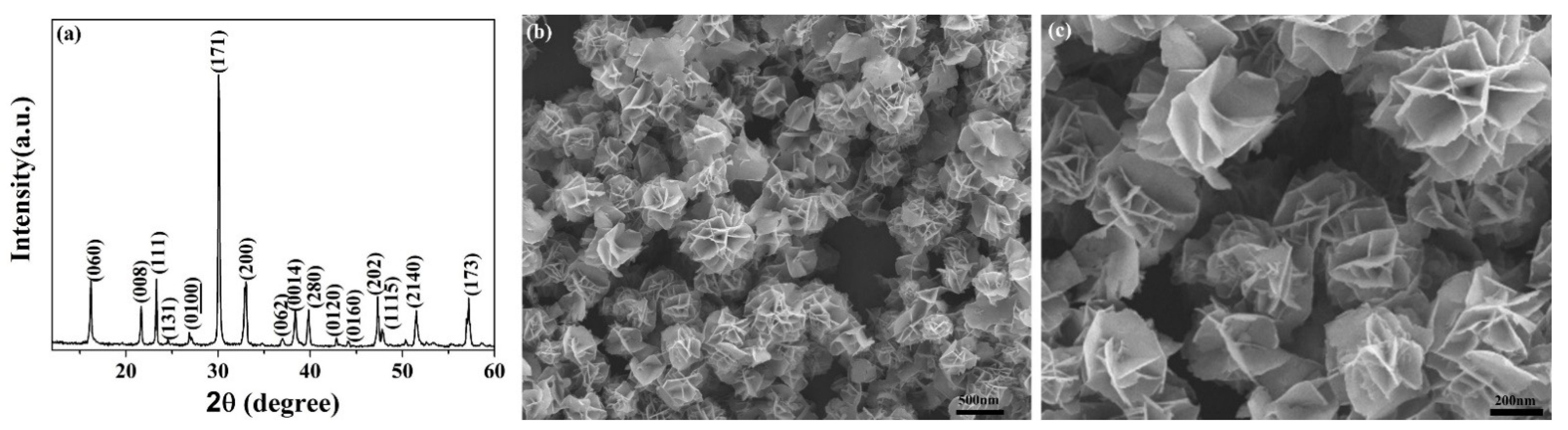
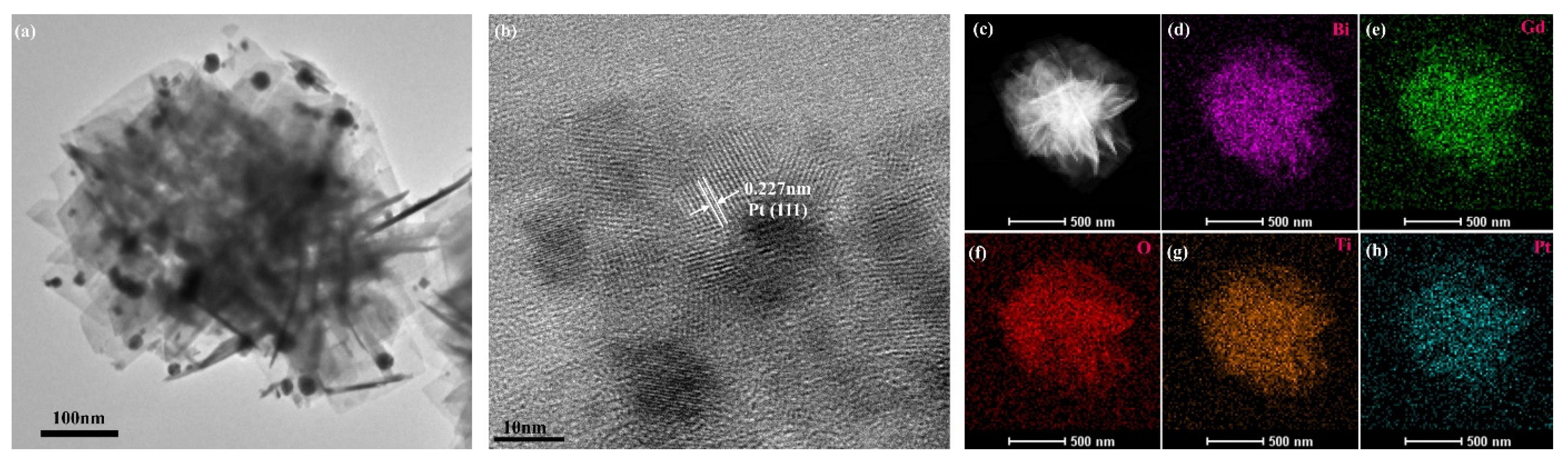
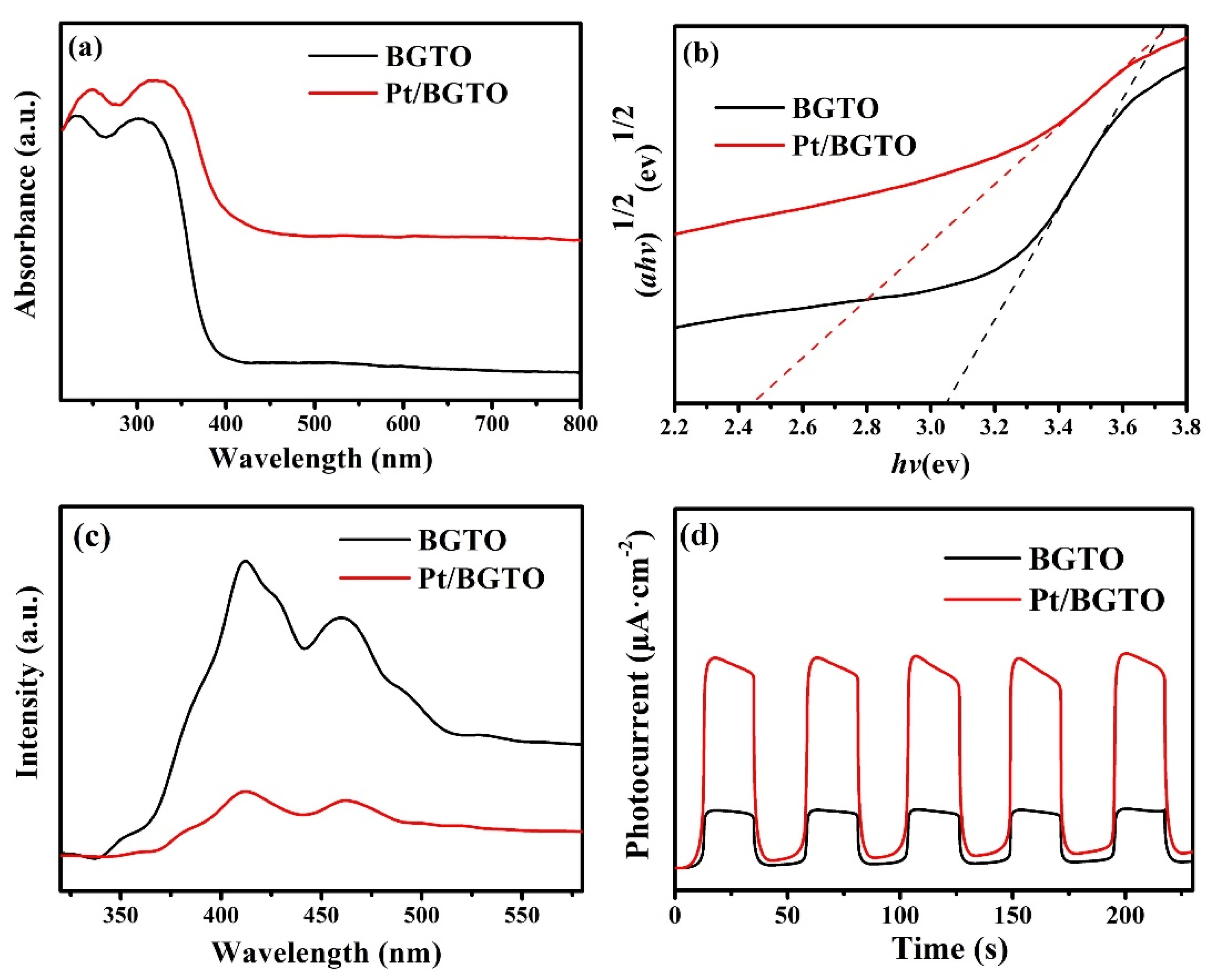
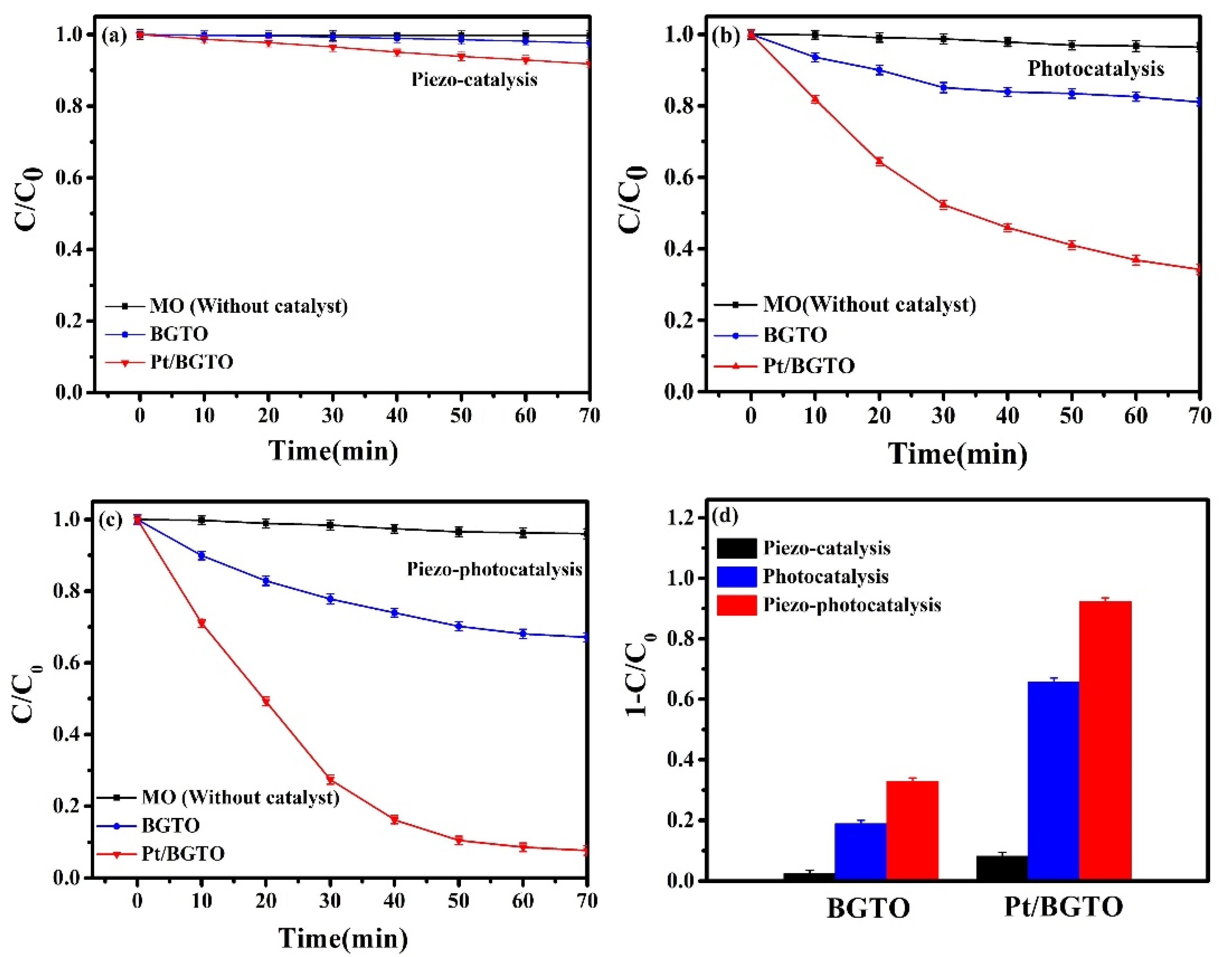
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pera-Titus, M.; García-Molina, V.; Baños, M.A.; Giménez, J.; Esplugas, S. Environmentally persistent free radicals: Insights on a new class of pollutants. Environ. Sci. Technol. 2018, 52, 2468–2481. [Google Scholar]
- Dong, Q.; Chen, Z.W.; Zhao, B.; Zhang, Y.Z.; Lu, Z.Y.; Wang, X.; Li, J.L.; Chen, W. In situ fabrication of niobium pentoxide/graphitic carbon nitride type-II heterojunctions for enhanced photocatalytic hydrogen evolution reaction. J. Colloid Interf. Sci. 2022, 608, 1951–1959. [Google Scholar] [CrossRef]
- Chen, Z.W.; Jiang, X.Y.; Zhu, C.B.; Shi, C.K. Chromium-modified Bi4Ti3O12 photocatalyst: Application forhydrogen evolution and pollutant degradation. Appl. Catal. B 2016, 199, 241–251. [Google Scholar] [CrossRef]
- Liu, D.; Li, C.L.; Zhao, C.Y.; Nie, E.R.; Wang, J.Q.; Zhou, J.; Zhao, Q. Efficient dye contaminant elimination and simultaneously electricity production via a Bi-doped TiO2 photocatalytic fuel cell. Nanomaterials 2022, 12, 210. [Google Scholar] [CrossRef]
- Zhuge, Z.H.; Liu, X.J.; Chen, T.Q.; Gong, Y.Y.; Li, C.; Niu, L.Y.; Xu, S.Q.; Xu, X.T.; Alothman, Z.A.; Sun, C.Q.; et al. Highly efficient photocatalytic degradation of different hazardous contaminants by CaIn2S4-Ti3C2Tx Schottky heterojunction: An experimental and mechanism study. Chem. Eng. J. 2021, 421, 127838. [Google Scholar] [CrossRef]
- Li, S.; Ge, Z.; Zhang, B.; Yao, Y.; Wang, H.; Yang, J.; Li, Y.; Gao, C.; Lin, Y. Mechanochemically synthesized sub-5nm sized CuS quantum dots with high visible-light-driven photocatalytic activity. Appl. Surf. Sci. 2016, 384, 272–278. [Google Scholar] [CrossRef]
- Liu, B.B.; Liu, X.J.; Liu, J.Y.; Feng, C.J.; Li, Z.; Li, C.; Gong, Y.Y.; Pan, L.K.; Xu, S.Q.; Sun, C.Q. Efficient charge separation between UiO-66 and ZnIn2S4 flowerlike 3D microspheres for photoelectronchemical properties. Appl. Catal. B 2018, 226, 234–241. [Google Scholar] [CrossRef]
- Vu, N.-N.; Kaliaguine, S.; Do, T.-O. Critical Aspects and Recent Advances in Structural Engineering of Photocatalysts for Sunlight-Driven Photocatalytic Reduction of CO2 into Fuels. Adv. Funct. Mater. 2019, 29, 1901825. [Google Scholar] [CrossRef]
