Synchronous Defect and Interface Engineering of NiMoO4 Nanowire Arrays for High-Performance Supercapacitors
Abstract
:1. Introduction
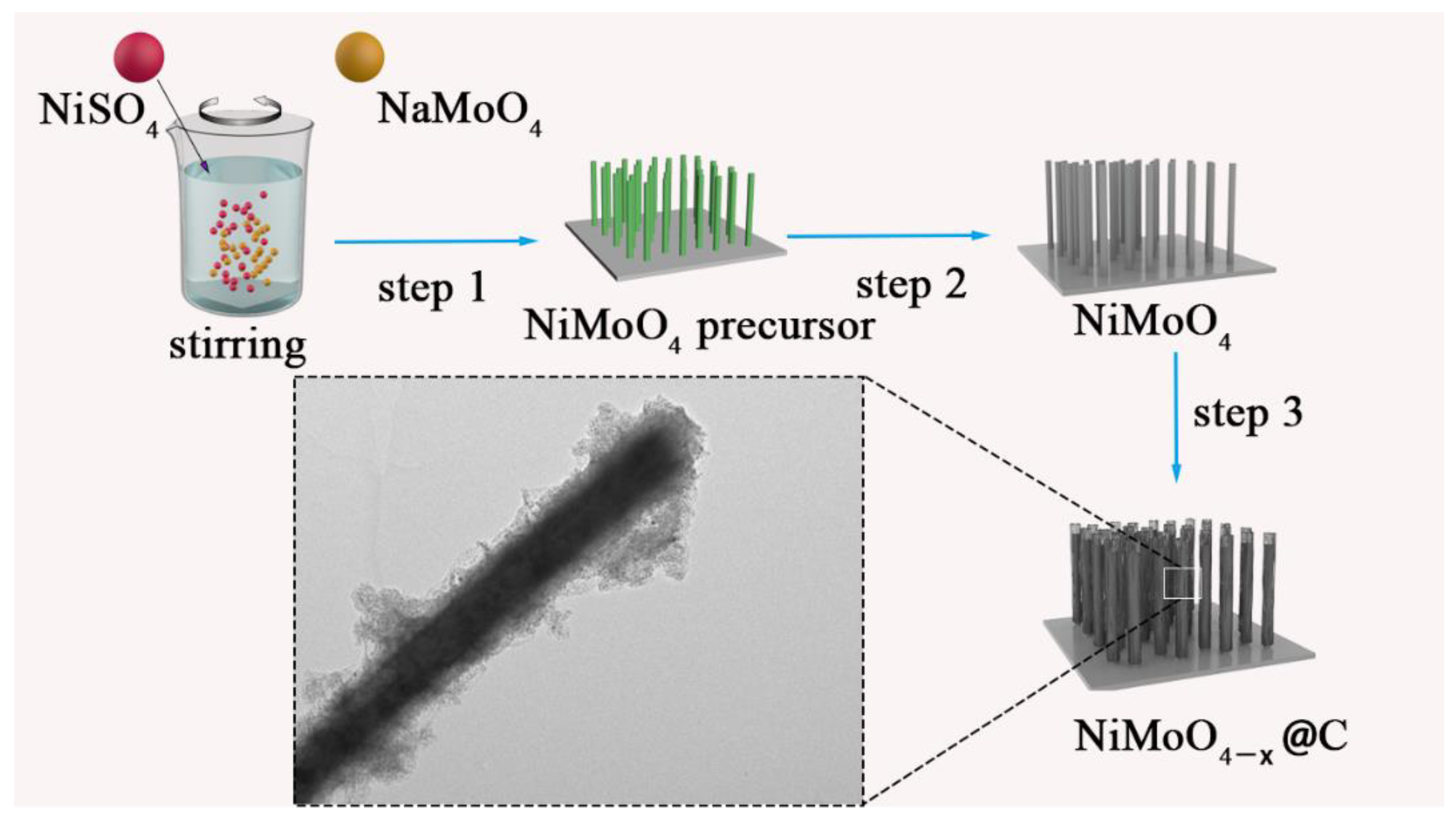
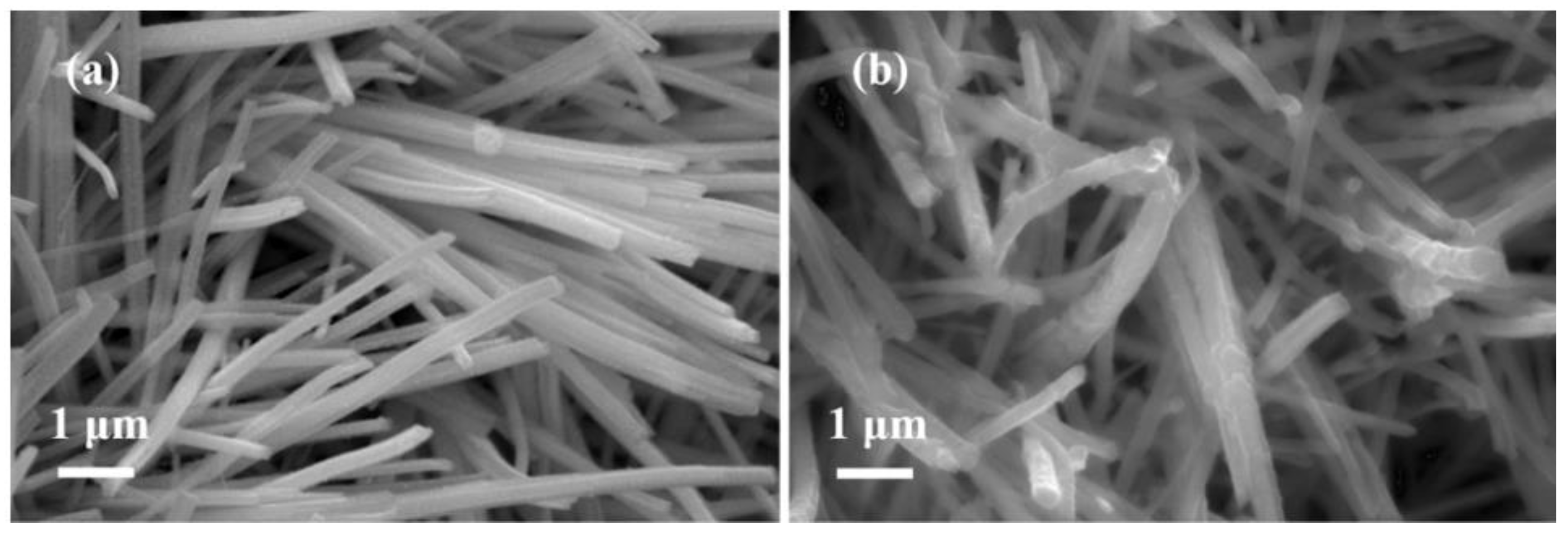
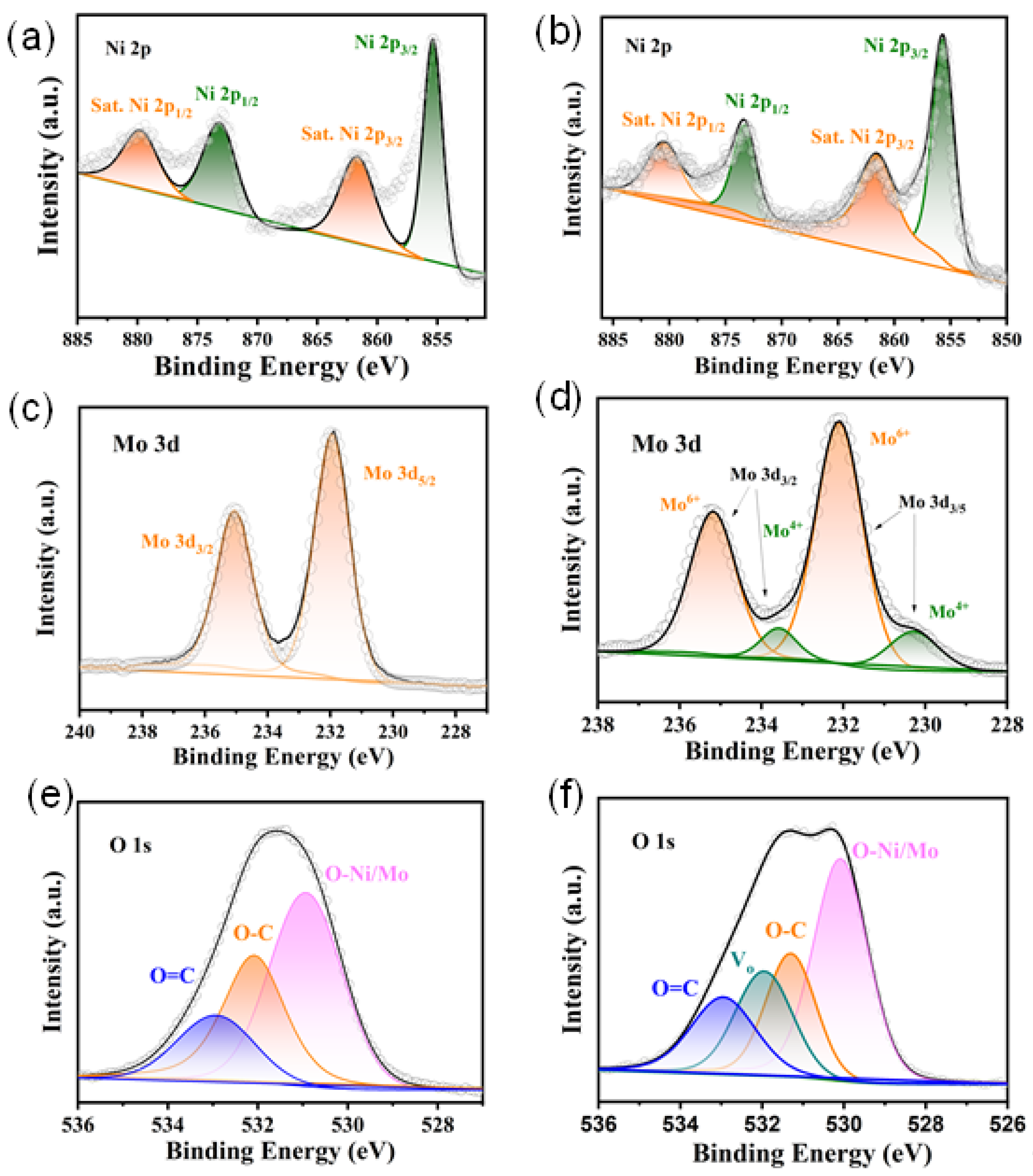
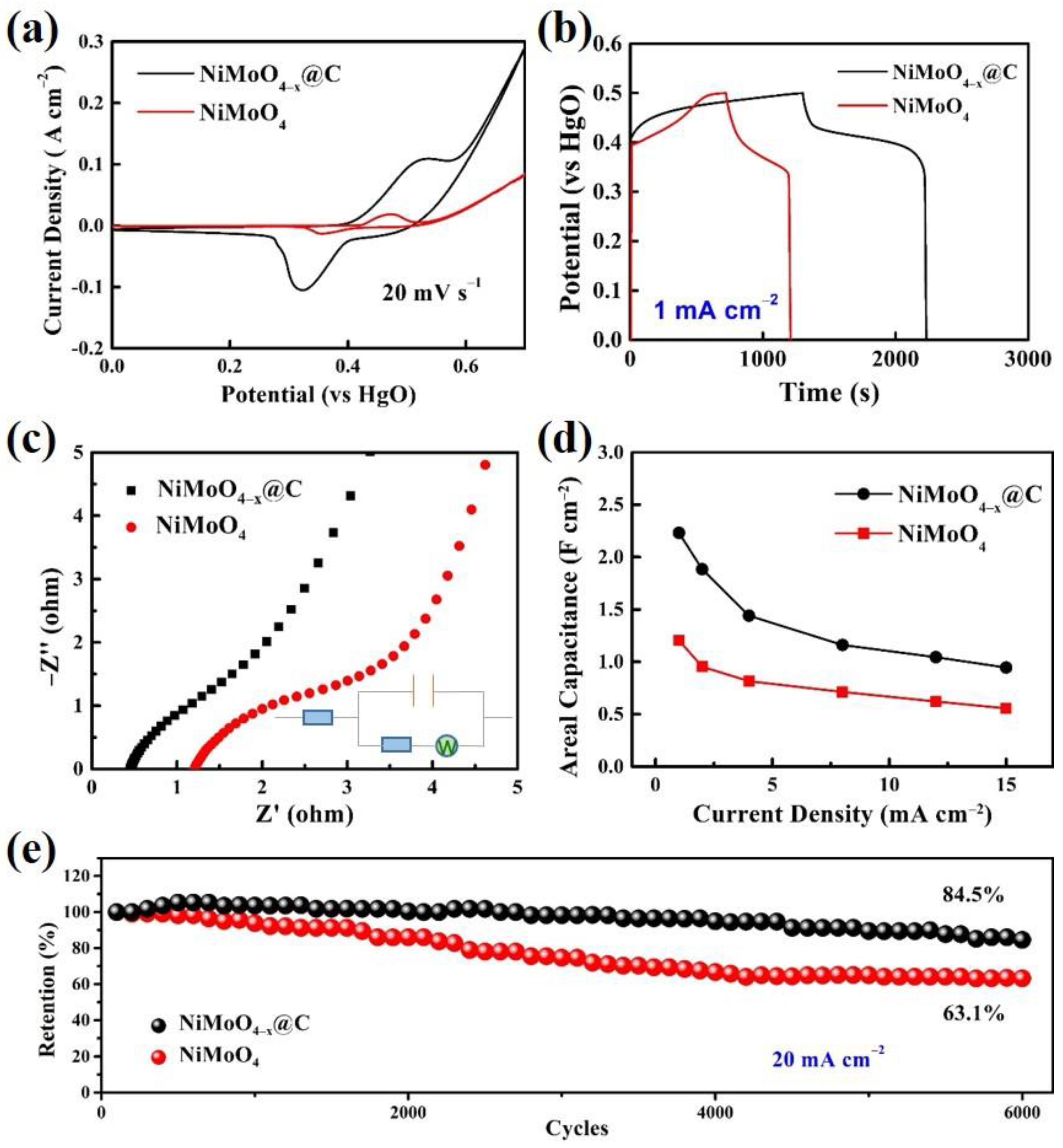
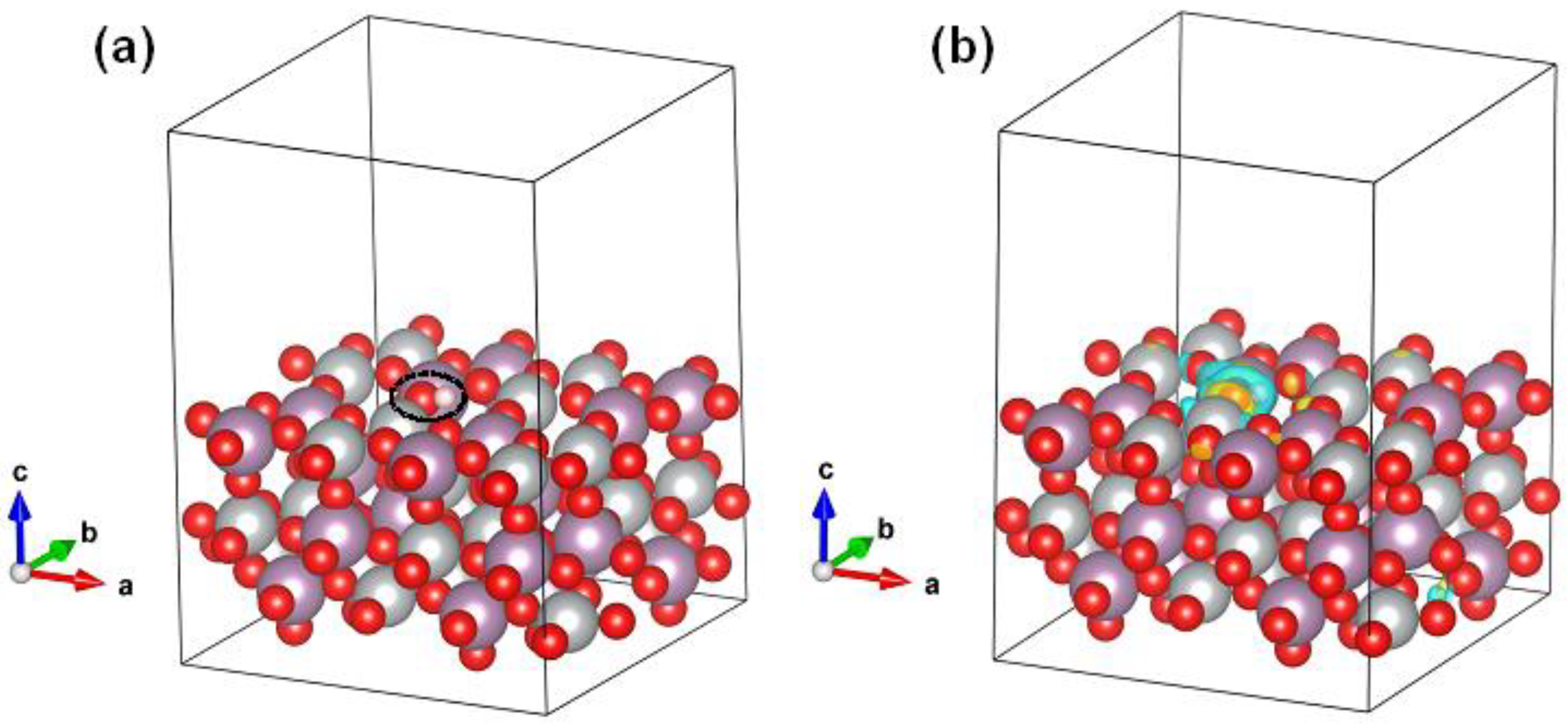
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.N.; He, J.; Xiong, D.B.; Wu, J.S.; Li, Q.Q.; Dravid, V.; Zhao, Y.F. Nickel Cobalt Hydroxide Reduced Graphene Oxide Hybrid Nanolayers for High Performance Asymmetric Supercapacitors with Remarkable Cycling Stability. ACS Appl. Mater. Interfaces 2016, 8, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Yun, Q.B.; Lu, Q.P.; Zhang, X.; Tan, C.L.; Zhang, H. Three-Dimensional Architectures Constructed from Transition-Metal Dichalcogenide Nanomaterials for Electrochemical Energy Storage and Conversion. Angew. Chem. Int. Ed. 2018, 57, 626–646. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.X.; Zhou, C.L.; Wang, H.E.; Zhu, K.; Ye, K.; Wang, Q.; Yan, J.; Cao, D.X.; Li, N.; Wang, G.L. Hollow Co-Mo-Se nanosheet arrays derived from metal-organic framework for high-performance supercapacitors. J. Power Sources 2021, 490, 229532. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Kota, S.; Lin, Z.F.; Zhao, M.Q.; Shpigel, N.; Levi, M.D.; Halim, J.; Taberna, P.L.; Barsoum, M.; Simon, P.; et al. Ultra-high-rate pseudocapacitive energy storage in two-dimensional transition metal carbides. Nat. Energy 2017, 2, 17105. [Google Scholar] [CrossRef]
- Ren, J.; Shen, M.; Li, Z.L.; Yang, C.M.; Liang, Y.; Wang, H.E.; Li, J.H.; Li, N.; Qian, D. Towards high-performance all-solid-state asymmetric supercapacitors: A hierarchical doughnut-like Ni3S2 PPy core-shell heterostructure on nickel foam electrode and density functional theory calculations. J. Power Sources 2021, 501, 230003. [Google Scholar] [CrossRef]
- Sun, L.; Sun, Y.D.; Fu, Q.; Pan, C.X. Facile preparation of NiO nanoparticles anchored on N/P-codoped 3D carbon nanofibers network for high-performance asymmetric supercapacitors. J. Alloys Compd. 2021, 888, 161488. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.F.; Meng, Y.; Plamthottam, R.; Tjiu, W.W.; Zhang, C.; Liu, T.X. Ultrathin Polypyrrole Layers Boosting MoO3 as Both Cathode and Anode Materials for a 2.0 V High-Voltage Aqueous Supercapacitor. ACS Appl. Mater. Interfaces 2022, 14, 4490–4499. [Google Scholar] [CrossRef]
