Formulation and In Vitro Efficacy Assessment of Teucrium marum Extract Loading Hyalurosomes Enriched with Tween 80 and Glycerol
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Material Collection
2.3. Extraction Process
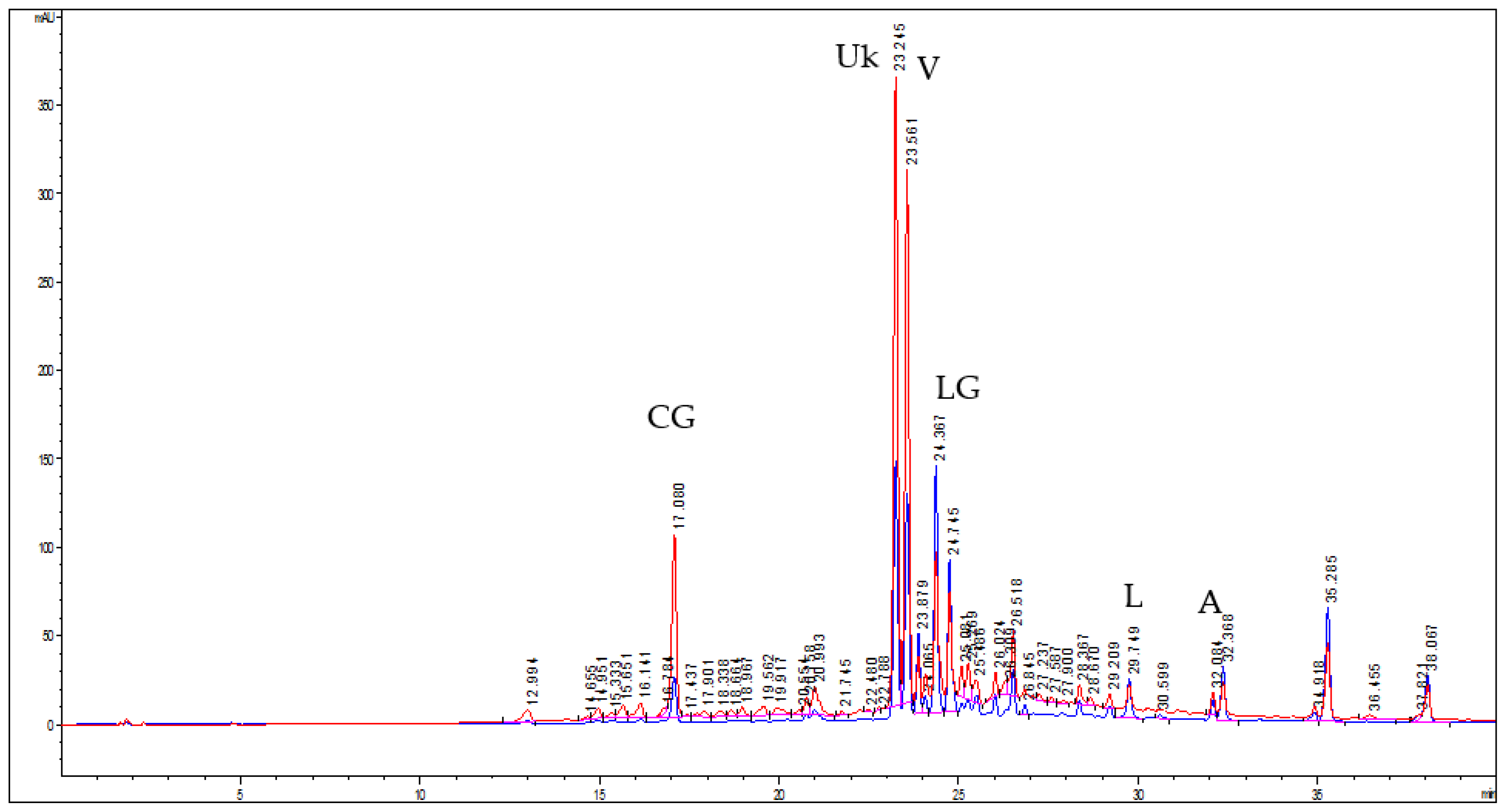
2.4. HPLC–DAD Analysis of the Extract
2.5. Vesicle Preparation
2.6. Vesicle Characterization
2.7. DPPH• Assay
2.8. Biocompatibility of the Formulations
2.9. Protective Effect of the Formulations against Cell Damages Induced by Hydrogen Peroxide
2.10. Scratch Assay
2.11. Statistical Analysis of Data
3. Results
3.1. Extraction of the Bioactives and Characterization of the Extract
3.2. Vesicle Preparation and Characterization
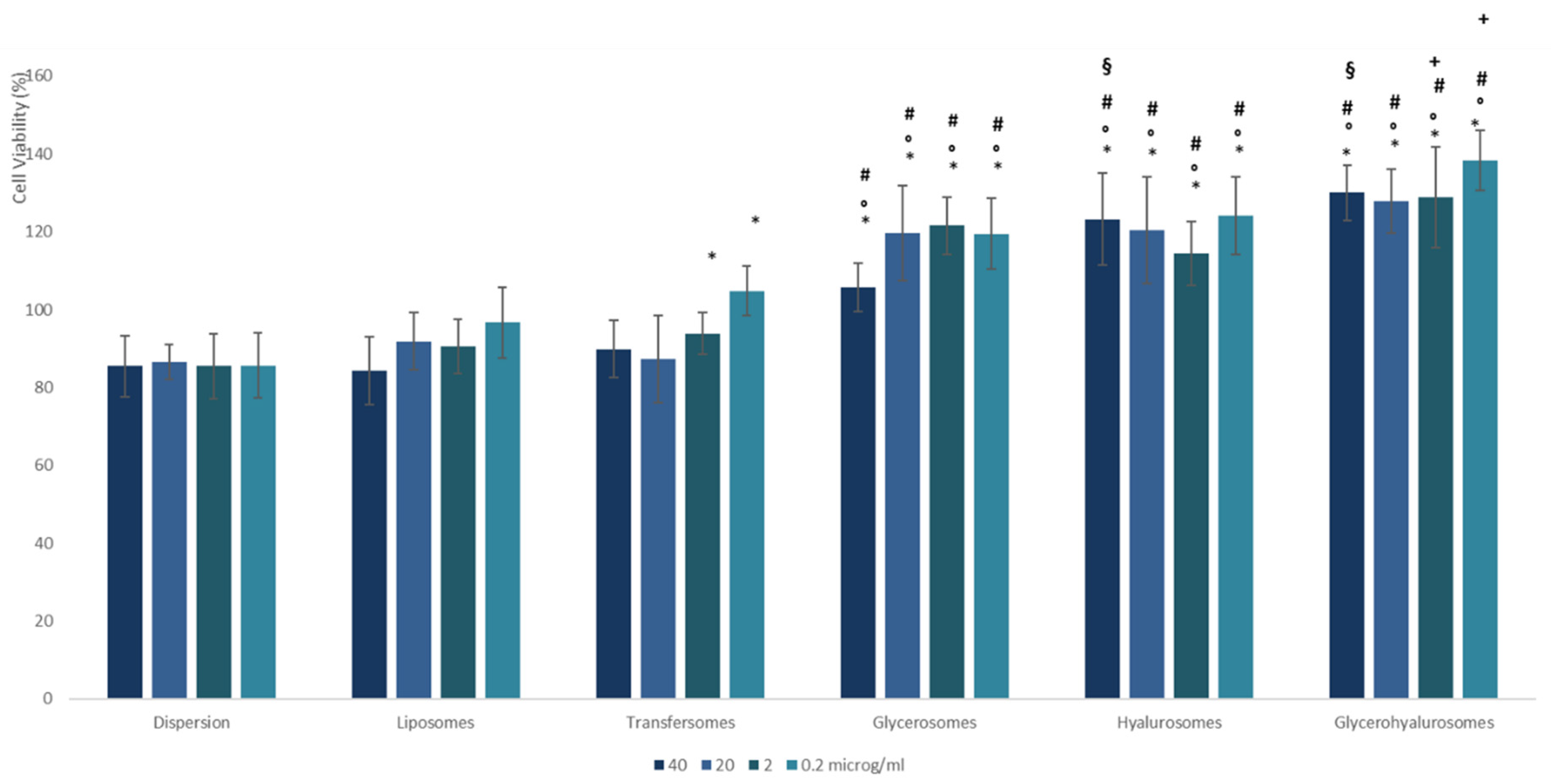
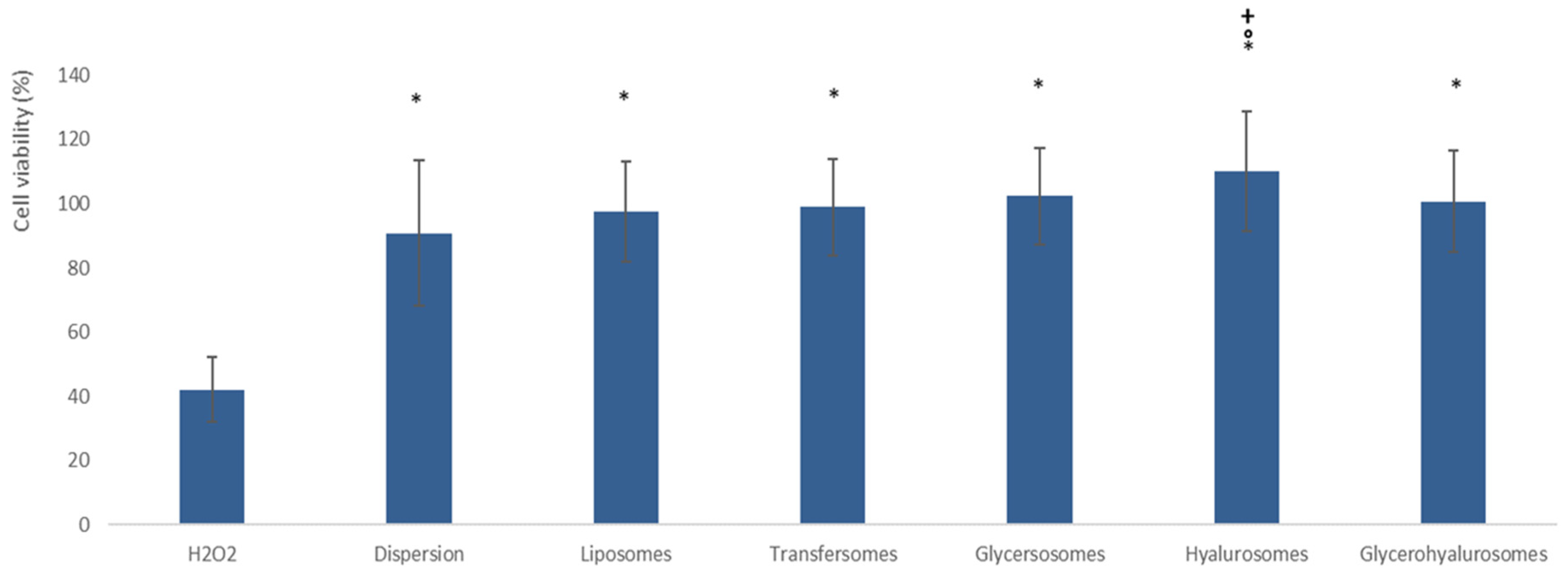
3.3. Biocompatibility of the Formulations
3.4. Protective Effect of Formulations against Damages Induced by Hydrogen Peroxide in Fibroblasts
3.5. Effect of Formulations on Proliferation and Migration of Fibroblasts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Mesquita, L.S.S.; Luz, T.R.S.A.; de Mesquita, J.W.C.; Coutinho, D.F.; do Amaral, F.M.M.; de Sousa Ribeiro, M.N.; Malik, S. Exploring the anticancer properties of essential oils from family Lamiaceae. Food Rev. Int. 2019, 35, 105–131. [Google Scholar] [CrossRef]
- Maccioni, A.; Falconieri, D.; Sanna, C.; Porcedda, S.; Piras, A.; Maxia, A. Characterization of Essential Oils from Different Taxa Belonging to the Genus Teucrium in Sardinia Island, Italy. Plants 2021, 10, 1359. [Google Scholar] [CrossRef] [PubMed]
- Djabou, N.; Muselli, A.; Allali, H.; Dib, M.E.A.; Tabti, B.; Varesi, L.; Costa, J. Chemical and genetic diversity of two Mediterranean subspecies of Teucrium polium L. Phytochemistry 2012, 83, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J. Volatile components variation in the Teucrium marum complex (Lamiaceae) from the Balearic Islands. Bot. J. Linn. Soc. 2000, 132, 253–261. [Google Scholar] [CrossRef]
- Ricci, D.; Fraternale, D.; Giamperi, L.; Bucchini, A.; Epifano, F.; Burini, G.; Curini, M. Chemical composition, antimicrobial and antioxidant activity of the essential oil of Teucrium marum (Lamiaceae). J. Ethnopharmacol. 2005, 98, 195–200. [Google Scholar] [CrossRef]