- Du, C.; Yan, B.; Lin, Z.; Yang, G. Enhanced carrier separation and increased electron density in 2D heavily N-doped ZnIn2S4 for photocatalytic hydrogen production. J. Mater. Chem. A 2020, 8, 207–217. [Google Scholar] [CrossRef]
- Ju, L.; Shang, J.; Tang, X.; Kou, L.Z. Tunable photocatalytic water splitting by the ferroelectric switch in a 2D AgBiP2Se6 monolayer. J. Am. Chem. Soc. 2020, 142, 1492–1500. [Google Scholar] [CrossRef]
- Rong, X.; Chen, H.; Rong, J.; Zhang, X.; Wei, J.; Liu, S.; Zhou, X.; Xu, J.; Qiu, F.; Wu, Z. An all-solid-state Z-scheme TiO2/ZnFe2O4 photocatalytic system for the N2 photofixation enhancement. Chem. Eng. J. 2019, 371, 286–293. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Lan, S.; Zeng, X.; Rather, R.A.; Lo, I.M.C. Enhanced trimethoxypyrimidine degradation by piezophotocatalysis of BaTiO3/Ag3PO4 using mechanical vibration and visible light simultaneously. Environ. Sci. Nano 2019, 6, 554–564. [Google Scholar] [CrossRef]
- Wu, J.; Qin, N.; Bao, D. Effective enhancement of piezocatalytic activity of BaTiO3 nanowires under ultrasonic vibration. Nano Energy 2018, 45, 44–51. [Google Scholar] [CrossRef]
- Feng, Y.; Li, H.; Ling, L.; Yan, S.; Pan, D.; Ge, H.; Li, H.; Bian, Z. Enhanced photocatalytic degradation performance by fluid-induced piezoelectric field. Environ. Sci. Technol. 2018, 52, 7842–7848. [Google Scholar] [CrossRef]
- You, H.; Wu, Z.; Zhang, L.; Ying, Y.; Liu, Y.; Fei, L.; Chen, X.; Jia, Y.; Wang, Y.; Wang, F.; et al. Harvesting the vibration energy of BiFeO3 nanosheets for hydrogen evolution. Angew. Chem. 2019, 58, 11779–11784. [Google Scholar] [CrossRef] [PubMed]
- Starr, M.B.; Shi, J.; Wang, X. Piezopotential-driven redox reactions at the surface of piezoelectric materials. Angew. Chem. Int. Ed. 2012, 51, 5962–5966. [Google Scholar] [CrossRef]
- Ma, W.; Yao, B.; Zhang, W.; He, Y.; Yu, Y.; Niu, J.; Wang, C. A novel multi-flaw MoS2 nanosheet piezocatalyst with superhigh degradation efficiency for ciprofloxacin. Environ. Sci. Nano 2018, 5, 2876–2887. [Google Scholar] [CrossRef]
- You, H.; Ma, X.; Wu, Z.; Fei, L.; Chen, X.; Yang, J.; Liu, Y.; Jia, Y.; Li, H.; Wang, F.; et al. Piezoelectrically/pyroelectrically-driven vibration/cold-hot energy harvesting for mechano-/pyro-bi-catalytic dye decomposition of NaNbO3 nanofibers. Nano Energy 2018, 52, 351–359. [Google Scholar] [CrossRef]
- Hong, K.; Xu, H.; Konishi, H.; Li, X. Direct water splitting through vibrating piezoelectric microfibers in water. J. Phys. Chem. Lett. 2010, 1, 997–1002. [Google Scholar] [CrossRef]
