Low-Cost Inorganic Strontium Ferrite a Novel Hole Transporting Material for Efficient Perovskite Solar Cells
Abstract
:1. Introduction
2. Experimental
2.1. Material and Methods
2.1.1. Materials
2.1.2. Preparation of Sol of SrFe2O4
2.1.3. Preparation of ZnO Sol
2.1.4. Device Fabrications
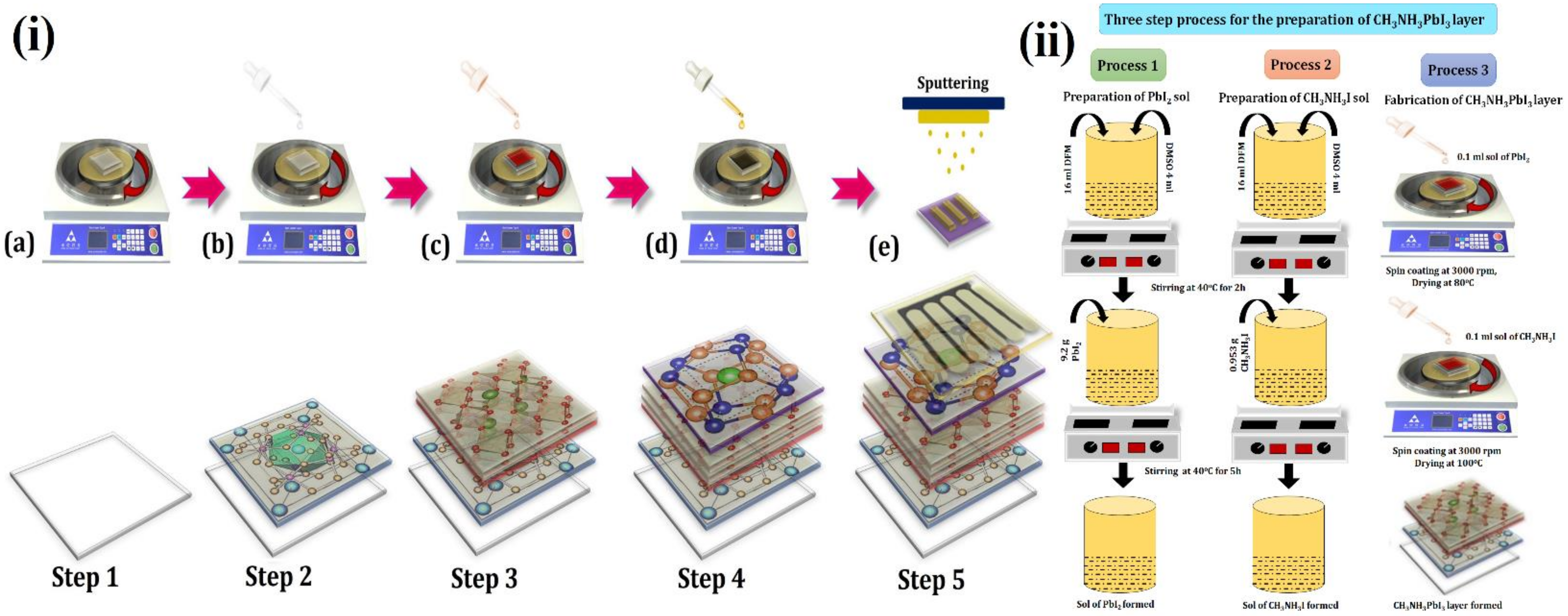
- i.
- Step 1: The first step for the fabrication of a solar cell device is to place the FTO on the substrate holder of the spin-coater. A total of 0.1 mL SrFe2O4 sol (40 mg/mL) was poured onto the FTO, and spin coating at 5000 rpm was conducted for 60 s. Then, the substrate was annealed at 100 °C for 20 min. This SrFe2O4 layer acts as a HTL.
- ii.
- Step 2: A three-step process, Figure 1ii, was adopted for the coating of the perovskite layer (CH3NH3PbI3) [8]. Process 1 involved the preparation of PbI2 sol. For the preparation of PbI2 sol, 16 mL of N,N-Dimethylformamide (DMF) and 4 mL of dimethyl sulfoxide (DMSO) were mixed and allowed to stir for 2 h at 40 °C. Afterward, 9.2 g of PbI2 was added to this mixture, and this was allowed to stir for 5 h at 40 °C. This led to the formation of PbI2 sol. Process 2 involved the preparation of CH3NH3I sol. For this, again, 16 mL of DMF and 4 mL of DMSO were mixed and allowed to stir for 2 h at 40 °C. Subsequently, 0.953 g CH3NH3I was added, and this was allowed to stir for 5 h at 40 °C. The formation of CH3NH3I sol took place.
- iii.
- Step 3: As explained in Figure 1i (Step 3) and Figure 1ii, (Process 3) involved the deposition of the CH3NH3PbI3 layer by spin coating technique onto SrFe2O4 coated FTO. The 0.1 mL of PbI2 sol (450 mg/mL) was poured onto SrFe2O4 and spin-coated at 3000 rpm. This was dried at 80 °C for 15 min, then 0.1 mL sol of CH3NH3I (50 mg/mL) was poured onto it, and spin-coating was carried out at 3000 rpm. Afterward, it was dried at 100 °C for 60 min. This would led to the formation of a perovskite layer (CH3NH3PbI3) on the surface of FTO. CH3NH3PbI3 is used as a perovskite layer because it is an ideal and superlative perovskite material since it absorbs light in the visible light regime; has a high dielectric constant, high carrier diffusion, and high Bohr radius; dissociates free charge carriers easily; allows tunable band engineering; has a low exciton binding energy ~2 meV and long carrier lifetimes [2].
- iv.
- Step 4: The 0.1 mL ZnO sol (40 mg/mL) was poured on the above-fabricated substrate for the deposition of the ZnO layer that will act as ETL. The spin-coating was conducted at 3000 rpm and was dried for 10 min at 200 °C.
- v.
