Porous TiO2/Carbon Dot Nanoflowers with Enhanced Surface Areas for Improving Photocatalytic Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of TiO2 Mesoporous Particles
2.2. Preparation of Carbon Dots
2.3. Preparation of TiO2/CDs Photocatalysts
2.4. Characterization of the Photocatalysts
2.5. Photocatalytic Degradation Experiments
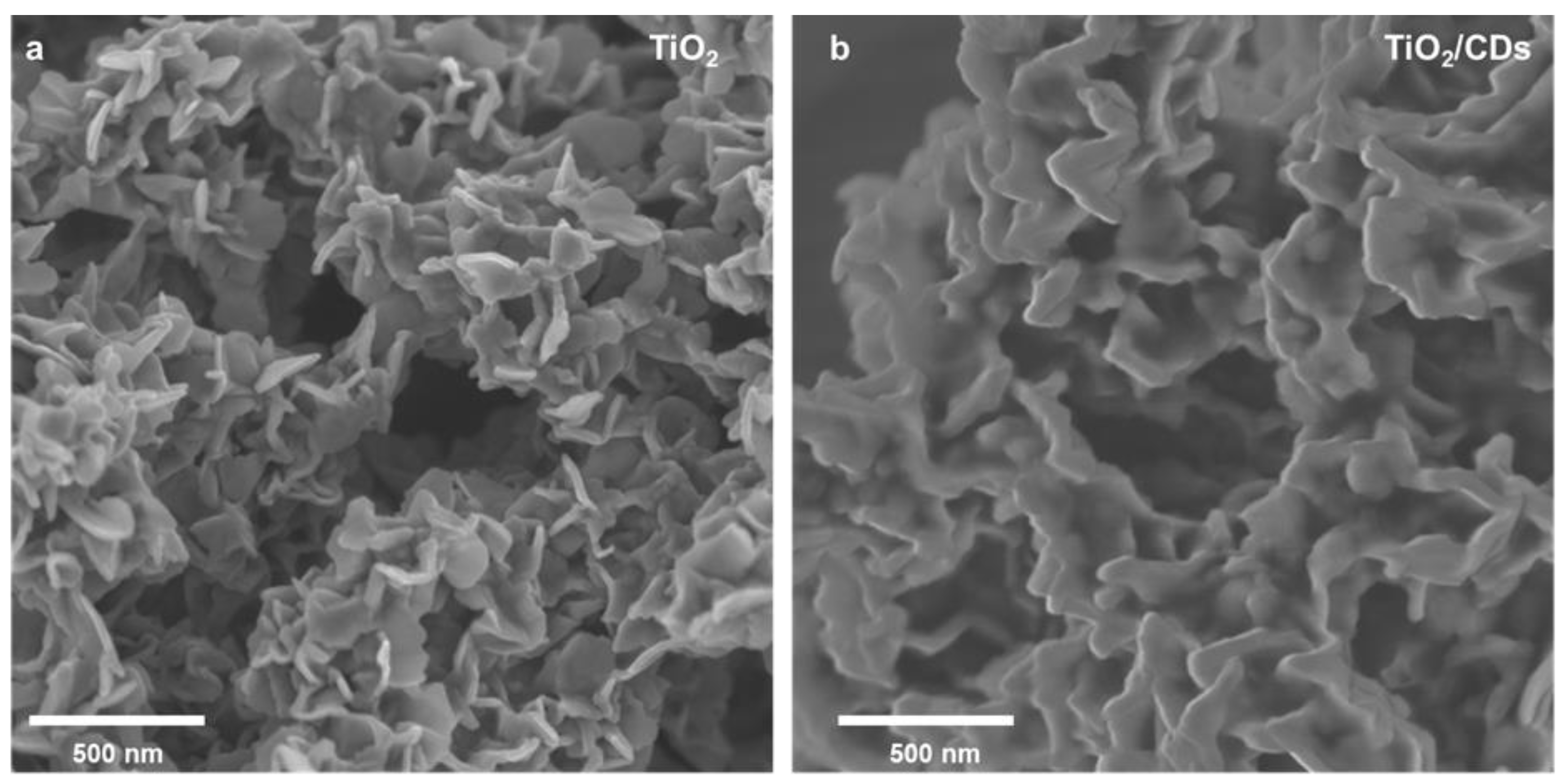
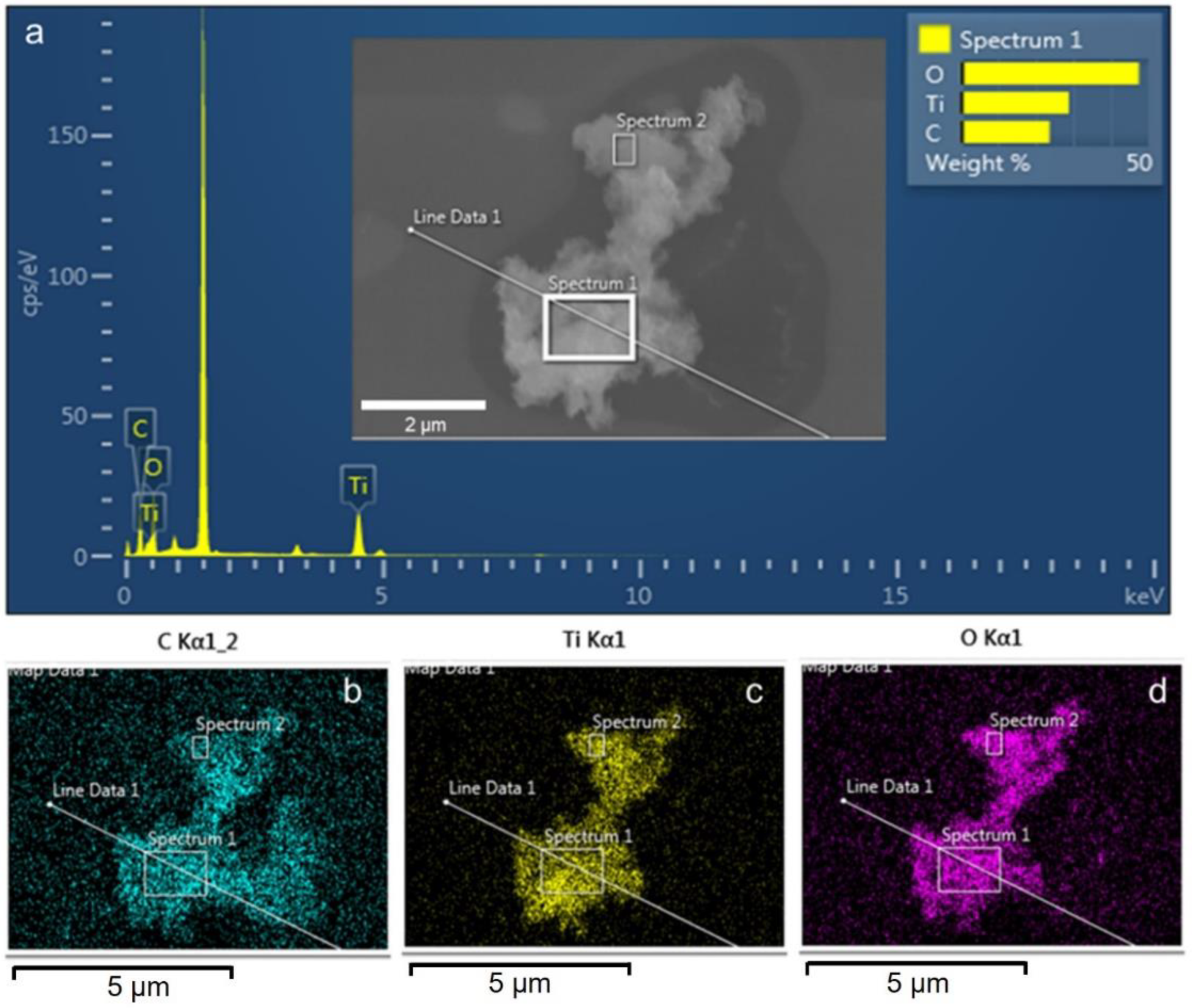
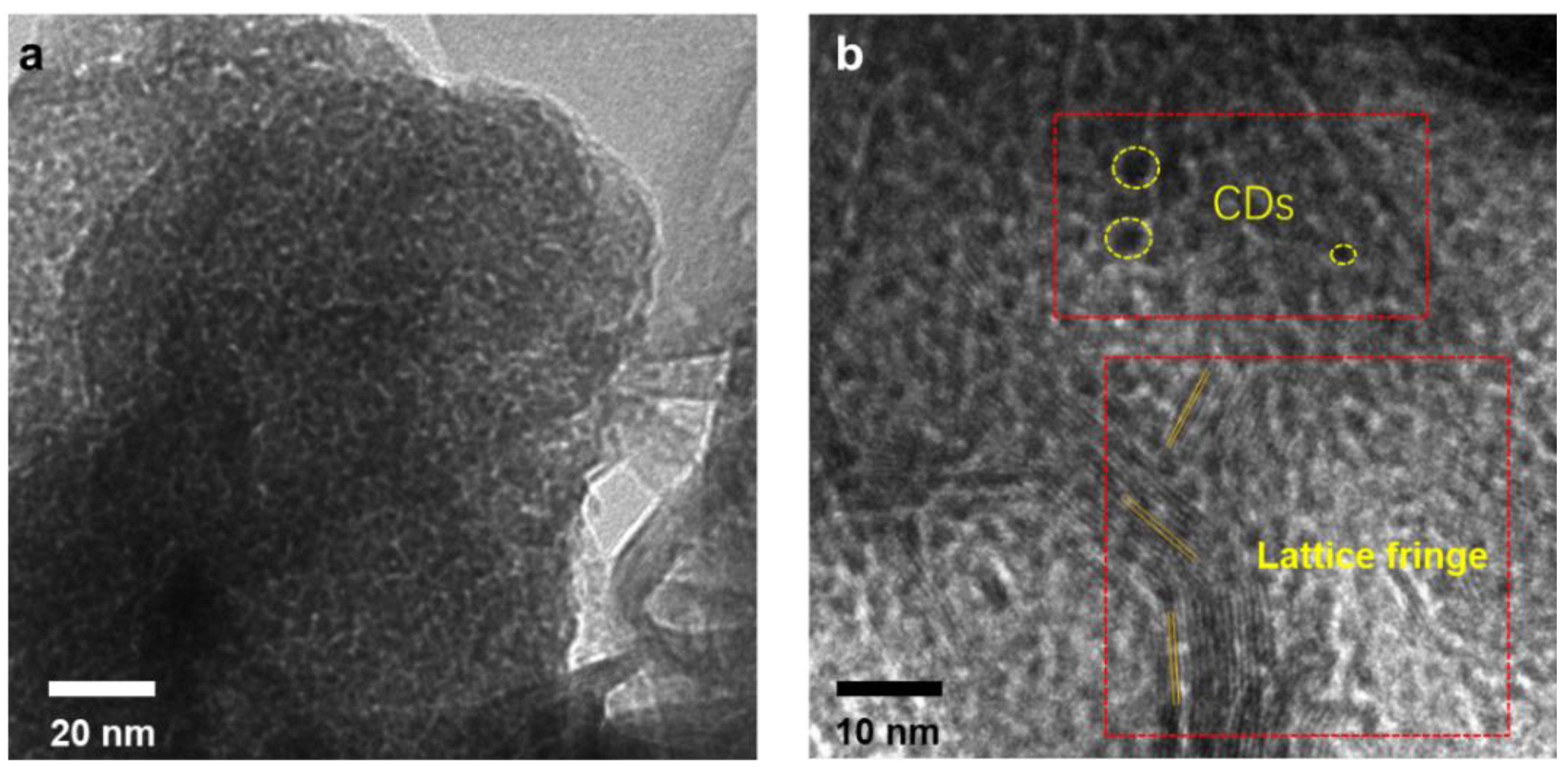
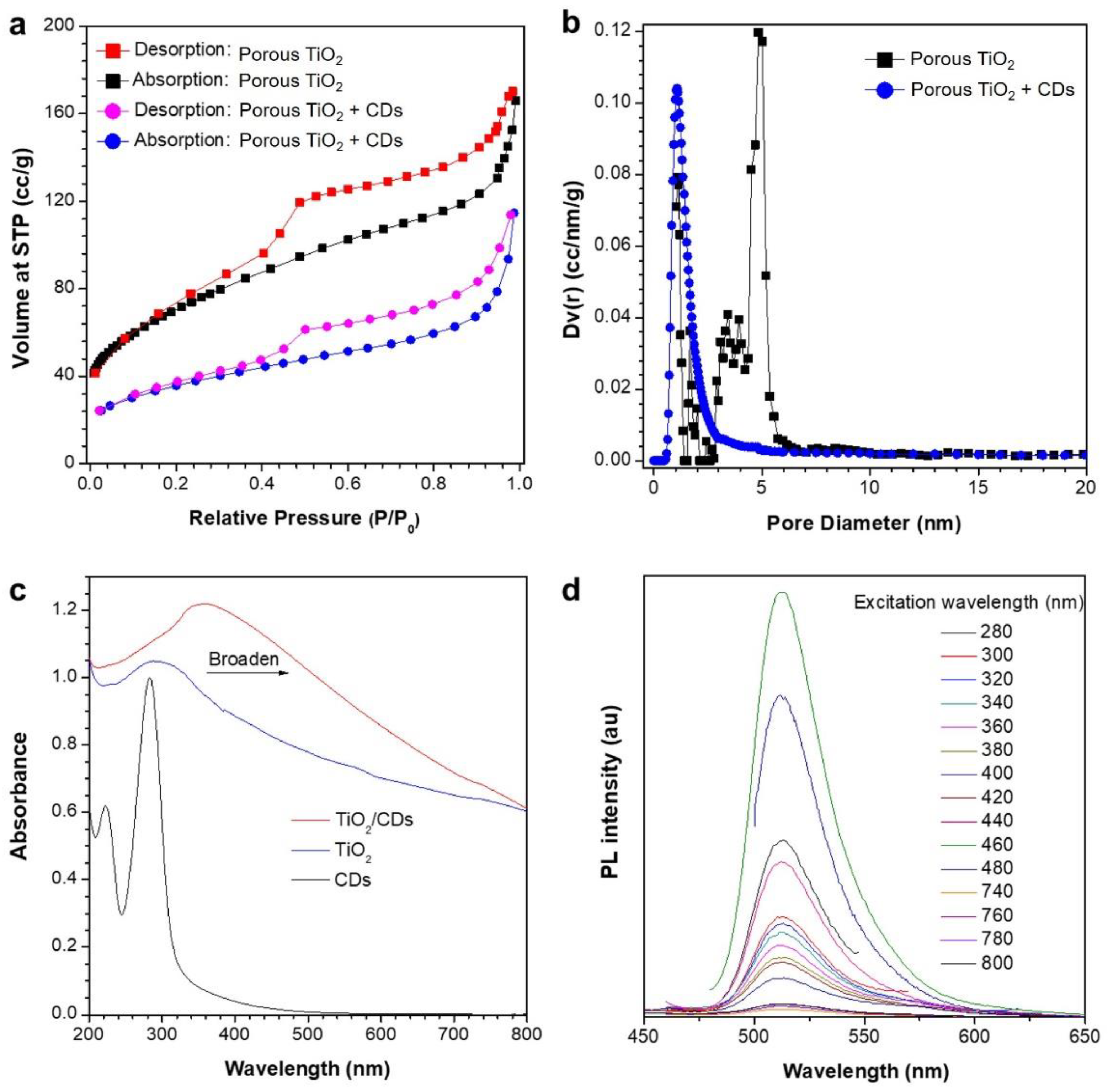
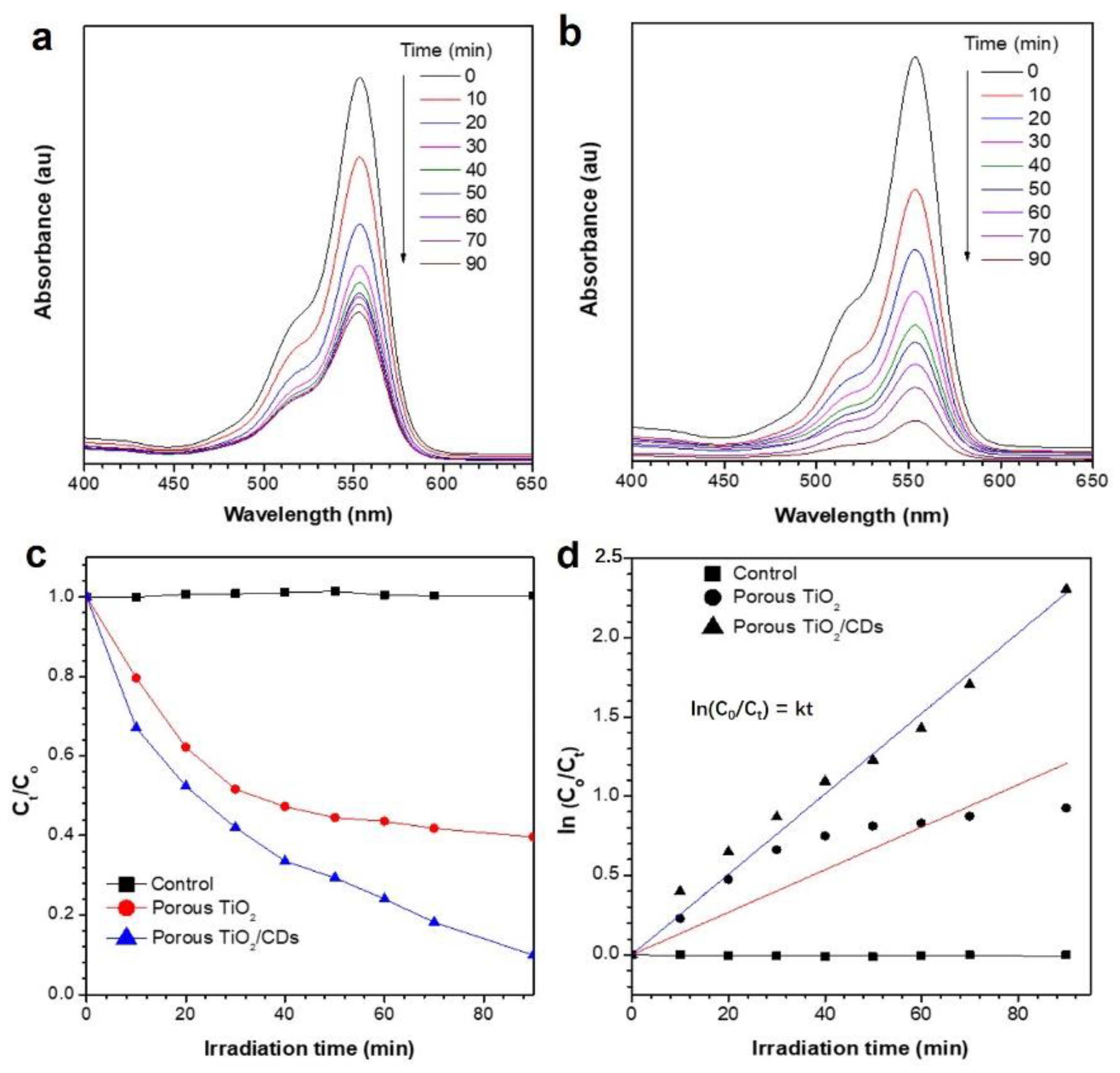
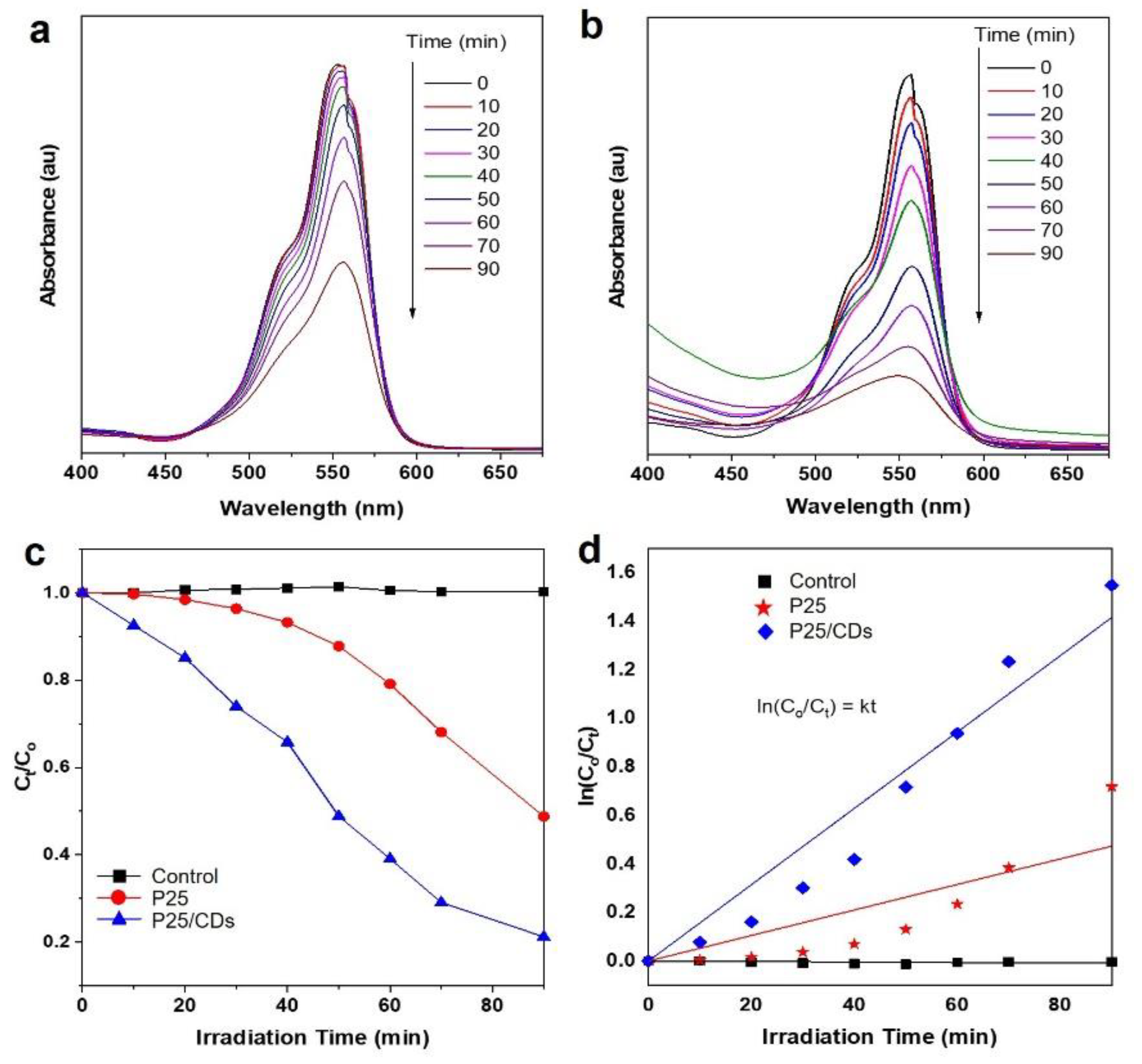
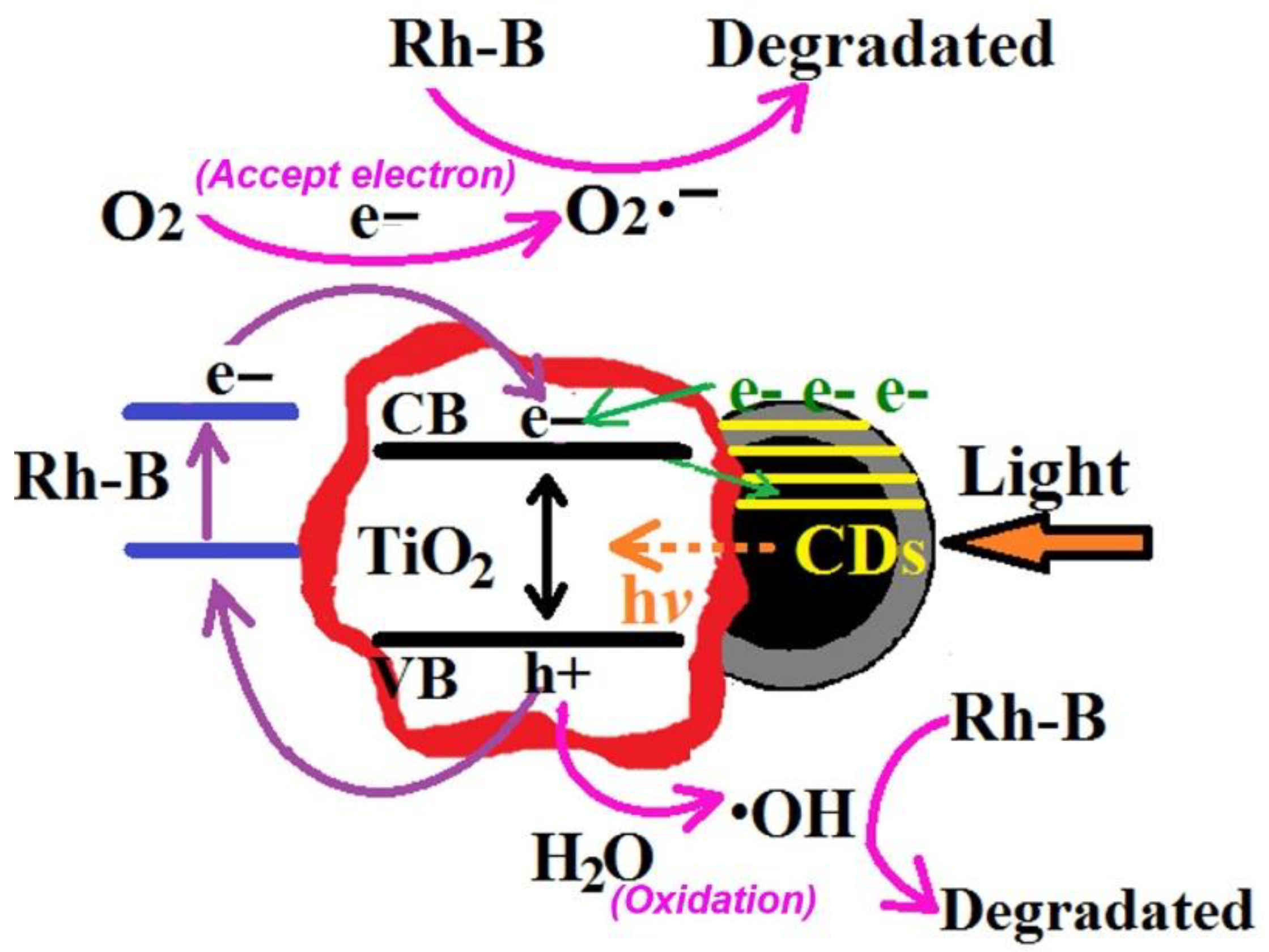
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martins, N.C.T.; Ângelo, J.; Girão, A.V.; Trindade, T.; Andrade, L.; Mendes, A. N-doped carbon quantum dots/TiO2 composite with improved photocatalytic activity. Appl. Catal. B 2016, 193, 67–74. [Google Scholar] [CrossRef]
- Zhuo, S.; Shao, M.; Lee, S.-T. Upconversion and Downconversion Fluorescent Graphene Quantum Dots: Ultrasonic Preparation and Photocatalysis. ACS Nano 2012, 6, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Zhang, D.; Pan, D.; Zhu, W.; Liu, P.; Cai, Y.; Li, G.; Li, H. A chloroplast structured photocatalyst enabled by microwave synthesis. Nat. Commun. 2019, 10, 1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, Y.; Furube, A.; Murai, M.; Hara, K.; Katoh, R.; Tachiya, M. Direct Observation of Reactive Trapped Holes in TiO2 Undergoing Photocatalytic Oxidation of Adsorbed Alcohols: Evaluation of the Reaction Rates and Yields. J. Am. Chem. Soc. 2006, 128, 416–417. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, X.; Wang, X.; Zhang, Y.; Wei, W.; Sun, Y.; Antonietti, M.; Titirici, M.M. One-step solvothermal synthesis of a carbon@TiO(2) dyade structure effectively promoting visible-light photocatalysis. Adv. Mater. 2010, 22, 3317–3321. [Google Scholar] [CrossRef]
- Rao, N.N.; Dube, S. Photocatalytic degradation of mixed surfactants and some commercial soap/detergent products using suspended TiO2 catalysts. J. Mol. Catal. A Chem. 1996, 104, L197–L199. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, X.-L.; He, L.; Zhang, Y.-X.; Hu, Z.-Y.; Tian, G.; Cheng, X.; Wu, S.-M.; Li, Y.-Z.; Yang, X.-H.; et al. Spatial Heterojunction in Nanostructured TiO2 and Its Cascade Effect for Efficient Photocatalysis. Nano Lett. 2020, 20, 3122–3129. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Zhang, P.; Shao, C.; Zhang, Z.; Zhang, M.; Mu, J.; Guo, Z.; Liu, Y. TiO(2)@carbon core/shell nanofibers: Controllable preparation and enhanced visible photocatalytic properties. Nanoscale 2011, 3, 2943–2949. [Google Scholar] [CrossRef]
- Wang, J.; Shen, Y.; Liu, S.; Zhang, Y. Single 2D MXene precursor-derived TiO2 nanosheets with a uniform decoration of amorphous carbon for enhancing photocatalytic water splitting. Appl. Catal. B 2020, 270, 118885. [Google Scholar] [CrossRef]