- Xu, X.; Jia, Y.; Xiao, L.; Wu, Z. Strong vibration-catalysis of ZnO nanorods for dye wastewater decolorization via piezoelectro-chemical coupling. Chemosphere 2018, 193, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Sun, J.; Liu, X.; Zhu, J.; Xiong, Y.; Tian, S. Enhancement and mechanism of nano-BaTiO3 piezocatalytic degradation of tricyclazole by co-loading Pt and RuO2. Environ. Sci. Nano 2019, 6, 2241–2252. [Google Scholar] [CrossRef]
- Xu, X.; Wu, Z.; Xiao, L.; Jia, Y.; Ma, J.; Wang, F.; Lang, W.; Wang, M.; Huang, H. Strong piezo-electro-chemical effect of piezoelectric BaTiO3 nanofibers for vibration-catalysis. J. Alloys Compd. 2018, 762, 915–921. [Google Scholar] [CrossRef]
- Lin, H.; Wu, Z.; Jia, Y.; Li, W.; Zheng, R.; Luo, H. Piezoelectrically induced mechano-catalytic effect for degradation of dye wastewater through vibrating Pb(Zr0.52-Ti0.48)O3 fibers. Appl. Phys. Lett. 2014, 104, 162907. [Google Scholar] [CrossRef]
- Feng, Y.; Ling, L.; Wang, Y.; Xu, Z.; Cao, F.; Li, H.; Bian, Z. Engineering spherical lead zirconate titanate to explore the essence of piezo-catalysis. Nano Energy 2017, 40, 481–486. [Google Scholar] [CrossRef]
- You, H.; Jia, Y.; Wu, Z.; Xu, X.; Qian, W.; Xia, Y.; Ismail, M. Strong piezo-electrochemical effect of multiferroic BiFeO3 square micro-sheets for mechanocatalysis. Electrochem. Commun. 2017, 79, 55–58. [Google Scholar] [CrossRef]
- You, H.; Wu, Z.; Jia, Y.; Xu, X.; Xia, Y.; Han, Z.; Wang, Y. High-efficiency and mechano-/photo-bi-catalysis of piezoelectric-ZnO@photoelectric-TiO2 core-shell nanofibers for dye decomposition. Chemosphere 2017, 183, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, J. Synergistically catalytic activities of BiFeO3/TiO2 core-shell nanocomposites for degradation of organic dye molecule through piezophototronic effect. Nano Energy 2019, 56, 74–81. [Google Scholar] [CrossRef]
- Li, H.; Sang, Y.; Chang, S.; Huang, X.; Zhang, Y.; Yang, R.; Jiang, H.; Liu, H.; Wang, Z.L. Enhanced Ferroelectric-Nanocrystal-Based Hybrid Photocatalysis by Ultrasonic-Wave-Generated Piezophototronic Effect. Nano Lett. 2015, 15, 2372–2379. [Google Scholar] [CrossRef]
- Fu, B.; Li, J.J.; Jiang, H.D.; He, X.L.; Ma, Y.M.; Wang, J.K.; Hu, C.Z. Modulation of electric dipoles inside electrospun BaTiO3@TiO2 core-shell nanofibers for enhanced piezo-photocatalytic degradation of organic pollutants. Nano Energy 2022, 93, 106841. [Google Scholar] [CrossRef]