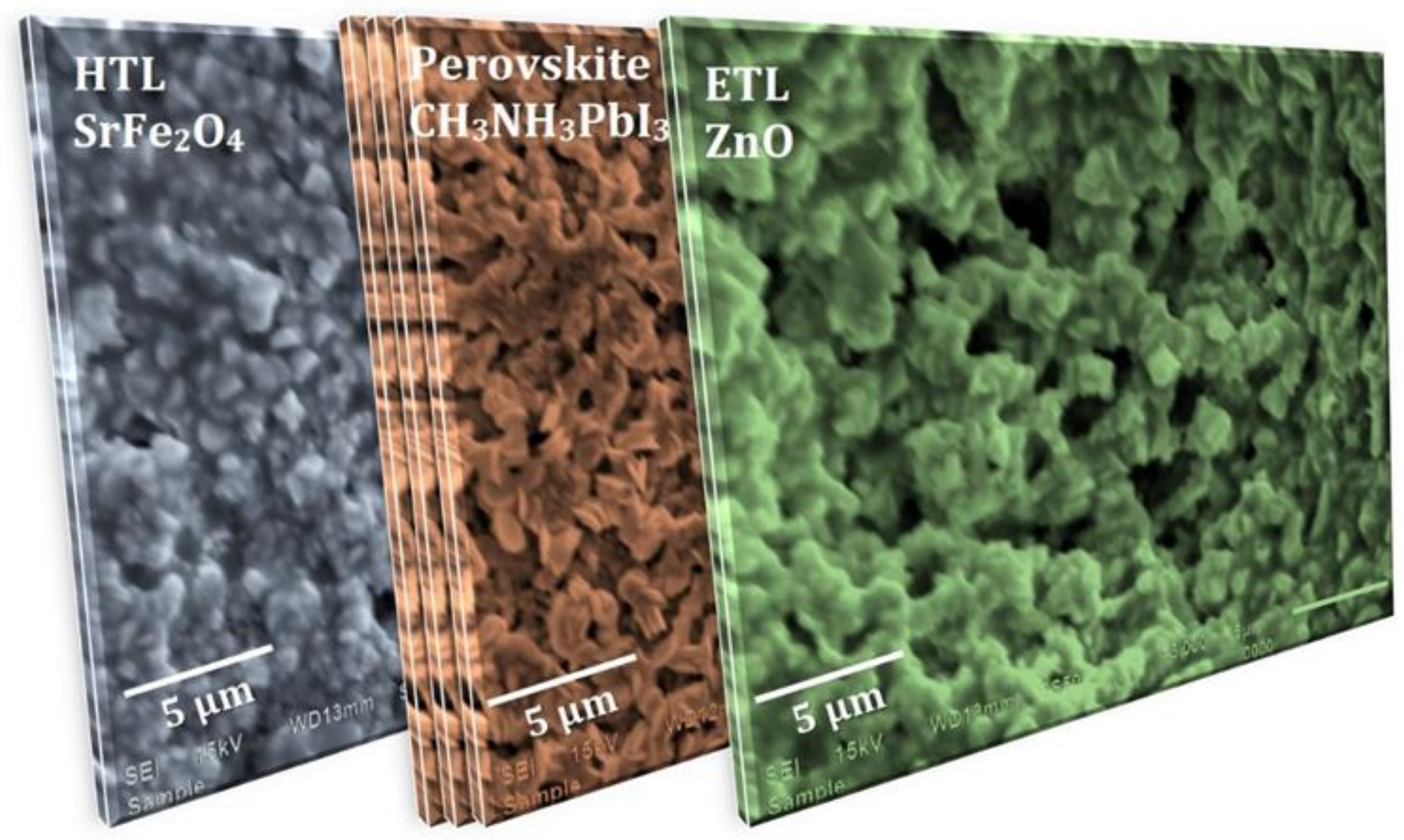
- Step 5: The deposition of the Pt electrode was carried out onto the above-fabricated substrate using a sputtering process. The surface morphology of each layer of the device is presented in Figure 2, showing the large grain size and presence of voids.
2.2. Instrumentation
2.3. First Principle Calculations
2.4. Device Simulation
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, A.D.; Sun, Q.; Goetz, K.P.; An, Q.; Schramm, T.; Hofstetter, Y.; Vaynzof, Y. A general approach to high-efficiency perovskite solar cells by any antisolvent. Nat. Commun. 2021, 12, 1878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, H.; Wang, W.; Zhang, J.; Xu, B.; Karen, K.L.; Sun, X.W. Hybrid Perovskite Light-Emitting Diodes Based on Perovskite Nanocrystals with Organic–Inorganic Mixed Cations. Adv. Mater. 2017, 29, 1606405. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.H.; Im, S.H.; Heo, J.H.; Mandal, T.N.; Seok, S.I. Chemical Management for Colorful, Efficient, and Stable Inorganic–Organic Hybrid Nanostructured Solar Cells. Nano Lett. 2013, 13, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- Urbina, A.J. The balance between efficiency, stability and environmental impacts in perovskite solar cells: A review. J. Phys. Energy 2020, 2, 022001. [Google Scholar] [CrossRef]
- You, J.; Meng, L.; Song, T.B.; Guo, T.F.; Yang, Y.M.; Chang, W.H.; Yang, Y. Improved air stability of perovskite solar cells via solution-processed metal oxide transport layers. Nat. Nanotechnol. 2016, 11, 75–81. [Google Scholar] [CrossRef]