- Fois, M.; Farris, E.; Calvia, G.; Campus, G.; Fenu, G.; Porceddu, M.; Bacchetta, G. The Endemic Vascular Flora of Sardinia: A Dynamic Checklist with an Overview of Biogeography and Conservation Status. Plants 2022, 11, 601. [Google Scholar] [CrossRef] [PubMed]
- Ulubelen, A.; Topu, G.; Sönmez, U. Chemical and biological evaluation of Genus teucrium. Stud. Nat. Prod. Chem. 2000, 23, 591–648. [Google Scholar] [CrossRef]
- Servettaz, O.; Maleci, L.B.; Pinetti, A. Micromorphological and phytochemical characters of Teucrium marum and T. subspinosum (Labiatae) from Sardinia and Balearic Islands. Plant Syst. Evol. 1992, 179, 129–139. [Google Scholar] [CrossRef]
- Coll, J.; Tandrón, Y. Isolation and structure elucidation of three neo-clerodane diterpenes from Teucrium fruticans L. (LABIATAE). Phytochemistry 2005, 66, 2298–2303. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.L.; Muñoz-Acevedo, A.; Rai, M. Ethnobotany: Local Knowledge and Traditions; CRC Press: Boca Raton, FL, USA, 2019; ISBN 9781138388987. [Google Scholar]
- Eisner, T.; Eisner, M.; Aneshansley, D.J.; Wu, C.-L.; Meinwald, J. Chemical defense of the mint plant, Teucrium marum (Labiatae). Chemoecology 2000, 10, 211–216. [Google Scholar] [CrossRef]
- Poli, F.; Serrilli, A.M.; Scartezzini, P.; Muzzoli, M.; Maxia, A.; Ballero, M.; Serafini, M.; Bianco, A. Endemic species of sardo-corso-balearic area: Molecular composition and biological assay of Teucrium. Nat. Prod. Res. 2007, 21, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A Review of Its Occurrence, (Bio) Synthesis and Pharmacological Significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Manconi, M.; Petretto, G.; D’Hallewin, G.; Escribano, E.; Milia, E.; Pinna, R.; Palmieri, A.; Firoznezhad, M.; Peris, J.E.; Usach, I.; et al. Thymus essential oil extraction, characterization and incorporation in phospholipid vesicles for the antioxidant/antibacterial treatment of oral cavity diseases. Colloids Surf. B Biointerfaces 2018, 171, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, G.K.; Carvalho, E.L.S.; Von Poser, G.L.; Teixeira, H.F. On the use of nanotechnology-based strategies for association of complex matrices from plant extracts. Rev. Bras. Farm. 2015, 25, 426–436. [Google Scholar] [CrossRef]