- Xue, X.; Zang, W.; Deng, P.; Wang, Q.; Xing, L.; Zhang, Y.; Wang, Z. Piezo-potential enhanced photocatalytic degradation of organic dye using ZnO nanowires. Nano Energy 2015, 13, 414–422. [Google Scholar] [CrossRef]
- Jia, S.F.; Su, Y.P.; Zhang, B.P.; Zhao, Z.C.; Li, S.; Zhang, Y.F.; Li, P.C.; Xu, M.Y.; Ren, R. Few-layer MoS2 nanosheet-coated KNbO3 nanowire heterostructures: Piezo-photocatalytic effect enhanced hydrogen production and organic pollutant degradation. Nanoscale 2019, 11, 7690–7700. [Google Scholar] [CrossRef]
- Suljo, L.; Phillip, C.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar]
- Zhang, N.; Liu, S.; Xu, Y.-J. Recent Progress on Metal Core@Semiconductor Shell Nanocomposites as a Promising Type of Photocatalyst. Nanoscale 2012, 4, 2227–2238. [Google Scholar] [CrossRef] [PubMed]
- Quhe, R.G.; Liu, J.C.; Wu, J.; Yang, J.; Wang, Y.; Li, Q.; Li, T.; Yang, J.; Peng, H.; Lei, M.; et al. High-performance sub-10 nm monolayer Bi2O2Se transistors. Nanoscale 2019, 11, 532–540. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Chen, W. Plasmonic Ag/AgBr nanohybrid: Synergistic effect of SPR with photographic sensitivity for enhanced photocatalytic activity and stability. Dalton Trans. 2012, 41, 4866–4870. [Google Scholar] [CrossRef]
- Chen, J.J.; Wu, J.C.S.; Wu, P.C.; Tsai, D.P. Plasmonic Photocatalyst for H2 Evolution in Photocatalytic Water Splitting. J. Phys. Chem. C 2011, 115, 210–216. [Google Scholar] [CrossRef]
- Koichi, A.; Makoto, F.; Carsten, R.; Junji, T.; Hirotaka, M.; Yoshimichi, O.; Naoya, Y.; Toshiya, W. A Plasmonic Photocatalyst Consisting of Silver Nanoparticles Embedded in Titanium Dioxide. J. Am. Chem. Soc. 2008, 130, 1676–1680. [Google Scholar]
- Wang, P.; Huang, B.; Dai, Y.; Whangbo, M.H. Plasmonic photocatalysts: Harvesting visible light with noble metal nanoparticles. Phys. Chem. Chem. Phys. 2012, 14, 9813–9825. [Google Scholar] [CrossRef]
- Li, R.; Han, H.; Zhang, F.; Wang, D.; Li, C. Highly efficient photocatalysts constructed by rational assembly of dual-cocatalysts separately on different facets of BiVO4. Energy Environ. Sci. 2014, 7, 1369–1376. [Google Scholar] [CrossRef]
- Wang, M.; Chen, J.; Liao, X.; Liu, Z.; Zhang, J.; Gao, L.; Li, Y. Highly efficient photocatalytic hydrogen production of platinum nanoparticle-decorated SiC nanowires under simulated sunlight irradiation. Int. J. Hydrogen Energy 2012, 39, 14581–14587. [Google Scholar] [CrossRef]
