Optimization of Platinum Nanoparticles (PtNPs) Synthesis by Acid Phosphatase Mediated Eco-Benign Combined with Photocatalytic and Bioactivity Assessments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Acid Phosphatase Enzyme
2.3. Synthesis of PtNPs Using Acid Phosphatase
2.4. Photocatalytic Activity of PtNPs
2.5. Antibacterial Test
2.5.1. Minimum Inhibitory Concentration (MIC)
2.5.2. Reactive Oxygen Species (ROS) Production
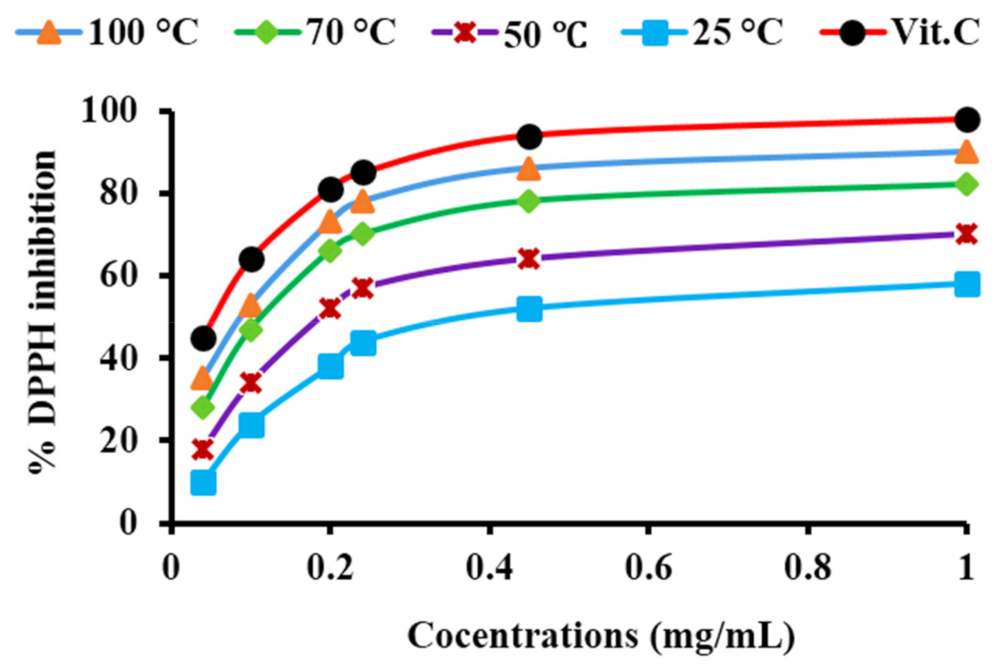
2.6. Antioxidant Activity of PtNPs
2.7. Surface Characterization
3. Results and Discussion
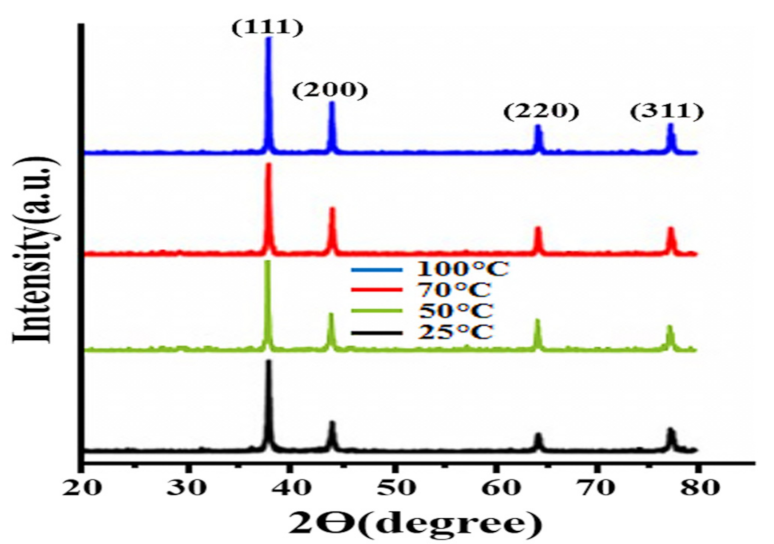
3.1. XRD Analysis
3.2. FT-IR Analysis
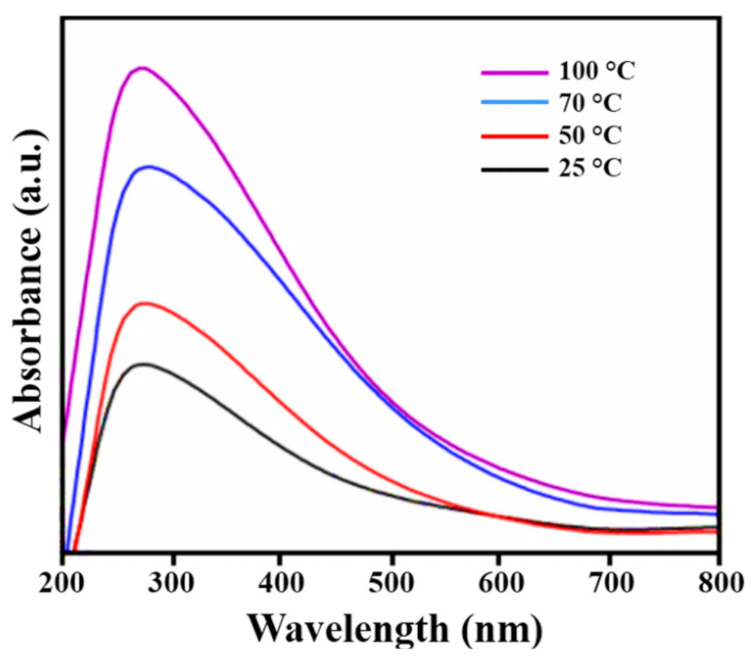
3.3. UV-Visible Analysis
3.4. HRTEM, SAED and DLS Analysis
3.5. X-ray Photoelectron Spectroscopic (XPS) Analysis
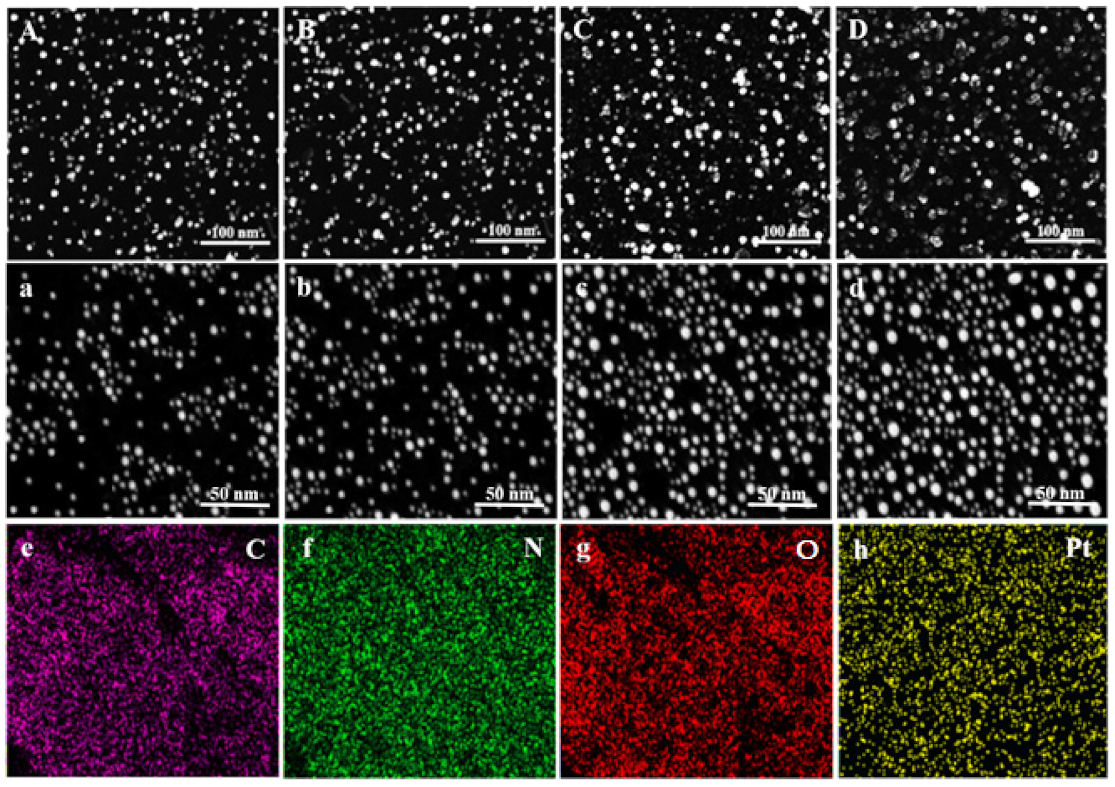
3.6. SEM and EDX Analysis
3.7. Zeta Potential Analysis
3.8. Applications of PtNPs
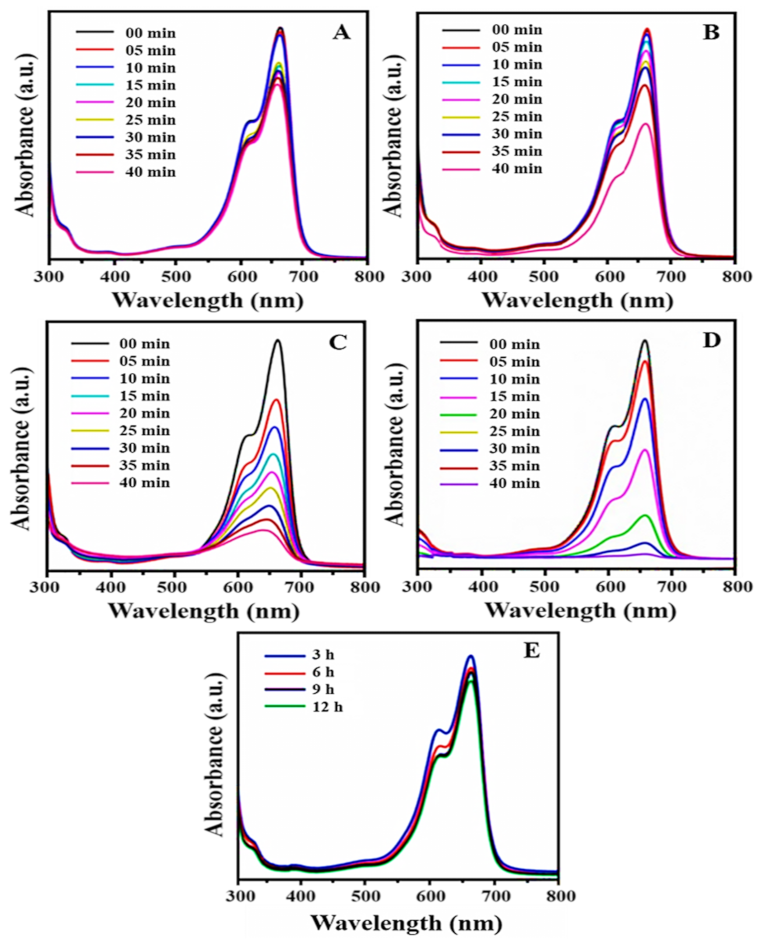
3.8.1. Photocatalytic Degradation of MB by PtNPs
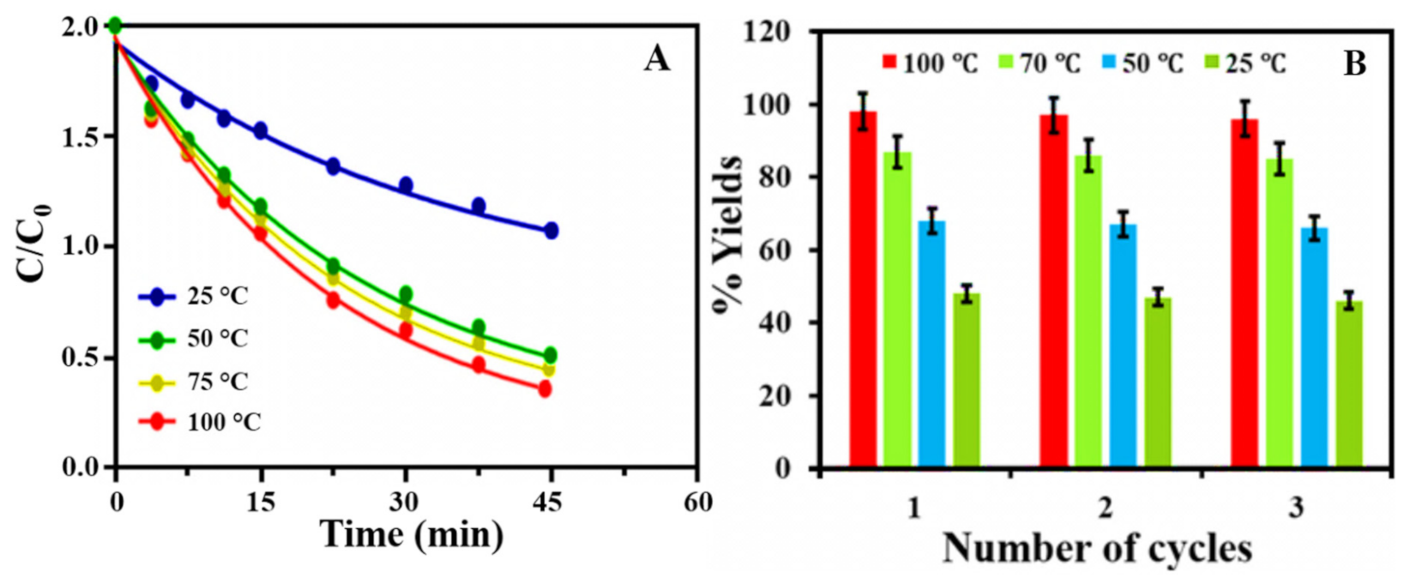
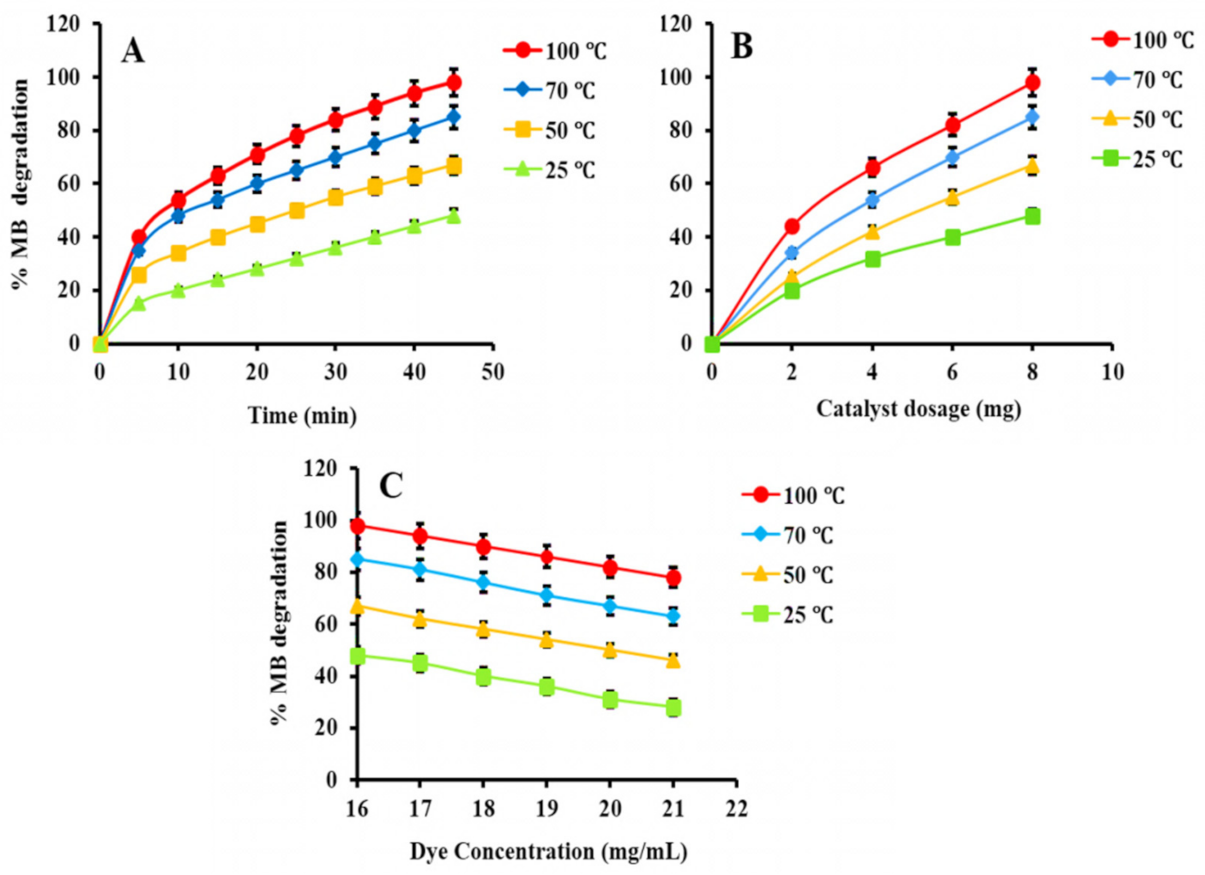
(1) Factors Affecting the Photocatalytic Activity of MB
(2) Effect of Irradiation Time
(3) Effect of Catalyst Dosage
(4) Effect of Dye Concentration
(5) Reusability
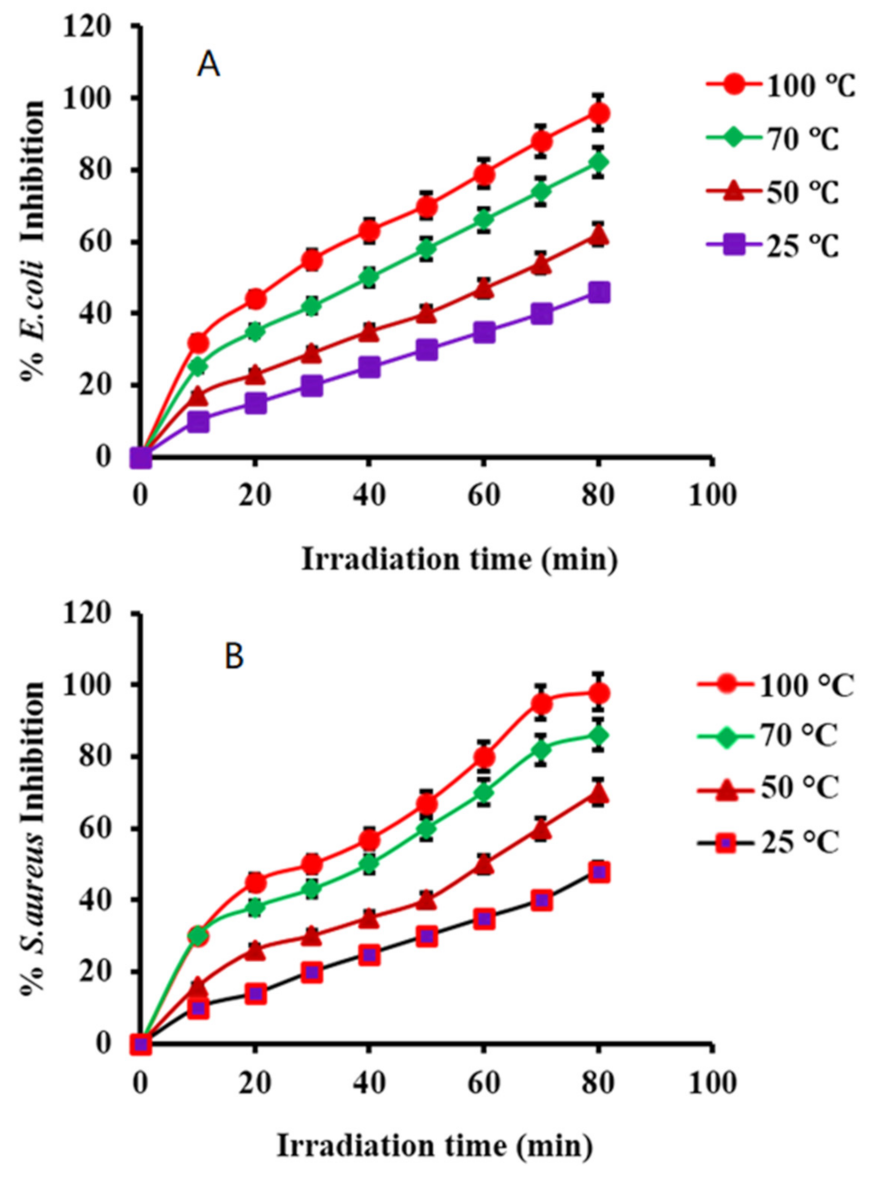
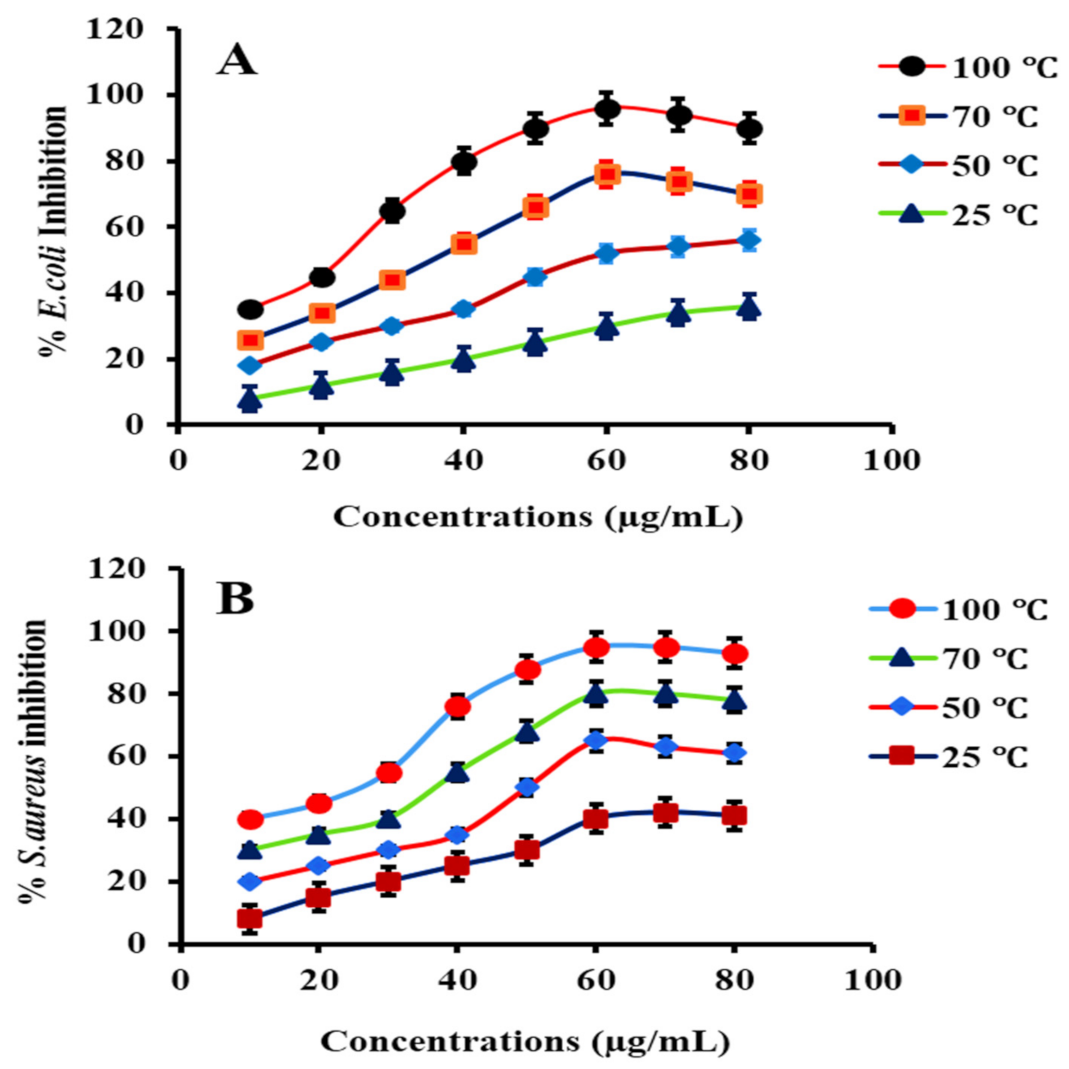
3.8.2. Antibacterial Activity
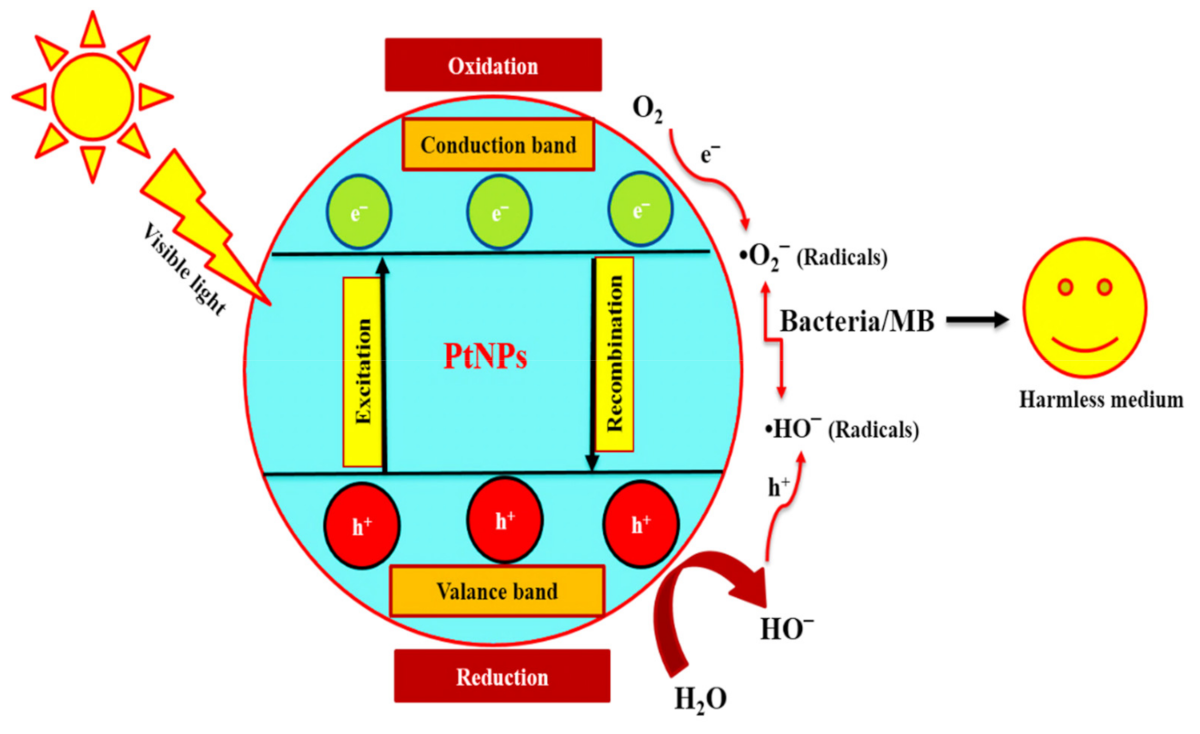
(1) Mechanism of Bacterial Inhibition
(2) Generation of ROS
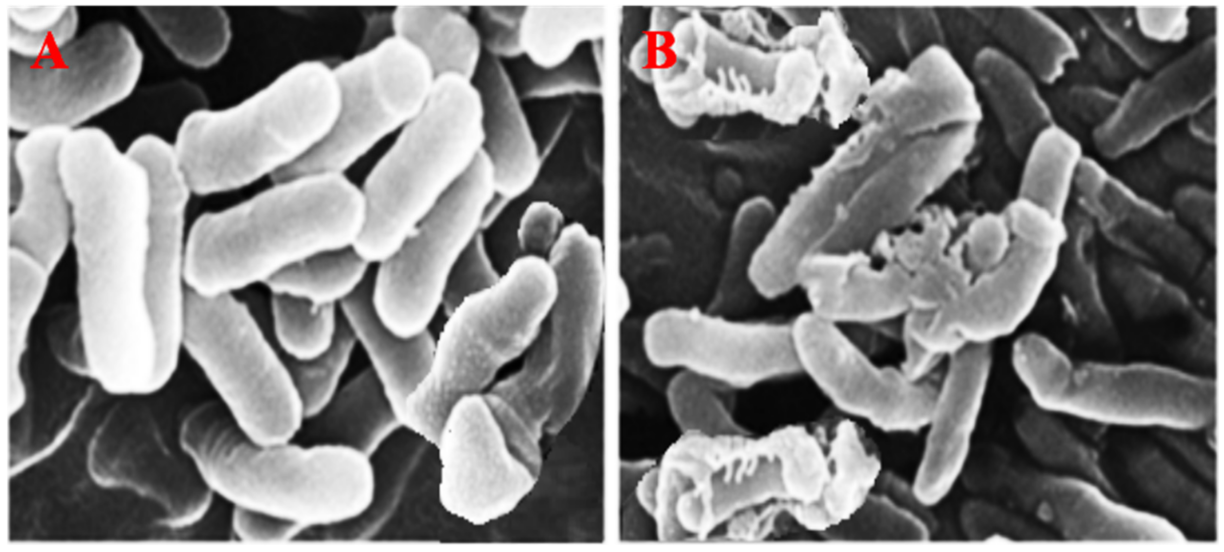
(3) Effect of PtNPs on the Surface Morphology of E. coli Cell
(4) Determination of MIC
(5) Efficacy of PtNPs with Irradiation Time
(6) Concentration Effect of PtNPs
3.8.3. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdelfatah, A.M.; Fawzy, M.; Eltaweil, A.S.; El-Khouly, M.E. Green synthesis of nano-zero-valent iron using ricinus communis seeds extract: Characterization and application in the treatment of methylene blue-polluted water. ACS Omega 2021, 6, 25397–25411. [Google Scholar] [CrossRef] [PubMed]
- Mallikarjuna, K.; Bathula, C.; Buruga, K.; Shrestha, N.K.; Noh, Y.-Y.; Kim, H. Green synthesis of palladium nanoparticles using fenugreek tea and their catalytic applications in organic reactions. Mater. Lett. 2017, 205, 138–141. [Google Scholar] [CrossRef]
- Bathula, C.; Subalakshmi, K.; Kumar, A.; Yadav, H.; Ramesh, S.; Shinde, S.; Shrestha, N.K.; Mallikarjuna, K.; Kim, H. Ultrasonically driven green synthesis of palladium nanoparticles by coleus amboinicus for catalytic reduction and suzuki-miyaura reaction. Colloids Surf. B Biointerfaces 2020, 192, 111026. [Google Scholar] [CrossRef] [PubMed]
- Eltaweil, A.S.; Elgarhy, G.S.; El-Subruiti, G.M.; Omer, A.M. Carboxymethyl cellulose/carboxylated graphene oxide composite microbeads for efficient adsorption of cationic methylene blue dye. Int. J. Biol. Macromol. 2020, 154, 307–318. [Google Scholar] [CrossRef]
- Jameel, M.S.; Aziz, A.A.; Dheyab, M.A.; Mehrdel, B.; Khaniabadi, P.M. Rapid sonochemically-assisted green synthesis of highly stable and biocompatible platinum nanoparticles. Surf. Interfaces 2020, 20, 100635. [Google Scholar] [CrossRef]
- Şahin, B.; Aygün, A.; Gündüz, H.; Şahin, K.; Demir, E.; Akocak, S.; Şen, F. Cytotoxic effects of platinum nanoparticles obtained from pomegranate extract by the green synthesis method on the mcf-7 cell line. Colloids Surf. B Biointerfaces 2018, 163, 119–124. [Google Scholar] [CrossRef]
- Mallikarjuna, K.; Bathula, C.; Reddy, G.D.; Shrestha, N.K.; Kim, H.; Noh, Y.-Y. Au-pd bimetallic nanoparticles embedded highly porous fenugreek polysaccharide based micro networks for catalytic applications. Int. J. Biol. Macromol. 2019, 126, 352–358. [Google Scholar] [CrossRef]
- Dong, L.; Li, R.; Wang, L.; Lan, X.; Sun, H.; Zhao, Y.; Wang, L. Green synthesis of platinum nanoclusters using lentinan for sensitively colorimetric detection of glucose. Int. J. Biol. Macromol. 2021, 172, 289–298. [Google Scholar] [CrossRef]
