Modified Breath Figure Methods for the Pore-Selective Functionalization of Honeycomb-Patterned Porous Polymer Films
Abstract
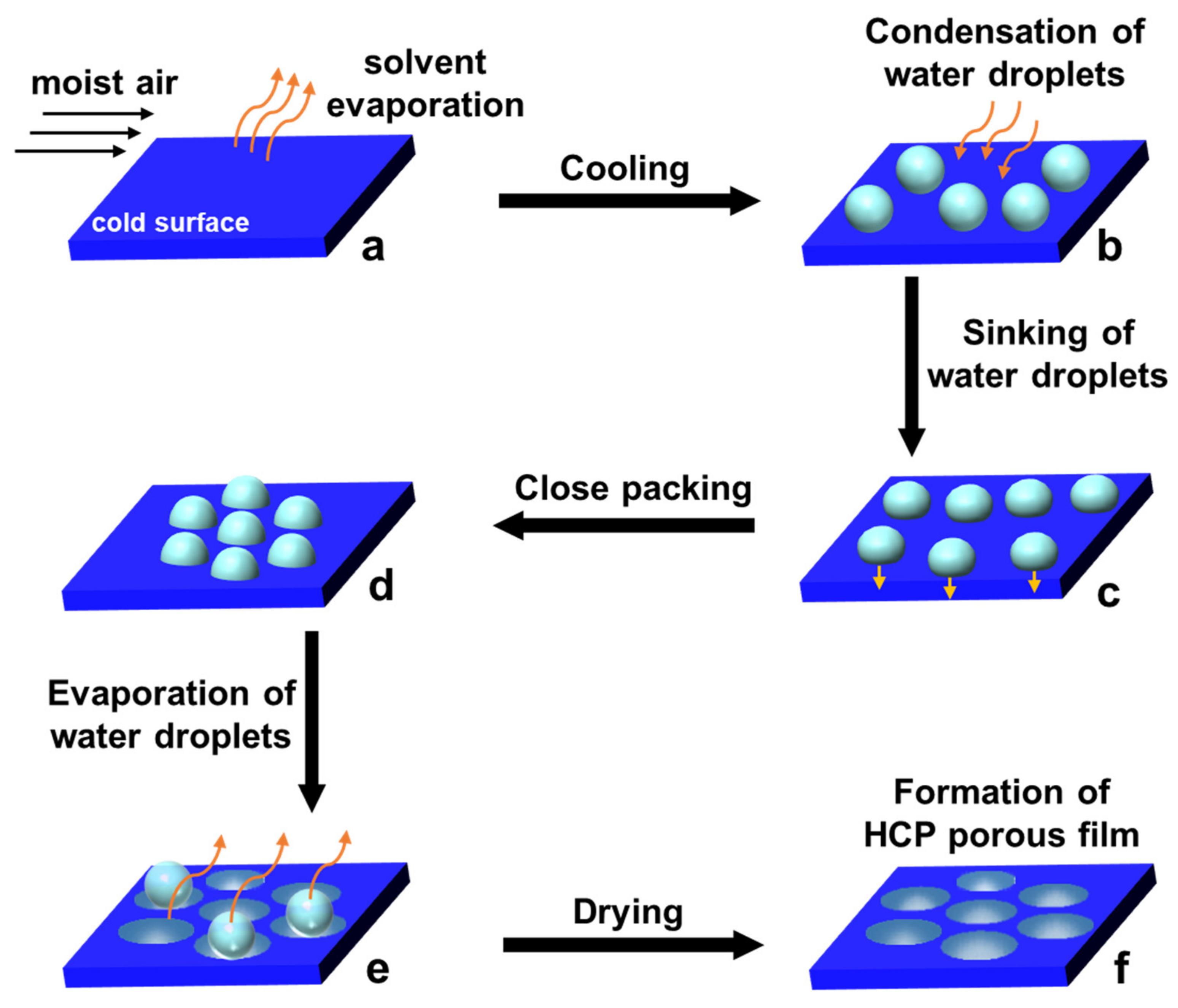
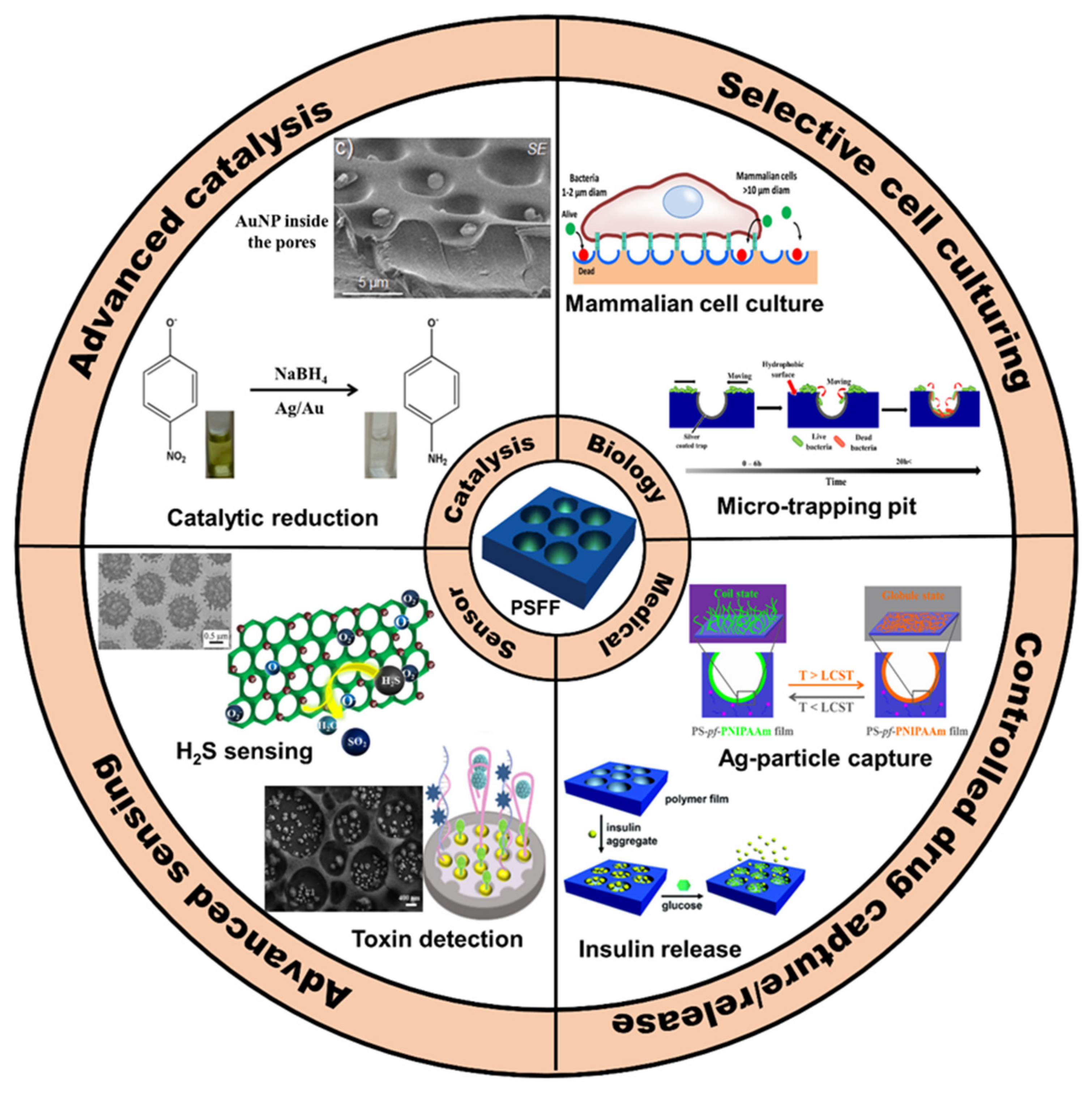
:1. Introduction
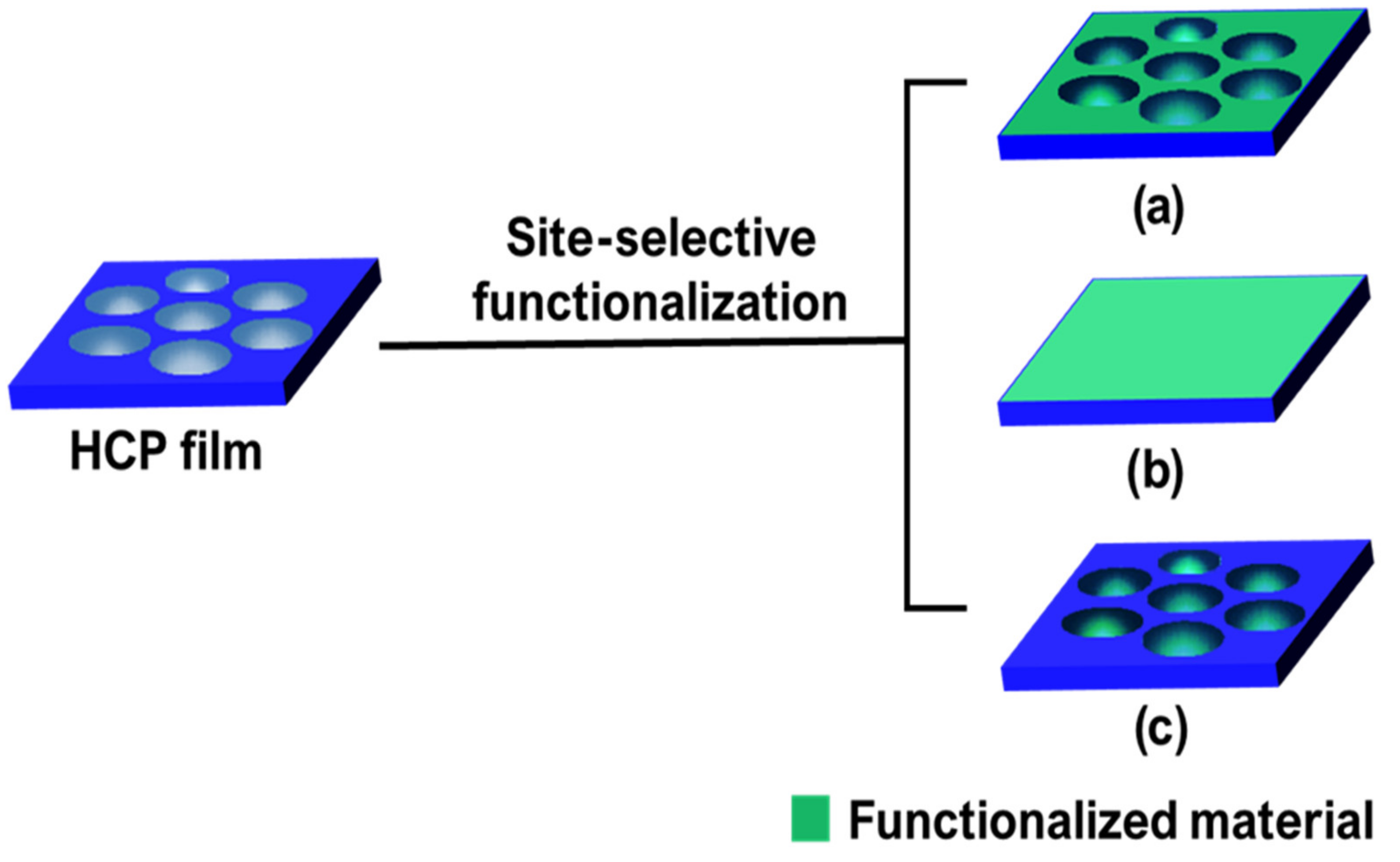
2. Pore-Selective Functionalization of HCP Films
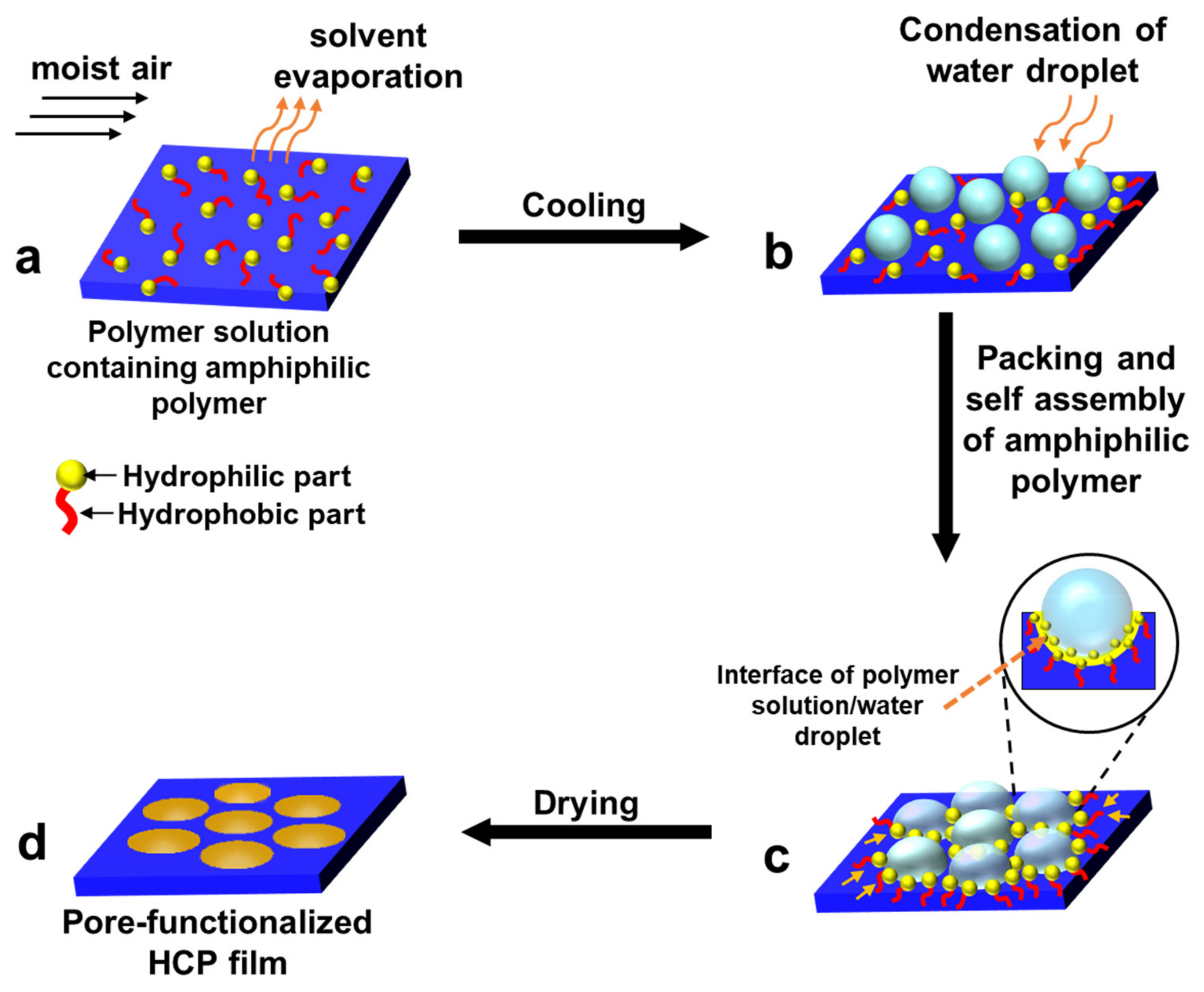
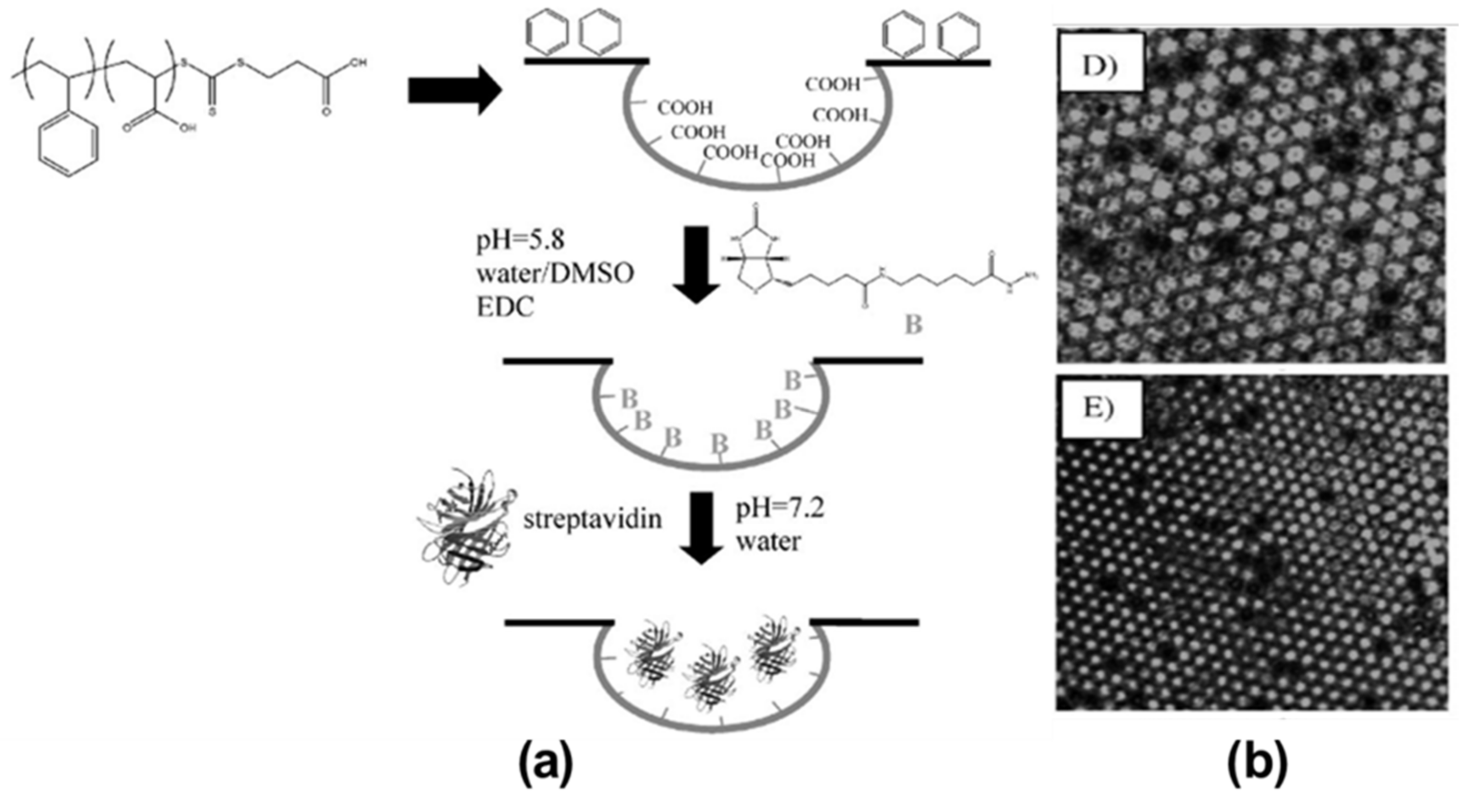
2.1. Pore-Selective Functionalization of HCP Films by Self-Assembly
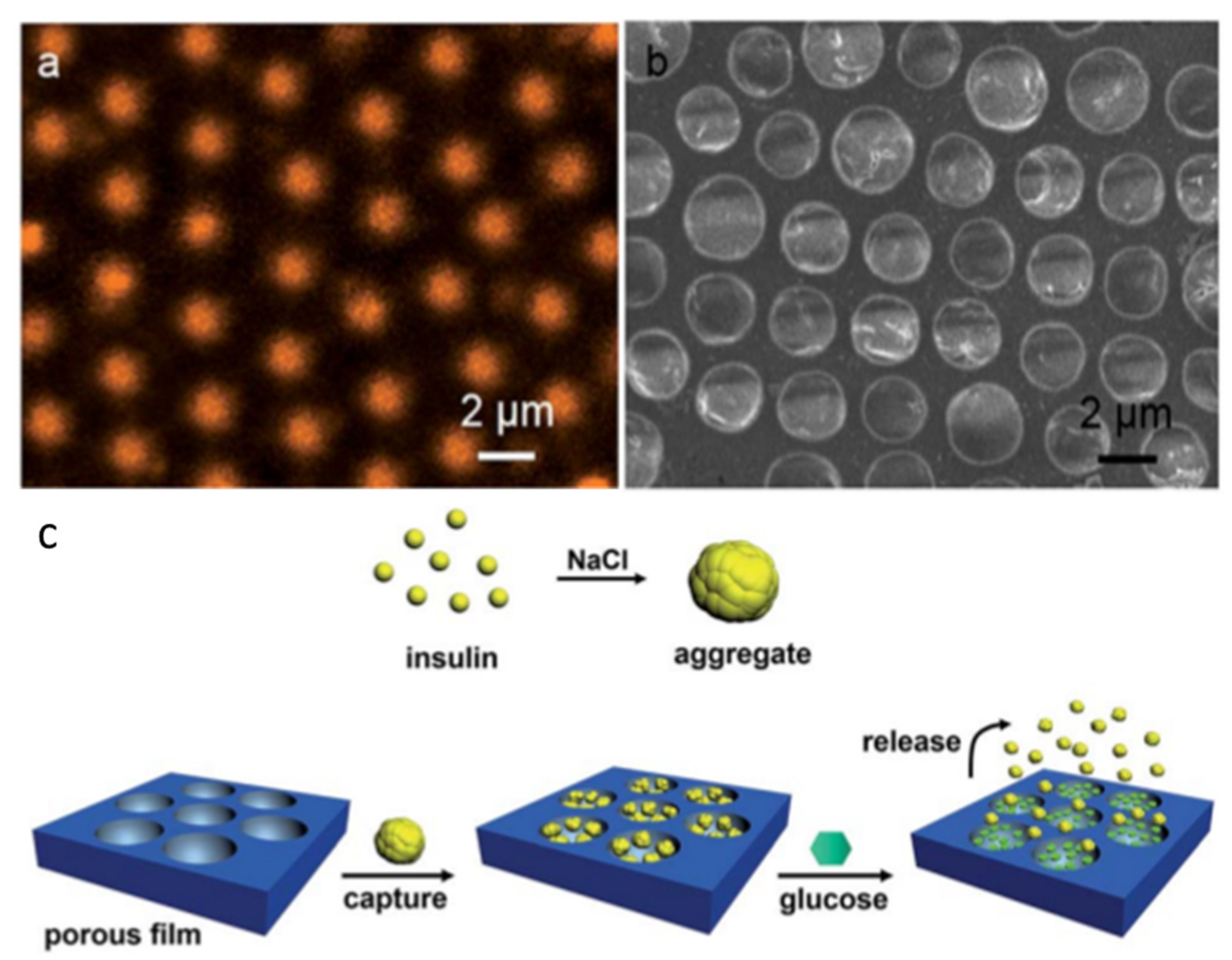
2.2. Pore-Functionalization by Self-Assembly Followed by Additional Treatment
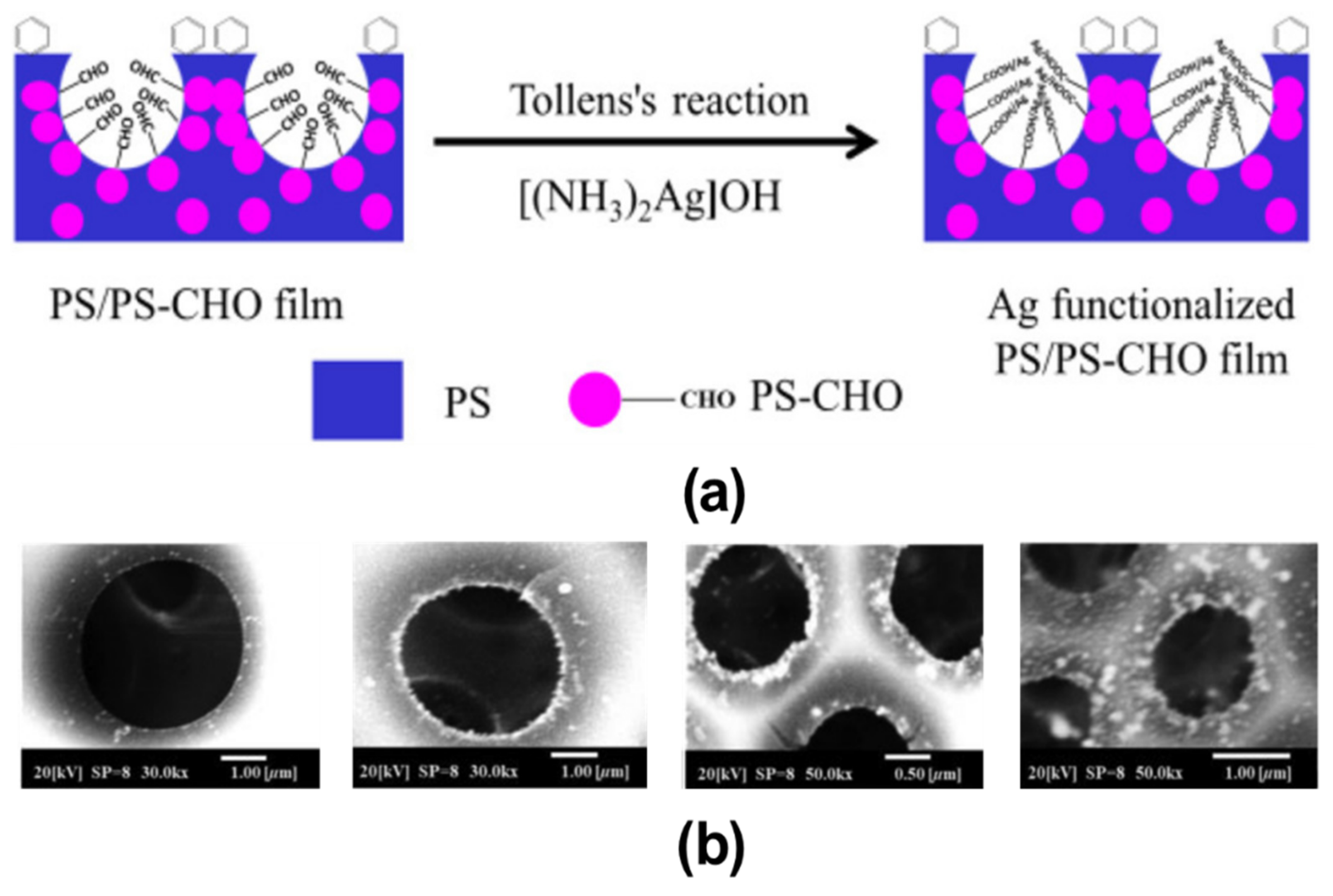
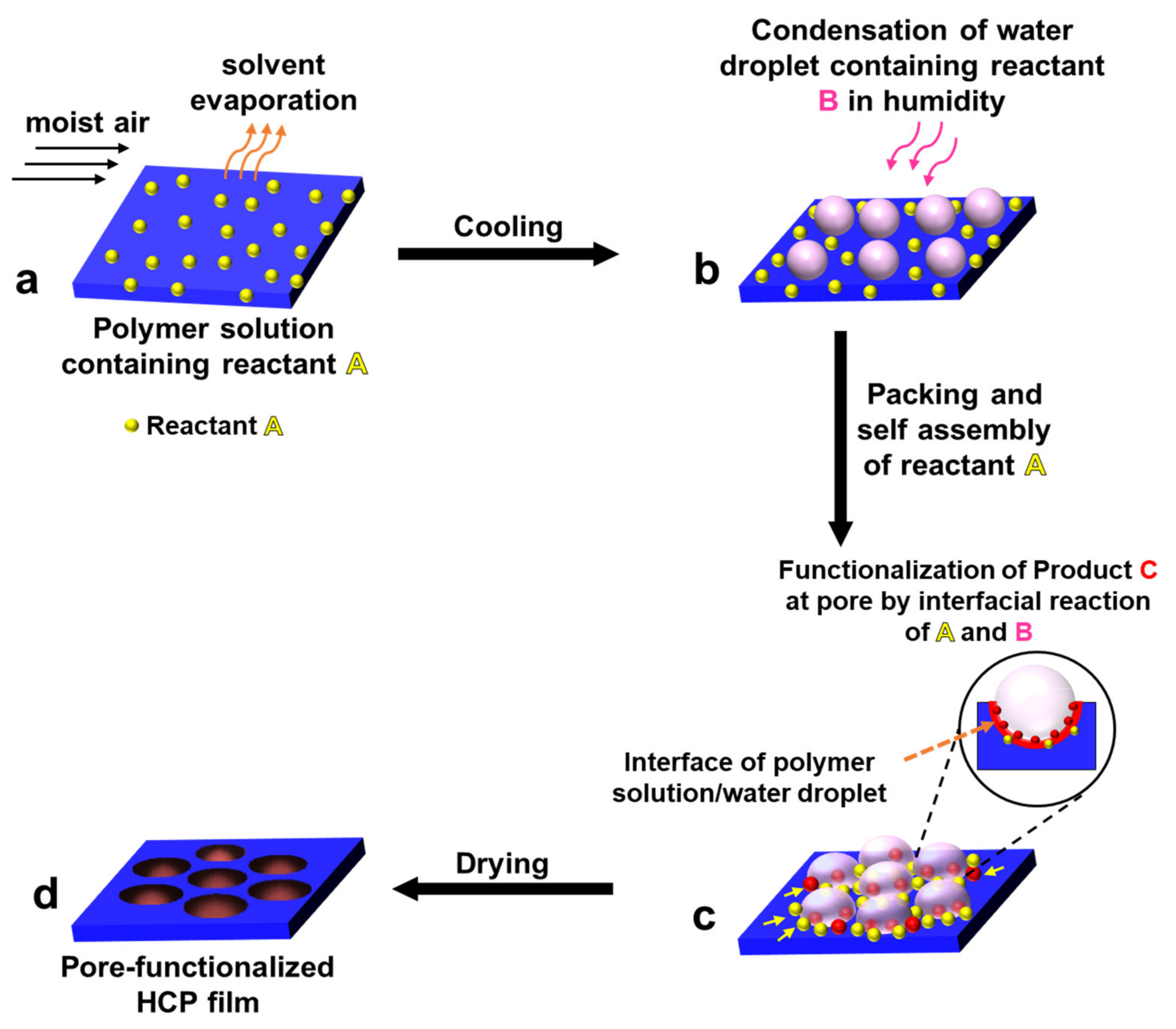
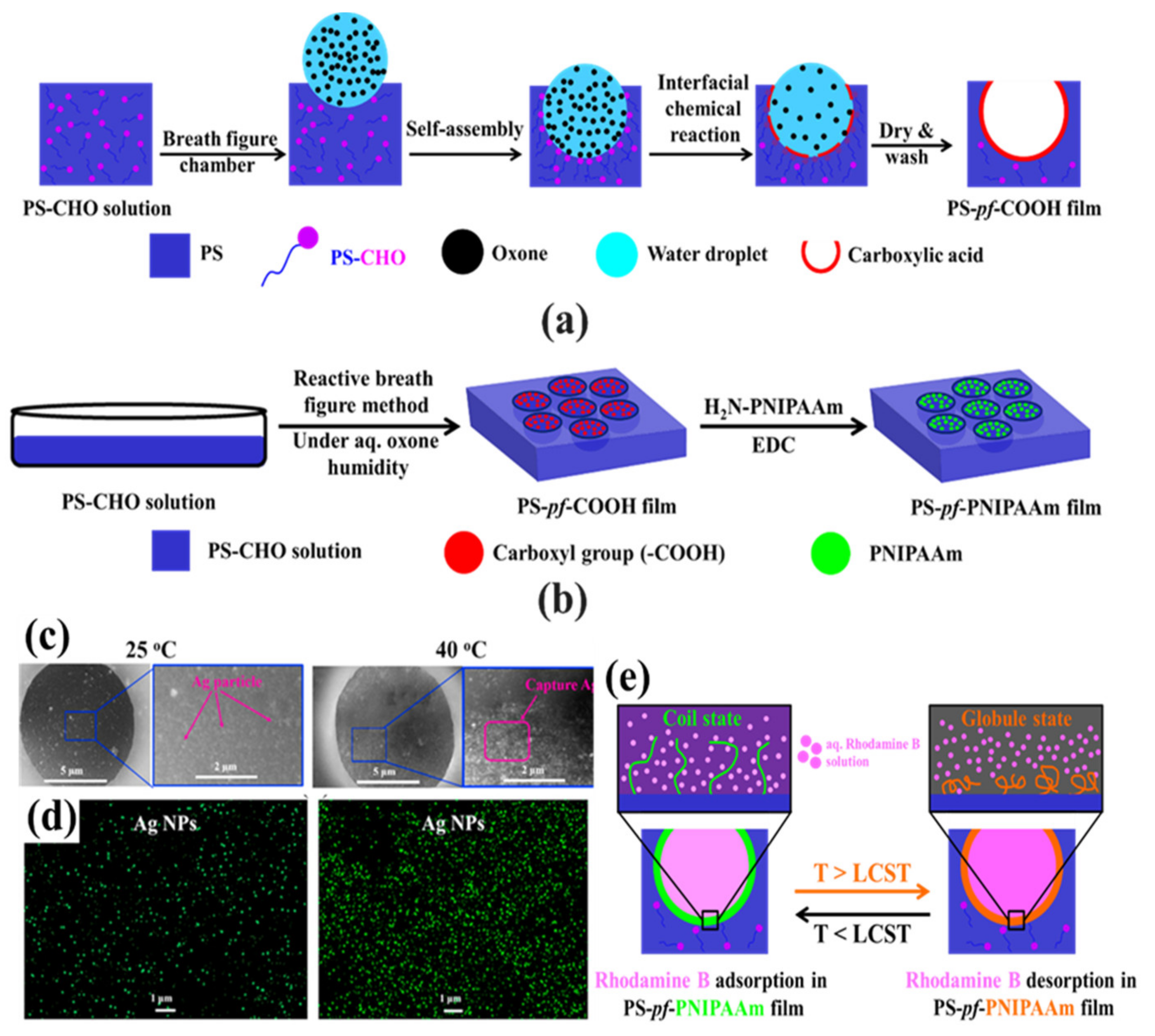
2.3. Pore-Selective Functionalization of HCP Films Accompanying Chemical Reaction
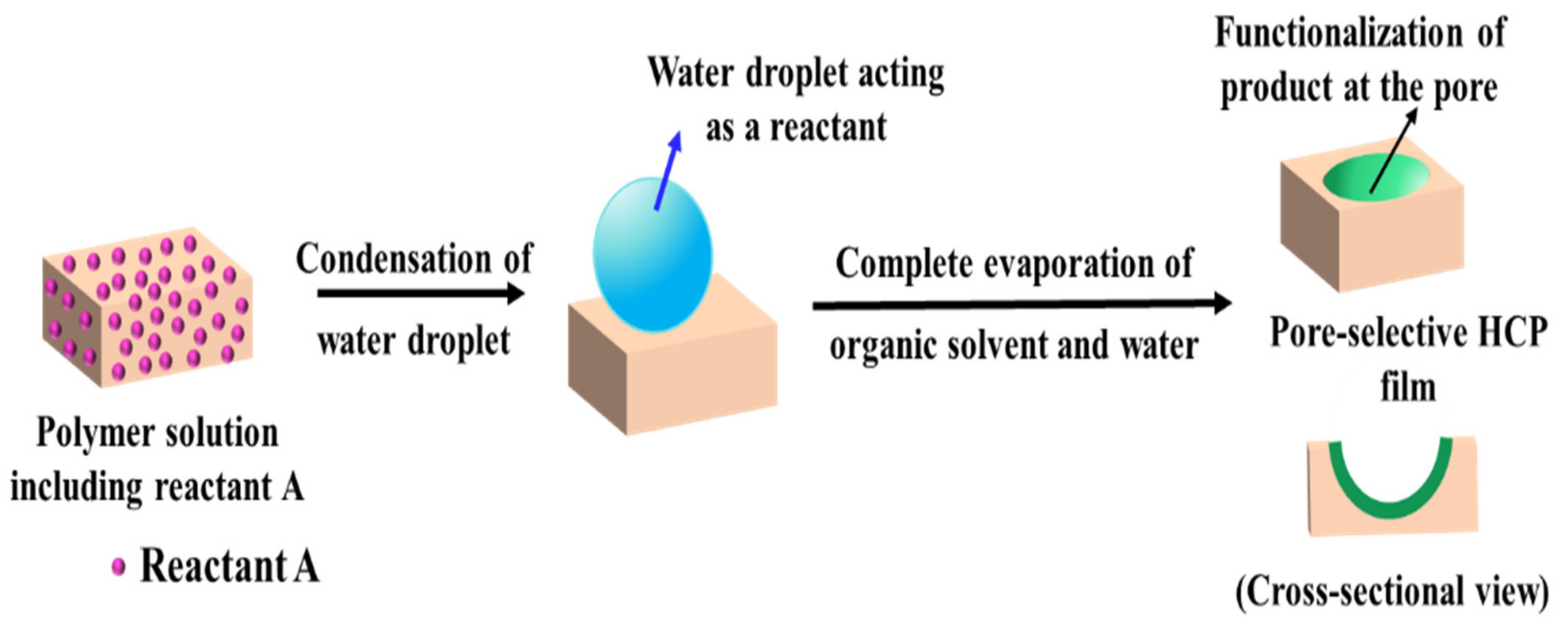
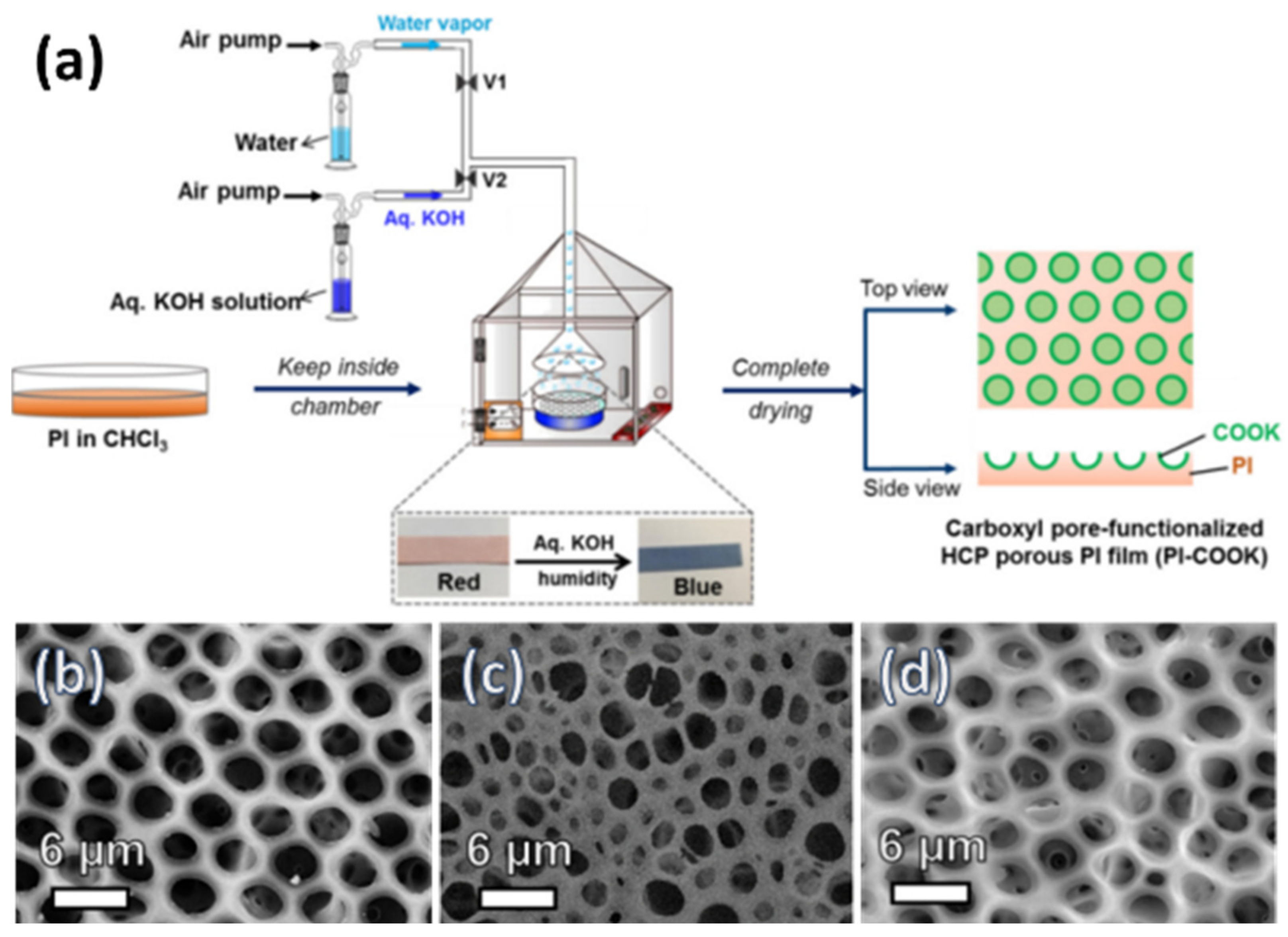
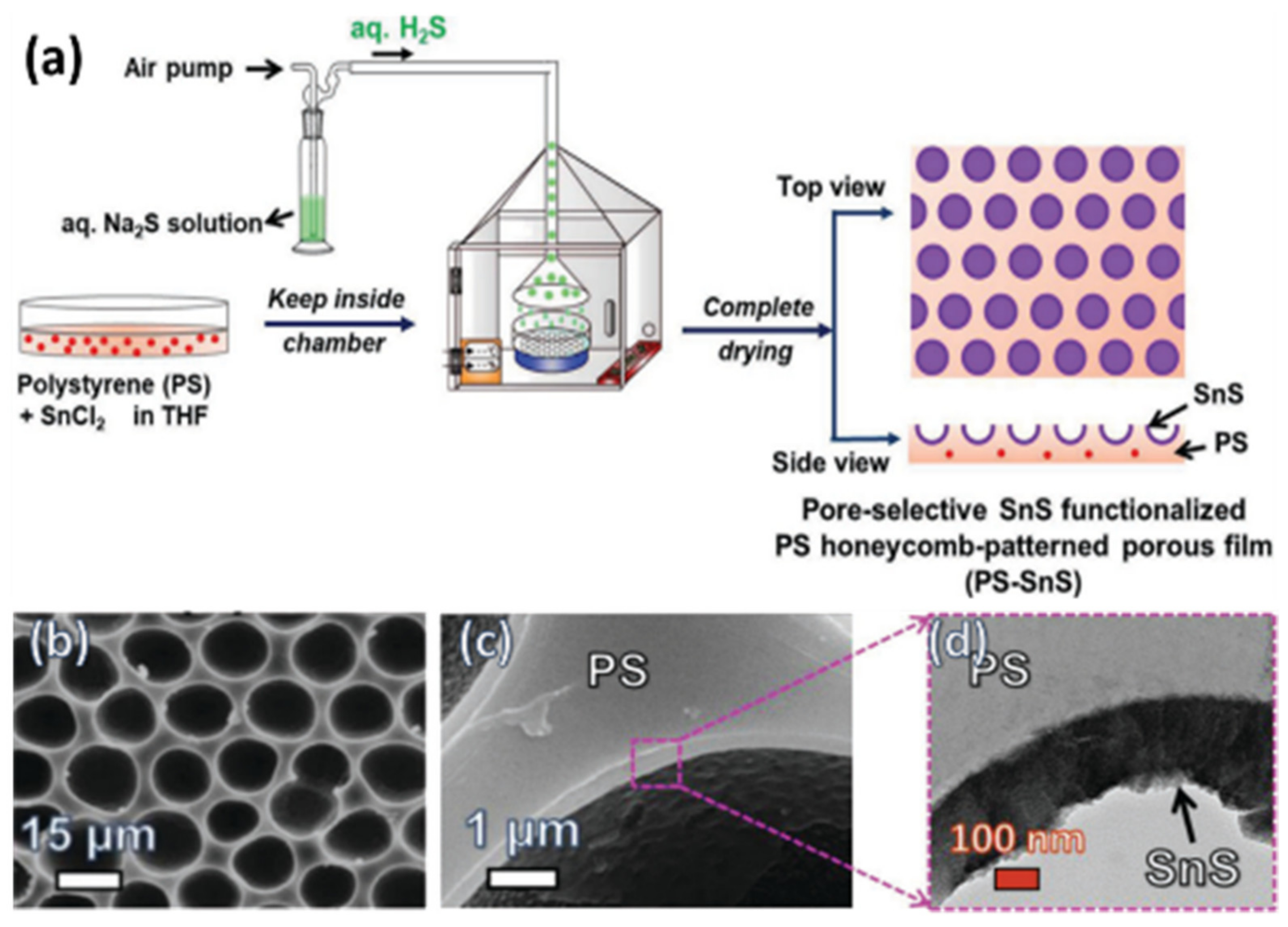
2.3.1. Pore-Functionalization by BF Process Using Water as a Reactant
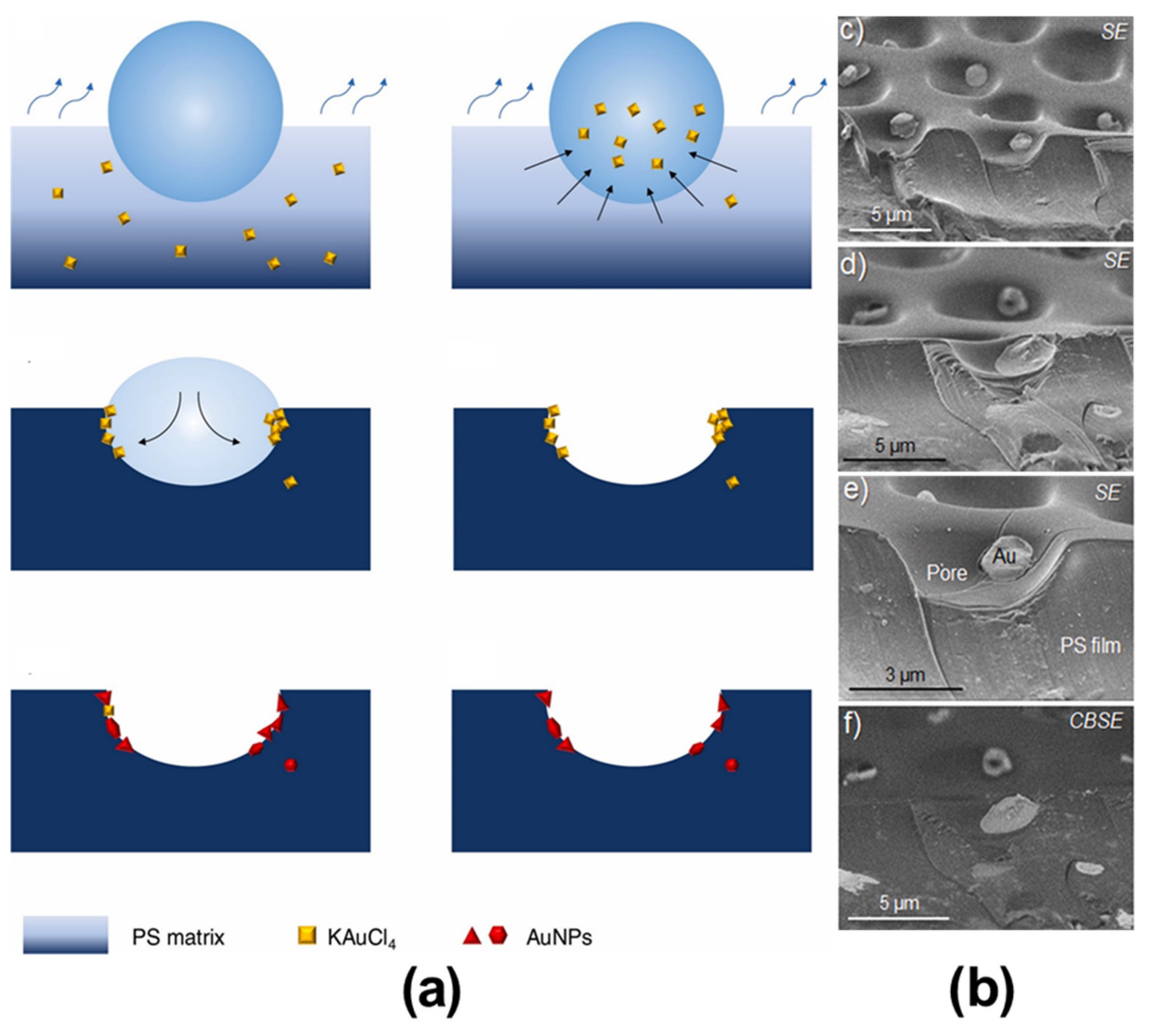
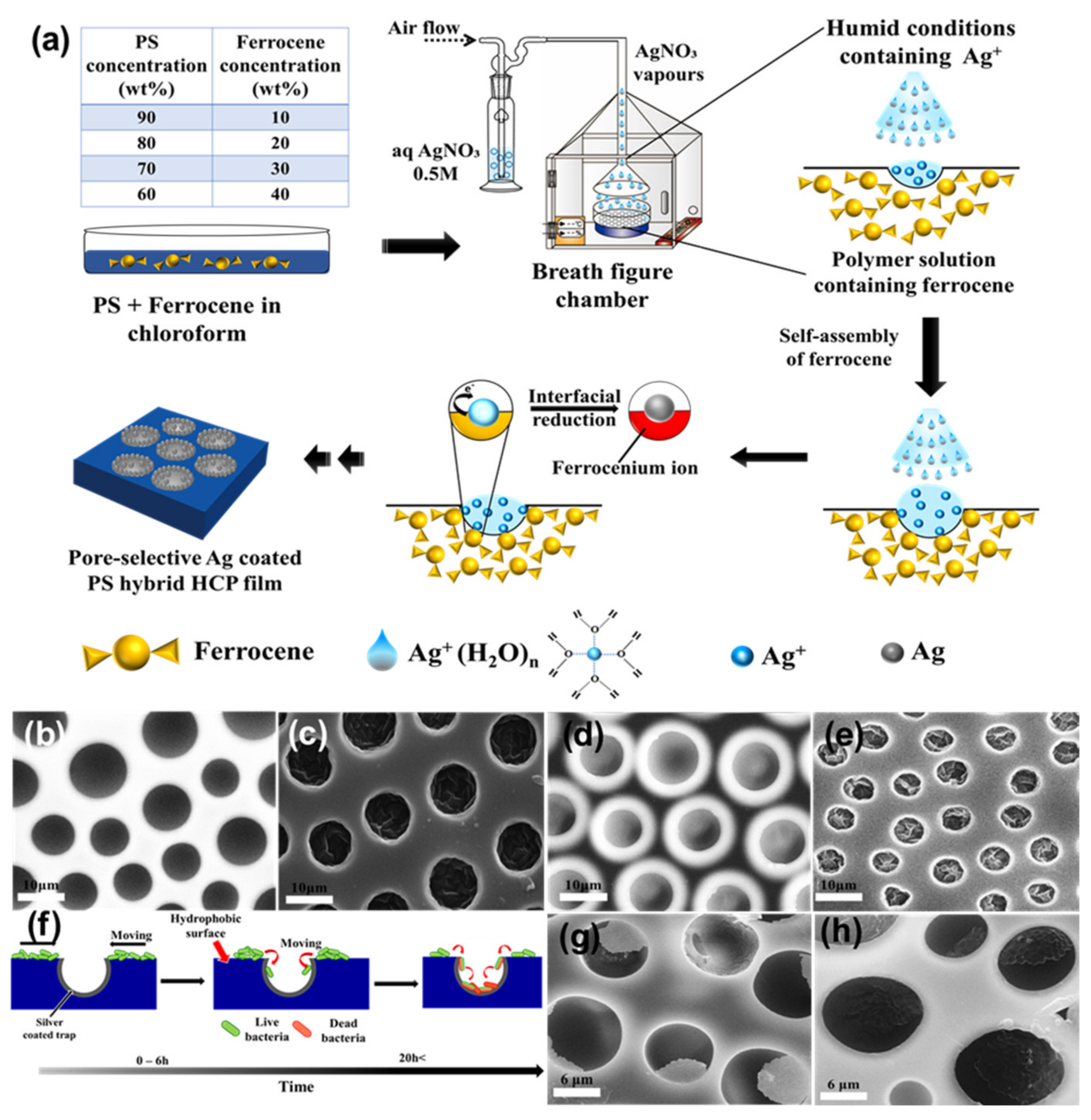
2.3.2. BF Process Accompanying Chemical Reactions with a Non-Aqueous Reactant
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, A.; Bai, H.; Li, L. Breath figure: A nature-inspired preparation method for ordered porous films. Chem. Rev. 2015, 115, 9801–9868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yabu, H. Fabrication of honeycomb films by the breath figure technique and their applications. Sci. Technol. Adv. Mater. 2018, 19, 802–822. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Li, G.; Dai, E.; Lu, G.; Huang, X.; Hao, L.; Tan, Y. Ordered Honeycomb-Pattern Membrane. Chin. J. Chem. 2020, 38, 1767–1779. [Google Scholar] [CrossRef]
- Mural, P.K.S.; Madras, G.; Bose, S. Polymeric membranes derived from immiscible blends with hierarchical porous structures, tailored bio-interfaces and enhanced flux: Potential and key challenges. Nano-Struct. Nano-Objects 2018, 14, 149–165. [Google Scholar] [CrossRef]
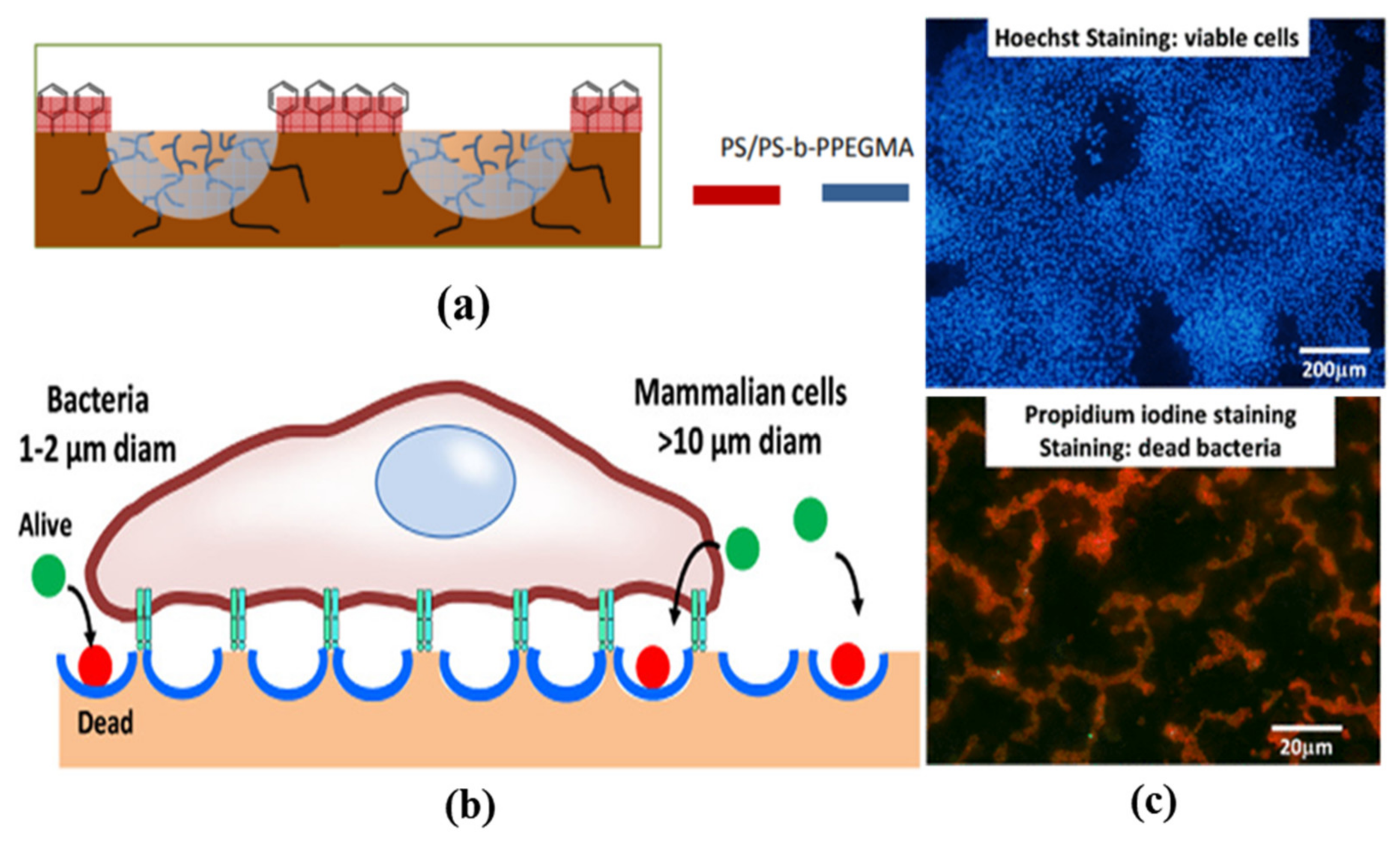
- Vargas-Alfredo, N.; Santos-Coquillat, A.; Martínez-Campos, E.; Dorronsoro, A.; Cortajarena, A.L.; del Campo, A.; Rodríguez-Hernández, J. Highly Efficient Antibacterial Surfaces Based on Bacterial/Cell Size Selective Microporous Supports. ACS Appl. Mater. Interfaces 2017, 9, 44270–44280. [Google Scholar] [CrossRef] [PubMed]
- Dehsari, H.S.; Michels, J.J.; Asadi, K. Processing of ferroelectric polymers for microelectronics: From morphological analysis to functional devices. J. Mater. Chem. C 2017, 5, 10490–10497. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Brückner, C.; Nieh, M.-P.; Lei, Y. A fluorescent polymer film with self-assembled three-dimensionally ordered nanopores: Preparation, characterization and its application for explosives detection. J. Mater. Chem. A 2014, 2, 14613–14621. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, J.; Liu, G.; Jia, X.; Zhao, J.; Chen, L.; Yang, P. Hexameric to Trimeric Lanthanide-Included Selenotungstates and Their 2D Honeycomb Organic–Inorganic Hybrid Films Used for Detecting Ochratoxin A. ACS Appl. Mater. Interfaces 2021, 13, 35997–36010. [Google Scholar] [CrossRef]
- Liu, G.; Liu, L.; Gong, T.; Li, Y.; Chen, L.; Zhao, J. Nicotinic-Acid-Ornamented Tetrameric Rare-Earth-Substituted Phospho(III)tungstates with the Coexistence of Mixed Keggin/Dawson Building Blocks and Its Honeycomb Nanofilm for Detecting Toxins. Inorg. Chem. 2021, 60, 14457–14466. [Google Scholar] [CrossRef]
- Li, C.; Qiao, X.; Jian, J.; Feng, F.; Wang, H.; Jia, L. Ordered porous BiVO4 based gas sensors with high selectivity and fast-response towards H2S. Chem. Eng. J. 2019, 375, 121924. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, L.; Yang, C.; Zheng, W.; Liu, X.; Zhang, J. Oxygen Vacancies Enabled Porous SnO2 Thin Films for Highly Sensitive Detection of Triethylamine at Room Temperature. ACS Appl. Mater. Interfaces 2020, 12, 20704–20713. [Google Scholar] [CrossRef] [PubMed]
