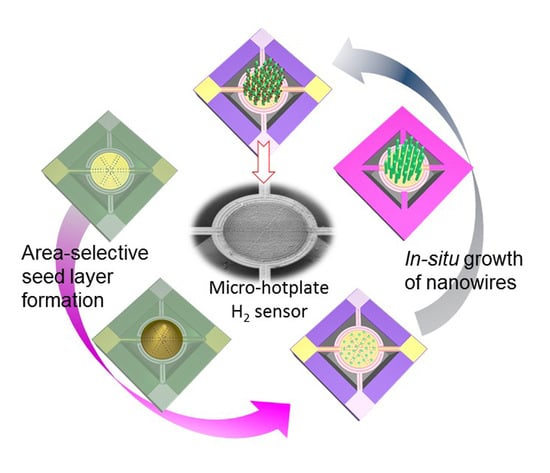
Area-Selective, In-Situ Growth of Pd-Modified ZnO Nanowires on MEMS Hydrogen Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Fabrication of Micro-Hotplate with Patterned Hydrophobic Layer
2.3. In-Situ Preparation of Pd-Modified ZnO Nanowires on Sensor Chip
2.4. Material Characterization
2.5. Temperature Calibration and Gas Sensing Measurement Setup
3. Results and Discussions
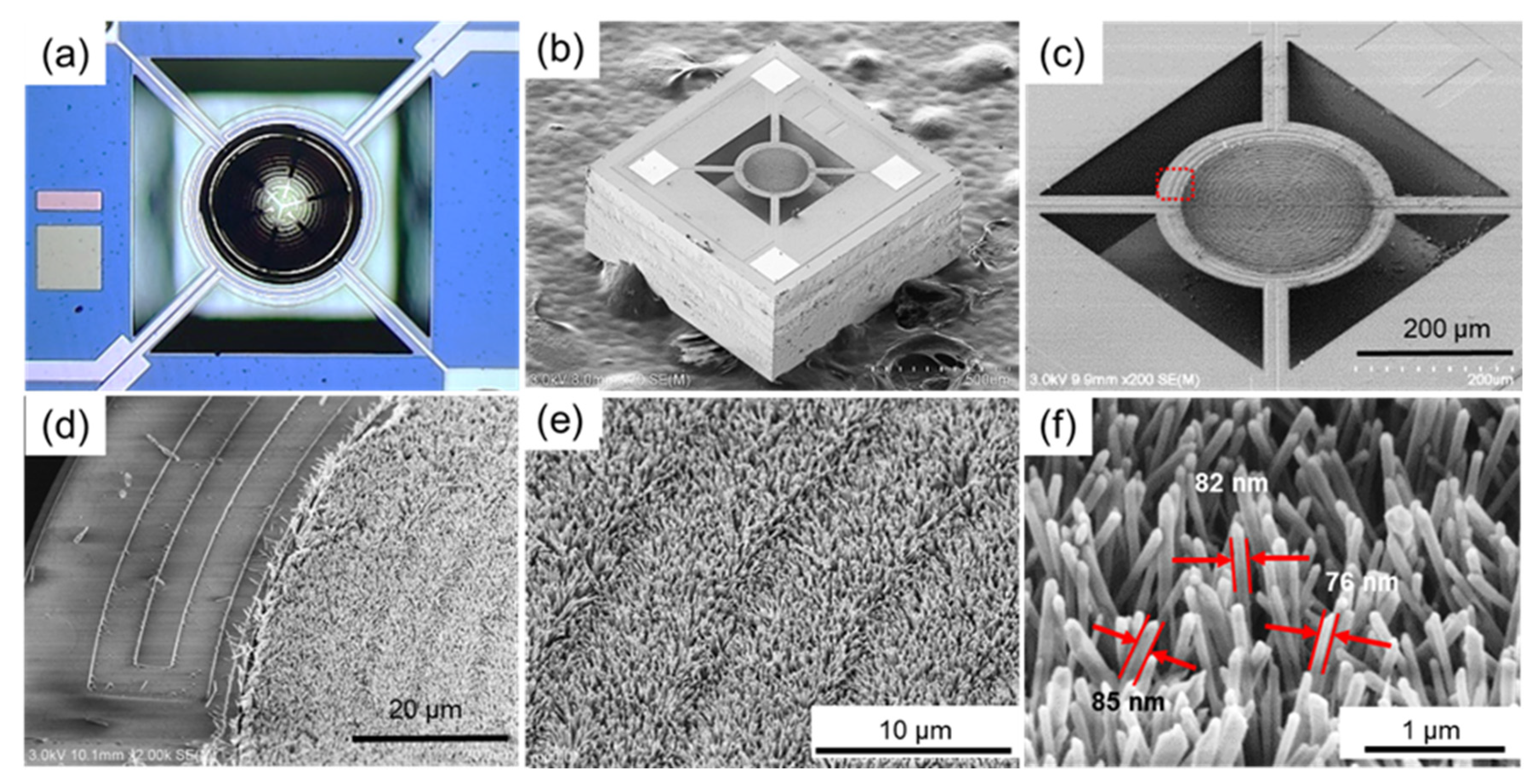
3.1. Micro-Hotplate Sensors
3.2. In-Situ Growth of Pd-Modified ZnO NWs on Micro-Hotplate Chip
3.3. Characterization of Pd-Modified ZnO Nanowires
3.4. Gas Sensing Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bai, J.; Zhou, B. Titanium Dioxide Nanomaterials for Sensor Applications. Chem. Rev. 2014, 114, 10131–10176. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xue, Z.; Chen, X.; Huang, C.; Bai, W.; Lu, Z.; Wang, T. Nanomaterial-based gas sensors used for breath diagnosis. J. Mater. Chem. B 2020, 8, 3231–3248. [Google Scholar] [CrossRef] [PubMed]
- Llobet, E. Gas sensors using carbon nanomaterials: A review. Sens. Actuators B Chem. 2013, 179, 32–45. [Google Scholar] [CrossRef]
- Korotcenkov, G. Current Trends in Nanomaterials for Metal Oxide-Based Conductometric Gas Sensors: Advantages and Limitations. Part 1: 1D and 2D Nanostructures. Nanomaterials 2020, 10, 1392. [Google Scholar] [CrossRef] [PubMed]
- Yamazoe, N.; Shimanoe, K. New perspectives of gas sensor technology. Sens. Actuators B Chem. 2009, 138, 100–107. [Google Scholar] [CrossRef]
- Hsueh, T.-J.; Lu, C.-L. A hybrid YSZ/SnO2/MEMS SO2 gas sensor. RSC Adv. 2019, 9, 27800–27806. [Google Scholar] [CrossRef] [Green Version]
- Asri, M.I.A.; Hasan, M.N.; Fuaad, M.R.A.; Yunos, Y.M.; Ali, M.S.M. MEMS Gas Sensors: A Review. IEEE Sens. J. 2021, 21, 18381–18397. [Google Scholar] [CrossRef]
- Kumar, N.; Haviar, S.; Zeman, P. Three-Layer PdO/CuWO4/CuO System for Hydrogen Gas Sensing with Reduced Humidity Interference. Nanomaterials 2021, 11, 3456. [Google Scholar] [CrossRef]
- Xu, P.; Yu, H.; Li, X. In situ growth of noble metal nanoparticles on graphene oxide sheets and direct construction of functionalized porous-layered structure on gravimetric microsensors for chemical detection. Chem. Commun. 2012, 48, 10784–10786. [Google Scholar] [CrossRef]
- Tripathy, A.; Pramanik, S.; Cho, J.; Santhosh, J.; Abu Osman, N. Role of Morphological Structure, Doping, and Coating of Different Materials in the Sensing Characteristics of Humidity Sensors. Sensors 2014, 14, 16343–16422. [Google Scholar] [CrossRef]
- Chen, X.; Wong, C.K.Y.; Yuan, C.A.; Zhang, G. Nanowire-based gas sensors. Sens. Actuators B Chem. 2013, 177, 178–195. [Google Scholar] [CrossRef]
