Scalable Production of High-Quality Silver Nanowires via Continuous-Flow Droplet Synthesis
Abstract
:1. Introduction
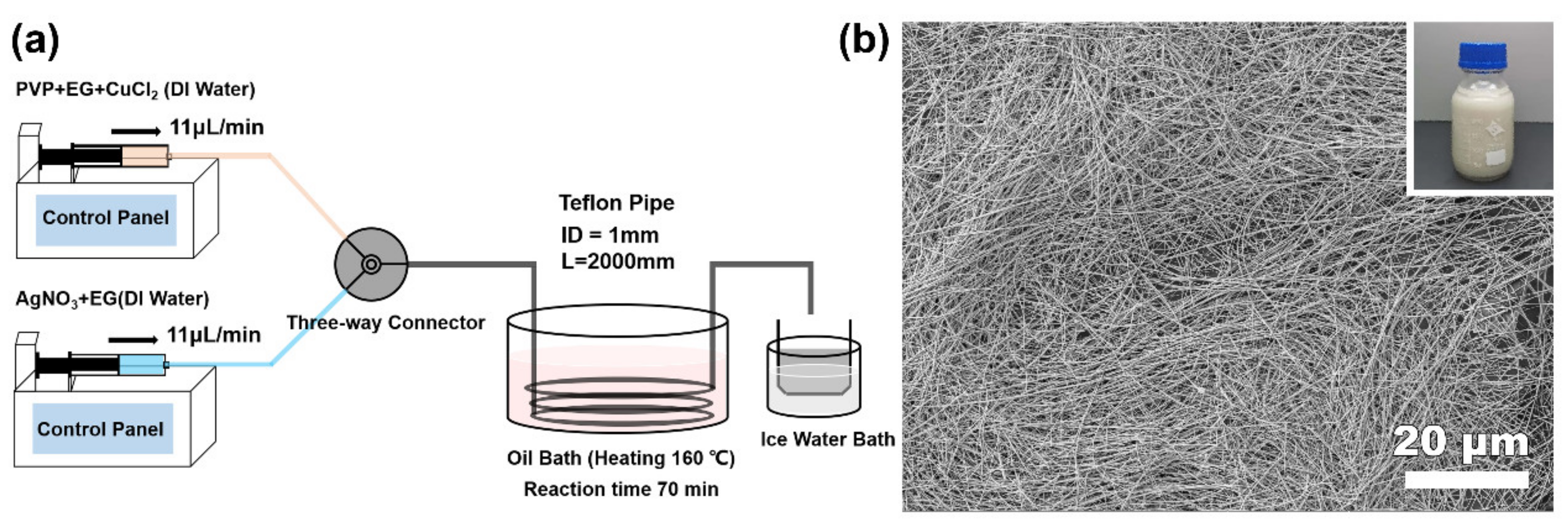
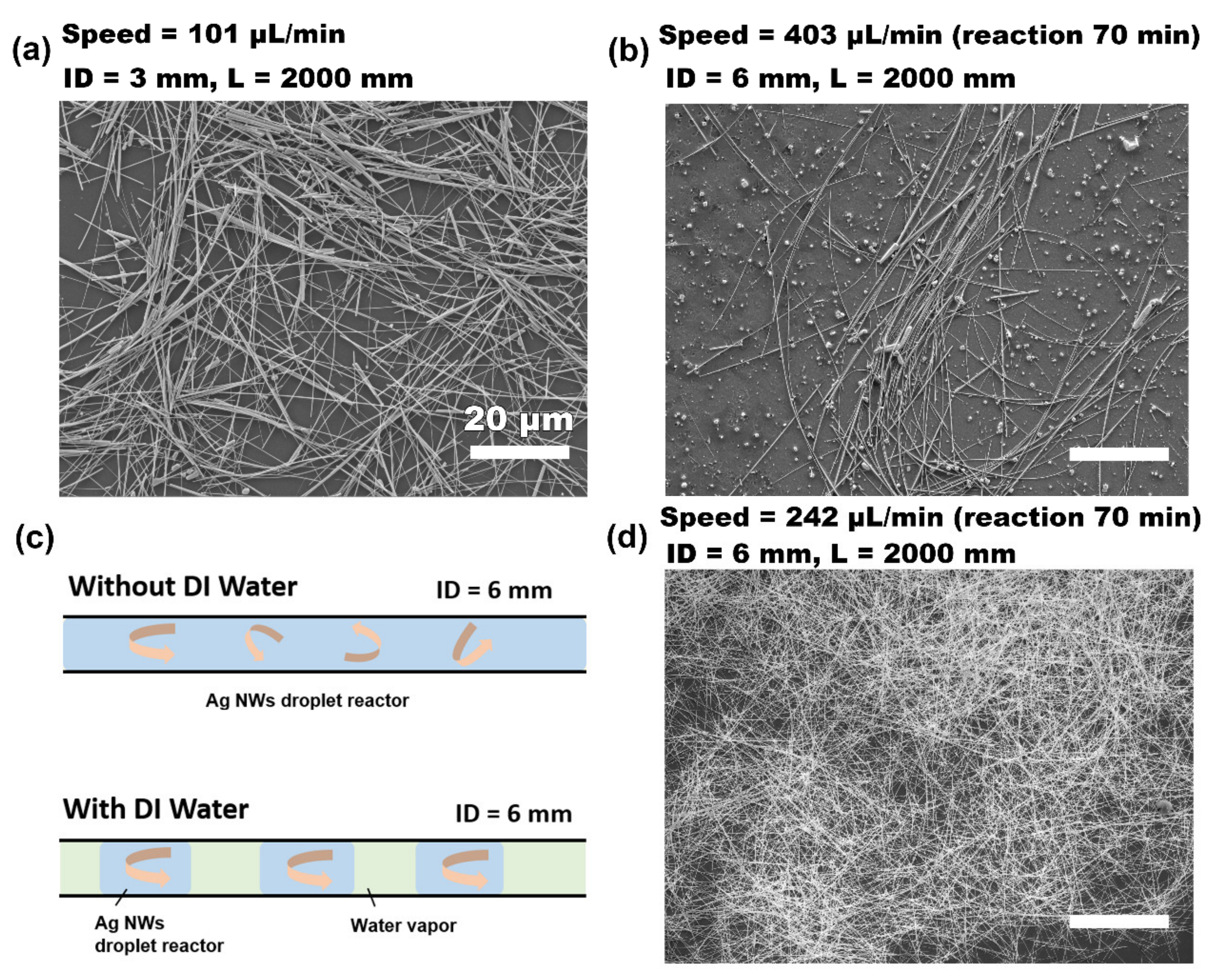
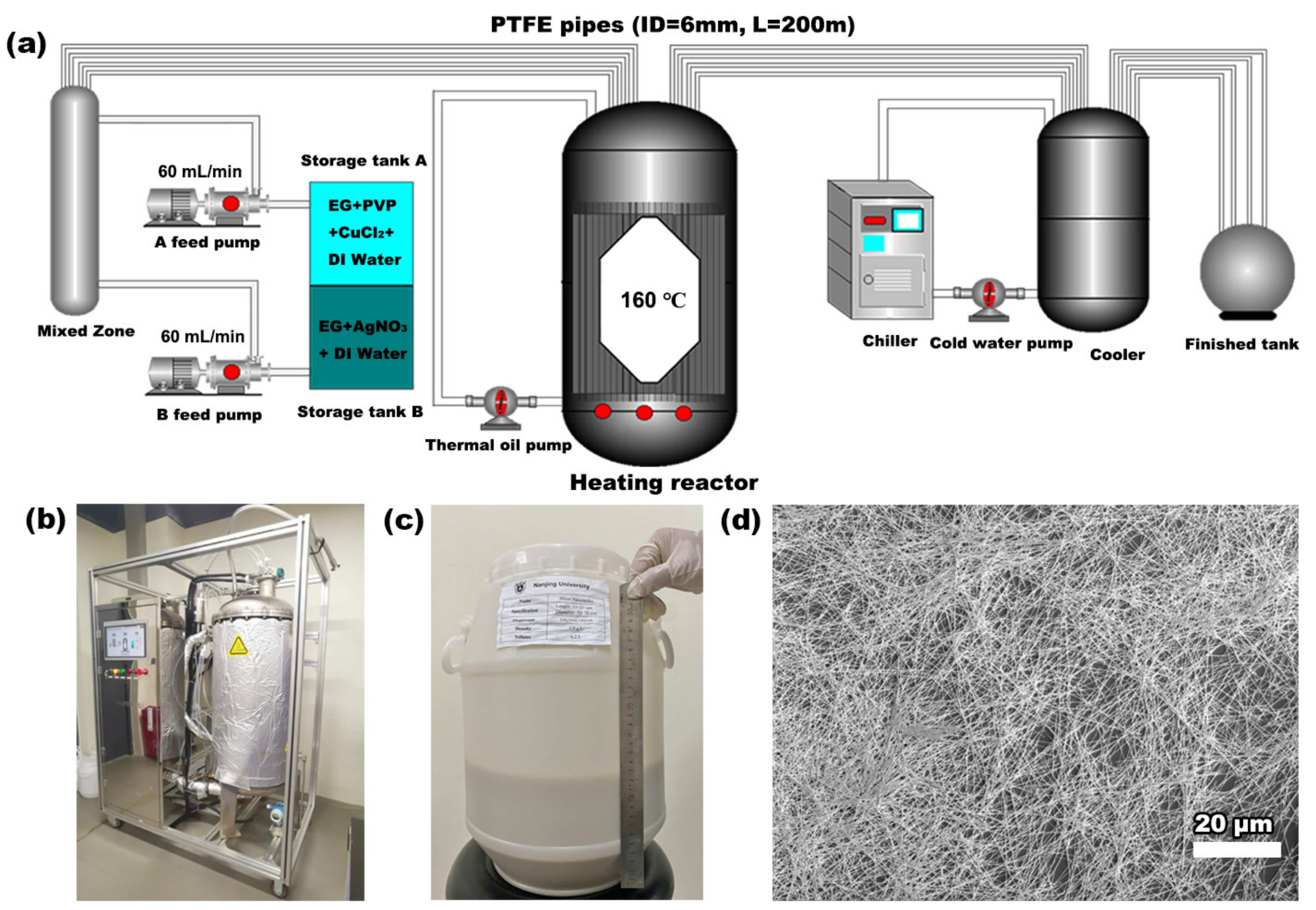
2. Experimental Section
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoang, P.H.; Park, H.; Kim, D.-P. Ultrafast and Continuous Synthesis of Unaccommodating Inorganic Nanomaterials in Droplet- and Ionic Liquid-Assisted Microfluidic System. J. Am. Chem. Soc. 2011, 133, 14765–14770. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, Z.; Zhu, M.; Lu, Y. Polyacrylic Acid Assisted Assembly of Oxide Particles and Carbon Nanotubes for High-Performance Flexible Battery Anodes. Adv. Energy Mater. 2014, 5, 1401207. [Google Scholar] [CrossRef]
- Epps, R.W.; Felton, K.C.; Coley, C.W.; Abolhasani, M. Automated microfluidic platform for systematic studies of colloidal perovskite nanocrystals: Towards continuous nano-manufacturing. Lab Chip 2017, 17, 4040–4047. [Google Scholar] [CrossRef] [PubMed]
- Chen, O.; Riedemann, L.; Etoc, F.; Herrmann, H.; Coppey, M.; Barch, M.; Farrar, C.; Zhao, J.; Bruns, O.; Wei, H.; et al. Magneto-fluorescent core-shell supernanoparticles. Nat. Commun. 2014, 5, 5093. [Google Scholar] [CrossRef] [Green Version]
- Choi, A.; Seo, K.D.; Kim, B.C.; Kim, D.S. Recent advances in engineering microparticles and their nascent uti-lization in biomedical delivery and diagnostic applications. Lab Chip 2017, 17, 591–613. [Google Scholar] [CrossRef]