- Allaw, M.; Manca, M.L.; Castangia, I.; Manconi, M. From plants to phospholipid vesicles: A comprehensive review on the incorporation of phytochemicals into phospholipid vesicles designed for skin applications with special focus on scalability and in vitro and in vivo efficacy. J. Drug Deliv. Sci. Technol. 2021, 67, 103049. [Google Scholar] [CrossRef]
- Celia, C.; Cilurzo, F.; Trapasso, E.; Cosco, D.; Fresta, M.; Paolino, D. Ethosomes® and transfersomes® containing linoleic acid: Physicochemical and technological features of topical drug delivery carriers for the potential treatment of melasma disorders. Biomed. Microdevices 2011, 14, 119–130. [Google Scholar] [CrossRef]
- Tavano, L.; Muzzalupo, R.; Trombino, S.; Cassano, R.; Pingitore, A.; Picci, N. Effect of formulations variables on the in vitro percutaneous permeation of Sodium Diclofenac from new vesicular systems obtained from Pluronic triblock copolymers. Colloids Surf. B Biointerfaces 2010, 79, 227–234. [Google Scholar] [CrossRef]
- Jain, S.; Patel, N.; Shah, M.K.; Khatri, P.; Vora, N. Recent Advances in Lipid-Based Vesicles and Particulate Carriers for Topical and Transdermal Application. J. Pharm. Sci. 2017, 106, 423–445. [Google Scholar] [CrossRef]
- Sinico, C.; Fadda, A.M. Vesicular carriers for dermal drug delivery. Expert Opin. Drug Deliv. 2009, 6, 813–825. [Google Scholar] [CrossRef]
- Tavano, L.; Gentile, L.; Rossi, C.O.; Muzzalupo, R. Novel gel-niosomes formulations as multicomponent systems for transdermal drug delivery. Colloids Surf. B Biointerfaces 2013, 110, 281–288. [Google Scholar] [CrossRef]
- Manca, M.L.; Zaru, M.; Manconi, M.; Lai, F.; Valenti, D.; Sinico, C.; Fadda, A.M. Glycerosomes: A new tool for effective dermal and transdermal drug delivery. Int. J. Pharm. 2013, 455, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Cosco, D.; Paolino, D.; Maiuolo, J.; Di Marzio, L.; Carafa, M.; Ventura, C.A.; Fresta, M. Ultradeformable liposomes as multidrug carrier of resveratrol and 5-fluorouracil for their topical delivery. Int. J. Pharm. 2015, 489, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.L.; Marongiu, F.; Castangia, I.; Catalán-Latorre, A.; Caddeo, C.; Bacchetta, G.; Ennas, G.; Zaru, M.; Fadda, A.M.; Manconi, M. Protective effect of grape extract phospholipid vesicles against oxidative stress skin damages. Ind. Crop. Prod. 2016, 83, 561–567. [Google Scholar] [CrossRef]