- Shu, T.; Wang, H.; Li, Q.; Feng, Z.P.; Wei, F.X.; Yao, K.X.; Sun, Z.; Qi, J.Q.; Sui, Y.W. Highly stable Co3O4 nanoparticles/carbon nanosheets array derived from flake-like ZIF-67 as an advanced electrode for supercapacacitor. Chem. Eng. J. 2021, 419, 129631. [Google Scholar] [CrossRef]
- Pandit, B.; Goda, E.S.; Abu Elella, M.H.; Rehman, A.U.; Hong, S.E.; Rondiya, S.R.; Barkataki, P.; Shaikh, S.F.; Al-Enizi, A.M.; El-Bahy, S.M.; et al. One-pot hydrothermal preparation of hierarchical manganese oxide nanorods for high-performance symmetric supercapacitors. J. Energy Chem. 2022, 65, 116–126. [Google Scholar] [CrossRef]
- Wei, S.; Wan, C.C.; Zhang, L.Y.; Liu, X.Y.; Tian, W.Y.; Su, J.H.; Cheng, W.J.; Wu, Y.Q. N-doped and oxygen vacancy-rich NiCo2O4 nanograss for supercapacitor electrode. Chem. Eng. J. 2022, 429, 132242. [Google Scholar] [CrossRef]
- Liu, C.X.; Zhao, Z.; Liu, Y.; Lu, Q.S. Carbon dots decorated zinc cobaltite nanowires-assembled hierarchical arrays supported on nickel foam as binder-free electrodes for high performance supercapacitors. J. Power Sources 2022, 519, 230780. [Google Scholar] [CrossRef]
- Liu, H.Q.; Dai, M.Z.; Zhao, D.P.; Wu, X.; Wang, B. Realizing Superior Electrochemical Performance of Asymmetric Capacitors through Tailoring Electrode Architectures. ACS Appl. Energy Mater. 2020, 3, 7004–7010. [Google Scholar] [CrossRef]
- Li, P.X.; Ruan, C.H.; Xu, J.; Xie, Y.B. Supercapacitive performance of CoMoO4 with oxygen vacancy porous nanosheet. Electrochim. Acta 2020, 330, 135334. [Google Scholar] [CrossRef]
- Zhu, Z.; Sun, Y.; Li, C.S.; Yang, C.; Li, L.; Zhu, J.H.; Chou, S.L.; Wang, M.M.; Wang, D.D.; Li, Y.L. Mini-review: Progress on micro/nanoscale MnMoO4 as an electrode material for advanced supercapacitor applications. Mater. Chem. Front. 2021, 5, 7403–7418. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, P.; Zhang, H.M.; Yu, X.Z.; Zhu, J.; Li, Q.H.; Wang, T.H. NiMoO4 nanowires supported on Ni foam as novel advanced electrodes for supercapacitors. J. Mater. Chem. A 2013, 1, 9024–9027. [Google Scholar] [CrossRef]
- Denis, D.K.; Sun, X.; Zhang, J.Y.; Wang, Y.Y.; Hou, L.R.; Li, J.; Yuan, C.Z. Solid Solution Engineering of Co-Ni-Based Ternary Molybdate Nanorods toward Hybrid Supercapacitors and Lithium-Ion Batteries as High-Performance Electrodes. ACS Appl. Energy Mater. 2020, 3, 3955–3965. [Google Scholar] [CrossRef]