- Fan, L.; Ji, X.; Lin, G.; Liu, K.; Chen, S.; Ma, G.; Xue, W.; Zhang, X.; Wang, L. Green synthesis of stable platinum nanoclusters with enhanced peroxidase-like activity for sensitive detection of glucose and glutathione. Microchem. J. 2021, 166, 106202. [Google Scholar] [CrossRef]
- Al-Radadi, N.S. Green synthesis of platinum nanoparticles using saudi’s dates extract and their usage on the cancer cell treatment. Arab. J. Chem. 2019, 12, 330–349. [Google Scholar] [CrossRef]
- Naseer, A.; Ali, A.; Ali, S.; Mahmood, A.; Kusuma, H.; Nazir, A.; Yaseen, M.; Khan, M.; Ghaffar, A.; Abbas, M. Biogenic and eco-benign synthesis of platinum nanoparticles (Pt NPs) using plants aqueous extracts and biological derivatives: Environmental, biological and catalytic applications. J. Mater. Res. Technol. 2020, 9, 9093–9107. [Google Scholar] [CrossRef]
- Duan, H.; Wang, D.; Li, Y. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef] [PubMed]
- Gouda, M.; Chen, K.; Li, X.; Liu, Y.; He, Y. Detection of microalgae single-cell antioxidant and electrochemical potentials by gold microelectrode and raman micro-spectroscopy combined with chemometrics. Sens. Actuators B Chem. 2021, 329, 129229. [Google Scholar] [CrossRef]
- Gouda, M.; El-Din Bekhit, A.; Tang, Y.; Huang, Y.; Huang, L.; He, Y.; Li, X. Recent innovations of ultrasound green technology in herbal phytochemistry: A review. Ultrason. Sonochemistry 2021, 73, 105538. [Google Scholar] [CrossRef]
- El-Borady, O.M.; Fawzy, M.; Hosny, M. Antioxidant, anticancer and enhanced photocatalytic potentials of gold nanoparticles biosynthesized by common reed leaf extract. Appl. Nanosci. 2021, 1–12. [Google Scholar] [CrossRef]
- Muthu, K.; Priya, S. Green synthesis, characterization and catalytic activity of silver nanoparticles using cassia auriculata flower extract separated fraction. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 179, 66–72. [Google Scholar] [CrossRef]
- Shammout, M.; Awwad, A. A novel route for the synthesis of copper oxide nanoparticles using bougainvillea plant flowers extract and antifungal activity evaluation. Chem. Int. 2021, 7, 71–78. [Google Scholar]
- Aygun, A.; Gülbagca, F.; Ozer, L.Y.; Ustaoglu, B.; Altunoglu, Y.C.; Baloglu, M.C.; Atalar, M.N.; Alma, M.H.; Sen, F. Biogenic platinum nanoparticles using black cumin seed and their potential usage as antimicrobial and anticancer agent. J. Pharm. Biomed. Anal. 2020, 179, 112961. [Google Scholar] [CrossRef]
- Mohamadi, A.R.; Nami, N.; Norouzi, B. Bio-directed synthesis of platinum nanoparticles by nymphaea alba extract: Fabrication of a novel non-enzymatic hydrogen peroxide sensor. J. Mater.Sci.: Mater. Electron. 2020, 31, 18721–18731. [Google Scholar] [CrossRef]
- Selvi, A.M.; Palanisamy, S.; Jeyanthi, S.; Vinosha, M.; Mohandoss, S.; Tabarsa, M.; You, S.; Kannapiran, E.; Prabhu, N.M. Synthesis of tragia involucrata mediated platinum nanoparticles for comprehensive therapeutic applications: Antioxidant, antibacterial and mitochondria-associated apoptosis in hela cells. Process Biochem. 2020, 98, 21–33. [Google Scholar] [CrossRef]
- Hosny, M.; Fawzy, M.; El-Fakharany, E.M.; Omer, A.M.; Abd El-Monaem, E.M.; Khalifa, R.E.; Eltaweil, A.S. Biogenic synthesis, characterization, antimicrobial, antioxidant, antidiabetic, and catalytic applications of platinum nanoparticles synthesized from polygonum salicifolium leaves. J. Environ. Chem. Eng. 2022, 10, 106806. [Google Scholar] [CrossRef]
- El-Monaem, E.M.A.; El-Latif, M.M.A.; Eltaweil, A.S.; El-Subruiti, G.M. Cobalt nanoparticles supported on reduced amine-functionalized graphene oxide for catalytic reduction of nitroanilines and organic dyes. Nano 2021, 16, 2150039. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; El-Tawil, A.M.; Abd El-Monaem, E.M.; El-Subruiti, G.M. Zero valent iron nanoparticle-loaded nanobentonite intercalated carboxymethyl chitosan for efficient removal of both anionic and cationic dyes. ACS Omega 2021, 6, 6348–6360. [Google Scholar] [CrossRef] [PubMed]