- Choi, W.Y.; Termin, A.; Hoffmann, M.R. The Role of Metal-Ion Dopants in Quantum-Sized TiO2—Correlation between Photoreactivity and Charge-Carrier Recombination Dynamics. J. Phys. Chem. US 1994, 98, 13669–13679. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Synergetic Effect of MoS2 and Graphene as Cocatalysts for Enhanced Photocatalytic H2 Production Activity of TiO2 Nanoparticles. J. Am. Chem. Soc. 2012, 134, 6575–6578. [Google Scholar] [CrossRef] [PubMed]
- Maarisetty, D.; Mahanta, S.; Sahoo, A.K.; Mohapatra, P.; Baral, S.S. Steering the Charge Kinetics in Dual-Functional Photocatalysis by Surface Dipole Moments and Band Edge Modulation: A Defect Study in TiO2-ZnS-rGO Composites. ACS Appl. Mater. Interfaces 2020, 12, 11679–11692. [Google Scholar] [CrossRef] [PubMed]
- Osterloh, F.E. Inorganic Materials as Catalysts for Photochemical Splitting of Water. Chem. Mater. 2008, 20, 35–54. [Google Scholar] [CrossRef]
- Lv, X.-J.; Zhou, S.-X.; Zhang, C.; Chang, H.-X.; Chen, Y.; Fu, W.-F. Synergetic effect of Cu and graphene as cocatalyst on TiO2 for enhanced photocatalytic hydrogen evolution from solar water splitting. J. Mater. Chem. 2012, 22, 18542–18549. [Google Scholar] [CrossRef]
- Fessi, N.; Nsib, M.F.; Cardenas, L.; Guillard, C.; Dappozze, F.; Houas, A.; Parrino, F.; Palmisano, L.; Ledoux, G.; Amans, D.; et al. Surface and Electronic Features of Fluorinated TiO2 and Their Influence on the Photocatalytic Degradation of 1-Methylnaphthalene. J. Phys. Chem. C 2020, 124, 11456–11468. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.; Fang, W.-H.; Fernandez-Alberti, S.; Long, R. Unraveling the quantum dynamics origin of high photocatalytic activity in nitrogen-doped anatase TiO2: Time-domain ab initio analysis. J. Mater. Chem. A 2020, 8, 25235–25244. [Google Scholar] [CrossRef]
- Wang, Y.; Saitow, K.-I. Mechanochemical Synthesis of Red-Light-Active Green TiO2 Photocatalysts with Disorder: Defect-Rich, with Polymorphs, and No Metal Loading. Chem. Mater. 2020, 32, 9190–9200. [Google Scholar] [CrossRef]
- Wang, J.; Gao, M.; Ho, G.W. Bidentate-complex-derived TiO2/carbon dot photocatalysts: In situ synthesis, versatile heterostructures, and enhanced H2 evolution. J. Mater. Chem. A 2014, 2, 5703–5709. [Google Scholar] [CrossRef]
- Kim, Y.K.; Sharker, S.M.; In, I.; Park, S.Y. Surface coated fluorescent carbon nanoparticles/TiO2 as visible-light sensitive photocatalytic complexes for antifouling activity. Carbon 2016, 103, 412–420. [Google Scholar] [CrossRef]
- Woan, K.; Pyrgiotakis, G.; Sigmund, W. Photocatalytic Carbon-Nanotube–TiO2 Composites. Adv. Mater. 2009, 21, 2233–2239. [Google Scholar] [CrossRef]
- Apostolopoulou, V.; Vakros, J.; Kordulis, C.; Lycourghiotis, A. Preparation and characterization of [60] fullerene nanoparticles supported on titania used as a photocatalyst. Colloids Surf. A 2009, 349, 189–194. [Google Scholar] [CrossRef]