- Guo, D.; Luo, Y.Z.; Yu, X.Z.; Li, Q.H.; Wang, T.H. High performance NiMoO4 nanowires supported on carbon cloth as advanced electrodes for symmetric supercapacitors. Nano Energy 2014, 8, 174–182. [Google Scholar] [CrossRef]
- Hong, J.; Lee, Y.W.; Hou, B.; Ko, W.; Lee, J.; Pak, S.; Hong, J.; Morris, S.M.; Cha, S.; Sohn, J.I.; et al. Solubility-Dependent NiMoO4 Nanoarchitectures: Direct Correlation between Rationally Designed Structure and Electrochemical Pseudokinetics. ACS Appl. Mater. Interfaces 2016, 8, 35227–35234. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.J.; Li, L.L.; Wu, H.B.; Madhavi, S.; Lou, X.W. Controlled Growth of NiMoO4 Nanosheet and Nanorod Arrays on Various Conductive Substrates as Advanced Electrodes for Asymmetric Supercapacitors. Adv. Energy Mater. 2015, 5, 1401172. [Google Scholar] [CrossRef]
- Yin, Z.X.; Zhang, S.; Chen, Y.J.; Gao, P.; Zhu, C.L.; Yang, P.P.; Qi, L.H. Hierarchical nanosheet-based NiMoO4 nanotubes: Synthesis and high supercapacitor performance. J. Mater. Chem. A 2015, 3, 739–745. [Google Scholar] [CrossRef]
- Lin, L.Y.; Liu, T.M.; Liu, J.L.; Sun, R.; Hao, J.H.; Ji, K.M.; Wang, Z.C. Facile synthesis of groove-like NiMoO4 hollow nanorods for high-performance supercapacitors. Appl. Surf. Sci. 2016, 360, 234–239. [Google Scholar] [CrossRef]
- Yao, M.M.; Hu, Z.H.; Liu, Y.F.; Liu, P.P. A novel synthesis of size-controllable mesoporous NiMoO4 nanospheres for supercapacitor applications. Ionics 2016, 22, 701–709. [Google Scholar] [CrossRef]
- Jinlong, L.; Meng, Y.; Tongxiang, L. Enhanced performance of NiMoO4 nanoparticles and quantum dots and reduced nanohole graphene oxide hybrid for supercapacitor applications. Appl. Surf. Sci. 2017, 419, 624–630. [Google Scholar] [CrossRef]
- Huang, L.; Xiang, J.W.; Zhang, W.; Chen, C.J.; Xu, H.H.; Huang, Y.H. 3D interconnected porous NiMoO4 nanoplate arrays on Ni foam as high-performance binder-free electrode for supercapacitors. J. Mater. Chem. A 2015, 3, 22081–22087. [Google Scholar] [CrossRef]
- Wang, Z.J.; Wei, G.J.; Du, K.; Zhao, X.X.; Liu, M.; Wang, S.T.; Zhou, Y.; An, C.H.; Zhang, J. Ni Foam-Supported Carbon-Sheathed NiMoO4 Nanowires as Integrated Electrode for High-Performance Hybrid Supercapacitors. ACS Sustain. Chem. Eng. 2017, 5, 5964–5971. [Google Scholar] [CrossRef]