- Chen, C.; Ma, W.; Zhao, J. Semiconductor-mediated photodegradation of pollutants under visible-light irradiation. Chem. Soc. Rev. 2010, 39, 4206–4219. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.; Huang, H.; Zhang, T.; Zhang, Y. Controllable synthesis of multi-responsive ferroelectric layered perovskite-like Bi4Ti3O12: Photocatalysis and piezoelectric-catalysis and mechanism insight. Appl. Catal. B Environ. 2017, 219, 550–562. [Google Scholar] [CrossRef]
- Li, J.; Dong, X.; Zhang, G.; Cui, W.; Cen, W.; Wu, Z.; Lee, S.C.; Dong, F. Probing ring-opening pathways for efficient photocatalytic toluene decomposition. J. Mater. Chem. A 2019, 7, 3366–3374. [Google Scholar] [CrossRef]
- Kim, S.S.; Bae, J.C.; Kim, W.J. Fabrication and ferroelectric studies of (Bi, Gd)4Ti3O12 thin films grown on Pt/Ti/SiO2/Si and p-type Si substrates. J. Cryst. Growth 2005, 274, 394–401. [Google Scholar] [CrossRef]
- Shimakawa, Y.; Kubo, Y.; Tauchi, Y.; Asano, H.; Kamiyama, T.; Izumi, F.; Hiroi, Z. Crystal and electronic structures of Bi4−xLaxTi3O12 ferroelectric materials. Appl. Phys. Lett. 2001, 79, 2791–2793. [Google Scholar] [CrossRef]
- Tomar, M.; Melgarejo, R.; Hidalgo, A.; Mazumder, S.; Katiyar, R. Structural and ferroelectric studies of Bi3.44La0.56Ti3O12 films. Appl. Phys. Lett. 2003, 83, 341–343. [Google Scholar] [CrossRef]
- Qi, L.F.; Yu, J.G.; Mietek, J. Preparation and enhanced visible-light photocatalytic H2-production activity of CdS-sensitized Pt/TiO2 nanosheets with exposed (001) facets. Phys. Chem. Chem. Phys. 2011, 13, 8915–8923. [Google Scholar] [CrossRef] [PubMed]
- Nagababu, P.; Ahmed, S.A.M.; Prabhu, Y.T.; Kularkar, A.; Bhowmick, S.; Rayalu, S.S. Synthesis of Ni2P/CdS and Pt/TiO2 nano-composite for photoreduction of CO2 into methanol. Sci. Rep. 2021, 11, 8084. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.M.; Wang, Y.Q.; Zhang, X.C.; Wang, R.X.; Kou, L.F.; Wang, J.C.; Li, R.; Fan, C.M. Millimeter-level nitrogen modified activated carbon spheres assisted Bi4Ti3O12 composites for bifunctional adsorption/photoreduction of CO2. Chem. Eng. J. 2021, 417, 128218. [Google Scholar] [CrossRef]
- Gao, T.; Chen, Z.; Zhu, Y.; Niu, F.; Huang, Q.; Qin, L.; Sun, X.; Huang, Y. Synthesis of BiFeO3 nanoparticles for the visible-light induced photocatalytic property. Mater. Res. Bull. 2014, 59, 6–12. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, H.; Jin, W.; Shi, C. Enhanced photocatalytic performance over Bi4Ti3O12 nanosheets with controllable size and exposed {001} facets for Rhodamine B degradation. Appl. Catal. B Environ. 2016, 180, 698–706. [Google Scholar] [CrossRef]