- Qi, K.; Selvaraj, R.; Al Fahdi, T.; Al-Kindy, S.; Kim, Y.; Wang, G.-C.; Tai, C.-W.; Sillanpää, M. Enhanced photocatalytic activity of anatase-TiO2 nanoparticles by fullerene modification: A theoretical and experimental study. Appl. Surf. Sci. 2016, 387, 750–758. [Google Scholar] [CrossRef]
- Zhang, L.-W.; Fu, H.-B.; Zhu, Y.-F. Efficient TiO2 Photocatalysts from Surface Hybridization of TiO2 Particles with Graphite-like Carbon. Adv. Funct. Mater. 2008, 18, 2180–2189. [Google Scholar] [CrossRef]
- Yang, H.; Zhai, L.; Li, K.; Liu, X.; Deng, B.; Xu, W. A highly efficient nano-graphite-doped TiO2 photocatalyst with a unique sea-island structure for visible-light degradation. Catal. Sci. Technol. 2020, 10, 1161–1170. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Y.; Cheng, W.; Li, Y.; Xu, X.; Lin, K. Mesoporous TiO2/Carbon Beads: One-Pot Preparation and Their Application in Visible-Light-Induced Photodegradation. Nano-Micro Lett. 2015, 7, 243–254. [Google Scholar] [CrossRef] [Green Version]
- Marinovic, A.; Kiat, L.S.; Dunn, S.; Titirici, M.-M.; Briscoe, J. Carbon-Nanodot Solar Cells from Renewable Precursors. ChemSusChem 2017, 10, 1004–1013. [Google Scholar] [CrossRef]
- Gerber, I.C.; Serp, P. A Theory/Experience Description of Support Effects in Carbon-Supported Catalysts. Chem. Rev. 2020, 120, 1250–1349. [Google Scholar] [CrossRef]
- Cheng, C.; Lu, D.; Shen, B.; Liu, Y.D.; Lei, J.Y.; Wang, L.Z.; Zhang, J.L.; Matsuoka, M. Mesoporous silica-based carbon dot/TiO2 photocatalyst for efficient organic pollutant degradation. Microporous Mesoporous Mater. 2016, 226, 79–87. [Google Scholar] [CrossRef]