- Eltaweil, A.S.; Mamdouh, I.M.; Abd El-Monaem, E.M.; El-Subruiti, G.M. Highly efficient removal for methylene blue and Cu2+ onto UiO-66 metal–organic framework/carboxylated graphene oxide-incorporated sodium alginate beads. ACS Omega 2021, 6, 23528–23541. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Arul, V.; Sethuraman, M.G. Ecofriendly synthesis of fluorescent nitrogen-doped carbon dots from coccinia grandis and its efficient catalytic application in the reduction of methyl orange. J. Fluoresc. 2020, 30, 103–112. [Google Scholar] [CrossRef]
- Arul, V.; Chandrasekaran, P.; Sivaraman, G.; Sethuraman, M.G. Efficient green synthesis of N, B co-doped bright fluorescent carbon nanodots and their electrocatalytic and bio-imaging applications. Diam. Relat. Mater. 2021, 116, 108437. [Google Scholar] [CrossRef]
- Arul, V.; Chandrasekaran, P.; Sethuraman, M. Reduction of congo red using nitrogen doped fluorescent carbon nanodots obtained from sprout extract of borassus flabellifer. Chem. Phys. Lett. 2020, 754, 137646. [Google Scholar] [CrossRef]
- Eltaweil, A.; Mohamed, H.A.; Abd El-Monaem, E.M.; El-Subruiti, G. Mesoporous magnetic biochar composite for enhanced adsorption of malachite green dye: Characterization, adsorption kinetics, thermodynamics and isotherms. Adv. Powder Technol. 2020, 31, 1253–1263. [Google Scholar] [CrossRef]
- Omer, A.M.; Tamer, T.M.; Khalifa, R.E.; Eltaweil, A.S.; Agwa, M.M.; Sabra, S.; Abd-Elmonem, M.S.; Mohy-Eldin, M.S.; Ziora, Z.M. Formulation and antibacterial activity evaluation of quaternized aminochitosan membrane for wound dressing applications. Polymers 2021, 13, 2428. [Google Scholar] [CrossRef]
- Giufrè, M.; Mazzolini, E.; Cerquetti, M.; Brusaferro, S.; Accogli, M.; Agnoletti, F.; Agodi, A.; Alborali, G.L.; Arghittu, M.; Auxilia, F. Extended-spectrum β-lactamase-producing escherichia coli from extraintestinal infections in humans and from food-producing animals in italy: A ‘one health’study. Int. J. Antimicrob. Agents 2021, 58, 106433. [Google Scholar] [CrossRef]
- Carezzano, M.E.; Sotelo, J.P.; Primo, E.; Reinoso, E.B.; Paletti Rovey, M.F.; Demo, M.S.; Giordano, W.F.; Oliva, M.d.l.M. Inhibitory effect of thymus vulgaris and origanum vulgare essential oils on virulence factors of phytopathogenic pseudomonas syringae strains. Plant Biol. 2017, 19, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.K.; Donato, J.; Wang, H.H.; Cloud-Hansen, K.A.; Davies, J.; Handelsman, J. Call of the wild: Antibiotic resistance genes in natural environments. Nat. Rev. Microbiol. 2010, 8, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lippard, S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005, 4, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.; Fang, G.; Cao, Z.; Shang, Y.; Alfarraj, S.; Alharbi, S.A.; Li, J.; Yang, S.; Duan, X. Green synthesis, characterization, cytotoxicity, antioxidant, and anti-human ovarian cancer activities of curcumae kwangsiensis leaf aqueous extract green-synthesized gold nanoparticles. Arab. J. Chem. 2021, 14, 103000. [Google Scholar] [CrossRef]
- Zaman, U.; Naz, R.; Khattak, N.S.; ur Rehman, K.; Saeed, A.; Farooq, M.; Sahar, J.; Iqbal, A. Kinetic and thermodynamic studies of novel acid phosphates extracted from cichorium intybus seedlings. Int. J. Biol. Macromol. 2021, 168, 195–204. [Google Scholar] [CrossRef] [PubMed]
- ur Rehman, K.; Tahir, K.; Al-Abdulkarim, H.A.; Saleh, E.A.M.; Alosaimi, A.M.; Hussein, M.A.; Khan, A.U.; Khan, Z.U.H.; Nazir, S.; Zaman, U. Photoinhibition and photocatalytic response of surfactant mediated Pt/ZnO nanocomposite. Photodiagnosis Photodyn. Ther. 2021, 35, 102458. [Google Scholar] [CrossRef] [PubMed]