- Neznalová, K.; Fajstavr, D.; Rimpelová, S.; Kasálková, N.S.; Kolská, Z.; Švorčík, V.; Slepička, P. Honeycomb-patterned poly(L-lactic) acid on plasma-activated FEP as cell culture scaffold. Polym. Degrad. Stab. 2020, 181, 109370. [Google Scholar] [CrossRef]
- Yin, H.; Zhan, F.; Li, Z.; Huang, H.; Marcasuzaa, P.; Luo, X.; Feng, Y.; Billon, L. CO2-Triggered ON/OFF Wettability Switching on Bioinspired Polylactic Acid Porous Films for Controllable Bioadhesion. Biomacromolecules 2021, 22, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, X.-R.; Huang, H.-D.; Xu, L.; Ji, X.; Zhong, G.-J.; Lin, H.; Li, Z.-M. Superhydrophobic, Self-Cleaning, and Robust Properties of Oriented Polylactide Imparted by Surface Structuring. ACS Sustain. Chem. Eng. 2021, 9, 6296–6304. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, Y.; Li, Z. Facile preparation of super-hydrophobic fabrics composed of fibres with microporous or microspherical coatings using the static breath figure method. Prog. Org. Coat. 2020, 149, 105938. [Google Scholar] [CrossRef]
- Naik, N.S.; Padaki, M.; Isloor, A.M.; Nagaraja, K.K.; Vishnumurthy, K.A. Poly(ionic liquid)-Based charge and size selective loose nanofiltration membrane for molecular separation. Chem. Eng. J. 2021, 418, 129372. [Google Scholar] [CrossRef]
- Jang, S.; Kang, S.M.; Choi, M. Multifunctional moth-eye TiO2/PDMS pads with high transmittance and UV filtering. ACS Appl. Mater. Interfaces 2017, 9, 44038–44044. [Google Scholar] [CrossRef]
- Li, Q.; Jonas, U.; Zhao, X.; Kappl, M. The forces at work in colloidal self-assembly: A review on fundamental interactions between colloidal particles. Asia-Pac. J. Chem. Eng. 2008, 3, 255–268. [Google Scholar] [CrossRef]
- Fan, B.; Wan, J.; Zhai, J.; Chen, X.; Thang, S.H. Triggered Degradable Colloidal Particles with Ordered Inverse Bicontinuous Cubic and Hexagonal Mesophases. ACS Nano 2021, 15, 4688–4698. [Google Scholar] [CrossRef]
- Pietsch, T.; Gindy, N.; Fahmi, A. Nano-and micro-sized honeycomb patterns through hierarchical self-assembly of metal-loaded diblock copolymer vesicles. Soft Matter 2009, 5, 2188–2197. [Google Scholar] [CrossRef]
- Werner, J.r.G.; Lee, H.; Wiesner, U.; Weitz, D.A. Ordered mesoporous microcapsules from double emulsion confined block copolymer self-assembly. ACS Nano 2021, 15, 3490–3499. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Bang, J.; Stein, G.E.; Fredrickson, G.H.; Hawker, C.J.; Kramer, E.J.; Sprung, M.; Wang, J. Square packing and structural arrangement of ABC triblock copolymer spheres in thin films. Macromolecules 2008, 41, 4328–4339. [Google Scholar] [CrossRef]
- Cummins, C.; Lundy, R.; Walsh, J.J.; Ponsinet, V.; Fleury, G.; Morris, M.A. Enabling future nanomanufacturing through block copolymer self-assembly: A review. Nano Today 2020, 35, 100936. [Google Scholar] [CrossRef]
- Bui, V.-T.; Lee, H.S.; Choi, J.-H.; Choi, H.-S. Highly ordered and robust honeycomb films with tunable pore sizes fabricated via UV crosslinking after applying improved phase separation. Polymer 2015, 74, 46–53. [Google Scholar] [CrossRef]
- Zhang, Y.; Almodovar-Arbelo, N.E.; Weidman, J.L.; Corti, D.S.; Boudouris, B.W.; Phillip, W.A. Fit-for-purpose block polymer membranes molecularly engineered for water treatment. NPJ Clean Water 2018, 1, 2. [Google Scholar] [CrossRef]
- Lombardo, D.; Calandra, P.; Pasqua, L.; Magazù, S. Self-Assembly of Organic Nanomaterials and Biomaterials: The Bottom-Up Approach for Functional Nanostructures Formation and Advanced Applications. Materials 2020, 13, 1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Bhushan, P.; Bhattacharya, S. Fabrication of Nanostructures with Bottom-up Approach and Their Utility in Diagnostics, Therapeutics, and Others. In Environmental, Chemical and Medical Sensors; Bhattacharya, S., Agarwal, A.K., Chanda, N., Pandey, A., Sen, A.K., Eds.; Springer: Singapore, 2018; pp. 167–198. [Google Scholar]
- Rayleigh, L. Breath figures. Nature 1911, 86, 416–417. [Google Scholar] [CrossRef]
- Aitken, J. Breath figures. Nature 1913, 90, 619–621. [Google Scholar] [CrossRef]