- Gao, H.; Sapelkin, A.V.; Titirici, M.M.; Sukhorukov, G.B. In Situ Synthesis of Fluorescent Carbon Dots/Polyelectrolyte Nanocomposite Microcapsules with Reduced Permeability and Ultrasound Sensitivity. ACS Nano 2016, 10, 9608–9615. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Ma, X.; Chen, X.; Sun, Y.; Cui, X.; Lin, Y. A nanocomposite of carbon quantum dots and TiO2 nanotube arrays: Enhancing photoelectrochemical and photocatalytic properties. RSC Adv. 2014, 4, 1120–1127. [Google Scholar] [CrossRef]
- Gao, C.; Wei, T.; Zhang, Y.; Song, X.; Huan, Y.; Liu, H.; Zhao, M.; Yu, J.; Chen, X. A Photoresponsive Rutile TiO2 Heterojunction with Enhanced Electron–Hole Separation for High-Performance Hydrogen Evolution. Adv. Mater. 2019, 31, 1806596. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shu, J.; Anqi, Z.; Juyuan, H.; Yan, Z.; Chen, J. Synthesis of carbon quantum dots/TiO2 nanocomposite for photo-degradation of Rhodamine B and cefradine. Diamond Relat. Mater. 2016, 70, 137–144. [Google Scholar] [CrossRef]
- Tian, J.; Leng, Y.; Zhao, Z.; Xia, Y.; Sang, Y.; Hao, P.; Zhan, J.; Li, M.; Liu, H. Carbon quantum dots/hydrogenated TiO2 nanobelt heterostructures and their broad spectrum photocatalytic properties under UV, visible, and near-infrared irradiation. Nano Energy 2015, 11, 419–427. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, J.; Lian, S.; Tsang, C.H.A.; Yang, X.; Lee, S.-T. Water-Soluble Fluorescent Carbon Quantum Dots and Photocatalyst Design. Angew. Chem. Int. Ed. 2010, 49, 4430–4434. [Google Scholar] [CrossRef]