- ur Rehman, K.; Zaman, U.; Khan, D.; Khan, W.U. Surfactant assisted CuO/MCM-41 nanocomposite: Ultra efficient photocatalyst for degradation of methylene blue dye and inactivation of highly drug resistant bacteria. Mater. Chem. Phys. 2022, 277, 125454. [Google Scholar] [CrossRef]
- Fouad, M.T.; Moustafa, A.; Hussein, L.; Romeilah, R.; Gouda, M. In-vitro antioxidant and antimicrobial activities of selected fruit and vegetable juices and fermented dairy products commonly consumed in Egypt. Res. J. Pharm. Biol. Chem. 2015, 6, 541–550. [Google Scholar]
- Eramabadi, P.; Masoudi, M.; Makhdoumi, A.; Mashreghi, M. Microbial cell lysate supernatant (CLS) alteration impact on platinum nanoparticles fabrication, characterization, antioxidant and antibacterial activity. Mater. Sci. Eng. C 2020, 117, 111292. [Google Scholar] [CrossRef]
- Roy, S.; Roy, S.; Neuenswander, B.; Hill, D.; Larock, R.C. Solution-phase synthesis of a diverse isocoumarin library. J. Comb. Chem. 2009, 11, 1128–1135. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.U.; Yuan, Q.; Wei, Y.; Tahir, K.; Khan, S.U.; Ahmad, A.; Khan, S.; Nazir, S.; Khan, F.U. Ultra-efficient photocatalytic deprivation of methylene blue and biological activities of biogenic silver nanoparticles. J. Photochem. Photobiol. B Biol. 2016, 159, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.P.; Patil, R.V.; Pawar, K.D. Green biogenic approach to optimized biosynthesis of noble metal nanoparticles with potential catalytic, antioxidant and antihaemolytic activities. Process Biochem. 2020, 98, 172–182. [Google Scholar] [CrossRef]
- Xie, D.; Ma, Y.; Gu, Y.; Zhou, H.; Zhang, H.; Wang, G.; Zhang, Y.; Zhao, H. Bifunctional NH2-MIL-88 (Fe) metal–organic framework nanooctahedra for highly sensitive detection and efficient removal of arsenate in aqueous media. J. Mater. Chem. A 2017, 5, 23794–23804. [Google Scholar] [CrossRef]
- Wei, K.; Rao, H.; Xue, X.; Luo, M.; Xue, Z. A facile photothermometric sensor of acid phosphatase based on coooh nanozymes-mediated 3, 3′, 5, 5′-tetramethylbenzidine photothermal system. Microchem. J. 2021, 170, 106736. [Google Scholar] [CrossRef]
- Farzi-Khajeh, H.; Safa, K.D.; Dastmalchi, S. Arsanilic acid modified superparamagnetic iron oxide nanoparticles for purification of alkaline phosphatase from hen’s egg yolk. J. Chromatogr. B 2017, 1061, 26–33. [Google Scholar] [CrossRef]
- Srivastava, P.K.; Anand, A. Immobilization of acid phosphatase from vigna aconitifolia seeds on chitosan beads and its characterization. Int. J. Biol. Macromol. 2014, 64, 150–154. [Google Scholar] [CrossRef]
- Shen, X.-C.; Liou, X.-Y.; Ye, L.-P.; Liang, H.; Wang, Z.-Y. Spectroscopic studies on the interaction between human hemoglobin and CdS quantum dots. J. Colloid Interface Sci. 2007, 311, 400–406. [Google Scholar] [CrossRef]
- Zada, S.; Ahmad, A.; Khan, S.; Iqbal, A.; Ahmad, S.; Ali, H.; Fu, P. Biofabrication of gold nanoparticles by lyptolyngbya jsc-1 extract as super reducing and stabilizing agents: Synthesis, characterization and antibacterial activity. Microb. Pathog. 2018, 114, 116–123. [Google Scholar] [CrossRef]
- Noruzi, M.; Zare, D.; Davoodi, D. A rapid biosynthesis route for the preparation of gold nanoparticles by aqueous extract of cypress leaves at room temperature. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 94, 84–88. [Google Scholar] [CrossRef]
- Oliveira, M.M.; Ugarte, D.; Zanchet, D.; Zarbin, A.J. Influence of synthetic parameters on the size, structure, and stability of dodecanethiol-stabilized silver nanoparticles. J. Colloid Interface Sci. 2005, 292, 429–435. [Google Scholar] [CrossRef]