- Widawski, G.; Rawiso, M.; François, B. Self-organized honeycomb morphology of star-polymer polystyrene films. Nature 1994, 369, 387–389. [Google Scholar] [CrossRef]
- Connal, L.A.; Vestberg, R.; Hawker, C.J.; Qiao, G.G. Dramatic morphology control in the fabrication of porous polymer films. Adv. Funct. Mater. 2008, 18, 3706–3714. [Google Scholar] [CrossRef]
- Bormashenko, E. Breath-figure self-assembly, a versatile method of manufacturing membranes and porous structures: Physical, chemical and technological aspects. Membranes 2017, 7, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasarao, M.; Collings, D.; Philips, A.; Patel, S. Three-dimensionally ordered array of air bubbles in a polymer film. Science 2001, 292, 79–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maruyama, N.; Koito, T.; Nishida, J.; Sawadaishi, T.; Cieren, X.; Ijiro, K.; Karthaus, O.; Shimomura, M. Mesoscopic patterns of molecular aggregates on solid substrates. Thin Solid Film. 1998, 327, 854–856. [Google Scholar] [CrossRef]
- Barner-Kowollik, C.; Dalton, H.; Davis, T.P.; Stenzel, M.H. Nano-and Micro-Engineering of Ordered Porous Blue-Light-Emitting Films by Templating Well-Defined Organic Polymers Around Condensing Water Droplets. Angew. Chem. 2003, 115, 3792–3796. [Google Scholar] [CrossRef]
- Connal, L.A.; Vestberg, R.; Gurr, P.A.; Hawker, C.J.; Qiao, G.G. Patterning on nonplanar substrates: Flexible honeycomb films from a range of self-assembling star copolymers. Langmuir 2008, 24, 556–562. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Q.-L.; Chen, J.-Z.; Li, L.; Huang, J.; Ma, Z.; Zhong, Y.-W. Highly ordered microporous films containing a polyolefin segment fabricated by the breath-figure method using well-defined polymethylene-b-polystyrene copolymers. Polym. Chem. 2010, 1, 164–167. [Google Scholar] [CrossRef]
- Kabuto, T.; Hashimoto, Y.; Karthaus, O. Thermally stable and solvent resistant mesoporous honeycomb films from a crosslinkable polymer. Adv. Funct. Mater. 2007, 17, 3569–3573. [Google Scholar] [CrossRef]
- Escalé, P.; Ting, S.S.; Khoukh, A.; Rubatat, L.; Save, M.; Stenzel, M.H.; Billon, L. Synthetic route effect on macromolecular architecture: From block to gradient copolymers based on acryloyl galactose monomer using RAFT polymerization. Macromolecules 2011, 44, 5911–5919. [Google Scholar] [CrossRef]
- Kojima, M.; Nakanishi, T.; Hirai, Y.; Yabu, H.; Shimomura, M. Photo-patterning of honeycomb films prepared from amphiphilic copolymer containing photochromic spiropyran. ChemComm 2010, 46, 3970–3972. [Google Scholar] [CrossRef]
- Zhao, B.; Li, C.; Lu, Y.; Wang, X.; Liu, Z.; Zhang, J. Formation of ordered macroporous membranes from random copolymers by the breath figure method. Polymer 2005, 46, 9508–9513. [Google Scholar] [CrossRef]
- Liu, X.; Monzavi, T.; Gitsov, I. Controlled ATRP synthesis of novel linear-dendritic block copolymers and their directed self-assembly in breath figure arrays. Polymer 2019, 11, 539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wang, C. Micropatterning of Proteins on 3D Porous Polymer Film Fabricated by Using the Breath-Figure Method. Adv. Mater. 2007, 19, 913–916. [Google Scholar] [CrossRef]
- Galeotti, F.; Calabrese, V.; Cavazzini, M.; Quici, S.; Poleunis, C.; Yunus, S.; Bolognesi, A. Self-functionalizing polymer film surfaces assisted by specific polystyrene end-tagging. Chem. Mater. 2010, 22, 2764–2769. [Google Scholar] [CrossRef]
- Hu, R.; Lam, J.W.; Li, M.; Deng, H.; Li, J.; Tang, B.Z. Homopolycyclotrimerization of A4-type tetrayne: A new approach for the creation of a soluble hyperbranched poly (tetraphenylethene) with multifunctionalities. J. Polym. Sci. A Polym. Chem. 2013, 51, 4752–4764. [Google Scholar] [CrossRef] [Green Version]
- Wan, L.-S.; Zhu, L.-W.; Ou, Y.; Xu, Z.-K. Multiple interfaces in self-assembled breath figures. ChemComm 2014, 50, 4024–4039. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, S. Regulating MC3T3-E1 cells on deformable poly (ε-caprolactone) honeycomb films prepared using a surfactant-free breath figure method in a water-miscible solvent. ACS Appl. Mater. Interfaces 2012, 4, 4966–4975. [Google Scholar] [CrossRef] [PubMed]
