Chromium(VI) Removal from Water by Lanthanum Hybrid Modified Activated Carbon Produced from Coconut Shells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Modified Activated Carbon
2.3. Analytical Determinations
2.4. Characterization Techniques,
2.5. Adsorption Experiments
2.5.1. Equilibrium Experiments
2.5.2. Kinetics Experiments
3. Results and Discussion
3.1. Characterization of COC-AC-La
3.1.1. Physical Properties
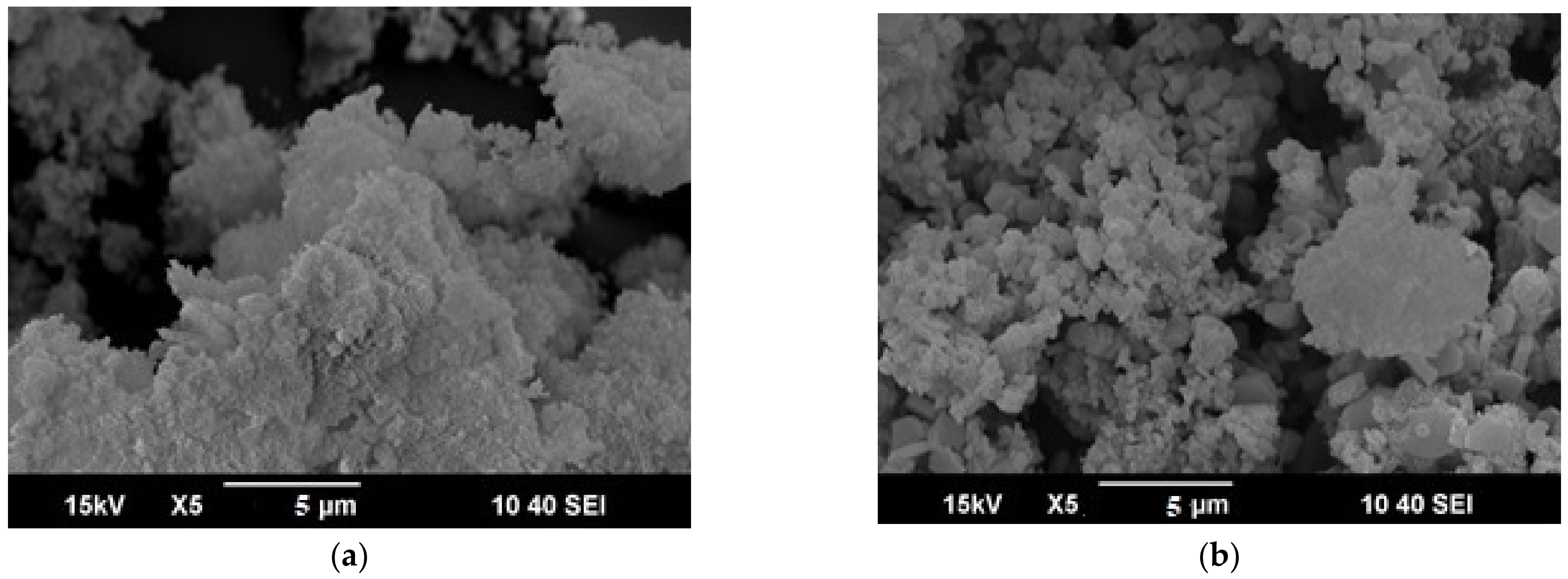
3.1.2. Scanning Electron Microscopy (SEM)
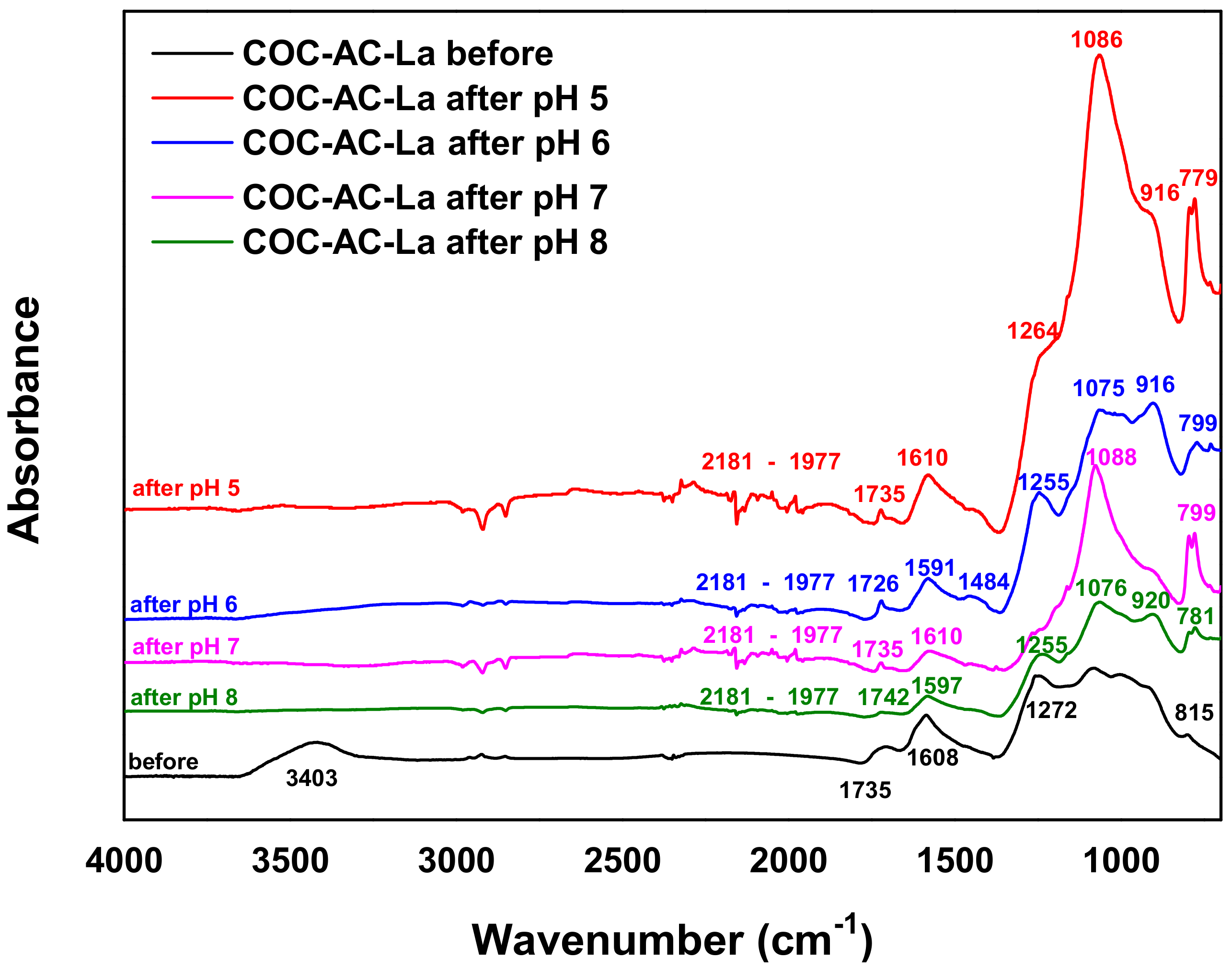
3.1.3. FTIR Analysis
3.2. Effect of Adsorbent Dose
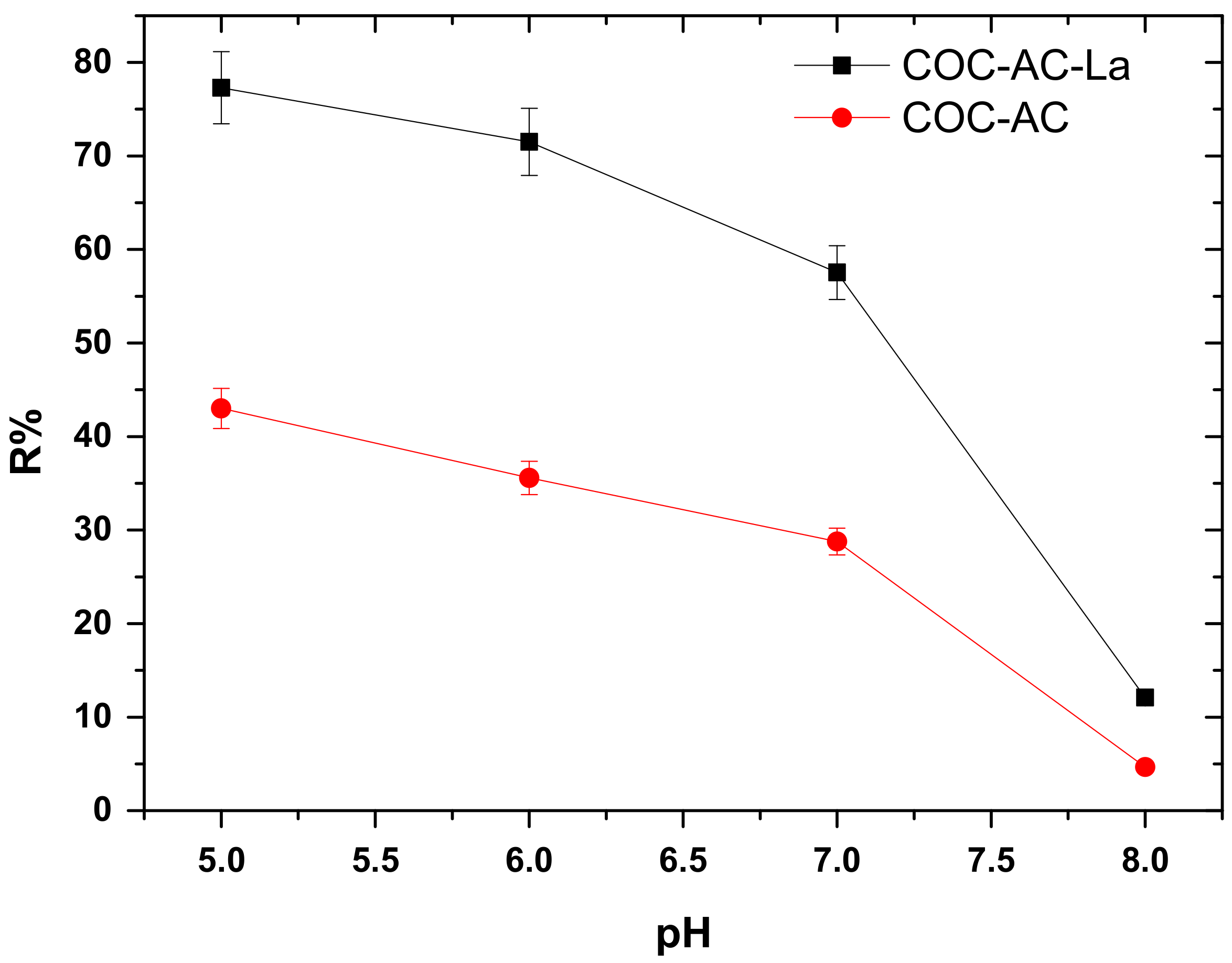
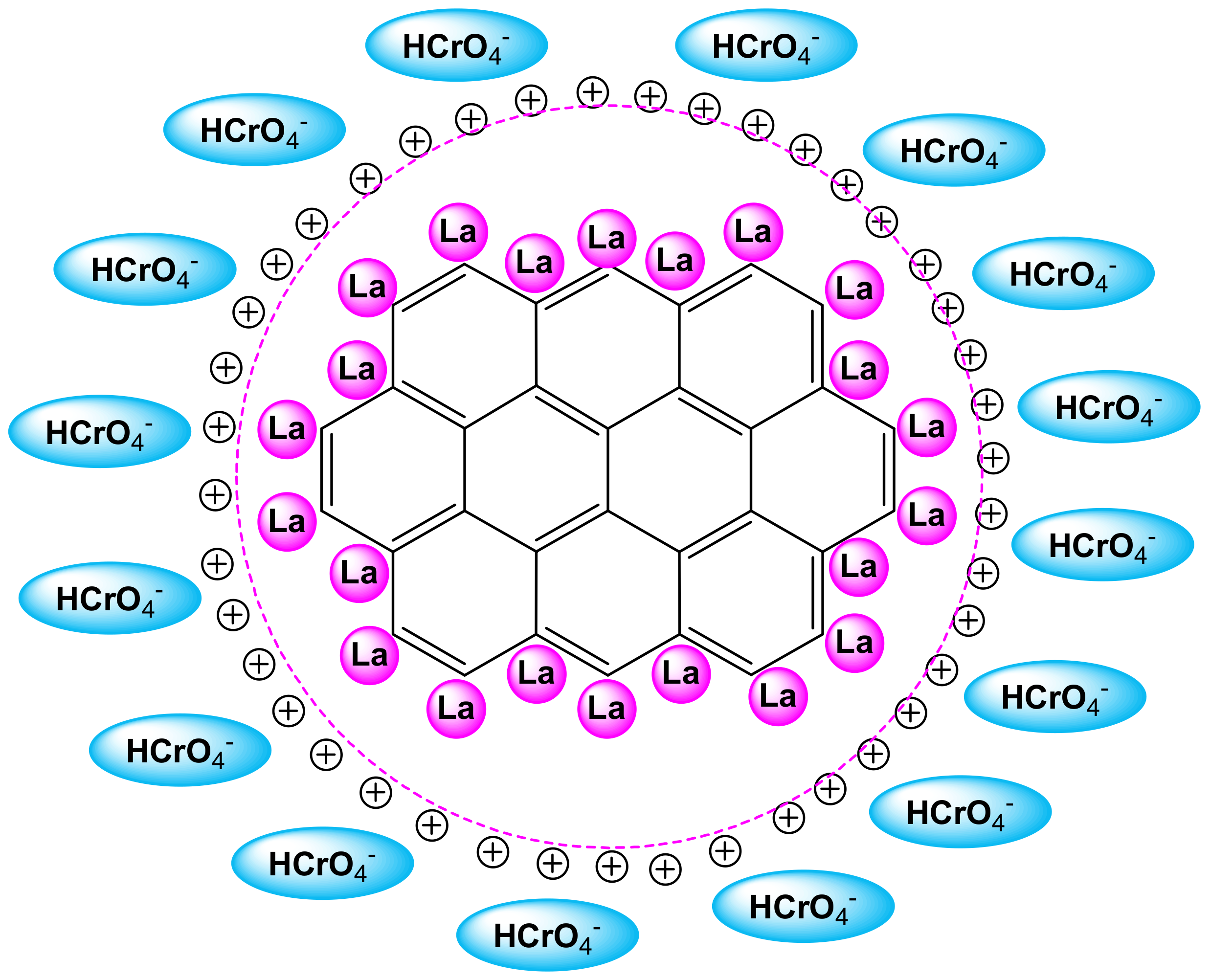
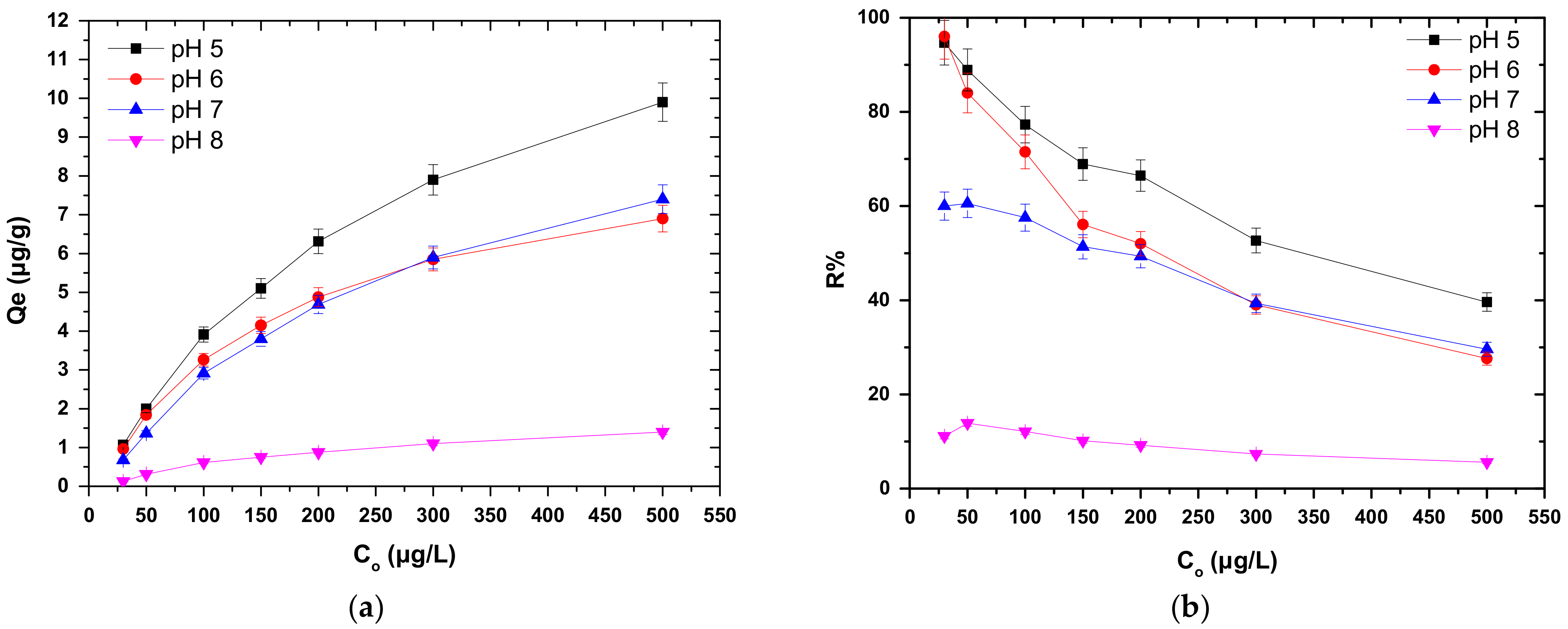
3.3. Effect of Initial Solution pH
3.4. Effect of Cr(VI) Initial Concentration
3.5. Effect of Contact Time
3.6. Adsorption Isotherms