- Xu, Y.K.; Xuan, H.C.; Gao, J.H.; Liang, T.; Han, X.K.; Yang, J.; Zhang, Y.Q.; Li, H.; Han, P.D.; Du, Y.W. Hierarchical three-dimensional NiMoO4-anchored rGO/Ni foam as advanced electrode material with improved supercapacitor performance. J. Mater. Sci. 2018, 53, 8483–8498. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Zhang, Z.; Qi, X.; Ren, X.H.; Xu, G.H.; Wan, P.B.; Sun, X.M.; Zhang, H. Wall-like hierarchical metal oxide nanosheet arrays grown on carbon cloth for excellent supercapacitor electrodes. Nanoscale 2016, 8, 13273–13279. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.P.; Cui, F.; Zhao, Y.; Lian, J.B.; Bao, J.; Liu, T.X.; Li, H.M. NiMoO4 nanorod deposited carbon sponges with ant-nest-like interior channels for high-performance pseudocapacitors. Inorg. Chem. Front. 2018, 5, 1594–1601. [Google Scholar] [CrossRef]
- Murugan, E.; Govindaraju, S.; Santhoshkumar, S. Hydrothermal synthesis, characterization and electrochemical behavior of NiMoO4 nanoflower and NiMoO4/rGO nanocomposite for high-performance supercapacitors. Electrochim. Acta 2021, 392, 138973. [Google Scholar] [CrossRef]
- Huang, B.J.; Yao, D.C.; Yuan, J.J.; Tao, Y.R.; Yin, Y.X.; He, G.Y.; Chen, H.Q. Hydrangea-like NiMoO4-Ag/rGO as Battery-type electrode for hybrid supercapacitors with superior stability. J. Colloid Interf. Sci. 2022, 606, 1652–1661. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.P.; Li, C.Y.; Yu, J.L.; Zhang, S.; Zhang, M.; Liu, H.C.; Ji, M.W.; Cong, G.T.; Zhang, T.; Zhu, C.Z.; et al. High performance flexible energy storage device based on copper foam supported NiMoO4 nanosheets-CNTs-CuO nanowires composites with core-shell holey nanostructure. J. Mater. Sci. Technol. 2021, 85, 87–94. [Google Scholar] [CrossRef]
- Zhu, D.; Sun, X.; Yu, J.; Liu, Q.; Liu, J.Y.; Chen, R.R.; Zhang, H.S.; Song, D.L.; Li, R.M.; Wang, J. Three-dimensional heterostructured polypyrrole/nickel molybdate anchored on carbon cloth for high-performance flexible supercapacitors. J. Colloid Interf. Sci. 2020, 574, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.Y.; Huang, H.; Wang, Q.Q.; Wang, Q.; Zhou, G.W. Nitrogen-doped carbon/NiMoO4 nanospheres assembled by nanosheets and ultrasmall nanoparticles for supercapacitors. Chem. Phys. Lett. 2019, 728, 215–223. [Google Scholar] [CrossRef]
- Tong, B.L.; Wei, W.T.; Chen, X.L.; Wang, J.; Ye, W.Y.; Cui, S.Z.; Chen, W.H.; Mi, L.W. Designed synthesis of porous NiMoO4/C composite nanorods for asymmetric supercapacitors. Cryst. Eng. Comm. 2019, 21, 5492–5499. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.M.; Ma, M.Y.; Mu, X.M.; Zhang, Y.X.; Du, J.W.; Hu, Q.; Huang, B.Y.; Hua, X.H.; Liu, G.; et al. Manganese-doped nickel molybdate nanostructures for high-performance asymmetric supercapacitors. Chem. Eng. J. 2019, 372, 452–461. [Google Scholar] [CrossRef]