- Ke, B.-B.; Wan, L.-S.; Zhang, W.-X.; Xu, Z.-K. Controlled synthesis of linear and comb-like glycopolymers for preparation of honeycomb-patterned films. Polymer 2010, 51, 2168–2176. [Google Scholar] [CrossRef]
- Tsai, H.; Xu, Z.; Pai, R.K.; Wang, L.; Dattelbaum, A.M.; Shreve, A.P.; Wang, H.-L.; Cotlet, M. Structural dynamics and charge transfer via complexation with fullerene in large area conjugated polymer honeycomb thin films. Chem. Mater. 2011, 23, 759–761. [Google Scholar] [CrossRef]
- Chiu, Y.-C.; Kuo, C.-C.; Lin, C.-J.; Chen, W.-C. Highly ordered luminescent microporous films prepared from crystalline conjugated rod-coil diblock copolymers of PF-b-PSA and their superhydrophobic characteristics. Soft Matter 2011, 7, 9350–9358. [Google Scholar] [CrossRef]
- Nakamichi, Y.; Hirai, Y.; Yabu, H.; Shimomura, M. Fabrication of patterned and anisotropic porous films based on photo-cross-linking of poly (1,2-butadiene) honeycomb films. J. Mater. Chem. 2011, 21, 3884–3889. [Google Scholar] [CrossRef]
- De León, A.S.; del Campo, A.; Fernández-García, M.; Rodríguez-Hernández, J.; Muñoz-Bonilla, A. Hierarchically structured multifunctional porous interfaces through water templated self-assembly of ternary systems. Langmuir 2012, 28, 9778–9787. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, H.; Wu, L. Micro-patterned polystyrene surfaces directed by surfactant-encapsulated polyoxometalate complex via breath figures. Polymer 2009, 50, 2113–2122. [Google Scholar] [CrossRef]
- Sun, W.; Shao, Z.; Ji, J. Particle-assisted fabrication of honeycomb-structured hybrid films via breath figures method. Polymer 2010, 51, 4169–4175. [Google Scholar] [CrossRef]
- Wang, J.; Wang, C.-F.; Shen, H.-X.; Chen, S. Quantum-dot-embedded ionomer-derived films with ordered honeycomb structures via breath figures. ChemComm 2010, 46, 7376–7378. [Google Scholar] [CrossRef] [PubMed]
- Deleuze, C.; Derail, C.; Delville, M.H.; Billon, L. Hierarchically structured hybrid honeycomb films via micro to nanosized building blocks. Soft Matter 2012, 8, 8559–8562. [Google Scholar] [CrossRef]
- Sun, W.; Zhou, Y.; Chen, Z. Fabrication of honeycomb-structured porous film from polystyrene via polymeric particle-assisted breath figures method. Macromol. Res. 2013, 21, 414–418. [Google Scholar] [CrossRef]
- Heng, L.; Qin, W.; Chen, S.; Hu, R.; Li, J.; Zhao, N.; Wang, S.; Tang, B.Z.; Jiang, L. Fabrication of small organic luminogens honeycomb-structured films with aggregation-induced emission features. J. Mater. Chem. 2012, 22, 15869–15873. [Google Scholar] [CrossRef]
- Dong, R.; Xu, J.; Yang, Z.; Wei, G.; Zhao, W.; Yan, J.; Fang, Y.; Hao, J. Preparation and Functions of Hybrid Membranes with Rings of Ag NPs Anchored at the Edges of Highly Ordered Honeycomb-Patterned Pores. Chem. Eur. J. 2013, 19, 13099–13104. [Google Scholar] [CrossRef]
- Lin, F.-W.; Xu, X.-L.; Wan, L.-S.; Wu, J.; Xu, Z.-K. Porphyrinated polyimide honeycomb films with high thermal stability for HCl gas sensing. RSC Adv. 2015, 5, 30472–30477. [Google Scholar] [CrossRef]
- Mao, Y.; Mei, Z.; Wen, J.; Li, G.; Tian, Y.; Zhou, B.; Tian, Y. Honeycomb structured porous films from a platinum porphyrin-grafted poly (styrene-co-4-vinylpyridine) copolymer as an optical oxygen sensor. Sens. Actuators B Chem. 2018, 257, 944–953. [Google Scholar] [CrossRef]
- Lu, Y.; Ren, Y.; Wang, L.; Wang, X.; Li, C. Template synthesis of conducting polyaniline composites based on honeycomb ordered polycarbonate film. Polymer 2009, 50, 2035–2039. [Google Scholar] [CrossRef]
- Galeotti, F.; Andicsova, A.; Yunus, S.; Botta, C. Precise surface patterning of silk fibroin films by breath figures. Soft Matter 2012, 8, 4815–4821. [Google Scholar] [CrossRef]