3.6.1. Freundlich Isotherm
3.6.2. Langmuir Isotherm
3.7. Adsorption Kinetics
Pseudo-Second Order Model
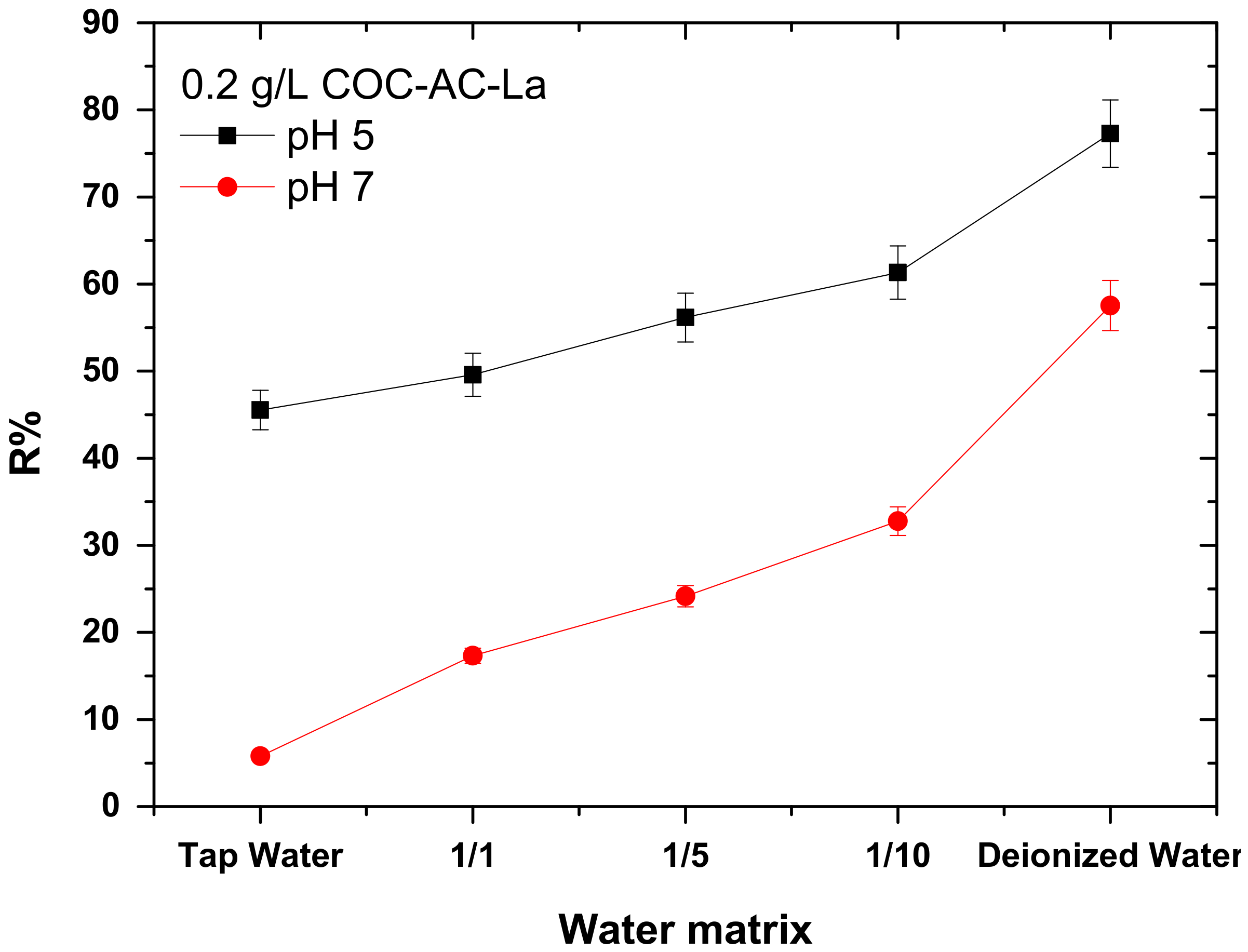
3.8. Effect of Water Matrix
3.9. Regeneration Study
3.10. Comparison with Other Materials in Literature
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delpla, I.; Jung, A.V.; Baures, E.; Clement, M.; Thomas, O. Impacts of climate change on surface water quality in relation to drinking water production. Environ. Int. 2009, 35, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Tolkou, A.K.; Zouboulis, A.I. Effect of climate change in WWTPs with a focus on MBR infrastructure. Desalin. Water Treat. 2016, 57. [Google Scholar] [CrossRef]
- Tumolo, M.; Ancona, V.; De Paola, D.; Losacco, D.; Campanale, C.; Massarelli, C.; Uricchio, V.F. Chromium pollution in European water, sources, health risk, and remediation strategies: An overview. Int. J. Environ. Res. Public Health 2020, 17, 5438. [Google Scholar] [CrossRef]