- Ansari, F.; Salavati-Niasari, M.; Amiri, O.; Mir, N.; Abdollahi Nejand, B.; Ahmadi, V. Magnetite as inorganic hole transport material for lead halide perovskite-based solar cells with enhanced stability. Ind. Eng. Chem. Res. 2020, 59, 743–750. [Google Scholar] [CrossRef]
- Bo, L.; Hu, Y.; Zhang, Z.; Tong, J. Efficient photocatalytic degradation of Rhodamine B catalyzed by SrFe2O4/g-C3N4 composite under visible light. Polyhedron 2019, 168, 94–100. [Google Scholar] [CrossRef]
- Jeng, J.Y.; Chen, K.C.; Chiang, T.Y.; Lin, P.Y.; Tsai, T.D.; Chang, Y.C.; Guo, T.F.; Chen, P.; Wen, T.C.; Hsu, Y.J. Nickel Oxide Electrode Interlayer in CH3NH3PbI3 Perovskite/PCBM Planar-Heterojunction Hybrid Solar Cells. Adv. Mater. 2014, 26, 4107–4113. [Google Scholar] [CrossRef]
- Smidstrup, S.; Markussen, T.; Vancraeyveld, P.; Wellendorff, J.; Schneider, J.; Gunst, T.; Verstichel, B.; Stradi, D.; Khomyakov, P.A.; Vej-Hansen, U.G.; et al. QuantumATK: An integrated platform of electronic and atomic-scale modelling tools. J. Phys. Condens. Matter. 2020, 32, 015901. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [Green Version]
- van Setten, M.J.; Giantomassi, M.; Bousquet, E.; Verstraete, M.J.; Hamann, D.R.; Gonze, X.; Rignanese, G.M. The PseudoDojo: Training and grading a 85 element optimized norm-conserving pseudopotential table. Comput. Phys. Commun. 2018, 226, 39–54. [Google Scholar] [CrossRef] [Green Version]
- Hong, N.H.; Thu Huong, N.; Kim, T.Y.; Goumri-Said, S.; Kanoun, M.B. Tuning Magnetic Properties of BiFeO3 Thin Films by Controlling Rare-Earth Doping: Experimental and First-Principles Studies. J. Phys. Chem. C 2015, 119, 14351–14357. [Google Scholar] [CrossRef]
- Burgelman, M.; Nollet, P.; Degrave, S. Modelling polycrystalline semiconductor solar cells. Thin Solid Films 2000, 361, 527–532. [Google Scholar] [CrossRef]