- Ting, S.S.; Min, E.H.; Escale, P.; Save, M.; Billon, L.; Stenzel, M.H. Lectin recognizable biomaterials synthesized via nitroxide-mediated polymerization of a methacryloyl galactose monomer. Macromolecules 2009, 42, 9422–9434. [Google Scholar] [CrossRef]
- Yabu, H.; Takebayashi, M.; Tanaka, M.; Shimomura, M. Superhydrophobic and lipophobic properties of self-organized honeycomb and pincushion structures. Langmuir 2005, 21, 3235–3237. [Google Scholar] [CrossRef]
- Yabu, H.; Shimomura, M. Single-step fabrication of transparent superhydrophobic porous polymer films. Chem. Mater. 2005, 17, 5231–5234. [Google Scholar] [CrossRef]
- Chen, P.-C.; Wan, L.-S.; Ke, B.-B.; Xu, Z.-K. Honeycomb-Patterned Film Segregated with Phenylboronic Acid for Glucose Sensing. Langmuir 2011, 27, 12597–12605. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wu, H.; Zhang, W.; Bai, X.; Chen, J.; Ikram, M.; Wang, R.; Shi, K. High-dispersed Fe2O3/Fe nanoparticles residing in 3D honeycomb-like N-doped graphitic carbon as high-performance room-temperature NO2 sensor. J. Hazard. Mater. 2021, 405, 124252. [Google Scholar] [CrossRef]
- Wan, L.-S.; Li, Q.-L.; Chen, P.-C.; Xu, Z.-K. Patterned biocatalytic films via one-step self-assembly. ChemComm 2012, 48, 4417–4419. [Google Scholar] [CrossRef]
- De León, A.S.; de la Mata, M.; Molina, S.I. Hybrid hierarchically structured materials combining breath figures and thermal decomposition of KAuCl4. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126766. [Google Scholar] [CrossRef]
- Rarima, R.; Unnikrishnan, G. Porous poly (lactic acid)/nano-silver composite membranes for catalytic reduction of 4-nitrophenol. Mater. Chem. Phys. 2020, 241, 122389. [Google Scholar] [CrossRef]
- Wan, L.-S.; Li, J.-W.; Ke, B.-B.; Xu, Z.-K. Ordered microporous membranes templated by breath figures for size-selective separation. J. Am. Chem. Soc. 2012, 134, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, G.; Liu, H.; Zhang, X. Fabrication of porous polymer coating layers with selective wettability on filter papers via the breath figure method and their applications in oil/water separation. RSC Adv. 2021, 11, 14276–14284. [Google Scholar] [CrossRef]
- Yu, B.; Luo, Y.; Zhang, X.; Usman, M.; Ahmed, A.; Shen, Y.; Cong, H. Preparation of pocket shaped microfiltration membranes with binary porous structures. Soft Matter 2018, 14, 8660–8665. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Guerrero, M.; Min, E.; Barner-Kowollik, C.; Müller, A.H.; Stenzel, M.H. Grafting thermoresponsive polymers onto honeycomb structured porous films using the RAFT process. J. Mater. Chem. 2008, 18, 4718–4730. [Google Scholar] [CrossRef]
- Ghannam, L.; Manguian, M.; François, J.; Billon, L. A versatile route to functional biomimetic coatings: Ionomers for honeycomb-like structures. Soft Matter 2007, 3, 1492–1499. [Google Scholar] [CrossRef]
- Hirai, Y.; Yabu, H.; Matsuo, Y.; Ijiro, K.; Shimomura, M. Arrays of triangular shaped pincushions for SERS substrates prepared by using self-organization and vapor deposition. ChemComm 2010, 46, 2298–2300. [Google Scholar] [CrossRef]
- Ma, H.; Gao, P.; Fan, D.; Li, G.; Wu, D.; Du, B.; Wei, Q. Radially aligned microchannels prepared from ordered arrays of cracks on colloidal films. Phys. Chem. Chem. Phys. 2013, 15, 9808–9811. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, Q.; Han, F.; Li, C.; Sun, J.; Lu, Y. Fabrication of conducting polypyrrole film with microlens arrays by combination of breath figures and replica molding methods. Polymer 2012, 53, 2599–2603. [Google Scholar] [CrossRef]
- Martínez-Campos, E.; Elzein, T.; Bejjani, A.; García-Granda, M.J.; Santos-Coquillat, A.; Ramos, V.; Muñoz-Bonilla, A.; Rodríguez-Hernández, J. Toward Cell Selective Surfaces: Cell Adhesion and Proliferation on Breath Figures with Antifouling Surface Chemistry. ACS Appl. Mater. Interfaces 2016, 8, 6344–6353. [Google Scholar] [CrossRef]
- Yeh, S.-C.; Wu, C.-H.; Huang, Y.-C.; Lee, J.-Y.; Jeng, R.-J. In Search of a Green Process: Polymeric Films with Ordered Arrays via a Water Droplet Technique. Polymers 2019, 11, 1473. [Google Scholar] [CrossRef] [Green Version]