- Wang, B.; Li, F.; Wang, L. Enhanced hexavalent chromium (Cr(VI)) removal from aqueous solution by Fe–Mn oxide-modified cattail biochar: Adsorption characteristics and mechanism. Chem. Ecol. 2020, 36, 138–154. [Google Scholar] [CrossRef]
- Sahu, S.; Sahu, U.K.; Patel, R.K. Synthesis of thorium-ethanolamine nanocomposite by the co-precipitation method and its application for Cr(VI) removal. New J. Chem. 2018, 42, 5556–5569. [Google Scholar] [CrossRef]
- Lewicki, S.; Zdanowski, R.; Krzyzowska, M.; Lewicka, A.; Debski, B.; Niemcewicz, M.; Goniewicz, M. The role of chromium III in the organism and its possible use in diabetes and obesity treatment. Ann. Agric. Environ. Med. 2014, 21, 331–335. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, S.; Kaur, N. “Food Security, Nutrition and Sustainable Agriculture-Emerging Technologies” Heavy metals toxicity and the environment. J. Pharmacogn. Phytochem. 2019, 1, 247–249. [Google Scholar]
- Dianyi, Y. Chromium (Cr) Toxicity; Agency for Toxic Substances and Disease Registry Case Studies in Environmental Medicine (CSEM): Neuchâtel, Switzerland, 2008; pp. 1–67. [Google Scholar]
- EPA. Chromium Compounds: Hazard Summary; National Center for Environmental Assessment (NCEA): Washington, DC, USA, 1973; pp. 639–700. [Google Scholar]
- WHO. World Health Organization, European Standards for Drinking-Water. 2nd ed.; World Health Organization: Geneva, Switzerland. 1970. Available online: https://apps.who.int/iris/bitstream/handle/10665/40025/European_standards_for_drinking-water.pdf?sequence=1&isAllowed=y (accessed on 10 February 2022).