- Manconi, M.; Marongiu, F.; Manca, M.L.; Caddeo, C.; Sarais, G.; Cencetti, C.; Pucci, L.; Longo, V.; Bacchetta, G.; Fadda, A.M. Nanoincorporation of bioactive compounds from red grape pomaces: In vitro and ex vivo evaluation of antioxidant activity. Int. J. Pharm. 2017, 523, 159–166. [Google Scholar] [CrossRef]
- Gil, K.A.; Jerković, I.; Marijanović, Z.; Manca, M.L.; Caddeo, C.; Tuberoso, C.I.G. Evaluation of an innovative sheep cheese with antioxidant activity enriched with different thyme essential oil lecithin liposomes. LWT 2021, 154, 112808. [Google Scholar] [CrossRef]
- Manca, M.L.; Cassano, R.; Valenti, D.; Trombino, S.; Ferrarelli, T.; Picci, N.; Fadda, A.M.; Manconi, M. Isoniazid-gelatin conjugate microparticles containing rifampicin for the treatment of tuberculosis. J. Pharm. Pharmacol. 2013, 65, 1302–1311. [Google Scholar] [CrossRef]
- Castangia, I.; Manca, M.L.; Caddeo, C.; Maxia, A.; Murgia, S.; Pons, R.; Demurtas, D.; Pando, D.; Falconieri, D.; Peris, J.E.; et al. Faceted phospholipid vesicles tailored for the delivery of Santolina insularis essential oil to the skin. Colloids Surfaces B Biointerfaces 2015, 132, 185–193. [Google Scholar] [CrossRef]
- Manca, M.L.; Manconi, M.; Nacher, A.; Carbone, C.; Valenti, D.; Maccioni, A.M.; Sinico, C.; Fadda, A.M. Development of novel diolein–niosomes for cutaneous delivery of tretinoin: Influence of formulation and in vitro assessment. Int. J. Pharm. 2014, 477, 176–186. [Google Scholar] [CrossRef]
- Manca, M.L.; Manconi, M.; Falchi, A.M.; Castangia, I.; Valenti, D.; Lampis, S.; Fadda, A.M. Close-packed vesicles for diclofenac skin delivery and fibroblast targeting. Colloids Surf. B Biointerfaces 2013, 111, 609–617. [Google Scholar] [CrossRef]
- Moulaoui, K.; Caddeo, C.; Manca, M.L.; Castangia, I.; Valenti, D.; Escribano, E.; Atmani, D.; Fadda, A.M.; Manconi, M. Identification and nanoentrapment of polyphenolic phytocomplex from Fraxinus angustifolia: In vitro and in vivo wound healing potential. Eur. J. Med. Chem. 2015, 89, 179–188. [Google Scholar] [CrossRef]