- Chiang, C.-Y.; Liu, T.-Y.; Su, Y.-A.; Wu, C.-H.; Cheng, Y.-W.; Cheng, H.-W.; Jeng, R.-J. Au Nanoparticles Immobilized on Honeycomb-Like Polymeric Films for Surface-Enhanced Raman Scattering (SERS) Detection. Polymers 2017, 9, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Male, U.; Shin, B.K.; Huh, D.S. Coupling of breath figure method with interfacial polymerization: Bottom-surface functionalized honeycomb-patterned porous films. Polymer 2017, 119, 206–211. [Google Scholar] [CrossRef]
- Uyen Thi, P.N.; Male, U.; Huh, D.S. In situ surface selective functionalization of honeycomb patterned porous poly(ε-caprolactone) films using reactive substrate. Polymer 2018, 147, 150–156. [Google Scholar] [CrossRef]
- Guo, T.; Han, K.; Heng, L.; Cao, M.; Jiang, L. Ordered porous structure hybrid films generated by breath figures for directional water penetration. RSC Adv. 2015, 5, 88471–88476. [Google Scholar] [CrossRef]
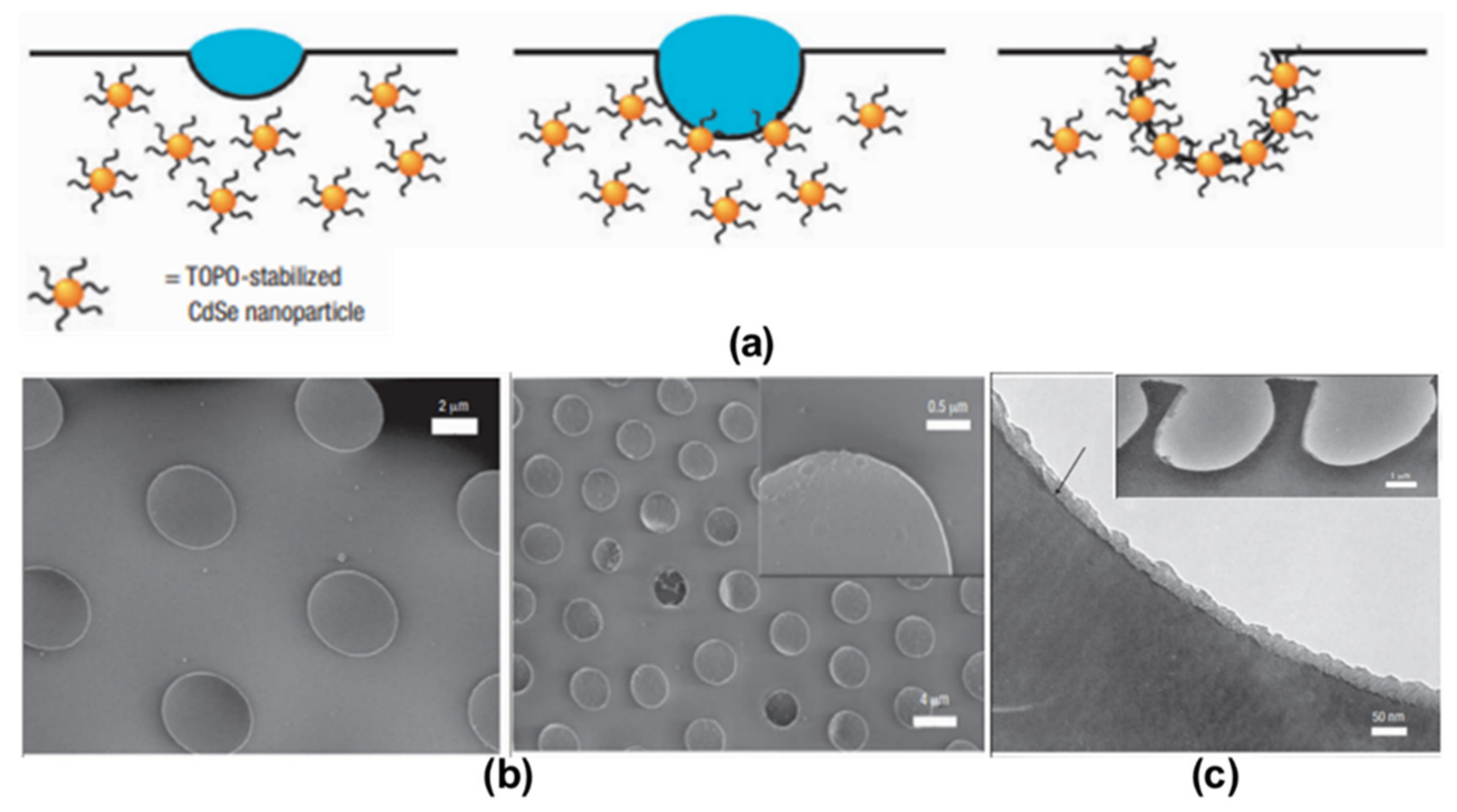
- Böker, A.; Lin, Y.; Chiapperini, K.; Horowitz, R.; Thompson, M.; Carreon, V.; Xu, T.; Abetz, C.; Skaff, H.; Dinsmore, A. Hierarchical nanoparticle assemblies formed by decorating breath figures. Nat. Mater. 2004, 3, 302–306. [Google Scholar] [CrossRef]
- Min, E.; Wong, K.H.; Stenzel, M.H. Microwells with patterned proteins by a self-assembly process using honeycomb-structured porous films. Adv. Mater. 2008, 20, 3550–3556. [Google Scholar] [CrossRef]
- Ke, B.-B.; Wan, L.-S.; Xu, Z.-K. Controllable Construction of Carbohydrate Microarrays by Site-Directed Grafting on Self-Organized Porous Films. Langmuir 2010, 26, 8946–8952. [Google Scholar] [CrossRef]
- Cha, T.; Guo, A.; Zhu, X.Y. Enzymatic activity on a chip: The critical role of protein orientation. Proteomics 2005, 5, 416–419. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Ibarboure, E.; Bordegé, V.; Fernández-García, M.; Rodríguez-Hernández, J. Fabrication of Honeycomb-Structured Porous Surfaces Decorated with Glycopolymers. Langmuir 2010, 26, 8552–8558. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, J.; Muñoz-Bonilla, A.; Ibarboure, E.; Bordegé, V.; Fernández-García, M. Honeycomb structured porous interfaces as templates for protein adhesion. J. Phys. Conf. Ser. 2010, 252, 012002. [Google Scholar] [CrossRef]
- Ma, H.; Cui, J.; Chen, J.; Hao, J. Self-Organized Polymer Nanocomposite Inverse Opal Films with Combined Optical Properties. Chem. Eur. J. 2011, 17, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Yu, B.; Cong, H.; Chi, M.; Cheng, Y.; Lv, C. Preparation of Hierarchical Highly Ordered Porous Films of Brominated Poly(phenylene oxide) and Hydrophilic SiO2/C Membrane via the Breath Figure Method. Materials 2018, 11, 481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Z.; Wang, L.; Yu, H. Fabrication of honeycomb-patterned film using hyperbranched polyethylene-based copolymer. Eur. Polym. J. 2017, 93, 428–435. [Google Scholar] [CrossRef]
- Liang, J.; Ma, Y.; Sims, S.; Wu, L. A patterned porous polymer film for localized capture of insulin and glucose-responsive release. J. Mater. Chem. B 2015, 3, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Yabu, H.; Hirai, Y.; Kojima, M.; Shimomura, M. Simple fabrication of honeycomb-and pincushion-structured films containing thermoresponsive polymers and their surface wettability. Chem. Mater. 2009, 21, 1787–1789. [Google Scholar] [CrossRef]
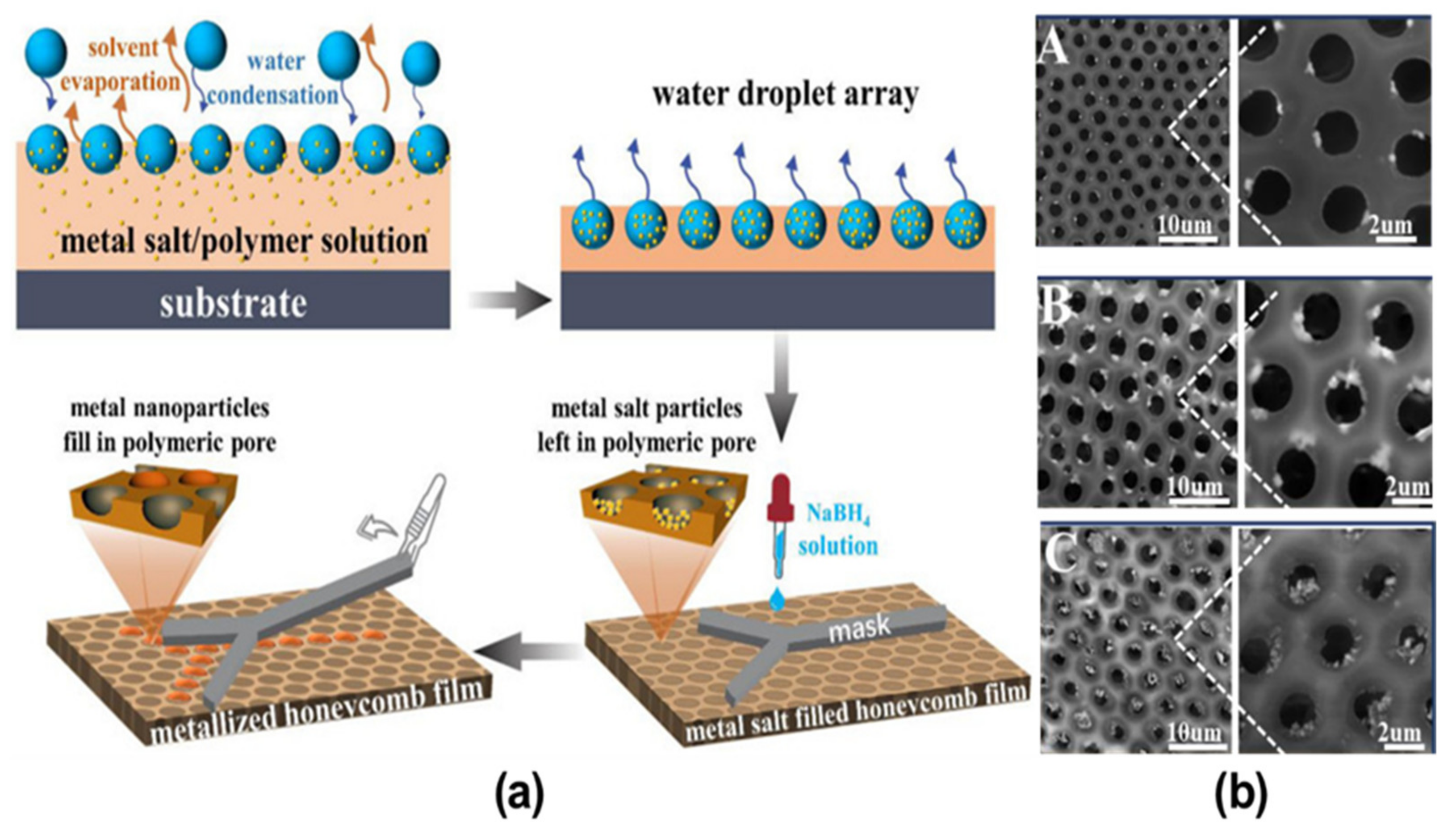
- Li, Y.; Ma, X.; Ma, J.; Zhang, Z.; Niu, Z.; Chen, F. Fabrication of Pore-Selective Metal-Nanoparticle-Functionalized Honeycomb Films via the Breath Figure Accompanied by In Situ Reduction. Polymers 2021, 13, 316. [Google Scholar] [CrossRef]
- Modigunta, J.K.R.; Male, U.; Huh, D.S. Formylated polystyrene for the fabrication of pore selective aldehyde group functionalized honeycomb patterned porous polystyrene films. J. Polym. Sci. B Polym. Phys. 2018, 56, 1181–1192. [Google Scholar] [CrossRef]