- Jovalekic, C.; Pavlovic, M.; Osmokrovic, P.; Atanasoska, L. X-ray photoelectron spectroscopy study of Bi4Ti3O12 ferroelectric ceramics. Appl. Phys. Lett. 1998, 72, 1051–1053. [Google Scholar] [CrossRef]
- Yang, K.L.; Li, J.; Peng, Y.; Lin, J. Enhanced visible light photocatalysis over Pt-loaded Bi2O3: An insight into its photogenerated charge separation, transfer and capture. Phys. Chem. Chem. Phys. 2017, 19, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.Z.; Wang, Z.B.; Chu, Y.Y.; Gu, D.M.; Yin, G.P. Ultrahigh stable carbon riveted Pt/TiO2-C catalyst prepared by in situ carbonized glucose for proton exchange membrane fuel cell. Energy Environ. Sci. 2011, 4, 728–735. [Google Scholar] [CrossRef]
- Li, R.; Chen, W.; Kobayashi, H.; Ma, C. Platinum-nanoparticle-loaded bismuth oxide: An efficient plasmonic photocatalyst active under visible light. Green Chem. 2010, 12, 212–215. [Google Scholar] [CrossRef]
- Yu, J.; Qi, L.; Jaroniec, M. Hydrogen production by photocatalytic water splitting over Pt/TiO2 nanosheets with exposed (001) facets. J. Phys. Chem. C 2010, 114, 13118–13125. [Google Scholar] [CrossRef]
- Wang, B.; Li, C.; Cui, H.; Zhang, J.; Zhai, J.; Li, Q. Fabrication and enhanced visible-light photocatalytic activity of Pt-deposited TiO2 hollow nanospheres. Chem. Eng. J. 2013, 223, 592–603. [Google Scholar] [CrossRef]
- Sayama, K.; Nomura, A.; Arai, T.; Sugita, T.; Abe, R.; Yanagida, M.; Oi, T.; Iwasaki, Y.; Abe, Y.; Sugihara, H. Photoelectrochemical decomposition of water into H2 and O2 on porous BiVO4 thin-film electrodes under visible light and significant effect of Ag ion treatment. J. Phys. Chem. B 2006, 110, 11352–11360. [Google Scholar] [CrossRef] [PubMed]
- Niu, F.; Chen, D.; Qin, L.; Gao, T.; Zhang, N.; Wang, S.; Chen, Z.; Wang, J.; Sun, X.; Huang, Y. Synthesis of Pt/BiFeO3 heterostructured photocatalysts for highly efficient visible-light photocatalytic performances. Sol. Energy Mater. Sol. Cells 2015, 143, 386–396. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Li, J.; Lu, Z.; Wang, X. Self-assembled synthesis of oxygen-doped g-C3N4 nanotubes in enhancement of visible-light photocatalytic hydrogen. J. Energy Chem. 2021, 54, 36–44. [Google Scholar] [CrossRef]