- Kim, J.H.; Seo, M.; Kim, S.Y. Lithographically Patterned Breath Figure of Photoresponsive Small Molecules: Dual-Patterned Honeycomb Lines from a Combination of Bottom-Up and Top-Down Lithography. Adv. Mater. 2009, 21, 4130–4133. [Google Scholar] [CrossRef]
- Karthik, J.; Damodaran, A.R.; Martin, L.W. Epitaxial Ferroelectric Heterostructures Fabricated by Selective Area Epitaxy of SrRuO3 Using an MgO Mask. Adv. Mater. 2012, 24, 1610–1615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virganavičius, D.; Cadarso, V.J.; Kirchner, R.; Stankevičius, L.; Tamulevičius, T.; Tamulevičius, S.; Schift, H. Patterning of diamond like carbon films for sensor applications using silicon containing thermoplastic resist (SiPol) as a hard mask. Appl. Surf. Sci. 2016, 385, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Barborini, E.; Vinati, S.; Leccardi, M.; Repetto, P.; Bertolini, G.; Rorato, O.; Lorenzelli, L.; Decarli, M.; Guarnieri, V.; Ducati, C.; et al. Batch fabrication of metal oxide sensors on micro-hotplates. J. Micromech. Microeng. 2008, 18, 055015. [Google Scholar] [CrossRef]
- Cui, S.; Dai, Z.; Tian, Q.; Liu, J.; Xiao, X.; Jiang, C.; Wu, W.; Roy, V.A.L. Wetting properties and SERS applications of ZnO/Ag nanowire arrays patterned by a screen printing method. J. Mater. Chem. C 2016, 4, 6371–6379. [Google Scholar] [CrossRef]
- Vincenzi, D.; Butturi, M.A.; Guidi, V.; Carotta, M.C.; Martinelli, G.; Guarnieri, V.; Brida, S.; Margesin, B.; Giacomozzi, F.; Zen, M.; et al. Development of a low-power thick-film gas sensor deposited by screen-printing technique onto a micromachined hotplate. Sens. Actuators B Chem. 2001, 77, 95–99. [Google Scholar] [CrossRef]
- Xu, J.; Strempel, K.; Zhou, H.; Waag, A.; Bertke, M.; Schmidt, A.; Peiner, E. Area-Selective Growth of Aligned ZnO Nanorod Arrays for MEMS Device Applications. Proceedings 2018, 2, 887. [Google Scholar]
- Lim, S.H.; Radha, B.; Chan, J.Y.; Saifullah, M.S.M.; Kulkarni, G.U.; Ho, G.W. Flexible Palladium-Based H2 Sensor with Fast Response and Low Leakage Detection by Nanoimprint Lithography. ACS Appl. Mater. Interfaces 2013, 5, 7274–7281. [Google Scholar] [CrossRef]
- Cai, Z.; Li, X.; Zeng, X. Direct fabrication of SnO2-based thick film gas sensor using Micropen direct writing and laser microcladding. Sens. Actuators B Chem. 2009, 137, 340–344. [Google Scholar] [CrossRef]
- Yoshimura, T.; Terasawa, N.; Kazama, H.; Naito, Y.; Suzuki, Y.; Asama, K. Selective growth of conjugated polymer thin films by the vapor deposition polymerization. Thin Solid Films 2006, 497, 182–184. [Google Scholar] [CrossRef]
- Fung, S.K.H.; Tang, Z.; Chan, P.C.H.; Sin, J.K.O.; Cheung, P.W. Thermal analysis and design of a micro-hotplate for integrated gas-sensor applications. Sens. Actuators A Phys. 1996, 54, 482–487. [Google Scholar] [CrossRef]