- Tolaymat, T.M.; El Badawy, A.M.; Genaidy, A.; Scheckel, K.G.; Luxton, T.P.; Suidan, M. An evidence-based environmental perspective of manufactured silver nanoparticle in syntheses and applications: A systematic review and critical appraisal of peer-reviewed scientific papers. Sci. Total Environ. 2010, 408, 999–1006. [Google Scholar] [CrossRef] [Green Version]
- Cao, T.T.; Yabu, H.; Huh, D.S. Flower-like ordered porous array by combination of breath figure and layer-by-layer technique. Polymer 2021, 233, 124206. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X. Facile preparation of honeycomb-structured TiO2 nanofilm via breath figures assembly and coffee ring effect. Mater. Lett. 2018, 227, 74–77. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Wang, Y.; Yang, X.; Zhao, N.; Zhang, X.; Xu, J. A Bottom-Up Approach To Fabricate Patterned Surfaces with Asymmetrical TiO2 Microparticles Trapped in the Holes of Honeycomblike Polymer Film. J. Am. Chem. Soc. 2011, 133, 3736–3739. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Pan, G.-B.; Zhang, M.; Li, F. Micro-patterned composite films with bowl-like SnO2 microparticles. Mater. Lett. 2013, 92, 284–286. [Google Scholar] [CrossRef]
- Shin, B.K.; Male, U.; Huh, D.S. In-situ pore filling of TiO2 nanoparticles in honeycomb patterned porous films: A modified breath figure method. Polymer 2018, 135, 1–8. [Google Scholar] [CrossRef]
- Uyen Thi, P.N.; Male, U.; Huh, D.S. Fabrication of photo-responsive moth eye-like patterned poly (vinyl alcohol) films selectively containing TiO2 nanoparticles in the microdome. Polymer 2018, 144, 103–110. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Zhang, L.; Tan, S.; Yu, X.; Zhao, N.; Chen, G.; Xu, J. Fabrication of honeycomb-patterned polyalkylcyanoacrylate films from monomer solution by breath figures method. J. Colloid Interface Sci. 2010, 350, 253–259. [Google Scholar] [CrossRef]
- Li, H.; Jia, Y.; Du, M.; Fei, J.; Zhao, J.; Cui, Y.; Li, J. Self-Organization of Honeycomb-like Porous TiO2 Films by means of the Breath-Figure Method for Surface Modification of Titanium Implants. Chem. Eur. J. 2013, 19, 5306–5313. [Google Scholar] [CrossRef]
- Zhao, H.; Shen, Y.; Zhang, S.; Zhang, H. A vapor phase hydrothermal modification method converting a honeycomb structured hybrid film into photoactive TiO2 film. Langmuir 2009, 25, 11032–11037. [Google Scholar] [CrossRef]
- Cao, T.T.; Modigunta, J.K.R.; Male, U.; Huh, D.S. Pore-Selective SnS Functionalization in Honeycomb-Patterned Films by a Breath Figure Process Accompanied by Chemical Reaction. Adv. Mater. Interfaces 2018, 5, 1801174. [Google Scholar] [CrossRef]
- Male, U.; Shin, B.K.; Huh, D.S. Polyaniline decorated honeycomb-patterned pores: Use of a reactive vapor in breath figure method. Polymer 2017, 121, 149–154. [Google Scholar] [CrossRef]
- Cao, T.T.; Male, U.; Huh, D.S. Fabrication of pore-selective carboxyl group functionalized polyimide honeycomb-patterned porous films using KOH humidity. Polymer 2018, 153, 86–94. [Google Scholar] [CrossRef]
- Modigunta, J.K.R.; Kim, J.M.; Cao, T.T.; Yabu, H.; Huh, D.S. Pore-selective modification of the honeycomb-patterned porous polystyrene film with poly(N-isopropylacrylamide) and application for thermo-responsive smart material. Polymer 2020, 201, 122630. [Google Scholar] [CrossRef]
- Falak, S.; Shin, B.K.; Huh, D.S. Single-Step Pore-Selective Silver-Functionalized Honeycomb-Patterned Porous Polystyrene Film Using a Modified Breath Figure Method. Macromol. Res. 2021, 29, 519–523. [Google Scholar] [CrossRef]
- Cao, T.T.; Uyen Thi, P.N.; Male, U.; Huh, D.S. Selective Coating of SnS on the Bio-Inspired Moth-Eye Patterned PEDOT: PSS Polymer Films. Macromol. Mater. Eng. 2019, 304, 1800727. [Google Scholar] [CrossRef]
- Kim, J.M.; Falak, S.; Huh, D.S. Thermo-responsive release of rhodamine B in the pore-selective poly(N-isopropylacrylamide) immobilized honeycomb-patterned porous film. Polym. Bull. 2021, 79, 1911–1928. [Google Scholar] [CrossRef]