- Jin, R.; Cao, Y.C.; Hao, E.; Métraux, G.S.; Schatz, G.C.; Mirkin, C.A. Controlling anisotropic nanoparticle growth through plasmon excitation. Nature 2003, 425, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Hosny, M.; Fawzy, M.; Abdelfatah, A.M.; Fawzy, E.E.; Eltaweil, A.S. Comparative study on the potentialities of two halophytic species in the green synthesis of gold nanoparticles and their anticancer, antioxidant and catalytic efficiencies. Adv. Powder Technol. 2021, 32, 3220–3233. [Google Scholar] [CrossRef]
- Cui, T.; Li, S.; Chen, S.; Liang, Y.; Sun, H.; Wang, L. “Stealth” dendrimers with encapsulation of indocyanine green for photothermal and photodynamic therapy of cancer. Int. J. Pharm. 2021, 600, 120502. [Google Scholar] [CrossRef] [PubMed]
- Jameel, M.S.; Aziz, A.A.; Dheyab, M.A.; Mehrdel, B. Rapid methanol-assisted amalgamation of high purity platinum nanoparticles utilizing sonochemical strategy and investigation on its catalytic activity. Surf. Interfaces 2020, 21, 100785. [Google Scholar] [CrossRef]
- Kora, A.J.; Rastogi, L. Peroxidase activity of biogenic platinum nanoparticles: A colorimetric probe towards selective detection of mercuric ions in water samples. Sens. Actuators B Chem. 2018, 254, 690–700. [Google Scholar] [CrossRef]
- Jameel, M.S.; Aziz, A.A.; Dheyab, M.A. Comparative analysis of platinum nanoparticles synthesized using sonochemical-assisted and conventional green methods. Nano-Struct. Nano-Objects 2020, 23, 100484. [Google Scholar] [CrossRef]
- Zou, F.; Zhou, J.; Zhang, J.; Li, J.; Tang, B.; Chen, W.; Wang, J.; Wang, X. Functionalization of silk with in-situ synthesized platinum nanoparticles. Materials 2018, 11, 1929. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.; Kongi, N.; Erikson, H.; Rähn, M.; Merisalu, M.; Matisen, L.; Paiste, P.; Aruväli, J.; Sammelselg, V.; Estudillo-Wong, L.A. Platinum nanoparticles photo-deposited on SnO2-C composites: An active and durable electrocatalyst for the oxygen reduction reaction. Electrochim. Acta 2019, 316, 162–172. [Google Scholar] [CrossRef]
- Celebioglu, A.; Ranjith, K.S.; Eren, H.; Biyikli, N.; Uyar, T. Surface decoration of pt nanoparticles via ald with TiO2 protective layer on polymeric nanofibers as flexible and reusable heterogeneous nanocatalysts. Sci. Rep. 2017, 7, 13401. [Google Scholar]
- Ali, N.H.; Mohammed, A.M. Biosynthesis and characterization of platinum nanoparticles using iraqi zahidi dates and evaluation of their biological applications. Biotechnol. Rep. 2021, 30, e00635. [Google Scholar] [CrossRef]
- Konishi, Y.; Ohno, K.; Saitoh, N.; Nomura, T.; Nagamine, S.; Hishida, H.; Takahashi, Y.; Uruga, T. Bioreductive deposition of platinum nanoparticles on the bacterium shewanella algae. J. Biotechnol. 2007, 128, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.R.; Bag, S.S.; Golder, A.K. Bio-inspired ptnps/graphene nanocomposite based electrocatalytic sensing of metabolites of dipyrone. Anal. Chim. Acta 2021, 1167, 338562. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, V.S.; Pugazhendhi, A.; Prakash, S.; Ahila, N.; Vinoj, G.; Selvam, S.; Kumar, G.; Kannapiran, E.; Rajendran, R.B. Synthesis of platinum nanoparticles using seaweed padina gymnospora and their catalytic activity as PVP/PtNPs nanocomposite towards biological applications. Biomed. Pharmacother. 2017, 92, 479–490. [Google Scholar] [CrossRef]
- Shah, R.; Eldridge, D.; Palombo, E.; Harding, I. Optimisation and stability assessment of solid lipid nanoparticles using particle size and zeta potential. J. Phys. Sci. 2014, 25, 59–75. [Google Scholar]
- Ullah, S.; Ahmad, A.; Wang, A.; Raza, M.; Jan, A.U.; Tahir, K.; Rahman, A.U.; Qipeng, Y. Bio-fabrication of catalytic platinum nanoparticles and their in vitro efficacy against lungs cancer cells line (A549). J. Photochem. Photobiol. B Biol. 2017, 173, 368–375. [Google Scholar] [CrossRef]
- Nishanthi, R.; Malathi, S.; Palani, P. Green synthesis and characterization of bioinspired silver, gold and platinum nanoparticles and evaluation of their synergistic antibacterial activity after combining with different classes of antibiotics. Mater. Sci. Eng. C 2019, 96, 693–707. [Google Scholar]