- WHO. OMS Chromium in Drinking-Water. In Guidelines for Drinking-Water Quality; WHO: Geneva, Switzerland, 1996; Volume 2. [Google Scholar]
- Kaprara, E.; Kazakis, N.; Simeonidis, K.; Coles, S.; Zouboulis, A.I.; Samaras, P.; Mitrakas, M. Occurrence of Cr(VI) in drinking water of Greece and relation to the geological background. J. Hazard. Mater. 2015, 281, 2–11. [Google Scholar] [CrossRef]
- Xanthopoulou, M.; Katsoyiannis, I. Chromium ions removal from groundwaters by functionalized ultra-filtration membranes. New Mater. Compd. Appl. 2019, 3, 38–46. [Google Scholar]
- Ball, J.W.; Izbicki, J.A. Occurrence of hexavalent chromium in ground water in the western Mojave Desert, California. Appl. Geochem. 2004, 19, 1123–1135. [Google Scholar] [CrossRef]
- Laskaridis, A.; Sarakatsianos, I.; Tzollas, N.; Katsoyiannis, I.A. Simultaneous removal of arsenate and chromate from ground- and surface- waters by iron-based redox assisted coagulation. Sustainability 2020, 12, 5394. [Google Scholar] [CrossRef]
- Hans, R.; Senanayake, G.; Dharmasiri, L.C.S.; Mathes, J.A.P.; Kim, D.J. A preliminary batch study of sorption kinetics of Cr(VI) ions from aqueous solutions by a magnetic ion exchange (MIEX®) resin and determination of film/pore diffusivity. Hydrometallurgy 2016, 164, 208–218. [Google Scholar] [CrossRef]
- Chang, I.S.; Kim, B.H. Effect of sulfate reduction activity on biological treatment of hexavalent chromium [Cr(VI)] contaminated electroplating wastewater under sulfate-rich condition. Chemosphere 2007, 68, 218–226. [Google Scholar] [CrossRef]
- Stylianou, S.; Simeonidis, K.; Mitrakas, M.; Zouboulis, A.; Ernst, M.; Katsoyiannis, I.A. Reductive precipitation and removal of Cr(VI) from groundwaters by pipe flocculation-microfiltration. Environ. Sci. Pollut. Res. 2018, 25, 12256–12262. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Lazaridis, N.K.; Kostoglou, M. On the simultaneous adsorption of a reactive dye and hexavalent chromium from aqueous solutions onto grafted chitosan. J. Colloid Interface Sci. 2013, 407, 432–441. [Google Scholar] [CrossRef]
- Labied, R.; Benturki, O.; Eddine Hamitouche, A.Y.; Donnot, A. Adsorption of hexavalent chromium by activated carbon obtained from a waste lignocellulosic material (Ziziphus jujuba cores): Kinetic, equilibrium, and thermodynamic study. Adsorpt. Sci. Technol. 2018, 36, 1066–1099. [Google Scholar] [CrossRef] [Green Version]
- Selvi, K.; Pattabhi, S.; Kadirvelu, K. Removal of Cr(VI) from aqueous solution by adsorption onto activated carbon. Bioresour. Technol. 2001, 80, 87–89. [Google Scholar] [CrossRef]
- Liu, J.; Yi, Z.; Ou, Z.; Yang, T. Removal of Cr(VI) and methyl orange by activated carbon fiber supported nanoscale zero-valent iron in a continuous fixed bed column. Water Sci. Technol. 2020, 82, 732–746. [Google Scholar] [CrossRef]
- Tolkou, A.K.; Katsoyiannis, I.A.; Zouboulis, A.I. Removal of arsenic, chromium and uranium from water sources by novel nanostructured materials including graphene-based modified adsorbents: A mini review of recent developments. Appl. Sci. 2020, 10, 3241. [Google Scholar] [CrossRef]
- Mahmood, T.; Saddique, M.T.; Naeem, A.; Mustafa, S.; Hussain, J.; Dilara, B. Cation exchange removal of Zn from aqueous solution by NiO. J. Non-Cryst. Solids 2011, 357, 1016–1020. [Google Scholar] [CrossRef]
- Samuel, M.S.; Bhattacharya, J.; Raj, S.; Santhanam, N.; Singh, H.; Pradeep Singh, N.D. Efficient removal of Chromium(VI) from aqueous solution using chitosan grafted graphene oxide (CS-GO) nanocomposite. Int. J. Biol. Macromol. 2019, 121, 285–292. [Google Scholar] [CrossRef]
- Setshedi, K.Z.; Bhaumik, M.; Onyango, M.S.; Maity, A. High-performance towards Cr(VI) removal using multi-active sites of polypyrrole-graphene oxide nanocomposites: Batch and column studies. Chem. Eng. J. 2015, 262, 921–931. [Google Scholar] [CrossRef]
- Tan, X.; Shaaban, M.; Yang, J.; Cai, Y.; Wang, B.; Peng, Q. Efficient removal of hexavalent chromium from an aquatic system using nanoscale zero-valent iron supported by ramie biochar. Nanomaterials 2021, 11, 2698. [Google Scholar] [CrossRef]
- Meez, E.; Tolkou, A.K.; Giannakoudakis, D.A.; Katsoyiannis, I.A.; Kyzas, G.Z. Activated Carbons for Arsenic Removal from Natural Waters and Wastewaters: A Review. Water 2021, 13, 2982. [Google Scholar]
- Rizzo, L.; Malato, S.; Antakyali, D.; Beretsou, V.G.; Đolić, M.B.; Gernjak, W.; Heath, E.; Ivancev-Tumbas, I.; Karaolia, P.; Lado Ribeiro, A.R.; et al. Consolidated vs new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater. Sci. Total Environ. 2019, 655, 986–1008. [Google Scholar] [CrossRef]