- Manca, M.; Castangia, I.; Zaru, M.; Nácher, A.; Valenti, D.; Fernàndez-Busquets, X.; Fadda, A.; Manconi, M. Development of curcumin loaded sodium hyaluronate immobilized vesicles (hyalurosomes) and their potential on skin inflammation and wound restoring. Biomaterials 2015, 71, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Castangia, I.; Caddeo, C.; Manca, M.L.; Casu, L.; Latorre, A.C.; Díez-Sales, O.; Ruiz-Saurí, A.; Bacchetta, G.; Fadda, A.M.; Manconi, M. Delivery of liquorice extract by liposomes and hyalurosomes to protect the skin against oxidative stress injuries. Carbohydr. Polym. 2015, 134, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Mitreski, I.; Stanoeva, J.P.; Stefova, M.; Stefkov, G.; Kulevanova, S. Polyphenols in Representative Teucrium Species in the Flora of R. Macedonia: LC/DAD/ESI-MSn Profile and Content. Nat. Prod. Commun. 2014, 9, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Catalan-Latorre, A.; Ravaghi, M.; Manca, M.L.; Caddeo, C.; Marongiu, F.; Ennas, G.; Escribano-Ferrer, E.; Peris, J.E.; Diez-Sales, O.; Fadda, A.M.; et al. Freeze-dried eudragit-hyaluronan multicompartment liposomes to improve the intestinal bioavailability of curcumin. Eur. J. Pharm. Biopharm. 2016, 107, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, M.; Manca, M.L.; Caddeo, C.; Escribano-Ferrer, E.; Carbone, C.; Peris, J.E.; Usach, I.; Diez-Sales, O.; Fadda, A.M.; Manconi, M. Co-Loading of Ascorbic Acid and Tocopherol in Eudragit-Nutriosomes to Counteract Intestinal Oxidative Stress. Pharmaceutics 2019, 11, 13. [Google Scholar] [CrossRef]
- Allaw, M.; Manca, M.L.; Gómez-Fernández, J.C.; Pedraz, J.L.; Terencio, M.C.; Sales, O.D.; Nacher, A.; Manconi, M. Oleuropein multicompartment nanovesicles enriched with collagen as a natural strategy for the treatment of skin wounds connected with oxidative stress. Nanomedicine 2021, 16, 2363–2376. [Google Scholar] [CrossRef]
- Yoneda, M.; Yamagata, M.; Suzuki, S.; Kimata, K. Hyaluronic acid modulates proliferation of mouse dermal fibroblasts in culture. J. Cell Sci. 1988, 90, 265–273. [Google Scholar] [CrossRef]
- Chidawanyika, T.; Supattapone, S. Hydrogen Peroxide-induced Cell Death in Mammalian Cells. J. Cell. Signal. 2021, 2, 2. [Google Scholar] [CrossRef]
- Ido, Y.; Duranton, A.; Lan, F.; Weikel, K.; Breton, L.; Ruderman, N.B. Resveratrol Prevents Oxidative Stress-Induced Senescence and Proliferative Dysfunction by Activating the AMPK-FOXO3 Cascade in Cultured Primary Human Keratinocytes. PLoS ONE 2015, 10, e0115341. [Google Scholar] [CrossRef]