- Enayati, M.; Chang, M.W.; Bragman, F.; Edirisinghe, M.; Stride, E. Electrohydrodynamic preparation of particles, capsules and bubbles for biomedical engineering applications. Colloids Surf. A Physicochem. Eng. Asp. 2011, 382, 154–164. [Google Scholar] [CrossRef]
- Zhao, C.-X.; He, L.; Qiao, S.Z.; Middelberg, A.P. Nanoparticle synthesis in microreactors. Chem. Eng. Sci. 2011, 66, 1463–1479. [Google Scholar] [CrossRef]
- Sui, J.; Yan, J.; Liu, D.; Wang, K.; Luo, G. Continuous Synthesis of Nanocrystals via Flow Chemistry Technology. Small 2019, 16, e1902828. [Google Scholar] [CrossRef]
- Jana, N.R. Gram-Scale Synthesis of Soluble, Near-Monodisperse Gold Nanorods and Other Anisotropic Nanoparticles. Small 2005, 1, 875–882. [Google Scholar] [CrossRef]
- Phillips, T.W.; Lignos, I.G.; Maceiczyk, R.M.; Demello, A.J.; Demello, J.C. Nanocrystal synthesis in microfluidic reactors: Where next? Lab Chip 2014, 14, 3172–3180. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Xia, Y. Scaling up the production of colloidal nanocrystals: Should we increase or decrease the reaction volume? Adv. Mater. 2014, 26, 2600–2606. [Google Scholar] [CrossRef]
- Lohse, S.E.; Eller, J.R.; Sivapalan, S.T.; Plews, M.R.; Murphy, C.J. A Simple Millifluidic Benchtop Reactor System for the High-Throughput Synthesis and Functionalization of Gold Nanoparticles with Different Sizes and Shapes. ACS Nano 2013, 7, 4135–4150. [Google Scholar] [CrossRef]
- Zhang, L.; Niu, G.; Lu, N.; Wang, J.; Tong, L.; Wang, L.; Kim, M.J.; Xia, Y. Continuous and Scalable Production of Well-Controlled Noble-Metal Nanocrystals in Milliliter-Sized Droplet Reactors. Nano Lett. 2014, 14, 6626–6631. [Google Scholar] [CrossRef]