- Enniya, I.; Rghioui, L.; Jourani, A. Adsorption of hexavalent chromium in aqueous solution on activated carbon prepared from apple peels. Sustain. Chem. Pharm. 2018, 7, 9–16. [Google Scholar] [CrossRef]
- Jha, M.K.; Joshi, S.; Sharma, R.K.; Kim, A.A.; Pant, B.; Park, M.; Pant, H.R. Surface modified activated carbons: Sustainable bio-based materials for environmental remediation. Nanomaterials 2021, 11, 3140. [Google Scholar] [CrossRef]
- Vargas, A.M.M.; Cazetta, A.L.; Garcia, C.A.; Moraes, J.C.G.; Nogami, E.M.; Lenzi, E.; Costa, W.F.; Almeida, V.C. Preparation and characterization of activated carbon from a new raw lignocellulosic material: Flamboyant (Delonix regia) pods. J. Environ. Manag. 2011, 92, 178–184. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Farhang, M.; Alimohammadi, M.; Afsharnia, M.; Mckay, G. Adsorptive removal of fluoride from water by activated carbon derived from CaCl2 -modified Crocus sativus leaves: Equilibrium adsorption isotherms, optimization, and influence of anions. Chem. Eng. Commun. 2018, 205, 955–965. [Google Scholar] [CrossRef]
- Demirbas, E.; Kobya, M.; Sulak, M.T. Adsorption kinetics of a basic dye from aqueous solutions onto apricot stone activated carbon. Bioresour. Technol. 2008, 99, 5368–5373. [Google Scholar] [CrossRef] [PubMed]
- Özçimen, D.; Ersoy-Meriçboyu, A. Adsorption of copper(II) ions onto hazelnut shell and apricot stone activated carbons. Adsorpt. Sci. Technol. 2010, 28, 327–340. [Google Scholar] [CrossRef]
- Ullah, I.; Nadeem, R.; Iqbal, M.; Manzoor, Q. Biosorption of chromium onto native and immobilized sugarcane bagasse waste biomass. Ecol. Eng. 2013, 60, 99–107. [Google Scholar] [CrossRef]
- Sarker, T.C.; Azam, S.M.G.G.; El-Gawad, A.M.A.; Gaglione, S.A.; Bonanomi, G. Sugarcane bagasse: A potential low-cost biosorbent for the removal of hazardous materials. Clean Technol. Environ. Policy 2017, 19, 2343–2362. [Google Scholar] [CrossRef]
- Yang, J.; Yu, M.; Chen, W. Adsorption of hexavalent chromium from aqueous solution by activated carbon prepared from longan seed: Kinetics, equilibrium and thermodynamics. J. Ind. Eng. Chem. 2015, 21, 414–422. [Google Scholar] [CrossRef]
- Shukla, A.; Zhang, Y.H.; Dubey, P.; Margrave, J.L.; Shukla, S.S. The role of sawdust in the removal of unwanted materials from water. J. Hazard. Mater. 2002, 95, 137–152. [Google Scholar] [CrossRef]
- Ihsanullah; Al-Khaldi, F.; Al-Khaldi, F.A.; Abu-Sharkh, B.; Abulkibash, A.M.; Qureshi, M.I.; Laoui, T.; Atieh, M.A. Effect of acid modification on adsorption of hexavalent chromium (Cr(VI)) from aqueous solution by activated carbon and carbon nanotubes. Desalin. Water Treat. 2016, 57, 7232–7244. [Google Scholar] [CrossRef]
- Gallios, G.P.; Tolkou, A.K.; Katsoyiannis, I.A.; Stefusova, K.; Vaclavikova, M.; Deliyanni, E.A. Adsorption of arsenate by nano scaled activated carbon modified by iron and manganese oxides. Sustainability 2017, 9, 1684. [Google Scholar] [CrossRef] [Green Version]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Mandal, S.; Sahu, M.K.; Giri, A.K.; Patel, R.K. Adsorption studies of chromium (VI) removal from water by lanthanum diethanolamine hybrid material. Environ. Technol. 2014, 35, 817–832. [Google Scholar] [CrossRef]
- Onoda, H.; Suzuki, R. Preparation of gel lanthanum–cerium phosphates and their fluorescence properties. Mater. Res. Innov. 2017, 21, 206–209. [Google Scholar] [CrossRef]
- Zhang, L.; Wan, L.; Chang, N.; Liu, J.; Duan, C.; Zhou, Q.; Li, X.; Wang, X. Removal of phosphate from water by activated carbon fiber loaded with lanthanum oxide. J. Hazard. Mater. 2011, 190, 848–855. [Google Scholar] [CrossRef]
- Yang, B.; Feng, Y.; Yu, Y.; He, S.; Liu, H.; Xue, L.; Yang, L. Lanthanum ferrite nanoparticles modification onto biochar: Derivation from four different methods and high performance for phosphate adsorption. Environ. Sci. Pollut. Res. 2019, 26, 22010–22020. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Bender, J.; Xu, S. Removal of Arsenate and Chromate by Lanthanum-modified Granular Ceramic Material: The Critical Role of Coating Temperature. Sci. Rep. 2019, 9, 7690. [Google Scholar] [CrossRef] [Green Version]
- Ali Atieh, M. Removal of chromium (VI) from polluted water using carbon nanotubes supported with activated carbon. Procedia Environ. Sci. 2011, 4, 281–293. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; An, D.; Sun, S.; Gao, J.; Qian, L. Reduction and removal of chromium VI in water by powdered activated carbon. Materials 2018, 11, 269. [Google Scholar] [CrossRef] [Green Version]
- Vo, A.T.; Nguyen, V.P.; Ouakouak, A.; Nieva, A. Efficient Removal of Cr(VI) from Water by Biochar and Activated Carbon Prepared through Hydrothermal Carbonization and Pyrolysis: Adsorption-Coupled Reduction Mechanism. Water 2019, 11, 1164. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1981. [Google Scholar]