- Pettersson, J.; Edoff, M.; Platzer-Björkman, C. Electrical modeling of Cu(In,Ga)Se2 cells with ALD-Zn1−xMgxO buffer layers. J. Appl. Phys. 2012, 111, 014509. [Google Scholar] [CrossRef]
- Kanoun, A.A.; Kanoun, M.B.; Merad, A.E.; Goumri-Said, S. Toward development of high-performance perovskite solar cells based on CH3NH3GeI3 using computational approach. Sol. Energy 2019, 182, 237–244. [Google Scholar] [CrossRef]
- Kanoun, M.B.; Goumri-Said, S. Exploring performances of hybrid perovskites tin-based photovoltaic solar cells: Non-equilibrium Green’s functions and macroscopic approaches. Phys. Rev. B Condens. Matter 2020, 591, 412247. [Google Scholar] [CrossRef]
- Kanoun, M.B.; Kanoun, A.A.; Merad, A.E.; Goumri-Said, S. Device design optimization with interface engineering for highly efficient mixed cations and halides perovskite solar cells. Results Phys. 2021, 20, 103707. [Google Scholar] [CrossRef]
- Hossain, M.I.; Qarony, W.; Jovanov, V.; Tsang, Y.H.; Knipp, D. Nanophotonic design of perovskite/silicon tandem solar cells. J. Mater. Chem. A 2018, 6, 3625–3633. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, Y.; Zeng, Q.; Jiang, J.; Li, Y. Large-Area Organic-Free Perovskite Solar Cells with High Thermal Stability. J. Phys. Chem. Lett. 2019, 10, 6382–6388. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Wang, H.; Ma, M.; Dong, S.; Xu, Q. Influence of drying temperature on morphology of MAPbI3 thin films and the performance of solar cells. J. Alloys Compd. 2019, 773, 511–518. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Molaei Imenabadi, R.; Vandenberghe, W.G.; Hsu, J.W. Minimizing performance degradation induced by interfacial recombination in perovskite solar cells through tailoring of the transport layer electronic properties. APL Mater. 