- Wang, Q.; Cai, J.; Biesold-McGee, G.V.; Huang, J.; Ng, Y.H.; Sun, H.; Wang, J.; Lai, Y.; Lin, Z. Silk fibroin-derived nitrogen-doped carbon quantum dots anchored on TiO2 nanotube arrays for heterogeneous photocatalytic degradation and water splitting. Nano Energy 2020, 78, 105313. [Google Scholar] [CrossRef]
- Thamaphat, K. Phase Characterization of TiO2 Powder by XRD and TEM. Kasetsart J. Nat. Sci. 2008, 42, 357–361. [Google Scholar]
- Hurum, D.C.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Recombination Pathways in the Degussa P25 Formulation of TiO2: Surface versus Lattice Mechanisms. J. Phys. Chem. B 2005, 109, 977–980. [Google Scholar] [CrossRef]
- Kopidakis, N.; Schiff, E.A.; Park, N.G.; van de Lagemaat, J.; Frank, A.J. Ambipolar Diffusion of Photocarriers in Electrolyte-Filled, Nanoporous TiO2. J. Phys. Chem. B 2000, 104, 3930–3936. [Google Scholar] [CrossRef]
- Liu, B.; Zeng, H.C. Carbon Nanotubes Supported Mesoporous Mesocrystals of Anatase TiO2. Chem. Mater. 2008, 20, 2711–2718. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Guillet-Nicolas, R.; García-Martínez, J.; Thommes, M. Recent advances in the textural characterization of hierarchically structured nanoporous materials. Chem. Soc. Rev. 2017, 46, 389–414. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Yu, H.; Zhao, Y.; Zhou, C.; Shang, L.; Peng, Y.; Cao, Y.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. Carbon quantum dots/TiO2 composites for efficient photocatalytic hydrogen evolution. J. Mater. Chem. A 2014, 2, 3344. [Google Scholar] [CrossRef]
- Tasviri, M.; Rafiee-Pour, H.-A.; Ghourchian, H.; Gholami, M.R. Amine functionalized TiO2–carbon nanotube composite: Synthesis, characterization and application to glucose biosensing. Appl. Nanosci. 2011, 1, 189–195. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, F.; Sun, H.; Ma, H.; Gao, H. Porous TiO2/Carbon Dot Nanoflowers with Enhanced Surface Areas for Improving Photocatalytic Activity. Nanomaterials 2022, 12, 2536. https://doi.org/10.3390/nano12152536
Song F, Sun H, Ma H, Gao H. Porous TiO2/Carbon Dot Nanoflowers with Enhanced Surface Areas for Improving Photocatalytic Activity. Nanomaterials. 2022; 12(15):2536. https://doi.org/10.3390/nano12152536
Chicago/Turabian StyleSong, Fengyan, Hao Sun, Hailong Ma, and Hui Gao. 2022. "Porous TiO2/Carbon Dot Nanoflowers with Enhanced Surface Areas for Improving Photocatalytic Activity" Nanomaterials 12, no. 15: 2536. https://doi.org/10.3390/nano12152536
APA StyleSong, F., Sun, H., Ma, H., & Gao, H. (2022). Porous TiO2/Carbon Dot Nanoflowers with Enhanced Surface Areas for Improving Photocatalytic Activity. Nanomaterials, 12(15), 2536. https://doi.org/10.3390/nano12152536