- Fischer, C.-H.; Giersig, M. Colloidal CdS preparation via flow techniques: Ultrasmall particles and the effect of a chromatoraphic column. Langmuir 1992, 8, 1475–1478. [Google Scholar] [CrossRef]
- Abou-Hassan, A.; Sandre, O.; Cabuil, V. Microfluidics in inorganic chemistry. Angew. Chem. Int. Ed. 2010, 49, 6268–6286. [Google Scholar] [CrossRef] [Green Version]
- Nightingale, A.M.; de Mello, J.C. Microscale synthesis of quantum dots. J. Mater. Chem. 2010, 20, 8454. [Google Scholar] [CrossRef]
- Akwi, F.M.; Watts, P. Continuous flow chemistry: Where are we now? Recent applications, challenges and limitations. Chem. Commun. 2018, 54, 13894–13928. [Google Scholar] [CrossRef]
- Hao, N.; Nie, Y.; Zhang, J.X.J. Microfluidic synthesis of functional inorganic micro-/nanoparticles and applications in biomedical engineering. Int. Mater. Rev. 2018, 63, 461–487. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y. Microfluidic Synthesis of Nanohybrids. Small 2017, 13, 1604084. [Google Scholar] [CrossRef]
- Huang, J.; Lin, L.; Li, Q.; Sun, D.; Wang, Y.; Lu, Y.; He, N.; Yang, K.; Yang, X.; Wang, H.; et al. Continuous-flow biosynthesis of silver nanoparticles by lixivium of sundried cinnamomum camphora Leaf in tubular microreactors. Ind. Eng. Chem. Res. 2008, 47, 6081–6090. [Google Scholar] [CrossRef]
- Nightingale, A.M.; Bannock, J.H.; Krishnadasan, S.H.; O’Mahony, F.T.F.; Haque, S.A.; Sloan, J.; Drury, C.; McIntyre, R.; Demello, J.C. Large-scale synthesis of nanocrystals in a multichannel droplet reactor. J. Mater. Chem. A 2013, 1, 4067–4076. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Chen, H.M.; Liu, C.; Andrews, S.C.; Hahn, C.; Yang, P. Large-scale synthesis of transition-metal-doped TiO2 nan-owires with controllable overpotential. J. Am. Chem. Soc. 2013, 135, 9995–9998. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Jing, Y.; Wang, K.; Wang, Y.; Luo, G. Reaction study of alpha-phase NaYF4:Yb,Er generation via a tubular micro-reactor: Discovery of an efficient synthesis strategy. Nanoscale 2019, 11, 8363–8371. [Google Scholar] [CrossRef] [PubMed]
- Ahrberg, C.D.; Choi, J.; Chung, B.G. Automated droplet reactor for the synthesis of iron oxide/gold core-shell nanoparticles. Sci. Rep. 2020, 10, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Zhou, M.; Yang, X.; Park, J.; Lu, N.; Wang, J.; Kim, M.J.; Wang, L.; Xia, Y. Synthesis of Pt-Ni octahedra in continuous-flow droplet reactors for the scalable production of highly active catalysts toward oxygen Reduction. Nano Lett. 2016, 16, 3850–3857. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Zhang, L.; Ruditskiy, A.; Wang, L.; Xia, Y. A Droplet-Reactor System Capable of Automation for the Continuous and Scalable Production of Noble-Metal Nanocrystals. Nano Lett. 2018, 18, 3879–3884. [Google Scholar] [CrossRef]
- Chou, K.-S.; Hsu, C.-Y.; Liu, B.-T. Salt-mediated polyol synthesis of silver nanowires in a continuous-flow tubular reactor. RSC Adv. 2015, 5, 29872–29877. [Google Scholar] [CrossRef]
- Yun, H.D.; Seo, D.M.; Lee, M.Y.; Kwon, S.Y.; Park, L.S. Effective synthesis and recovery of silver nanowires prepared by tapered con-tinuous flow reactor for flexible and transparent conducting electrode. Metals 2016, 6, 14. [Google Scholar] [CrossRef] [Green Version]