2018, 6, 036104. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Wang, Y.; Zhang, Q. Progress in perovskite solar cells based on ZnO nanostructures. Sol. Energy 2018, 163, 289–306. [Google Scholar] [CrossRef]
- Li, R.; Li, C.; Liu, M.; Vivo, P.; Zheng, M.; Dai, Z.; Zhan, J.; He, B.; Li, H.; Yang, W.; et al. Hydrogen-Bonded Dopant-Free Hole Transport Material Enables Efficient and Stable Inverted Perovskite Solar Cells. CCS Chem. 2021, 3, 3309–3319. [Google Scholar] [CrossRef]
- Cetin, C.; Chen, P.; Hao, M.; He, D.; Bai, Y.; Lyu, M.; Wang, L. Inorganic p-Type Semiconductors as Hole Conductor Building Blocks for Robust Perovskite Solar Cells. Adv. Sustain. Syst. 2018, 2, 1800032. [Google Scholar] [CrossRef]
- Ali, N.; Shehzad, N.; Uddin, S.; Ahmed, R.; Jabeen, M.; Kalam, A.; Al-Sehemi, A.G.; Alrobei, H.; Kanoun, M.B.; Khesro, A.; et al. A review on perovskite materials with solar cell prospective. Int. J. Energy Res. 2021, 45, 19729–19745. [Google Scholar] [CrossRef]
- Tangra, A.K.; Sharma, M.; Zainudeen, U.L.; Lotey, G.S. Investigation of Inorganic electron–hole transport material for high efficiency, stable and low-cost perovskite solar cell. J. Mater. Sci. Mater. Electron. 2020, 31, 13657–13666. [Google Scholar] [CrossRef]
- Spalla, M.; Perrin, L.; Planes, E.; Matheron, M.; Berson, S.; Flandin, L. Effect of the Hole Transporting/Active Layer Interface on the Perovskite Solar Cell Stability. ACS Appl. Energy Mater. 2020, 3, 3282–3292. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, J.; Chen, Q.; Wang, Y.; Zhou, Y.; Song, B.; Li, Y. High-efficiency planar p-i-n perovskite solar cells based on dopant-free dibenzo[b,d]furan-centred linear hole transporting material. J. Power Sources 2020, 449, 227488. [Google Scholar] [CrossRef]