- Yuan, J.J.; Yao, D.C.; Jiang, L.; Tao, Y.R.; Che, J.F.; He, G.Y.; Chen, H.Q. Mn-Doped NiMoO4 Mesoporous Nanorods/Reduced Graphene Oxide Composite for High-Performance All-Solid-State Supercapacitor. ACS Appl. Energy Mater. 2020, 3, 1794–1803. [Google Scholar] [CrossRef]
- Wang, F.F.; Ma, K.; Tian, W.; Dong, J.C.; Han, H.; Wang, H.P.; Deng, K.; Yue, H.R.; Zhang, Y.X.; Jiang, W.; et al. P-Doped NiMoO4 parallel arrays anchored on cobalt carbonate hydroxide with oxygen vacancies and mass transfer channels for supercapacitors and oxygen evolution. J. Mater. Chem. A 2019, 7, 19589–19596. [Google Scholar] [CrossRef]
- Sharma, P.; Sundaram, M.M.; Watcharatharapong, T.; Laird, D.; Euchner, H.; Ahuja, R. Zn Metal Atom Doping on the Surface Plane of One-Dimesional NiMoO4 Nanorods with Improved Redox Chemistry. ACS Appl. Mater. Interfaces 2020, 12, 44815–44829. [Google Scholar] [CrossRef]
- Cui, S.Z.; Wang, F.Q.; Sun, K.J.; Wang, X.; Hu, Q.Z.; Peng, H.; Ma, G.F.; Lei, Z.Q. High-Performance Hybrid Supercapacitors Based on Ce-Doped NiMoO4 Nanosheets and Fe3O4@Bi2O3 Nanoarrays. J. Phys. Chem. C 2021, 125, 18129–18140. [Google Scholar] [CrossRef]
- Qing, C.; Yang, C.X.; Chen, M.Y.; Li, W.H.; Wang, S.Y.; Tang, Y.W. Design of oxygen-deficient NiMoO4 nanoflake and nanorod arrays with enhanced supercapacitive performance. Chem. Eng. J. 2018, 354, 182–190. [Google Scholar] [CrossRef]
- Sivakumar, P.; Raj, C.J.; Park, J.; Jung, H. Synergistic effects of nanoarchitecture and oxygen vacancy in nickel molybdate hollow sphere towards a high-performance hybrid supercapacitor. Int. J. Energy Res. 2021, 45, 21516–21526. [Google Scholar] [CrossRef]
- Zhu, S.; Le, J.Y.; Mao, Y.J.; Chen, S.X.; Han, X.X.; Zeng, Z.L.; Wang, J.; Deng, S.G. Synergistic engineering of fluorine doping and oxygen vacancies towards high-energy and long-lifespan flexible solid-state asymmetric supercapacitor. Ionics 2021, 27, 2649–2658. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Wei, L.; Guo, X. Ultrathin mesoporous NiMoO4-modified MoO3 core/shell nanostructures: Enhanced capacitive storage and cycling performance for supercapacitors. Chem. Eng. J. 2018, 353, 615–625. [Google Scholar] [CrossRef]
- Shen, J.W.; Wang, Q.G.; Zhang, K.; Wang, S.M.; Li, L.; Dong, S.B.; Zhao, S.T.; Chen, J.; Sun, R.S.; Wang, Y.; et al. Flexible carbon cloth based solid-state supercapacitor from hierarchical holothurian-morphological NiCo2O4@NiMoO4/PANI. Electrochim. Acta 2019, 320, 134578. [Google Scholar] [CrossRef]