- Korkina, L.; Mikhal’Chik, E.; Suprun, M.; Pastore, S.; Del Toso, R. Molecular Mechanisms Underlying Wound Healing and Anti-Inflammatory Properties of Naturally Occurring Biotechnologically Produced Phenylpropanoid. Cell. Mol. Biol. 2007, 53, 84–91. [Google Scholar] [CrossRef]
- Vertuani, S.; Beghelli, E.; Scalambra, E.; Malisardi, G.; Copetti, S.; Toso, R.D.; Baldisserotto, A.; Manfredini, S. Activity and Stability Studies of Verbascoside, a Novel Antioxidant, in Dermo-Cosmetic and Pharmaceutical Topical Formulations. Molecules 2011, 16, 7068–7080. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, M.; Pastore, S.; Lulli, D.; Alipieva, K.; Kostyuk, V.; Potapovich, A.; Panetta, M.; Korkina, L. Verbascum xanthophoeniceum-derived phenylethanoid glycosides are potent inhibitors of inflammatory chemokines in dormant and interferon-gamma-stimulated human keratinocytes. J. Ethnopharmacol. 2012, 144, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Sperotto, N.D.D.M.; Steffens, L.; Veríssimo, R.M.; Henn, J.G.; Péres, V.F.; Vianna, P.; Chies, J.A.B.; Roehe, A.; Saffi, J.; Moura, D.J. Wound healing and anti-inflammatory activities induced by a Plantago australis hydroethanolic extract standardized in verbascoside. J. Ethnopharmacol. 2018, 225, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Ambrosone, L.; Guerra, G.; Cinelli, M.; Filippelli, M.; Mosca, M.; Vizzarri, F.; Giorgio, D.; Costagliola, C. Corneal Epithelial Wound Healing Promoted by Verbascoside-Based Liposomal Eyedrops. BioMed Res. Int. 2014, 2014, 471642. [Google Scholar] [CrossRef] [PubMed]
- Sogut, O.; Sezer, U.A.; Sezer, S. Liposomal delivery systems for herbal extracts. J. Drug Deliv. Sci. Technol. 2021, 61, 102147. [Google Scholar] [CrossRef]
- Vaillant, L.; Georgescou, G.; Rivollier, C.; Delarue, A. Combined effects of glycerol and petrolatum in an emollient cream: A randomized, double-blind, crossover study in healthy volunteers with dry skin. J. Cosmet. Dermatol. 2019, 19, 1399–1403. [Google Scholar] [CrossRef]
- Manca, M.L.; Castangia, I.; Caddeo, C.; Pando, D.; Escribano, E.; Valenti, D.; Lampis, S.; Zaru, M.; Fadda, A.M.; Manconi, M. Improvement of quercetin protective effect against oxidative stress skin damages by incorporation in nanovesicles. Colloids Surf. B Biointerfaces 2014, 123, 566–574. [Google Scholar] [CrossRef]