- Yuan, H.; Zhao, Y.; Duan, J.; Wang, Y.; Yang, X.; Tang, Q. All-inorganic CsPbBr3 perovskite solar cell with 10.26% efficiency by spectra engineering. J. Mater. Chem. A 2018, 6, 24324–24329. [Google Scholar] [CrossRef]
- Weller, M.T.; Weber, O.J.; Frost, J.M.; Walsh, A. Cubic Perovskite Structure of Black Formamidinium Lead Iodide, α-[HC(NH2)2]PbI3, at 298 K. J. Phys. Chem. Lett. 2015, 6, 3209–3212. [Google Scholar] [CrossRef]
- Pandey, K.; Singh, D.; Gupta, S.K.; Yadav, P.; Sonvane, Y.; Lukačević, I.; Kumar, M.; Kumar, M.; Ahuja, R. Improving electron transport in the hybrid perovskite solar cells using CaMnO3-based buffer layer. Nano Energy 2018, 45, 287–297. [Google Scholar] [CrossRef]
- Giorgi, G. Structural and electronic features of Si/CH3NH3PbI3 interfaces with optoelectronic applicability: Insights from first-principles. Nano Energy 2020, 67, 104166. [Google Scholar] [CrossRef]
- Hailegnaw, B.; Poscher, V.; Ulbricht, C.; Seelajaroen, H.; Teasdale, I.; Salinas, Y.; Scharber, M.C. Improving the Performance of Perovskite Solar Cells Using a Polyphosphazene Interfacing Layer. Phys. Status Solidi (a) 2019, 216, 1900436. [Google Scholar] [CrossRef]
- Gonzalez-Pedro, V.; Juarez-Perez, E.J.; Arsyad, W.S.; Barea, E.M.; Fabregat-Santiago, F.; Mora-Sero, I.; Bisquert, J. General Working Principles of CH3NH3PbX3 Perovskite Solar Cells. J. Nano Lett. 2014, 14, 888–893. [Google Scholar] [CrossRef]