- Gottesman, R.; Tangy, A.; Oussadon, I.; Zitoun, D. Silver nanowires and nanoparticles from a millifluidic reactor: Application to metal assisted silicon etching. New J. Chem. 2012, 36, 2456. [Google Scholar] [CrossRef]
- Nightingale, A.M.; Krishnadasan, S.H.; Berhanu, D.; Niu, X.; Drury, C.; McIntyre, R.; Valsami-Jones, E.; Demello, J.C. A stable droplet reactor for high temperature nanocrystal synthesis. Lab Chip 2010, 11, 1221–1227. [Google Scholar] [CrossRef]
- Sebastian, V.; Smith, C.D.; Jensen, K.F. Shape-controlled continuous synthesis of metal nanostructures. Nanoscale 2016, 8, 7534–7543. [Google Scholar] [CrossRef] [Green Version]
- Niu, G.; Ruditskiy, A.; Vara, M.; Xia, Y. Toward continuous and scalable production of colloidal nanocrystals by switching from batch to droplet reactors. Chem. Soc. Rev. 2015, 44, 5806–5820. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Connor, S.T.; Cui, A.Y.; Peumans, P. Solution-Processed Metal Nanowire Mesh Transparent Electrodes. Nano Lett. 2008, 8, 689–692. [Google Scholar] [CrossRef]
- Sun, Y.; Gates, B.; Mayers, B.; Xia, Y. Crystalline Silver Nanowires by Soft Solution Processing. Nano. Lett. 2002, 2, 165–168. [Google Scholar] [CrossRef]
- Yugang, S.; Younan, X. Large-scale synthesis of uniform silver nanowires through a soft, self-seeding, polyol process. Adv. Mater. 2002, 14, 833–837. [Google Scholar]
- Korte, K.E.; Skrabalak, S.E.; Xia, Y. Rapid synthesis of silver nanowires through a CuCl- or CuCl2-mediated polyol process. J. Mater. Chem. 2007, 18, 437–441. [Google Scholar] [CrossRef]