- Wu, H.; Yu, J.; Li, Z.; Yao, G.; Cao, R.; Li, X.; Zhu, H.; He, A.; Tang, Z. Microhotplate gas sensors incorporated with Al electrodes and 3D hierarchical structured PdO/PdO2-SnO2:Sb materials for sensitive VOC detection. Sens. Actuators B Chem. 2021, 329, 128984. [Google Scholar] [CrossRef]
- Lee, K.; Sahu, M.; Hajra, S.; Mohanta, K.; Kim, H.J. Effect of sintering temperature on the electrical and gas sensing properties of tin oxide powders. Ceram. Int. 2021, 47, 22794–22800. [Google Scholar] [CrossRef]
- Naisbitt, S.C.; Pratt, K.F.E.; Williams, D.E.; Parkin, I.P. A microstructural model of semiconducting gas sensor response: The effects of sintering temperature on the response of chromium titanate (CTO) to carbon monoxide. Sens. Actuators B Chem. 2006, 114, 969–977. [Google Scholar] [CrossRef]
- Delabie, L.; Honoré, M.; Lenaerts, S.; Huyberechts, G.; Roggen, J.; Maes, G. The effect of sintering and Pd-doping on the conversion of CO to CO2 on SnO2 gas sensor materials. Sens. Actuators B Chem. 1997, 44, 446–451. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Gao, Y.; Zheng, Z. Tunable Morphology and Ethanol-Sensing Performance by Sintering Temperature of WO3-Based Ceramics. Synth. React. Inorg. Met. Org. Nano Met. Chem. 2011, 41, 639–643. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, P.; Li, X.; Ren, Y.; Deng, Y. High-performance H2 sensors with selectively hydrophobic micro-plate for self-aligned upload of Pd nanodots modified mesoporous In2O3 sensing-material. Sens. Actuators B Chem. 2018, 267, 83–92. [Google Scholar] [CrossRef]
- Yamazaki, H.; Hayashi, Y.; Masunishi, K.; Ono, D.; Ikehashi, T. High sensitivity MEMS capacitive hydrogen sensor with inverted T-shaped electrode and ring-shaped palladium alloy for fast response and low power consumption. J. Micromech. Microeng. 2018, 28, 094001. [Google Scholar] [CrossRef]
- Luo, N.; Wang, C.; Zhang, D.; Guo, M.; Wang, X.; Cheng, Z.; Xu, J. Ultralow detection limit MEMS hydrogen sensor based on SnO2 with oxygen vacancies. Sens. Actuators B Chem. 2022, 354, 130982. [Google Scholar] [CrossRef]
- Vallejos, S.; Umek, P.; Stoycheva, T.; Annanouch, F.; Llobet, E.; Correig, X.; De Marco, P.; Bittencourt, C.; Blackman, C. Single-Step Deposition of Au- and Pt-Nanoparticle-Functionalized Tungsten Oxide Nanoneedles Synthesized Via Aerosol-Assisted CVD, and Used for Fabrication of Selective Gas Microsensor Arrays. Adv. Funct. Mater. 2013, 23, 1313–1322. [Google Scholar] [CrossRef]
- Cai, Z.; Park, S. Synthesis of Pd nanoparticle-decorated SnO2 nanowires and determination of the optimum quantity of Pd nanoparticles for highly sensitive and selective hydrogen gas sensor. Sens. Actuators B Chem. 2020, 322, 128651. [Google Scholar] [CrossRef]