- Rakocevic, L.; Gehlhaar, R.; Merckx, T.; Qiu, W.; Paetzold, U.W.; Fledderus, H.; Poortmans, J. Photovolt, Interconnection Optimization for Highly Efficient Perovskite Modules. IEEE J. 2017, 7, 404–408. [Google Scholar]
- Stuckelberger, M.; Nietzold, T.; Hall, G.N.; West, B.; Werner, J.; Niesen, B.; Ballif, C.; Rose, V.; Fenning, D.P.; Bertoni, M.I. Charge Collection in Hybrid Perovskite Solar Cells: Relation to the Nanoscale Elemental Distribution. IEEE J. Photovolt. 2017, 7, 590–597. [Google Scholar] [CrossRef]
- Pandey, R.; Chaujar, R.J. Technology computer aided design of 29.5% efficient perovskite/interdigitated back contact silicon heterojunction mechanically stacked tandem solar cell for energy-efficient applications. J. Photonics Energy 2017, 7, 022503. [Google Scholar] [CrossRef]
- Anwar, F.; Mahbub, R.; Satter, S.S.; Ullah, S.M. Effect of Different HTM Layers and Electrical Parameters on ZnO Nanorod-Based Lead-Free Perovskite Solar Cell for High-Efficiency Performance. Int. J. Photoenergy 2017, 2017, 9846310. [Google Scholar] [CrossRef]
- Kanoun, A.-A.; Goumri-Said, S.; Kanoun, M.B. Device design for high-efficiency monolithic two-terminal, four-terminal mechanically stacked, and four-terminal optically coupled perovskite-silicon tandem solar cells. Int. J. Energy Res. 2021, 45, 10538–10545. [Google Scholar] [CrossRef]
- Shockley, W.; Read, W.T. Statistics of the Recombinations of Holes and Electrons. Phys. Rev. 1952, 87, 835–842. [Google Scholar] [CrossRef]
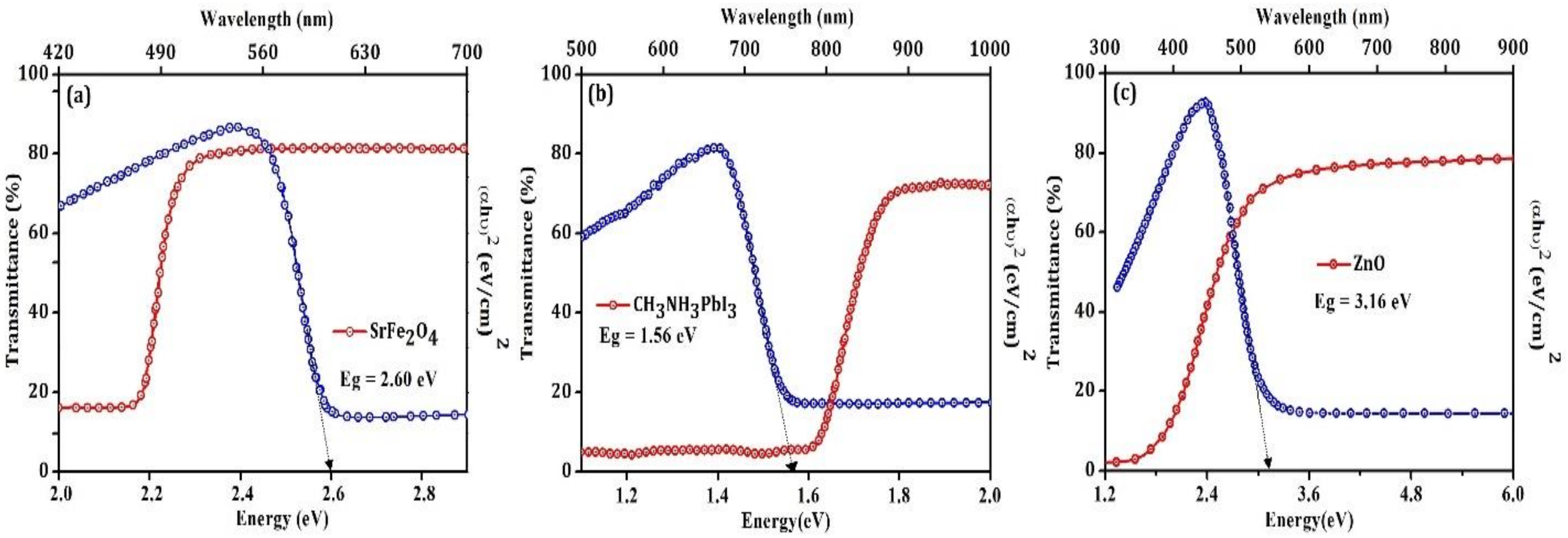
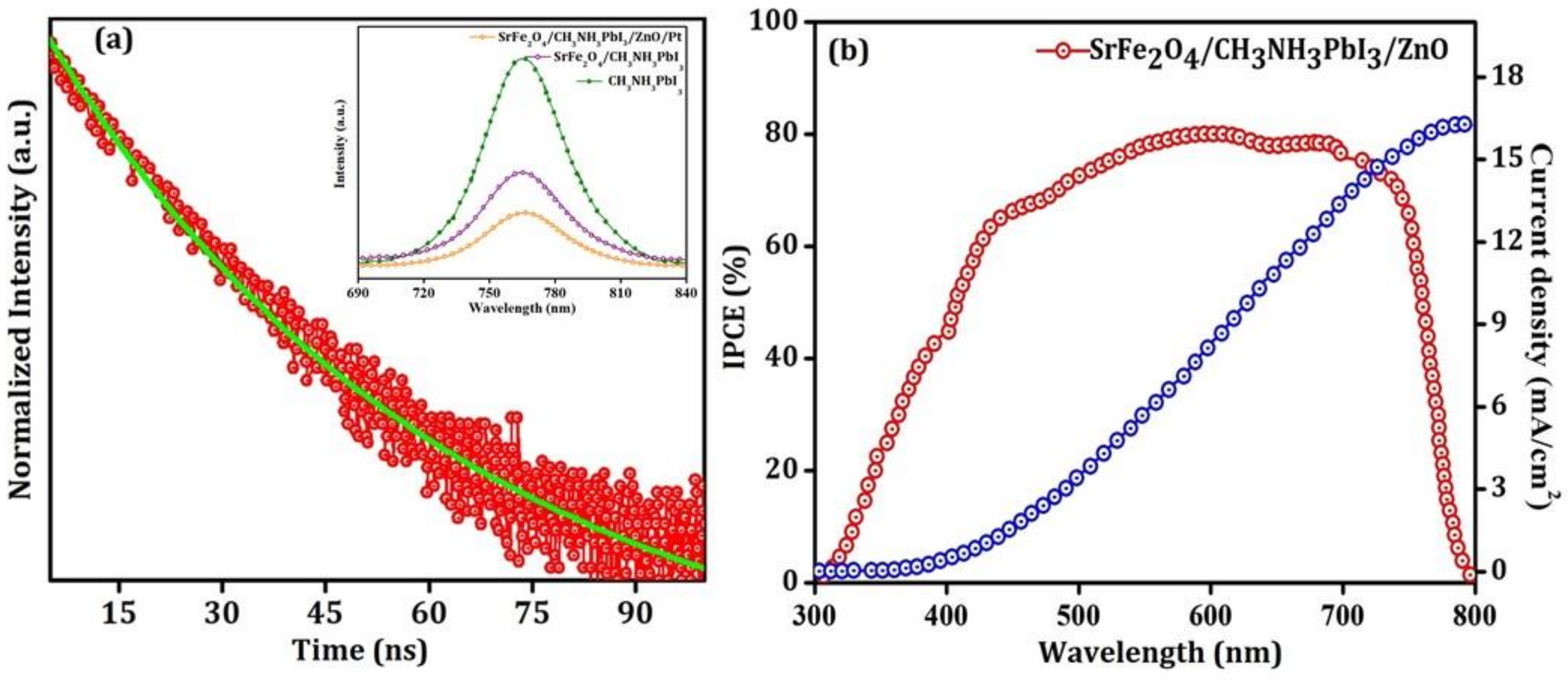
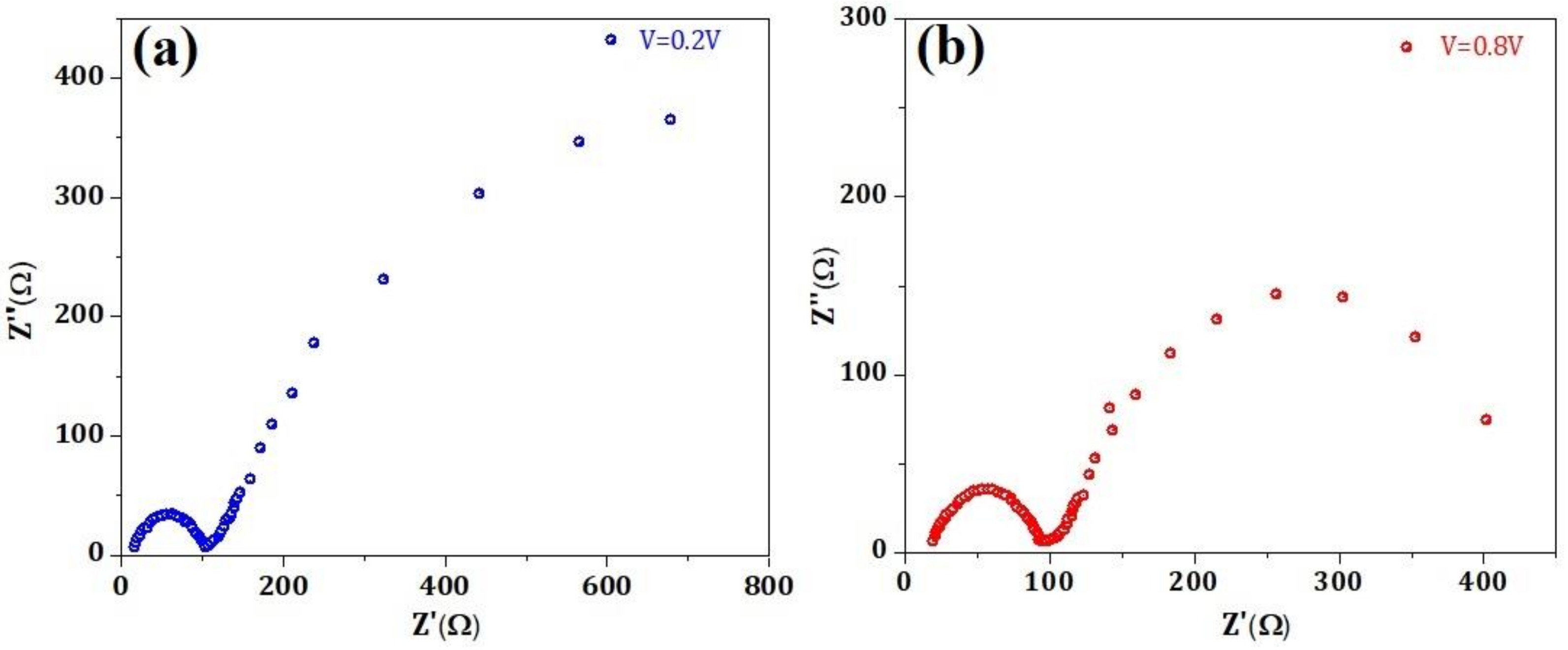
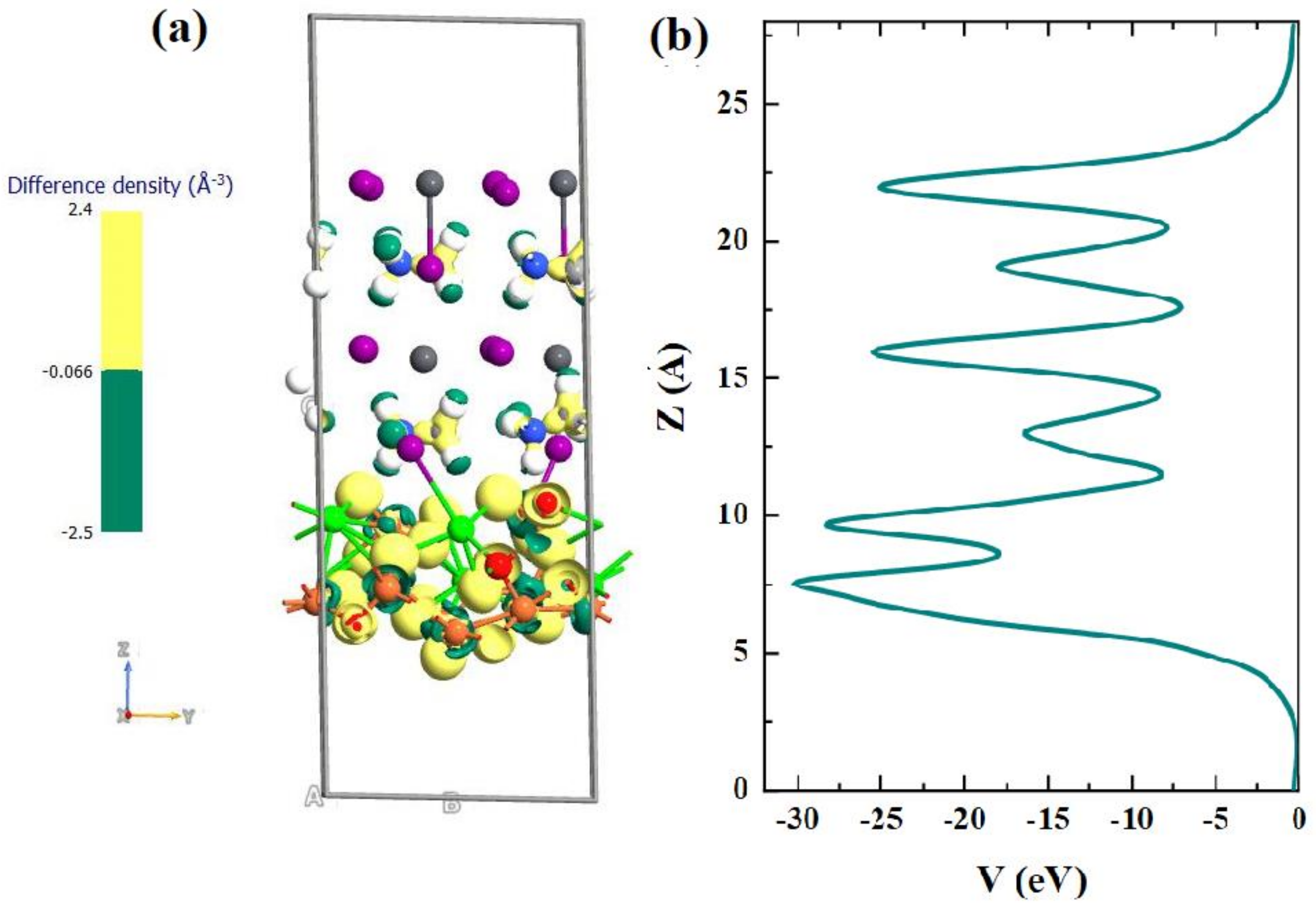
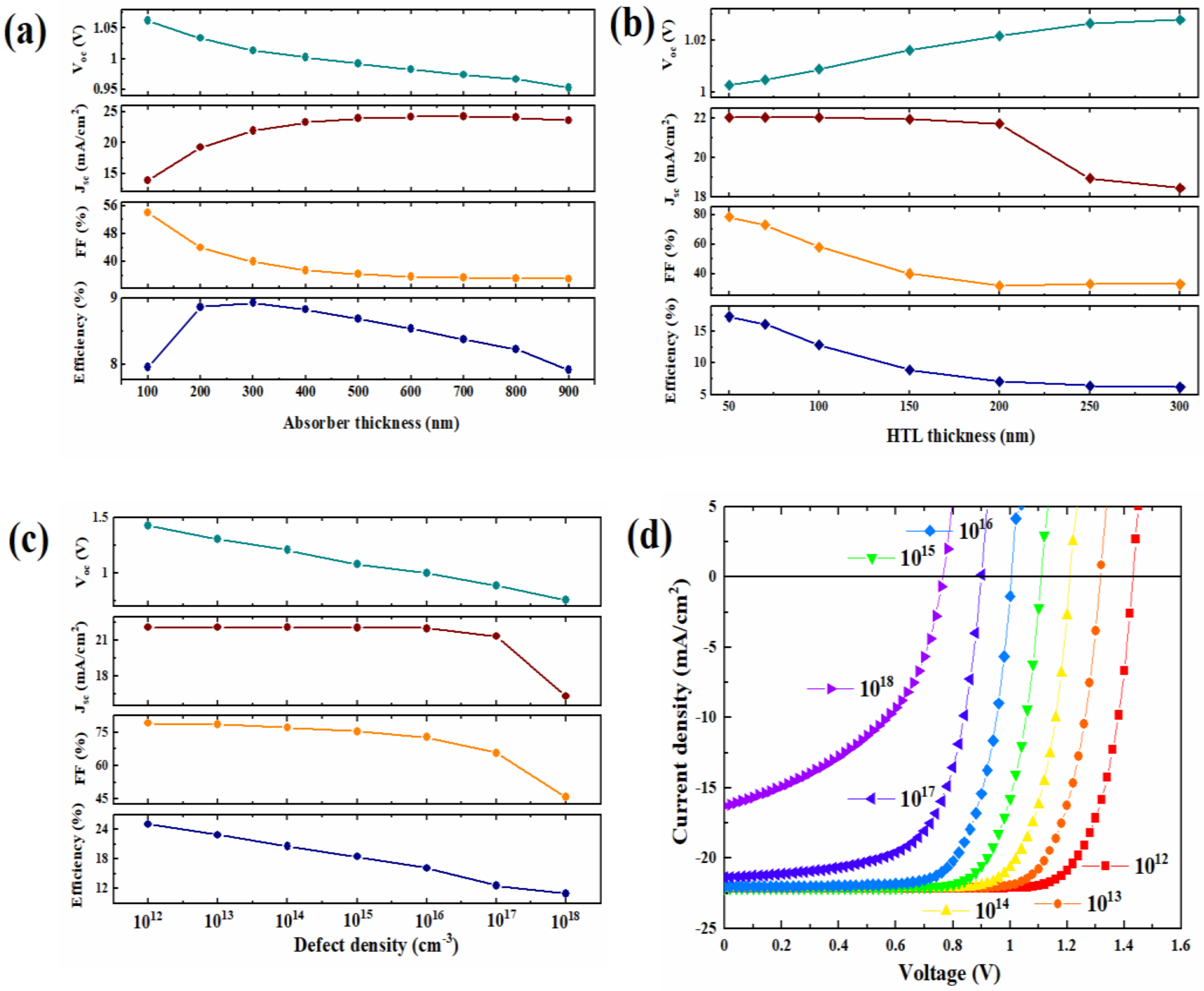
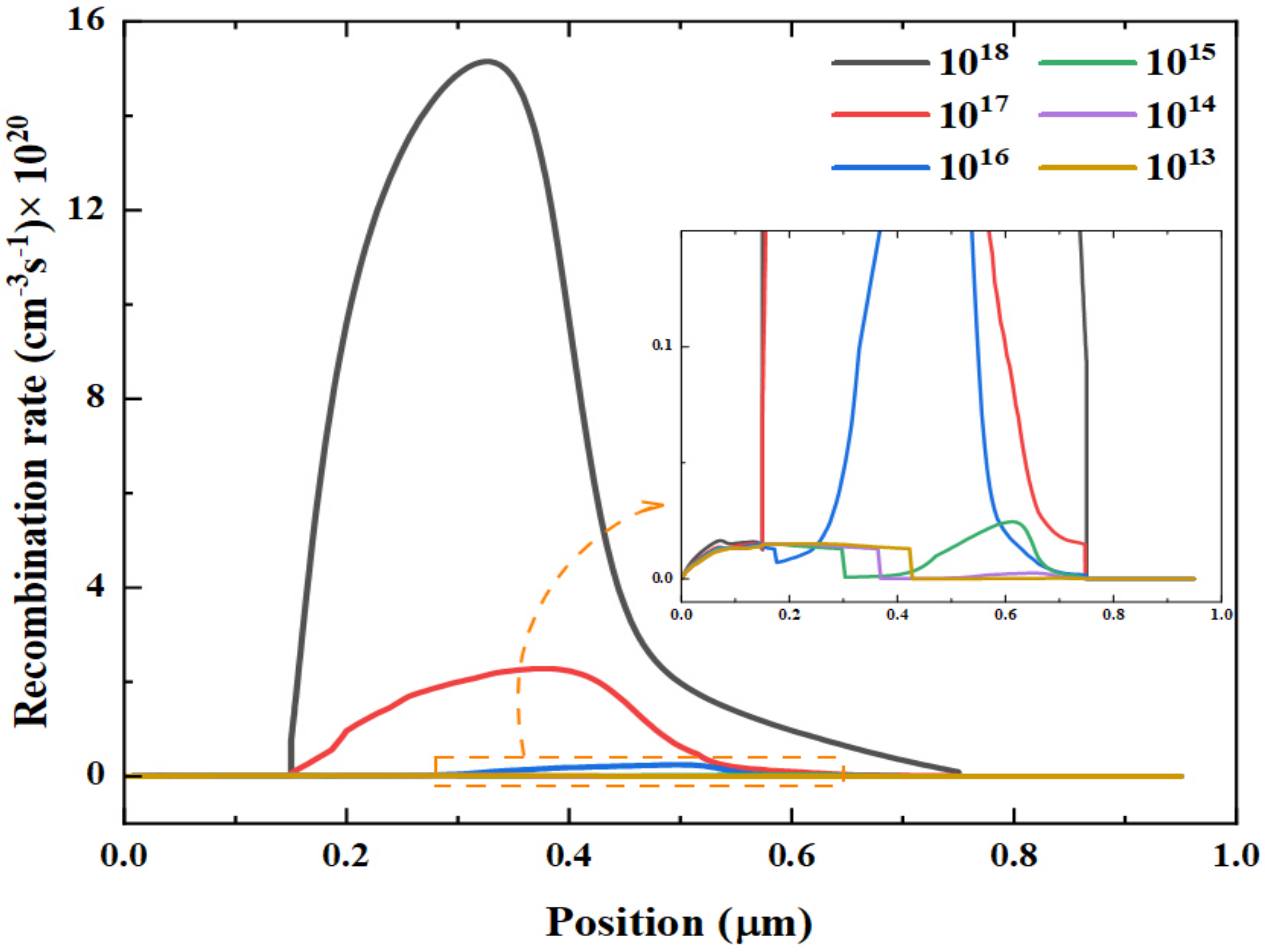