- Chambers, R.D.; Fox, M.A.; Holling, D.; Nakano, T.; Okazoe, T.; Sandford, G. Elemental fluorine. Part 16. Versatile thin-film gas-liquid multi-channel microreactors for effective scale-out. Lab Chip 2005, 5, 191–198. [Google Scholar] [CrossRef]
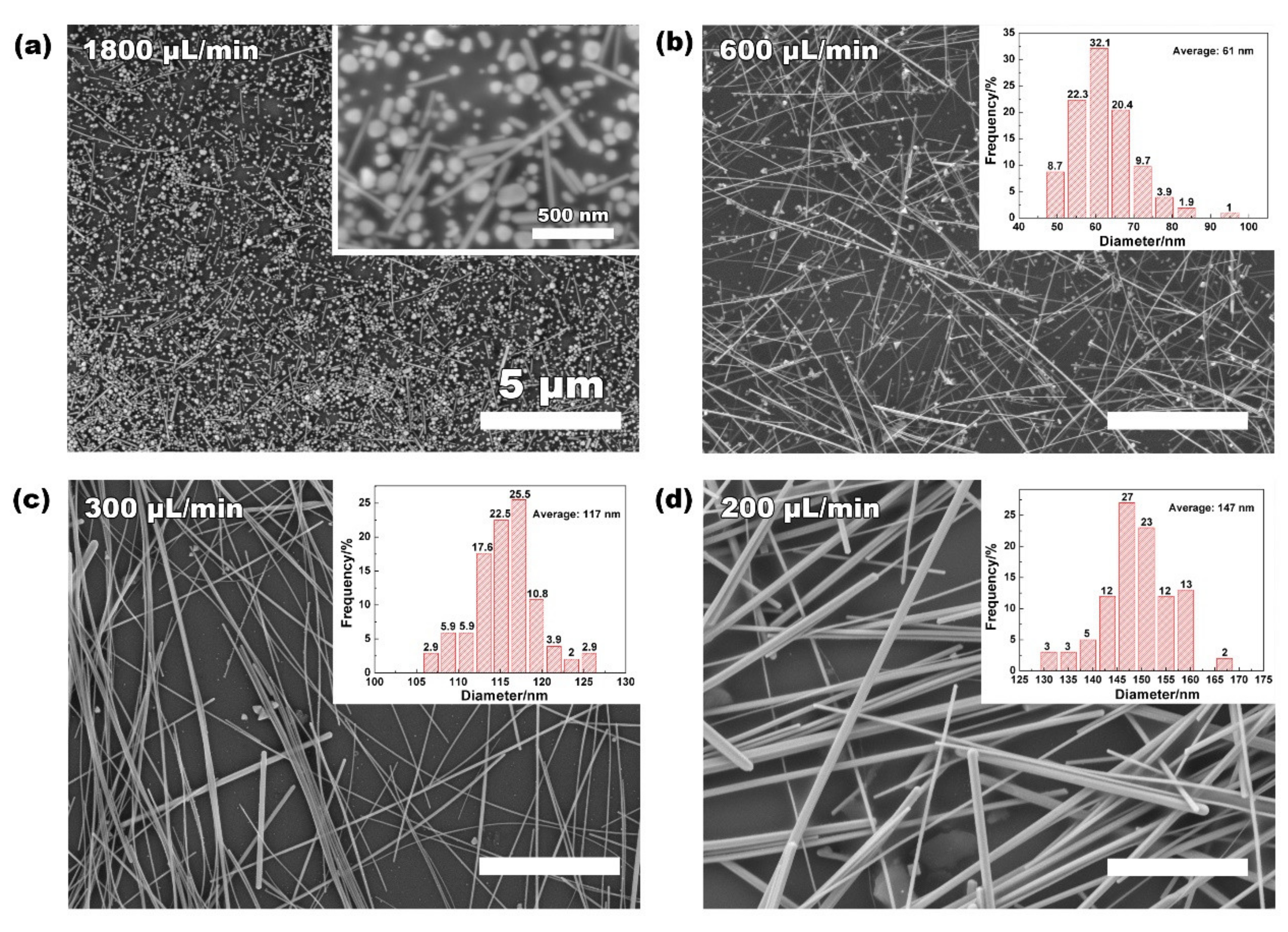
| Flow Rate (uL/min) | Reaction Residence (min) | L (um) | D (nm) | Aspect–Ratio (L/D) |
|---|---|---|---|---|
| 1800 | 10 | * | * | * |
| 600 | 30 | 28 ± 5 | 61 ± 10 | 459 |
| 300 | 60 | 47 ± 3 | 117 ± 10 | 402 |
| 200 | 90 | 51 ± 5 | 147 ± 43 | 347 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Yang, L.; Jiang, J.; Dong, X.; Cui, Z.; Wang, C.; Lu, Z. Scalable Production of High-Quality Silver Nanowires via Continuous-Flow Droplet Synthesis. Nanomaterials 2022, 12, 1018. https://doi.org/10.3390/nano12061018
Yu J, Yang L, Jiang J, Dong X, Cui Z, Wang C, Lu Z. Scalable Production of High-Quality Silver Nanowires via Continuous-Flow Droplet Synthesis. Nanomaterials. 2022; 12(6):1018. https://doi.org/10.3390/nano12061018
Chicago/Turabian StyleYu, Jianming, Lijie Yang, Jing Jiang, Xunyi Dong, Zhiyang Cui, Chao Wang, and Zhenda Lu. 2022. "Scalable Production of High-Quality Silver Nanowires via Continuous-Flow Droplet Synthesis" Nanomaterials 12, no. 6: 1018. https://doi.org/10.3390/nano12061018
APA StyleYu, J., Yang, L., Jiang, J., Dong, X., Cui, Z., Wang, C., & Lu, Z. (2022). Scalable Production of High-Quality Silver Nanowires via Continuous-Flow Droplet Synthesis. Nanomaterials, 12(6), 1018. https://doi.org/10.3390/nano12061018