- Fito, J.; Bultossa, G.; Kloos, H. Physicochemical and heavy metal constituents of the groundwater quality in Haramaya Woreda, Oromia Regional State, Ethiopia. Int. J. Energy Water Resour. 2019, 3, 23–32. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Golikand, A.N.; Ghaemi, M.; Yousefi, T. A novel lanthanum hydroxide nanostructure prepared by cathodic electrodeposition. Mater. Lett. 2011, 65, 1466–1468. [Google Scholar] [CrossRef]
- Jais, F.M.; Ibrahim, S.; Yoon, Y.; Jang, M. Enhanced arsenate removal by lanthanum and nano-magnetite composite incorporated palm shell waste-based activated carbon. Sep. Purif. Technol. 2016, 169, 93–102. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Deliyanni, E.A. Modified activated carbons from potato peels as green environmental-friendly adsorbents for the treatment of pharmaceutical effluents. Chem. Eng. Res. Des. 2015, 97, 135–144. [Google Scholar] [CrossRef]
- El-Hendawy, A.N.A. Variation in the FTIR spectra of a biomass under impregnation, carbonization and oxidation conditions. J. Anal. Appl. Pyrolysis 2006, 75, 159–166. [Google Scholar] [CrossRef]
- Madi, C.; Tabbal, M.; Christidis, T.; Isber, S.; Nsouli, B.; Zahraman, K. Microstructural characterization of chromium oxide thin films grown by remote plasma assisted pulsed laser deposition. J. Phys. Conf. Ser. 2007, 59, 600–604. [Google Scholar] [CrossRef]
- Bedada, D.; Angassa, K.; Tiruneh, A.; Kloos, H.; Fito, J. Chromium removal from tannery wastewater through activated carbon produced from Parthenium hysterophorus weed. Energy Ecol. Environ. 2020, 5, 184–195. [Google Scholar] [CrossRef]
- Kan, C.C.; Ibe, A.H.; Rivera, K.K.P.; Arazo, R.O.; de Luna, M.D.G. Hexavalent chromium removal from aqueous solution by adsorbents synthesized from groundwater treatment residuals. Sustain. Environ. Res. 2017, 27, 163–171. [Google Scholar] [CrossRef]
- Balan, C.; Volf, I.; Bilba, D. Uklanjanje hroma (VI) iz vodenih rastvora pomoću purolita—Bazne anjonske smole sa gel strukturom. Chem. Ind. Chem. Eng. Q. 2013, 19, 615–628. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Guo, P.; Sun, Y.; Cui, Y. Adsorption of hexavalent chromium by sodium alginate fiber biochar loaded with lanthanum. Materials 2021, 14, 2224. [Google Scholar] [CrossRef]
- Preethi, J.; Vigneshwaran, S.; Meenakshi, S. Performance of chitosan engraved iron and lanthanum mixed oxyhydroxide for the detoxification of hexavalent chromium. Int. J. Biol. Macromol. 2019, 130, 491–498. [Google Scholar] [CrossRef]
- Ajmani, A.; Shahnaz, T.; Subbiah, S.; Narayanasamy, S. Hexavalent chromium adsorption on virgin, biochar, and chemically modified carbons prepared from Phanera vahlii fruit biomass: Equilibrium, kinetics, and thermodynamics approach. Environ. Sci. Pollut. Res. 2019, 26, 32137–32150. [Google Scholar] [CrossRef]
- Moussavi, G.; Barikbin, B. Biosorption of chromium(VI) from industrial wastewater onto pistachio hull waste biomass. Chem. Eng. J. 2010, 162, 893–900. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Cr(VI) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and/or chitosan. Chemosphere 2004, 54, 951–967. [Google Scholar] [CrossRef] [PubMed]
- Beksissa, R.; Tekola, B.; Ayala, T.; Dame, B. Investigation of the adsorption performance of acid treated lignite coal for Cr (VI) removal from aqueous solution. Environ. Chall. 2021, 4, 100091. [Google Scholar] [CrossRef]
- Anah, L.; Astrini, N. Influence of pH on Cr(VI) ions removal from aqueous solutions using carboxymethyl cellulose-based hydrogel as adsorbent. In IOP Conference Series: Earth and Environmental Science; IOP: Bristol, UK, 2017; Volume 60. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Preethi, J.; Meenakshi, S. Fabrication of La3+ Impregnated Chitosan/β-Cyclodextrin Biopolymeric Materials for Effective Utilization of Chromate and Fluoride Adsorption in Single Systems. J. Chem. Eng. Data 2018, 63, 723–731. [Google Scholar] [CrossRef]
- Mullick, A.; Neogi, S. Acoustic cavitation induced synthesis of zirconium impregnated activated carbon for effective fluoride scavenging from water by adsorption. Ultrason. Sonochem. 2018, 45, 65–77. [Google Scholar] [CrossRef]
- Pakade, V.E.; Tavengwa, N.T.; Madikizela, L.M. Recent advances in hexavalent chromium removal from aqueous solutions by adsorptive methods. RSC Adv. 2019, 9, 26142–26164. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Wu, L.; Ou, M.; Wang, X.; Tang, Y. Sorption Studies of Chromium(VI) onto Cerium/Ferroferric Oxide Composites. J. Inorg. Organomet. Polym. Mater. 2021, 31, 2627–2637. [Google Scholar] [CrossRef]