- Lupan, O.; Postica, V.; Labat, F.; Ciofini, I.; Pauporté, T.; Adelung, R. Ultra-sensitive and selective hydrogen nanosensor with fast response at room temperature based on a single Pd/ZnO nanowire. Sens. Actuators B Chem. 2018, 254, 1259–1270. [Google Scholar] [CrossRef]
- Rashid, T.-R.; Phan, D.-T.; Chung, G.-S. Effect of Ga-modified layer on flexible hydrogen sensor using ZnO nanorods decorated by Pd catalysts. Sens. Actuators B Chem. 2014, 193, 869–876. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, P.; Zhang, P.; Li, X. Long-Term Stability Improvement of Micro-Hotplate Methane Sensor Product. In Proceedings of the 2020 IEEE 33rd International Conference on Micro Electro Mechanical Systems (MEMS), Vancouver, BC, Canada, 18–22 January 2020; pp. 1300–1303. [Google Scholar]
- Chen, C.; Xu, P.; Li, X. Regioselective Patterning of Multiple SAMs and Applications in Surface-Guided Smart Microfluidics. ACS Appl. Mater. Interfaces 2014, 6, 21961–21969. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, P.; Xu, T.; Zheng, D.; Li, X. ZnO-nanowire size effect induced ultra-high sensing response to ppb-level H2S. Sens. Actuators B Chem. 2017, 240, 264–272. [Google Scholar] [CrossRef]
- Zeng, J.; Zhu, C.; Tao, J.; Jin, M.; Zhang, H.; Li, Z.-Y.; Zhu, Y.; Xia, Y. Controlling the Nucleation and Growth of Silver on Palladium Nanocubes by Manipulating the Reaction Kinetics. Angew. Chem. Int. Ed. 2012, 51, 2354–2358. [Google Scholar] [CrossRef]
- Wang, X.; Yao, F.; Xu, P.; Li, M.; Yu, H.; Li, X. Quantitative Structure–Activity Relationship of Nanowire Adsorption to SO2 Revealed by In Situ TEM Technique. Nano Lett. 2021, 21, 1679–1687. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Zhang, T.; Chen, Y.; Xu, P.; Zheng, D.; Li, X. Area-Selective, In-Situ Growth of Pd-Modified ZnO Nanowires on MEMS Hydrogen Sensors. Nanomaterials 2022, 12, 1001. https://doi.org/10.3390/nano12061001
Hu J, Zhang T, Chen Y, Xu P, Zheng D, Li X. Area-Selective, In-Situ Growth of Pd-Modified ZnO Nanowires on MEMS Hydrogen Sensors. Nanomaterials. 2022; 12(6):1001. https://doi.org/10.3390/nano12061001
Chicago/Turabian StyleHu, Jiahao, Tao Zhang, Ying Chen, Pengcheng Xu, Dan Zheng, and Xinxin Li. 2022. "Area-Selective, In-Situ Growth of Pd-Modified ZnO Nanowires on MEMS Hydrogen Sensors" Nanomaterials 12, no. 6: 1001. https://doi.org/10.3390/nano12061001
APA StyleHu, J., Zhang, T., Chen, Y., Xu, P., Zheng, D., & Li, X. (2022). Area-Selective, In-Situ Growth of Pd-Modified ZnO Nanowires on MEMS Hydrogen Sensors. Nanomaterials, 12(6), 1001. https://doi.org/10.3390/nano12061001