- Harijan, D.K.; Gupta, S.; Ben, S.K.; Srivastava, A.; Singh, J.; Chandra, V. High photocatalytic efficiency of α-Fe2O3-ZnO composite using solar energy for methylene blue degradation. Phys. B Condens. Matter 2022, 627, 413567. [Google Scholar] [CrossRef]
- Hammouche, J.; Daoudi, K.; Columbus, S.; Ziad, R.; Ramachandran, K.; Gaidi, M. Structural and morphological optimization of ni doped ZnO decorated silicon nanowires for photocatalytic degradation of methylene blue. Inorg. Chem. Commun. 2021, 131, 108763. [Google Scholar] [CrossRef]
- Yadav, N.; Chaudhary, L.; Sakhare, P.; Dongale, T.; Patil, P.; Sheikh, A. Impact of collected sunlight on ZnFe2O4 nanoparticles for photocatalytic application. J. Colloid Interface Sci. 2018, 527, 289–297. [Google Scholar] [CrossRef]
- Dobrucka, R. Biofabrication of platinum nanoparticles using fumariae herba extract and their catalytic properties. Saudi J. Biol. Sci. 2019, 26, 31–37. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Fawzy, M.; Hosny, M.; Abd El-Monaem, E.M.; Tamer, T.M.; Omer, A.M. Green synthesis of platinum nanoparticles using atriplex halimus leaves for potential antimicrobial, antioxidant, and catalytic applications. Arab. J. Chem. 2022, 15, 103517. [Google Scholar] [CrossRef]
- Bogireddy, N.K.R.; Pal, U.; Kumar, M.K.; Domínguez, J.M.; Gomez, L.M.; Agarwal, V. Green fabrication of 2D platinum superstructures and their high catalytic activity for mitigation of organic pollutants. Catal. Today 2021, 360, 185–193. [Google Scholar] [CrossRef]
- Ramachandiran, D.; Elangovan, M.; Rajesh, K. Structural, optical, biological and photocatalytic activities of platinum nanoparticles using salixtetrasperma leaf extract via hydrothermal and ultrasonic methods. Optik 2021, 244, 167494. [Google Scholar] [CrossRef]
- Akter, J.; Sapkota, K.P.; Hanif, M.A.; Islam, M.A.; Abbas, H.G.; Hahn, J.R. Kinetically controlled selective synthesis of Cu2O and CuO nanoparticles toward enhanced degradation of methylene blue using ultraviolet and sun light. Mater. Sci. Semicond. Processing 2021, 123, 105570. [Google Scholar] [CrossRef]
- Riga, A.; Soutsas, K.; Ntampegliotis, K.; Karayannis, V.; Papapolymerou, G. Effect of system parameters and of inorganic salts on the decolorization and degradation of procion H-exl dyes. Comparison of H2O2/UV, Fenton, UV/Fenton, TiO2/UV and TiO2/UV/H2O2 processes. Desalination 2007, 211, 72–86. [Google Scholar] [CrossRef]
- Chiou, C.-H.; Wu, C.-Y.; Juang, R.-S. Influence of operating parameters on photocatalytic degradation of phenol in UV/TiO2 process. Chem. Eng. J. 2008, 139, 322–329. [Google Scholar] [CrossRef]
- Ahmed, J.; Mulla, M.Z.; Arfat, Y.A. Thermo-mechanical, structural characterization and antibacterial performance of solvent casted polylactide/cinnamon oil composite films. Food Control. 2016, 69, 196–204. [Google Scholar] [CrossRef]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Dellinger, E.P.; Goldstein, E.J.; Gorbach, S.L.; Hirschmann, J.V.; Kaplan, S.L.; Montoya, J.G.; Wade, J.C. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of america. Clin. Infect. Dis. 2014, 59, e10–e52. [Google Scholar] [CrossRef] [Green Version]
- Iyer, J.K.; Dickey, A.; Rouhani, P.; Kaul, A.; Govindaraju, N.; Singh, R.N.; Kaul, R. Nanodiamonds facilitate killing of intracellular uropathogenic e. Coli in an in vitro model of urinary tract infection pathogenesis. PLoS ONE 2018, 13, e0191020. [Google Scholar] [CrossRef] [Green Version]
- Chwalibog, A.; Sawosz, E.; Hotowy, A.; Szeliga, J.; Mitura, S.; Mitura, K.; Grodzik, M.; Orlowski, P.; Sokolowska, A. Visualization of interaction between inorganic nanoparticles and bacteria or fungi. Int. J. Nanomed. 2010, 5, 1085. [Google Scholar] [CrossRef] [Green Version]