- Xu, R.; Lin, J.M.; Wu, J.H.; Huang, M.L.; Fan, L.Q.; Xu, Z.D.; Song, Z.Y. A high-performance pseudocapacitive electrode material for supercapacitors based on the unique NiMoO4/NiO nanoflowers. Appl. Surf. Sci. 2019, 463, 721–731. [Google Scholar] [CrossRef]
- Yu, D.Y.; Zhang, Z.Q.; Teng, Y.F.; Meng, Y.N.; Wu, Y.P.; Liu, X.L.; Hua, Y.J.; Zhao, X.D.; Liu, X.Y. Fabrication of CuO@NiMoO4 core-shell nanowire arrays on copper foam and their application in high-performance all-solid-state asymmetric supercapacitors. J. Power Sources 2019, 440, 227164. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Z.L.; Xin, N.; Ying, Y.L.; Shi, W.D. High-performance supercapacitor based on highly active P-doped one-dimension/two-dimension hierarchical NiCo2O4/NiMoO4 for efficient energy storage. J. Colloid Interf. Sci. 2021, 601, 793–802. [Google Scholar] [CrossRef]
- Zeng, Y.; Liao, J.Z.; Wei, B.B.; Huang, Z.; Zhu, W.J.; Zheng, J.X.; Liang, H.F.; Zhang, Y.Z.; Wang, Z.C. Tuning the electronic structure of NiMoO4 by coupling with SnO2 for high-performance hybrid supercapacitors. Chem. Eng. J. 2021, 409, 128297. [Google Scholar] [CrossRef]
- Chen, C.; Yan, D.; Luo, X.; Gao, W.J.; Huang, G.J.; Han, Z.W.; Zeng, Y.; Zhu, Z.H. Construction of Core-Shell NiMoO4@Ni-Co-S Nanorods as Advanced Electrodes for High-Performance Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 4662–4671. [Google Scholar] [CrossRef]
- Chen, F.S.; Ji, S.; Liu, Q.B.; Wang, H.; Liu, H.; Brett, D.J.L.; Wang, G.X.; Wang, R.F. Rational Design of Hierarchically Core-Shell Structured Ni3S2@NiMoO4 Nanowires for Electrochemical Energy Storage. Small 2018, 14, 1800791. [Google Scholar] [CrossRef] [PubMed]
- Acharya, J.; Ojha, G.P.; Kim, B.S.; Pant, B.; Park, M. Modish Designation of Hollow-Tubular rGO-NiMoO4@Ni-Co-S Hybrid Core-shell Electrodes with Multichannel Superconductive Pathways for High-Performance Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2021, 13, 17487–17500. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.L.; Jeong, S.; Elumalai, G.; Kukunuri, S.; Fujita, J.; Ito, Y. Phase-Dependent Reactivity of Nickel Molybdates for Electrocatalytic Urea Oxidation. ACS Appl. Energy Mater. 2020, 3, 7535–7542. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Chen, X.; Kang, B.; Wang, H.-E.; Xiong, P.; Chen, Q.; Wei, M.; Li, N.; Qian, Q.; et al. Structural engineering of tin sulfides anchored on nitrogen/phosphorus dual-doped carbon nanofibres in sodium/potassium-ion batteries. Carbon 2022, 189, 46–56. [Google Scholar] [CrossRef]