- De León, A.S.; Molina, M.; Wedepohl, S.; Muñoz-Bonilla, A.; Rodríguez-Hernández, J.; Calderón, M. Immobilization of Stimuli-Responsive Nanogels onto Honeycomb Porous Surfaces and Controlled Release of Proteins. Langmuir 2016, 32, 1854–1862. [Google Scholar] [CrossRef]
- De León, A.S.; del Campo, A.; Rodríguez-Hernández, J.; Muñoz-Bonilla, A. Switchable and pH responsive porous surfaces based on polypeptide-based block copolymers. Mater. Des. 2017, 131, 121–126. [Google Scholar] [CrossRef]
- Jiang, T.; Moghaddam, S.Z.; Thormann, E. A pH-responsive polyelectrolyte multilayer film with tunable interfacial properties. Polymer 2021, 214, 123367. [Google Scholar] [CrossRef]
- Falak, S.; Shin, B.K.; Yabu, H.; Huh, D.S. Fabrication and characterization of pore-selective silver-functionalized honeycomb-patterned porous film and its application for antibacterial activity. Polymer 2022, 244, 124646. [Google Scholar] [CrossRef]
- Luo, T.; Bai, H.; Li, L. Breath figure in reactive vapor: A new route to nanopore array. Langmuir 2017, 33, 347–352. [Google Scholar] [CrossRef]
- Chen, S.; Gao, S.; Jing, J.; Lu, Q. Designing 3D Biological Surfaces via the Breath-Figure Method. Adv. Healthc. Mater. 2018, 7, 1701043. [Google Scholar] [CrossRef]
- Cao, T.T.; Yabu, H.; Huh, D.S. Hierarchical Nano/Micro Moth Eyelike Polymer Film Using Solid/Liquid Interfacial Reaction at Room Temperature. Langmuir 2020, 36, 9064–9073. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Shen, X.; Liu, X.; Chen, Z.; Shu, B.; Wan, L.; Liu, H.; He, J. Hybrid breath figure method: A new insight in Petri dishes for cell culture. J. Colloid Interface Sci. 2019, 541, 114–122. [Google Scholar] [CrossRef] [PubMed]
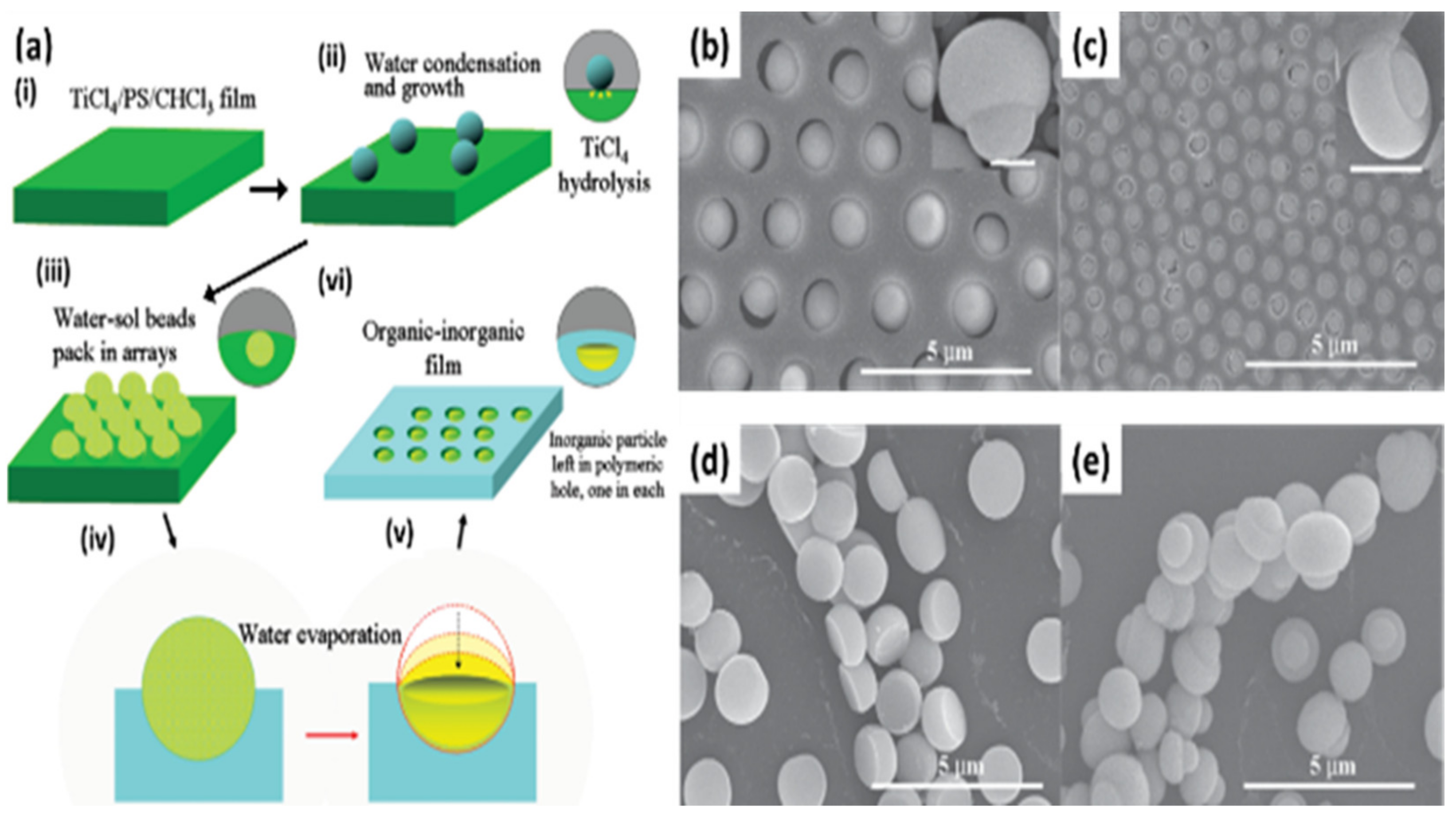
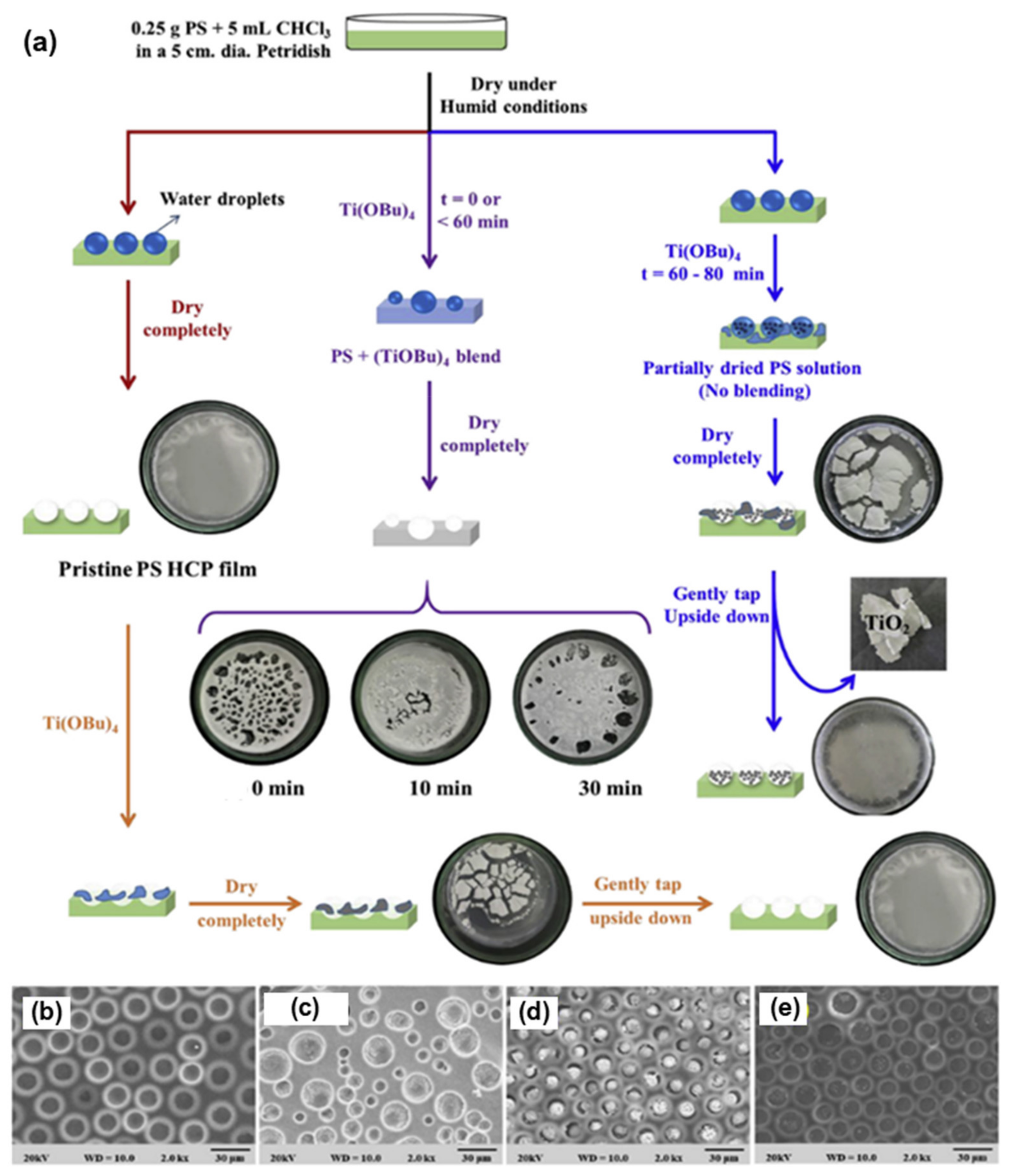
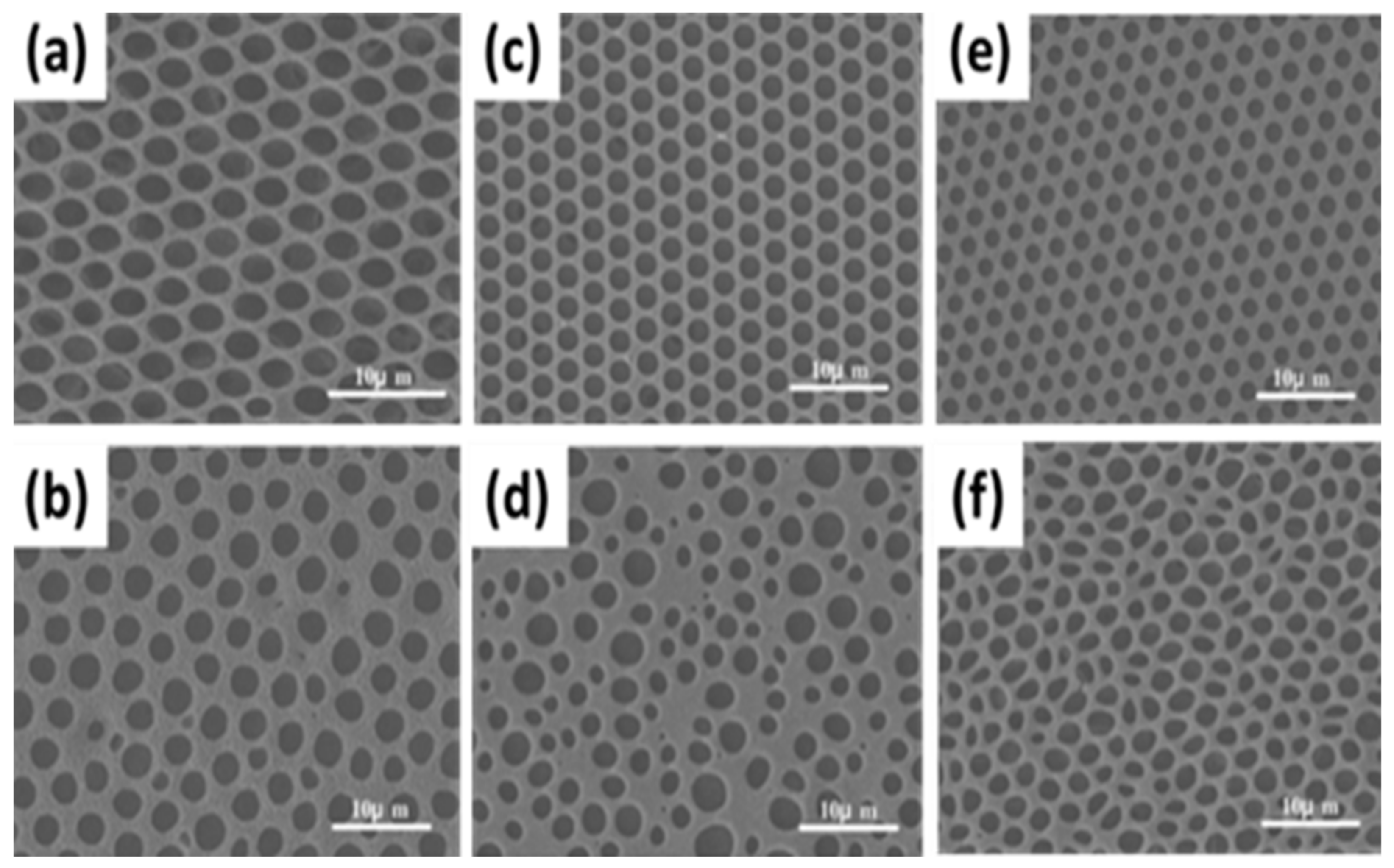
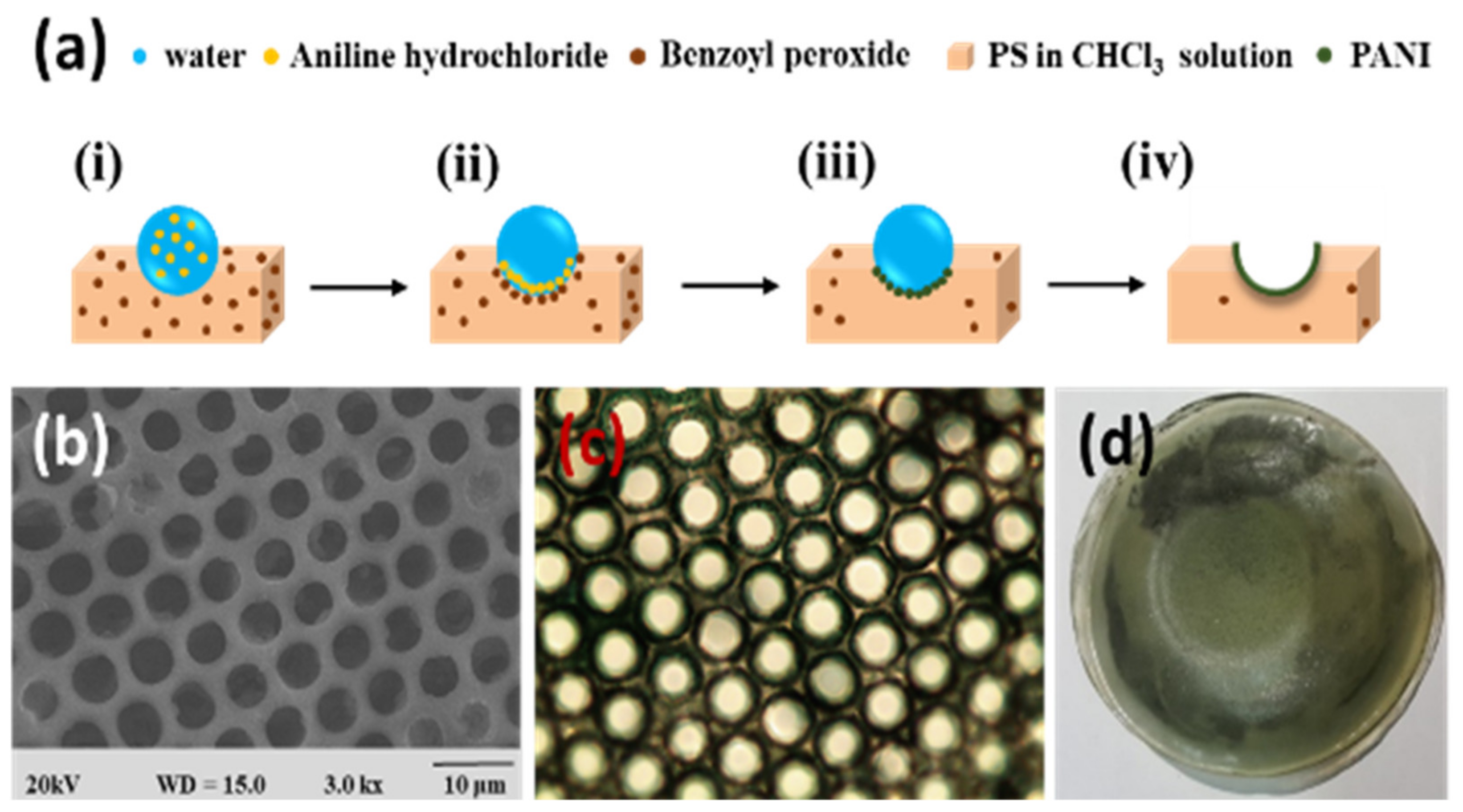
| Reactant A | Treatment | Product at Pore | Ref. |
|---|---|---|---|
| HAuCl4, AgNO3, and CuCl2 | NaBH4 | Au, Ag, and Cu NPs | [97] |
| PS-CHO | Tollen’s Reagent | Ag | [98] |
| KAuCl4 | Heat | Au NPs | [70] |
| Reactant A in the Polymer Solution | Humid Conditions | Product/Functionalization in Pore | Ref. |
|---|---|---|---|
| Titanium tetrachloride | Water | Titanium dioxide | [101] |
| Titanium tetrachloride | Water | Titanium dioxide | [102] |
| Tin tetrachloride | Water | Tin dioxide | [103] |
| Titanium butoxide | Water | Titanium dioxide NPs | [104] |
| Titanium n-butoxide | Water | Titanium dioxide | [107] |
| Titanium tetraisopropoxide | Water | Titanium dioxide | [108] |
| Alkylcyanoacrylate | Water | PACA | [106] |
| Reactant A in the Polymer Solution | Reactant B in Humid Conditions | Functionalization in Pore (Product C) | Ref. |
|---|---|---|---|
| Poly (4-vinylpyridine) | Formic acid | PVP-FA precipitate | [120] |
| Benzoyl peroxide | Aniline | Polyaniline | [110] |
| Tin dichloride | Hydrogen sulfide | Tin sulfide | [109] |
| Polyimide | Potassium hydroxide | Carboxylic group | [111] |
| PS aldehyde (PS-CHO) | Oxone | Carboxylic acid (–COOH) | [112] |
| Ferrocene | Silver Nitrate | Silver | [119] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falak, S.; Shin, B.; Huh, D. Modified Breath Figure Methods for the Pore-Selective Functionalization of Honeycomb-Patterned Porous Polymer Films. Nanomaterials 2022, 12, 1055. https://doi.org/10.3390/nano12071055
Falak S, Shin B, Huh D. Modified Breath Figure Methods for the Pore-Selective Functionalization of Honeycomb-Patterned Porous Polymer Films. Nanomaterials. 2022; 12(7):1055. https://doi.org/10.3390/nano12071055
Chicago/Turabian StyleFalak, Shahkar, Bokyoung Shin, and Dosung Huh. 2022. "Modified Breath Figure Methods for the Pore-Selective Functionalization of Honeycomb-Patterned Porous Polymer Films" Nanomaterials 12, no. 7: 1055. https://doi.org/10.3390/nano12071055
APA StyleFalak, S., Shin, B., & Huh, D. (2022). Modified Breath Figure Methods for the Pore-Selective Functionalization of Honeycomb-Patterned Porous Polymer Films. Nanomaterials, 12(7), 1055. https://doi.org/10.3390/nano12071055