- Guo, S.; Deng, Z.; Li, M.; Jiang, B.; Tian, C.; Pan, Q.; Fu, H. Phosphorus-Doped Carbon Nitride Tubes with a Layered Micro-nanostructure for Enhanced Visible-Light Photocatalytic Hydrogen Evolution. Angew. Chem. 2016, 55, 1830–1834. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.; Itzkan, I.; Dasari, R.; Feld, M. Single Molecule Detection Using Surface-Enhanced Raman Scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Wei, L.; Li, S.; Zhu, L.; Su, Y.; Liu, Y.; Bu, Y.; Lin, Y.; Liu, W.; Zhang, Z. Exclusive enhancement of catalytic activity in Bi0.5Na0.5TiO3 nanostructures: New insights into the design of efficient piezocatalysts and piezophotocatalysts. J. Mater. Chem. A 2020, 8, 16238–16245. [Google Scholar] [CrossRef]
- Chen, J.; Liao, B.; Liao, X.; Xie, H.; Yu, Y.; Hou, S.; Wang, C.; Fan, X. Strain-driven polarized electric field-promoted photocatalytic activity in borate-based CsCdBO3 bulk materials. ACS Appl. Mater. Interfaces 2021, 13, 34202–34212. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yu, J.; Jaroniec, M. All-Solid-State Z-Scheme Photocatalytic Systems. Adv. Mater. 2014, 26, 4920–4935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Y.; Liu, R.; Tasi, D. Plasmonic photocatalysis. Rep. Prog. Phys. 2013, 76, 046401. [Google Scholar] [CrossRef] [Green Version]
- Brongersma, M.L.; Halas, N.J.; Nordlander, P. Plasmon-induced hot carrier science and technology. Nat. Nanotechnol. 2015, 10, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.Z.; Wang, Z.L. Piezotronics and piezo-phototronics for adaptive electronics and optoelectronics. Nat. Rev. Mater. 2016, 1, 16031. [Google Scholar] [CrossRef]
- Hu, Y.; Pan, C.; Wang, Z. Recent progress in piezo-phototronics with extended materials, application areas and understanding. Semicond. Sci. Technol. 2017, 32, 053002. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Liu, S.; Wang, Z.; Zhou, Y.; Qin, Y.; Wang, Z. Piezotronic effect enhanced photocatalysis in strained anisotropic ZnO/TiO2 nanoplatelets via thermal stress. ACS Nano 2016, 10, 2636–2643. [Google Scholar] [CrossRef]
- Yu, X.; Wang, S.; Zhang, X.; Qi, A.; Qiao, X.; Liu, Z.; Wu, M.; Li, L.; Wang, Z. Heterostructured nanorod array with piezophototronic and plasmonic effect for photodynamic bacteria killing and wound healing. Nano Energy 2018, 46, 29–38. [Google Scholar] [CrossRef]
- Sharma, M.; Singh, G.; Vaish, R. Dye degradation and bacterial disinfection using multicatalytic BaZr0.02Ti0.98O3 ceramics. J. Am. Ceram. Soc. 2020, 13, 4778–4784. [Google Scholar] [CrossRef]