- Ravichandran, A.; Subramanian, P.; Manoharan, V.; Muthu, T.; Periyannan, R.; Thangapandi, M.; Ponnuchamy, K.; Pandi, B.; Marimuthu, P.N. Phyto-mediated synthesis of silver nanoparticles using fucoidan isolated from spatoglossum asperum and assessment of antibacterial activities. J. Photochem. Photobiol. B Biol. 2018, 185, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Vinosha, M.; Palanisamy, S.; Muthukrishnan, R.; Selvam, S.; Kannapiran, E.; You, S.; Prabhu, N.M. Biogenic synthesis of gold nanoparticles from halymenia dilatata for pharmaceutical applications: Antioxidant, anti-cancer and antibacterial activities. Process Biochem. 2019, 85, 219–229. [Google Scholar] [CrossRef]
- Manikandakrishnan, M.; Palanisamy, S.; Vinosha, M.; Kalanjiaraja, B.; Mohandoss, S.; Manikandan, R.; Tabarsa, M.; You, S.; Prabhu, N.M. Facile green route synthesis of gold nanoparticles using caulerpa racemosa for biomedical applications. J. Drug Deliv. Sci. Technol. 2019, 54, 101345. [Google Scholar] [CrossRef]
- Pedone, D.; Moglianetti, M.; De Luca, E.; Bardi, G.; Pompa, P.P. Platinum nanoparticles in nanobiomedicine. Chem. Soc. Rev. 2017, 46, 4951–4975. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; de Aberasturi, D.J.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Allaker, R.P. The use of nanoparticles to control oral biofilm formation. J. Dent. Res. 2010, 89, 1175–1186. [Google Scholar] [CrossRef]
- Fayaz, A.M.; Balaji, K.; Girilal, M.; Yadav, R.; Kalaichelvan, P.T.; Venketesan, R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: A study against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 103–109. [Google Scholar] [CrossRef]
- Zabaleta, M.E.; Forbes-Hernández, T.Y.; Simal-Gandara, J.; Quiles, J.L.; Cianciosi, D.; Bullon, B.; Giampieri, F.; Battino, M. Effect of polyphenols on HER2-positive breast cancer and related mirnas: Epigenomic regulation. Food Res. Int. 2020, 137, 109623. [Google Scholar] [CrossRef]
- Naaz, R.; Siddiqui, V.U.; Qadir, S.U.; Siddiqi, W.A. Green synthesis of silver nanoparticles using syngonium podophyllum leaf extract and its antibacterial activity. Mater. Today Proc. 2021, 46, 2352–2358. [Google Scholar] [CrossRef]
- Lv, H.; Cui, S.; Yang, Q.; Song, X.; Wang, D.; Hu, J.; Zhou, Y.; Liu, Y. Agnps-incorporated nanofiber mats: Relationship between agnps size/content, silver release, cytotoxicity, and antibacterial activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111331. [Google Scholar] [CrossRef]
- Chouhan, S.; Guleria, S. Green synthesis of agnps using cannabis sativa leaf extract: Characterization, antibacterial, anti-yeast and α-amylase inhibitory activity. Mater. Sci. Energy Technol. 2020, 3, 536–544. [Google Scholar] [CrossRef]
- Rosman, N.S.R.; Harun, N.A.; Idris, I.; Ismail, W.I.W. Eco-friendly silver nanoparticles (agnps) fabricated by green synthesis using the crude extract of marine polychaete, marphysa moribidii: Biosynthesis, characterisation, and antibacterial applications. Heliyon 2020, 6, e05462. [Google Scholar] [CrossRef] [PubMed]
- Holt, K.B.; Bard, A.J. Interaction of silver (I) ions with the respiratory chain of Escherichia coli: An electrochemical and scanning electrochemical microscopy study of the antimicrobial mechanism of micromolar Ag+. Biochemestry 2005, 44, 13214–13223. [Google Scholar] [CrossRef] [PubMed]
- Yun’an Qing, L.C.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oishi, M.; Miyagawa, N.; Sakura, T.; Nagasaki, Y. pH-responsive PEGylated nanogel containing platinum nanoparticles: Application to on–off regulation of catalytic activity for reactive oxygen species. React. Funct. Polym. 2007, 67, 662–668. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Singh, S.P.; Häder, D.-P.; Sinha, R.P. Detection of reactive oxygen species (ROS) by the oxidant-sensing probe 2′, 7′-dichlorodihydrofluorescein diacetate in the cyanobacterium anabaena variabilis PCC 7937. Biochem. Biophys. Res. Commun. 2010, 397, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Wei, Y.; Syed, F.; Tahir, K.; Rehman, A.U.; Khan, A.; Ullah, S.; Yuan, Q. The effects of bacteria-nanoparticles interface on the antibacterial activity of green synthesized silver nanoparticles. Microb. Pathog. 2017, 102, 133–142. [Google Scholar] [CrossRef]