- Nti, F.; Anang, D.A.; Han, J.I. Facilely synthesized NiMoO4/CoMoO4 nanorods as electrode material for high performance supercapacitor. J. Alloys Compd. 2018, 742, 342–350. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Huang, Z.; Ren, L.; Qi, X.; Wei, X.; Zhong, J. Facile hydrothermal synthesis of NiMoO4@CoMoO4 hierarchical nanospheres for supercapacitor applications. Phys. Chem. Chem. Phys. 2015, 17, 20795–20804. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, H.E.; Chen, X.X.; Cao, J.; Zhao, Y.D.; Neale, Z.G.; Cai, W.; Sui, J.H.; Cao, G.Z. Tubular MoO2 organized by 2D assemblies for fast and durable alkali-ion storage. Energy Storage Mater. 2018, 11, 161–169. [Google Scholar] [CrossRef]
- Wang, H.E.; Zhao, X.; Yin, K.L.; Li, Y.; Chen, L.H.; Yang, X.Y.; Zhang, W.J.; Su, B.L.; Cao, G.Z. Superior Pseudocapacitive Lithium-Ion Storage in Porous Vanadium Oxides C Heterostructure Composite. ACS Appl. Mater. Interfaces 2017, 9, 43665–43673. [Google Scholar] [CrossRef]
- Wang, H.E.; Yin, K.L.; Qin, N.; Zhao, X.; Xia, F.J.; Hu, Z.Y.; Guo, G.L.; Cao, G.Z.; Zhang, W.J. Oxygen-deficient titanium dioxide as a functional host for lithium-sulfur batteries. J. Mater. Chem. A 2019, 7, 10346–10353. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Cryst. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.C.; Tang, G.; Geng, W.T. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comp. Phys. Comm. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, P.; Fu, W.; Ma, X.; Zhou, J.; Zhang, X.; Li, J.; Xie, E.; Pan, X. Understanding the role of Co3O4 on stability between active hierarchies and scaffolds: An insight into NiMoO4 composites for supercapacitors. Appl. Surf. Sci. 2017, 416, 160–167. [Google Scholar] [CrossRef]
- Xuan, H.; Xu, Y.; Zhang, Y.; Li, H.; Han, P.; Du, Y. One-step combustion synthesis of porous CNTs/C/NiMoO4 composites for high-performance asymmetric supercapacitors. J. Alloys Compd. 2018, 745, 135–146. [Google Scholar] [CrossRef]
- Huang, Y.; Cui, F.; Zhao, Y.; Lian, J.; Bao, J.; Li, H. Controlled growth of ultrathin NiMoO4 nanosheets on carbon nanofiber membrane as advanced electrodes for asymmetric supercapacitors. J. Alloys Compd. 2018, 753, 176–185. [Google Scholar] [CrossRef]