- Rasti, B.; Jinap, S.; Mozafari, M.; Yazid, A. Comparative study of the oxidative and physical stability of liposomal and nanoliposomal polyunsaturated fatty acids prepared with conventional and Mozafari methods. Food Chem. 2012, 135, 2761–2770. [Google Scholar] [CrossRef]
- Manca, M.L.; Firoznezhad, M.; Caddeo, C.; Marongiu, F.; Escribano-Ferrer, E.; Sarais, G.; Peris, J.E.; Usach, I.; Zaru, M.; Manconi, M.; et al. Phytocomplexes extracted from grape seeds and stalks delivered in phospholipid vesicles tailored for the treatment of skin damages. Ind. Crop. Prod. 2019, 128, 471–478. [Google Scholar] [CrossRef]
- De Leo, V.; Milano, F.; Agostiano, A.; Catucci, L. Recent Advancements in Polymer/Liposome Assembly for Drug Delivery: From Surface Modifications to Hybrid Vesicles. Polymers 2021, 13, 1027. [Google Scholar] [CrossRef]
- Anadón, A.; Martínez, M.A.; Castellano, V.; Martínez-Larrañaga, M.R. The Role of in Vitro Methods as Alternatives to Animals in Toxicity Testing. Expert Opin. Drug Metab. Toxicol. 2014, 10, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Prosdocimi, M.; Bevilacqua, C. Exogenous hyaluronic acid and wound healing: An updated vision. Panminerva Med. 2012, 54, 129–135. [Google Scholar] [PubMed]
- De Paiva, W.K.V.; de Medeiros, W.R.D.B.; de Assis, C.F.; dos Santos, E.S.; Júnior, F.C.D.S. Physicochemical characterization and in vitro antioxidant activity of hyaluronic acid produced by Streptococcus zooepidemicus CCT 7546. Prep. Biochem. Biotechnol. 2021, 52, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Paolino, D.; Cosco, D.; Cilurzo, F.; Trapasso, E.; Morittu, V.M.; Celia, C.; Fresta, M. Improved in vitro and in vivo collagen biosynthesis by asiaticoside-loaded ultradeformable vesicles. J. Control. Release 2012, 162, 143–151. [Google Scholar] [CrossRef]
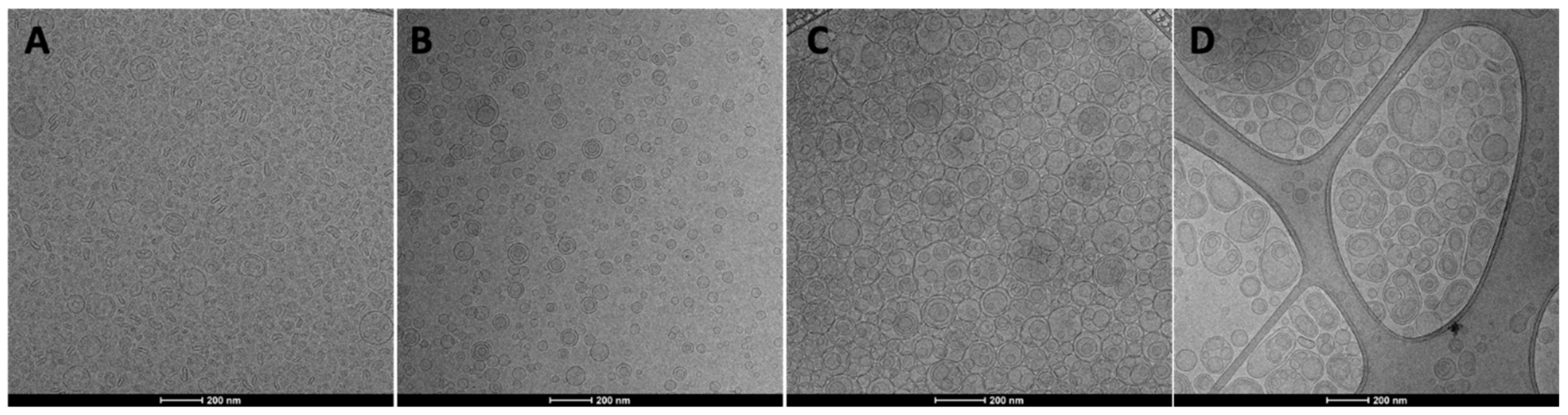
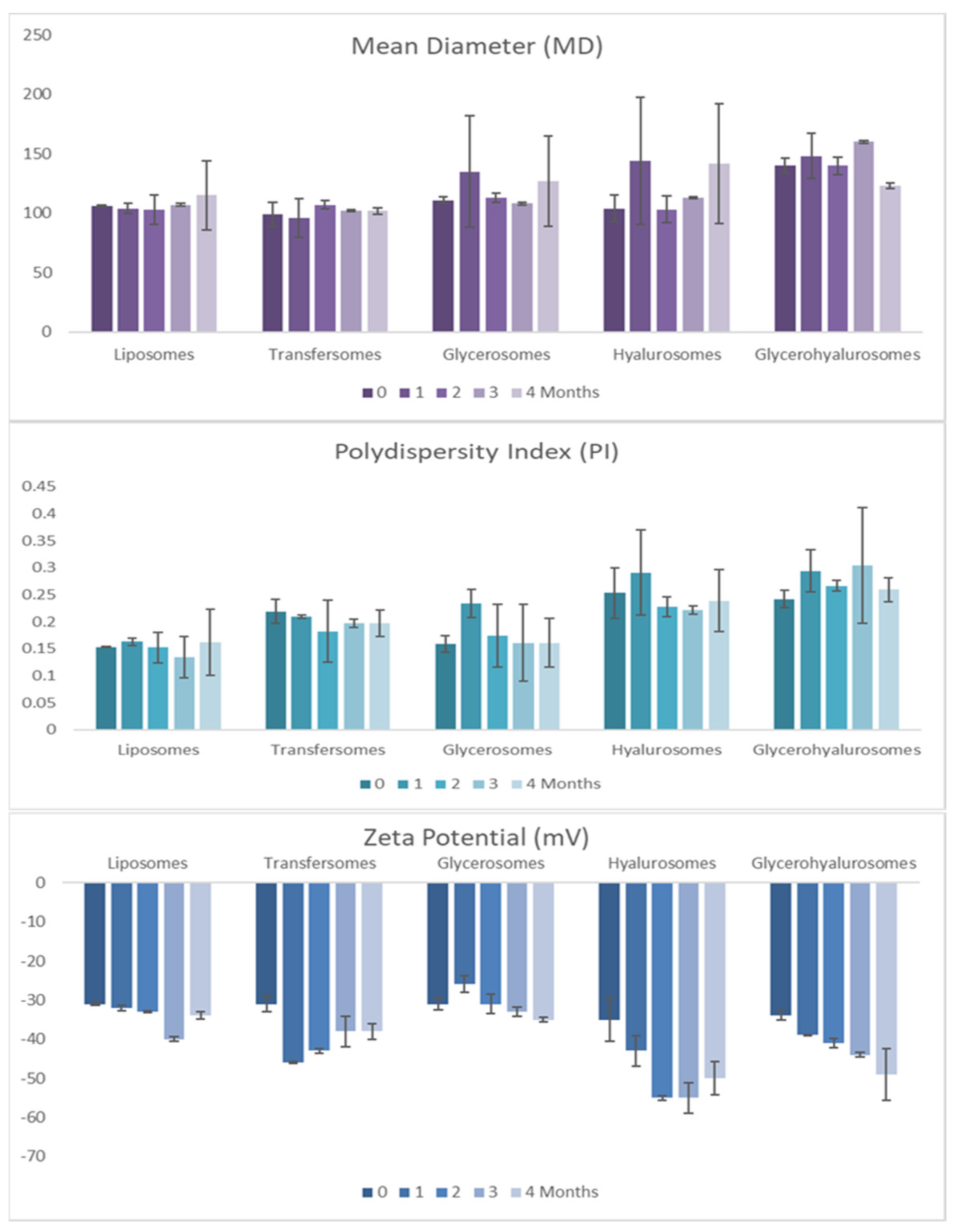

| S75 | Extract | Tween 80 | Sodium Hyaluronate | Glycerol | Water | |
|---|---|---|---|---|---|---|
| mg | mg | mg | mg | mL | mL | |
| Liposomes | 180 | 40 | - | - | - | 1 |
| Transfersomes | 180 | 40 | 15 | - | - | 1 |
| Glycerosomes | 180 | 40 | - | - | 0.25 | 0.75 |
| Hyalurosomes | 180 | 40 | - | 5 | - | 1 |
| Glycerohyalurosomes | 180 | 40 | 15 | 5 | 0.25 | 0.75 |