- Abuzalat, O.; Wong, D.; Elsayed, M.A. Nano-Porous Composites of Activated Carbon–Metal Organic Frameworks (Fe-BDC@AC) for Rapid Removal of Cr (VI): Synthesis, Adsorption, Mechanism, and Kinetics Studies. J. Inorg. Organomet. Polym. Mater. 2022. [Google Scholar] [CrossRef]
- Mortazavian, S.; An, H.; Chun, D.; Moon, J. Activated carbon impregnated by zero-valent iron nanoparticles (AC/nZVI) optimized for simultaneous adsorption and reduction of aqueous hexavalent chromium: Material characterizations and kinetic studies. Chem. Eng. J. 2018, 353, 781–795. [Google Scholar] [CrossRef]
- Mishra, S.P.; Ghosh, M.R. Use of silver impregnated activated carbon (SAC) for Cr(VI) removal. J. Environ. Chem. Eng. 2020, 8, 103641. [Google Scholar] [CrossRef]
- Emamy, F.H.; Bumajdad, A.; Lukaszewicz, J.P. Adsorption of hexavalent chromium and divalent lead ions on the nitrogen-enriched chitosan-based activated carbon. Nanomaterials 2021, 11, 1907. [Google Scholar] [CrossRef]





| Elemental Analysis (wt.%) | |
|---|---|
| Carbon | 59.42 |
| Hydrogen | 6.31 |
| Nitrogen | 0.56 |
| Oxygen | 33.63 |
| Sulfur | 0.08 |
| Proximate Analysis (wt.%) | |
| Moisture content | 9.41 |
| Volatile constant | 74.22 |
| Fixed carbon | 13.28 |
| Ash | 3.09 |
| Parameters | COC-AC-La |
|---|---|
| BET Surface area, SBET (m2/g) | 139 |
| Micropore volume, Vmicro (cm3/g) | 0.035 |
| Mesopore volume, Vmeso (cm3/g) | 0.144 |
| Total pore volume, VT (cm3/g) | 1.121 |
| pH | Freundlich Isotherm Model | |||
|---|---|---|---|---|
| 1/n | n | KF (μg/g) | R2 | |
| 5 | 0.3649 | 2.7408 | 1.2683 | 0.9886 |
| 6 | 0.3091 | 3.2351 | 1.1485 | 0.9907 |
| 7 | 0.4908 | 2.0376 | 0.4368 | 0.9659 |
| 8 | 0.5623 | 1.7783 | 0.0455 | 0.9735 |
| pH | Qe,exp (μg/g) | Pseudo-Second Order Model | ||
|---|---|---|---|---|
| K2 (L/μg·min) | Qe,cal (μg/g) | R2 | ||
| 5 | 3.9125 | 0.0036 | 4.4954 | 0.9895 |
| 7 | 2.9123 | 0.0184 | 2.5821 | 0.9941 |
| Water Type | pHinit | Conductivity (μS/cm) | Ca2+ (mg/L) | Mg2+ (mg/L) | |
|---|---|---|---|---|---|
| Tap water | 1 L tap water | 7.3 | 398.5 | 190 | 30 |
| 1/1 | 0.5 L tap water and 0.5 L deionized water | 7.2 | 209.3 | 80 | 15 |
| 1/5 | 0.2 L tap water and 0.8 L deionized water | 7.2 | 87.3 | 30 | 5 |
| 1/10 | 0.1 L tap water and 0.9 L deionized water | 7.2 | 45.4 | 20 | 2 |
| Dist. Water | 1 L deionized water | 6.8 | 1.2 | - | - |
| Adsorbent | [Cr]o (mg/L) | Dosage (g/L) | pHinit | Contact Time (min) | Adsorption Capacity (mg/g) | R% |
Recycling Cycles | Ref. |
|---|---|---|---|---|---|---|---|---|
| ALC | 100 | 3.5 | 1.0 | 240 | 4.3 | 76 | - | [66] |
| CMC-g-PAA | 10 | 1.0 | 1.0 | 600 | 6.5 | 64 | - | [67] |
| CS-GO | 50 | 2.0 | 2.0 | 420 | 104.0 | 96 | 10 | [25] |
| La-DEA | 10 | 8.0 | 5.6 | 50 | 357.1 | 99 | difficult to be regenerated | [43] |
| La-modified ceramic materials | 3.0 | 0.5 | 4.0 | 1440 | 13.0 | - | - | [47] |
| CS–La–βCD | 100 | 2.0 | 4.0 | 30 | 48.4 | 98 | 5 | [69] |
| CSFLMOH | 100 | 2.0 | 4.0 | 60 | 48.3 | - | 5 | [62] |
| Ce/Fe3O4 | 20 | 4.0 | 2.0 | 120 | 9.6 | 99 | 4 | [72] |
| Fe-BDC@AC | 25 | 1.0 | 5.5 | 50 | 79.1 | 61 | 5 | [73] |
| AC/nZVI | 10 | 1.5 | 4.0 | 720 | 6.7 | 63 | 5 | [74] |
| SAC | 10 | 5.0 | 5.0 | 150 | 2.65 | 73 | 2 | [75] |
| Ch-ACs | 10 | 10.0 | 2.0 | 60 | 20.0 | 99 | - | [76] |
| COC-AC-La | 0.1 | 0.2 | 5.0 | 240 | 6.3 μg/g | 78 | 5 | Present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolkou, A.K.; Trikalioti, S.; Makrogianni, O.; Xanthopoulou, M.; Deliyanni, E.A.; Katsoyiannis, I.A.; Kyzas, G.Z. Chromium(VI) Removal from Water by Lanthanum Hybrid Modified Activated Carbon Produced from Coconut Shells. Nanomaterials 2022, 12, 1067. https://doi.org/10.3390/nano12071067
Tolkou AK, Trikalioti S, Makrogianni O, Xanthopoulou M, Deliyanni EA, Katsoyiannis IA, Kyzas GZ. Chromium(VI) Removal from Water by Lanthanum Hybrid Modified Activated Carbon Produced from Coconut Shells. Nanomaterials. 2022; 12(7):1067. https://doi.org/10.3390/nano12071067
Chicago/Turabian StyleTolkou, Athanasia K., Soultana Trikalioti, Olina Makrogianni, Maria Xanthopoulou, Eleni A. Deliyanni, Ioannis A. Katsoyiannis, and George Z. Kyzas. 2022. "Chromium(VI) Removal from Water by Lanthanum Hybrid Modified Activated Carbon Produced from Coconut Shells" Nanomaterials 12, no. 7: 1067. https://doi.org/10.3390/nano12071067
APA StyleTolkou, A. K., Trikalioti, S., Makrogianni, O., Xanthopoulou, M., Deliyanni, E. A., Katsoyiannis, I. A., & Kyzas, G. Z. (2022). Chromium(VI) Removal from Water by Lanthanum Hybrid Modified Activated Carbon Produced from Coconut Shells. Nanomaterials, 12(7), 1067. https://doi.org/10.3390/nano12071067