- Hussain, S.; Javed, M.S.; Asim, S.; Shaheen, A.; Khan, A.J.; Abbas, Y.; Ullah, N.; Iqbal, A.; Wang, M.; Qiao, G. Novel gravel-like NiMoO4 nanoparticles on carbon cloth for outstanding supercapacitor applications. Ceram. Int. 2020, 46, 6406–6412. [Google Scholar] [CrossRef]
- Feng, X.; Ning, J.; Wang, D.; Zhang, J.; Xia, M.; Wang, Y.; Hao, Y. Heterostructure arrays of NiMoO4 nanoflakes on N-doping of graphene for high-performance asymmetric supercapacitors. J. Alloys Compd. 2020, 816, 152625. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, T.; Zhang, A.; Cui, L.; Liu, X.; Zheng, R.; Liu, J. Controllable synthesis of Ni1-xCoxMoO4 with tunable morphologies for high-performance asymmetric supercapacitors. J. Alloys Compd. 2021, 850, 156734. [Google Scholar] [CrossRef]
- Chen, L.; Deng, W.; Chen, Z.; Wang, X. Hetero-architectured core–shell NiMoO4@Ni9S8/MoS2 nanorods enabling high-performance supercapacitors. J. Mater. Res. 2022, 37, 284–293. [Google Scholar] [CrossRef]
- Muthu, D.; Vargheese, S.; Haldorai, Y.; Kumar, R.T.R. NiMoO4/reduced graphene oxide composite as an electrode material for hybrid supercapacitor. Mater. Sci. Semicond. Proc. 2021, 135, 106078. [Google Scholar] [CrossRef]
- Yi, T.-F.; Qiu, L.-Y.; Mei, J.; Qi, S.-Y.; Cui, P.; Luo, S.; Zhu, Y.-R.; Xie, Y.; He, Y.-B. Porous spherical NiO@NiMoO4@PPy nanoarchitectures as advanced electrochemical pseudocapacitor materials. Sci. Bull. 2020, 65, 546–556. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Ding, X.; Zhe, R.; Zhu, T.; Qing, C.; Liu, Y.; Wang, H.-E. Synchronous Defect and Interface Engineering of NiMoO4 Nanowire Arrays for High-Performance Supercapacitors. Nanomaterials 2022, 12, 1094. https://doi.org/10.3390/nano12071094
Wang P, Ding X, Zhe R, Zhu T, Qing C, Liu Y, Wang H-E. Synchronous Defect and Interface Engineering of NiMoO4 Nanowire Arrays for High-Performance Supercapacitors. Nanomaterials. 2022; 12(7):1094. https://doi.org/10.3390/nano12071094
Chicago/Turabian StyleWang, Pengcheng, Xinying Ding, Rongjie Zhe, Ting Zhu, Chen Qing, Yingkai Liu, and Hong-En Wang. 2022. "Synchronous Defect and Interface Engineering of NiMoO4 Nanowire Arrays for High-Performance Supercapacitors" Nanomaterials 12, no. 7: 1094. https://doi.org/10.3390/nano12071094
APA StyleWang, P., Ding, X., Zhe, R., Zhu, T., Qing, C., Liu, Y., & Wang, H.-E. (2022). Synchronous Defect and Interface Engineering of NiMoO4 Nanowire Arrays for High-Performance Supercapacitors. Nanomaterials, 12(7), 1094. https://doi.org/10.3390/nano12071094