| Materials | Structure | Optical Band Gap (eV) | UPS Spectra | Solar Cell Performance | |||
|---|---|---|---|---|---|---|---|
| LUMO | HOMO | Parameters | Experimental | Theoretical | |||
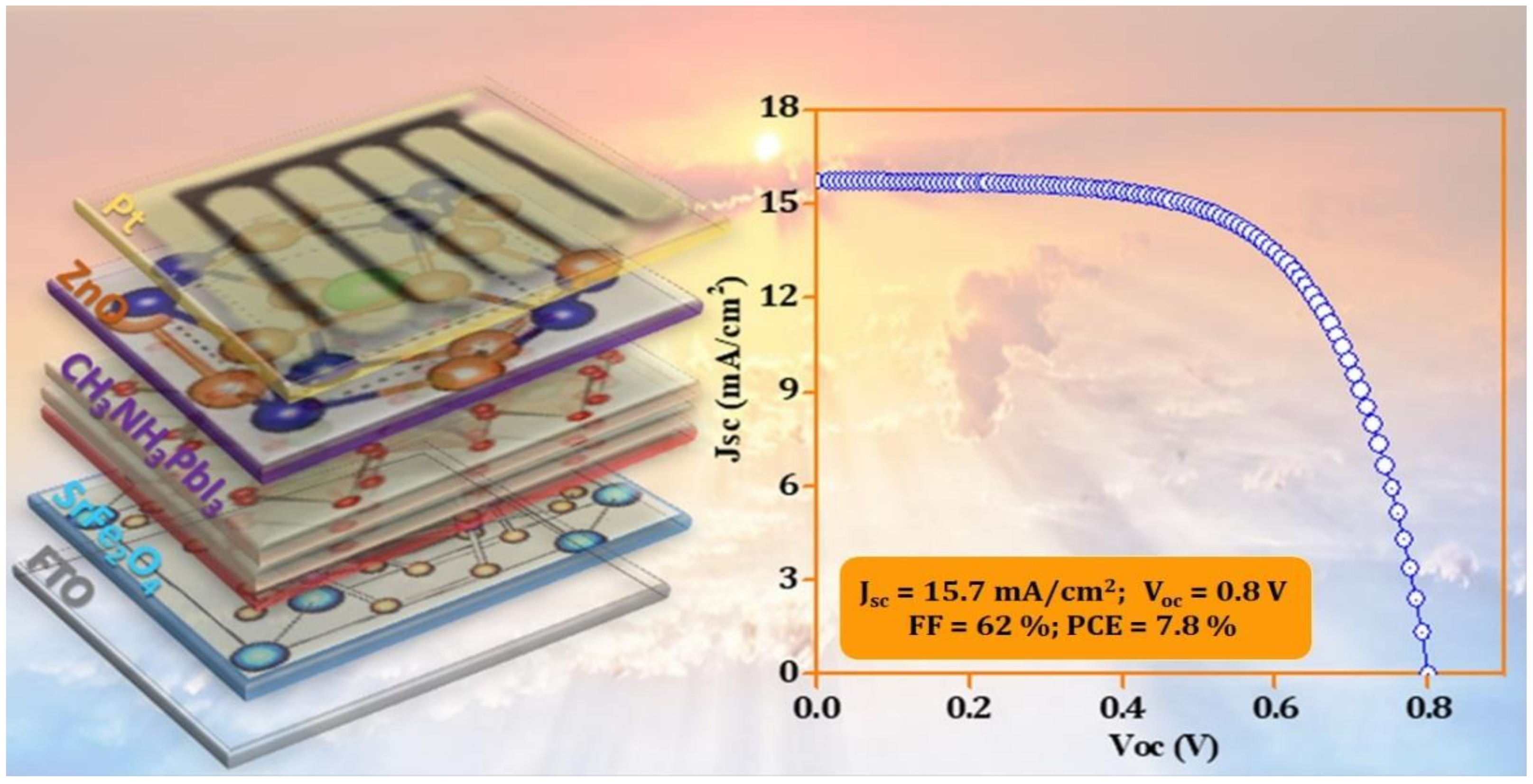
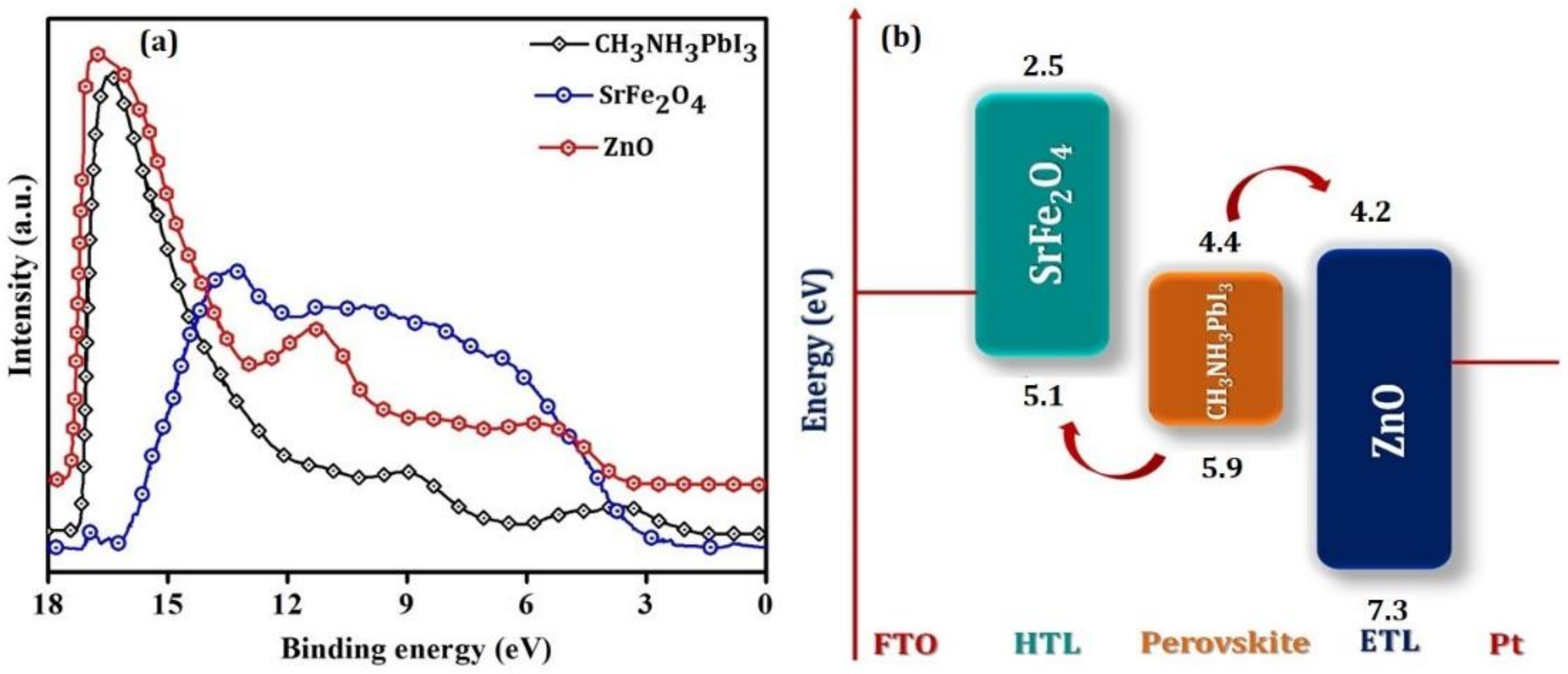
| SrFe2O4 | Monoclinic | 2.6 | 2.5 | 5.1 | Jsc (mA/cm2) | 15.7 | 23.3 |
| CH3NH3PbI3 | Cubic | 1.6 | 4.4 | 5.9 | Voc (V) | 0.8 | 1.0 |
| ZnO | Cubic | 3.2 | 4.2 | 7.3 | FF (%) | 62.0 | 40.0 |
| - | - | - | - | - | PCE (%) | 7.8 | 8.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tangra, A.K.; Kanoun, M.B.; Goumri-Said, S.; Kanoun, A.-A.; Musselman, K.; Kaur, J.; Lotey, G.S. Low-Cost Inorganic Strontium Ferrite a Novel Hole Transporting Material for Efficient Perovskite Solar Cells. Nanomaterials 2022, 12, 826. https://doi.org/10.3390/nano12050826
Tangra AK, Kanoun MB, Goumri-Said S, Kanoun A-A, Musselman K, Kaur J, Lotey GS. Low-Cost Inorganic Strontium Ferrite a Novel Hole Transporting Material for Efficient Perovskite Solar Cells. Nanomaterials. 2022; 12(5):826. https://doi.org/10.3390/nano12050826
Chicago/Turabian StyleTangra, Ankush Kumar, Mohammed Benali Kanoun, Souraya Goumri-Said, Ahmed-Ali Kanoun, Kevin Musselman, Jaspinder Kaur, and Gurmeet Singh Lotey. 2022. "Low-Cost Inorganic Strontium Ferrite a Novel Hole Transporting Material for Efficient Perovskite Solar Cells" Nanomaterials 12, no. 5: 826. https://doi.org/10.3390/nano12050826
APA StyleTangra, A. K., Kanoun, M. B., Goumri-Said, S., Kanoun, A.-A., Musselman, K., Kaur, J., & Lotey, G. S. (2022). Low-Cost Inorganic Strontium Ferrite a Novel Hole Transporting Material for Efficient Perovskite Solar Cells. Nanomaterials, 12(5), 826. https://doi.org/10.3390/nano12050826