- Zayed, M.F.; Mahfoze, R.A.; El-kousy, S.M.; Al-Ashkar, E.A. In-vitro antioxidant and antimicrobial activities of metal nanoparticles biosynthesized using optimized pimpinella anisum extract. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124167. [Google Scholar] [CrossRef]
- Patil, M.P.; Kang, M.-j.; Niyonizigiye, I.; Singh, A.; Kim, J.-O.; Seo, Y.B.; Kim, G.-D. Extracellular synthesis of gold nanoparticles using the marine bacterium paracoccus haeundaensis BC74171T and evaluation of their antioxidant activity and antiproliferative effect on normal and cancer cell lines. Colloids Surf. B Biointerfaces 2019, 183, 110455. [Google Scholar] [CrossRef]






| Catalyst | MB Concentration | Degradation Efficiency (%) | Time (min) | Reference |
|---|---|---|---|---|
| PtNPs | 4 mL | 100 | 15 | [70] |
| At-PtNPs | 100 PPm | 100 | 5 | [71] |
| PtNPs | 1.5 mL | 100 | 15 | [54] |
| SA-PtNPs | 2 mL | 39.87 | 1 | [72] |
| PtNPs | 500 mL | 90.28 | 50 | [73] |
| ACP-PtNPs | 80 mL | 99 | 28 | [This work] |
| Sample | Zone of Inhibition (mm) | |||
|---|---|---|---|---|
| Light | Dark | |||
| E. coli | S. aureus | E. coli | S. aureus | |
| Standard | 33 | 31 | 27 | 26 |
| PtNPs (100 °C) | 31 | 30 | 25 | 22 |
| PtNPs (70 °C) | 26 | 24 | 19 | 18 |
| PtNPs (50 °C) | 18 | 17 | 15 | 13 |
| PtNPs (25 °C) | 13 | 12 | 9 | 7 |
| Sample | Concentration | Bacterial Strain | Zone of Inhibition (mm) | Reference |
|---|---|---|---|---|
| AgNPs | 75 µL | E. coli/S. aureus | 18.5/14.9 | [88] |
| AgNPs | 20 µL | E. coli/S. aureus | 14/13 | [89] |
| AgNPs | 2 µL | E. coli/S. aureus | 8.8/8.5 | [90] |
| AgNPs | 20 µL | E. coli/S. aureus | n.a/n.a | [91] |
| AgNPs | 100 µL | E. coli/S. aureus | 8.39/8.54 | [92] |
| PtNPs | 50 µL | E. coli/S. aureus | 31/30 | [This work] |
| Bacteria | PtNPs (µg/mL) | |||
|---|---|---|---|---|
| 100 °C | 70 °C | 50 °C | 25 °C | |
| E. coli | 10 | 20 | 20 | 30 |
| S. aureus | 10 | 20 | 20 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, K.u.; Gouda, M.; Zaman, U.; Tahir, K.; Khan, S.U.; Saeed, S.; Khojah, E.; El-Beltagy, A.; Zaky, A.A.; Naeem, M.; et al. Optimization of Platinum Nanoparticles (PtNPs) Synthesis by Acid Phosphatase Mediated Eco-Benign Combined with Photocatalytic and Bioactivity Assessments. Nanomaterials 2022, 12, 1079. https://doi.org/10.3390/nano12071079
Rehman Ku, Gouda M, Zaman U, Tahir K, Khan SU, Saeed S, Khojah E, El-Beltagy A, Zaky AA, Naeem M, et al. Optimization of Platinum Nanoparticles (PtNPs) Synthesis by Acid Phosphatase Mediated Eco-Benign Combined with Photocatalytic and Bioactivity Assessments. Nanomaterials. 2022; 12(7):1079. https://doi.org/10.3390/nano12071079
Chicago/Turabian StyleRehman, Khalil ur, Mostafa Gouda, Umber Zaman, Kamran Tahir, Shahid Ullah Khan, Sumbul Saeed, Ebtihal Khojah, Alaa El-Beltagy, Ahmed A. Zaky, Mohamed Naeem, and et al. 2022. "Optimization of Platinum Nanoparticles (PtNPs) Synthesis by Acid Phosphatase Mediated Eco-Benign Combined with Photocatalytic and Bioactivity Assessments" Nanomaterials 12, no. 7: 1079. https://doi.org/10.3390/nano12071079
APA StyleRehman, K. u., Gouda, M., Zaman, U., Tahir, K., Khan, S. U., Saeed, S., Khojah, E., El-Beltagy, A., Zaky, A. A., Naeem, M., Khan, M. I., & Khattak, N. S. (2022). Optimization of Platinum Nanoparticles (PtNPs) Synthesis by Acid Phosphatase Mediated Eco-Benign Combined with Photocatalytic and Bioactivity Assessments. Nanomaterials, 12(7), 1079. https://doi.org/10.3390/nano12071079