| T. marum Extract | mg/g dr |
|---|---|
| Total phenolic compounds | 221.72 ± 8.44 |
| Hydroxycinnamic acids | 151.70 ± 2.46 |
| Chlorogenic acid | 20.26 ± 0.10 |
| Unknown | 58.97 ± 0.52 |
| Verbascoside | 48.50 ± 0.49 |
| Others | 34.45 ± 1.57 |
| Flavonols | 70.02 ± 0.83 |
| Luteolin-7-O-glucoside | 16.67 ± 0.19 |
| Luteolin | 3.56 ± 0.04 |
| Apigenin | 2.01 ± 0.01 |
| Others | 47.78 ± 0.44 |
| MD (nm) | PI | ZP (mV) | EE% | AA% | |
|---|---|---|---|---|---|
| Liposomes | 106 ± 11 | 0.15 | −31 ± 3 | 95 ± 2 | 95 ± 3 |
| Transfersomes | 99 ± 9 | 0.22 | −31 ± 5 | 96 ± 5 | 136 ± 9 |
| Glycerosomes | 111 ± 13 | 0.16 | −31 ± 7 | 97 ± 4 | 97 ± 4 |
| Hyalurosomes | 104 ± 7 | 0.25 | −35 ± 6 | 99 ± 3 | 100 ± 6 |
| Glycerohyalurosomes | 140 ± 15 | 0.24 | −34 ± 4 | 98 ± 2 | 98 ± 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Firoznezhad, M.; Castangia, I.; Tuberoso, C.I.G.; Cottiglia, F.; Marongiu, F.; Porceddu, M.; Usach, I.; Escribano-Ferrer, E.; Manca, M.L.; Manconi, M. Formulation and In Vitro Efficacy Assessment of Teucrium marum Extract Loading Hyalurosomes Enriched with Tween 80 and Glycerol. Nanomaterials 2022, 12, 1096. https://doi.org/10.3390/nano12071096
Firoznezhad M, Castangia I, Tuberoso CIG, Cottiglia F, Marongiu F, Porceddu M, Usach I, Escribano-Ferrer E, Manca ML, Manconi M. Formulation and In Vitro Efficacy Assessment of Teucrium marum Extract Loading Hyalurosomes Enriched with Tween 80 and Glycerol. Nanomaterials. 2022; 12(7):1096. https://doi.org/10.3390/nano12071096
Chicago/Turabian StyleFiroznezhad, Mohammad, Ines Castangia, Carlo Ignazio Giovanni Tuberoso, Filippo Cottiglia, Francesca Marongiu, Marco Porceddu, Iris Usach, Elvira Escribano-Ferrer, Maria Letizia Manca, and Maria Manconi. 2022. "Formulation and In Vitro Efficacy Assessment of Teucrium marum Extract Loading Hyalurosomes Enriched with Tween 80 and Glycerol" Nanomaterials 12, no. 7: 1096. https://doi.org/10.3390/nano12071096
APA StyleFiroznezhad, M., Castangia, I., Tuberoso, C. I. G., Cottiglia, F., Marongiu, F., Porceddu, M., Usach, I., Escribano-Ferrer, E., Manca, M. L., & Manconi, M. (2022). Formulation and In Vitro Efficacy Assessment of Teucrium marum Extract Loading Hyalurosomes Enriched with Tween 80 and Glycerol. Nanomaterials, 12(7), 1096. https://doi.org/10.3390/nano12071096