- Sharma, M.; Singhal, T.; Vaish, R. Effect of ferroelectric polarization on piezo/photo-catalysis in Ag nanoparticles loaded 0.5(Ba0.7Ca0.3)TiO3-0.5Ba(Zr0.1Ti0.9)O3 composites towards the degradation of organic pollutants. J. Am. Ceram. Soc. 2022, 105, 3165–3176. [Google Scholar] [CrossRef]
- Liu, X.F.; Xiao, L.Y.; Zhang, Y.; Sun, H.J. Significantly enhanced piezo-photocatalytic capability in BaTiO3 nanowires for degrading organic dye. J. Mater. 2020, 6, 256–262. [Google Scholar] [CrossRef]
- Li, Y.J.; Wang, Q.Q.; Wang, H.X.; Tian, J.; Cui, H.Z. Novel Ag2O nanoparticles modified MoS2 nanoflowers for piezoelectric-assisted full solar spectrum photocatalysis. J. Colloid Interface Sci. 2019, 537, 206–214. [Google Scholar] [CrossRef]
- Zhou, X.F.; Sun, Q.W.; Zhai, D.; Xue, G.L.; Luo, H.; Zhang, D. Excellent catalytic performance of molten-salt-synthesized Bi0.5Na0.5TiO3 nanorods by the piezo-phototronic coupling effect. Nano Energy 2021, 84, 105936. [Google Scholar] [CrossRef]
- Lu, L.Z.; Liang, N.; Li, X.F.; Sun, H.Q.; Zhang, Q.W.; Hao, X.H. Highly efficient synergetic piezo/photocatalytic degradation in novel M0.5Bi2.5Nb2O9 (M=Li, Na, K) ferroelectric nanosheets. Ceram. Int. 2021, 47, 8573–8583. [Google Scholar] [CrossRef]
- Bai, Y.; Zhao, J.Z.; Li, Y.Y.; Lv, Z.L.; Lu, K. Preparation and photocatalytic performance of TiO2/PbTiO3 fiber composite enhanced by external force induced piezoelectric field. J. Am. Ceram. Soc. 2019, 102, 5415–5423. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, F.; Wu, S.; Chen, Z.; Lu, Z. Piezoelectric Effect Enhanced Photocatalytic Activity of Pt/Bi3.4Gd0.6Ti3O12 Plasmonic Photocatalysis. Nanomaterials 2022, 12, 1170. https://doi.org/10.3390/nano12071170
Liang F, Wu S, Chen Z, Lu Z. Piezoelectric Effect Enhanced Photocatalytic Activity of Pt/Bi3.4Gd0.6Ti3O12 Plasmonic Photocatalysis. Nanomaterials. 2022; 12(7):1170. https://doi.org/10.3390/nano12071170
Chicago/Turabian StyleLiang, Fengjuan, Shijun Wu, Zhiwu Chen, and Zhenya Lu. 2022. "Piezoelectric Effect Enhanced Photocatalytic Activity of Pt/Bi3.4Gd0.6Ti3O12 Plasmonic Photocatalysis" Nanomaterials 12, no. 7: 1170. https://doi.org/10.3390/nano12071170
APA StyleLiang, F., Wu, S., Chen, Z., & Lu, Z. (2022). Piezoelectric Effect Enhanced Photocatalytic Activity of Pt/Bi3.4Gd0.6Ti3O12 Plasmonic Photocatalysis. Nanomaterials, 12(7), 1170. https://doi.org/10.3390/nano12071170




