Preparation and Characterization of Photoluminescent Graphene Quantum Dots from Watermelon Rind Waste for the Detection of Ferric Ions and Cellular Bio-Imaging Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of GQDs
2.2. Quantum Yield Evaluation of GQDs
2.3. Detection of Metal Ions in Aqueous Solution by GQDs
2.4. Detection of Fe in Tap and Drinking Water Samples by GQDs
2.5. Cytotoxicity Test and Cellular Bio-Imaging
3. Results and Discussions
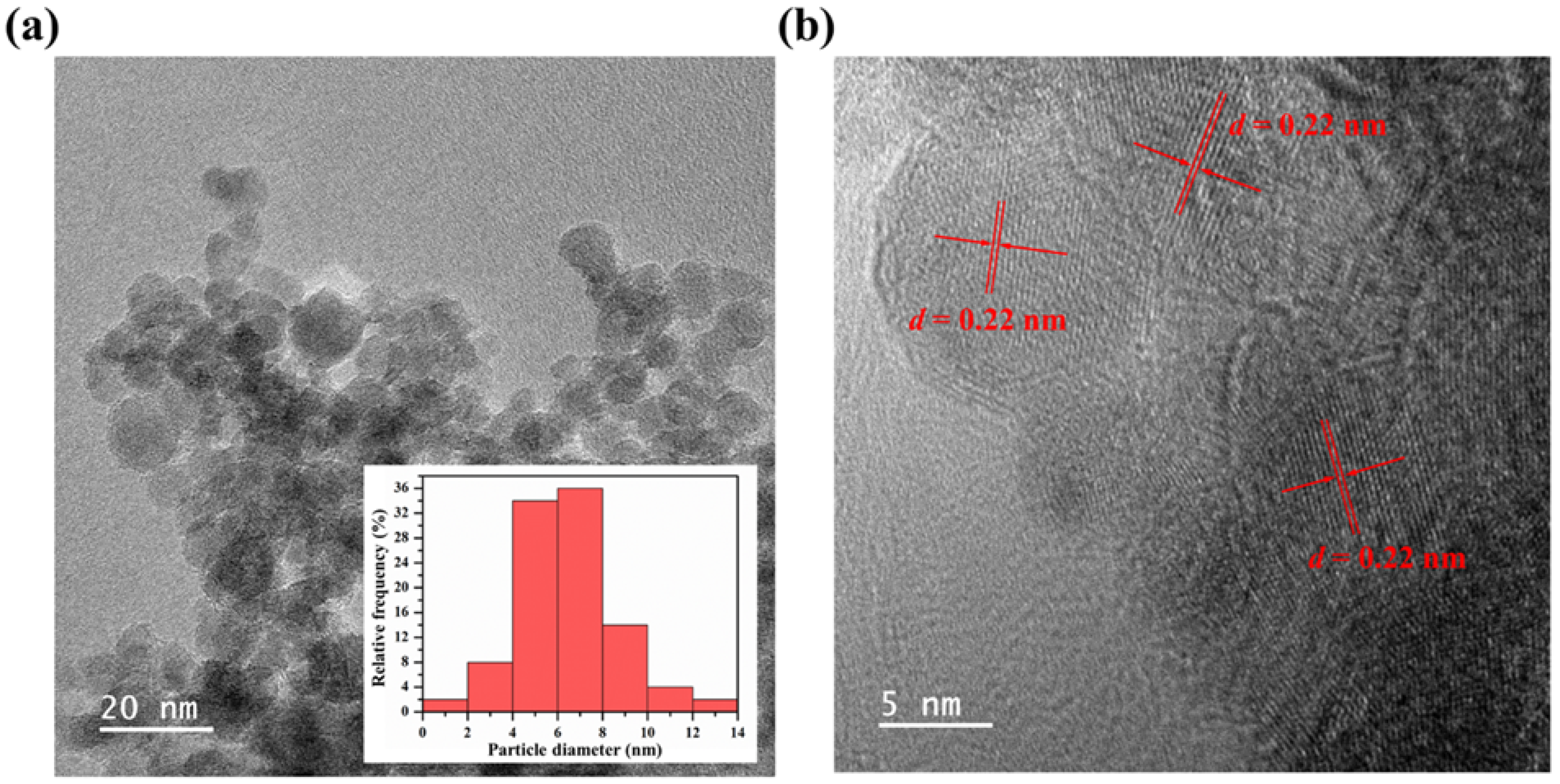
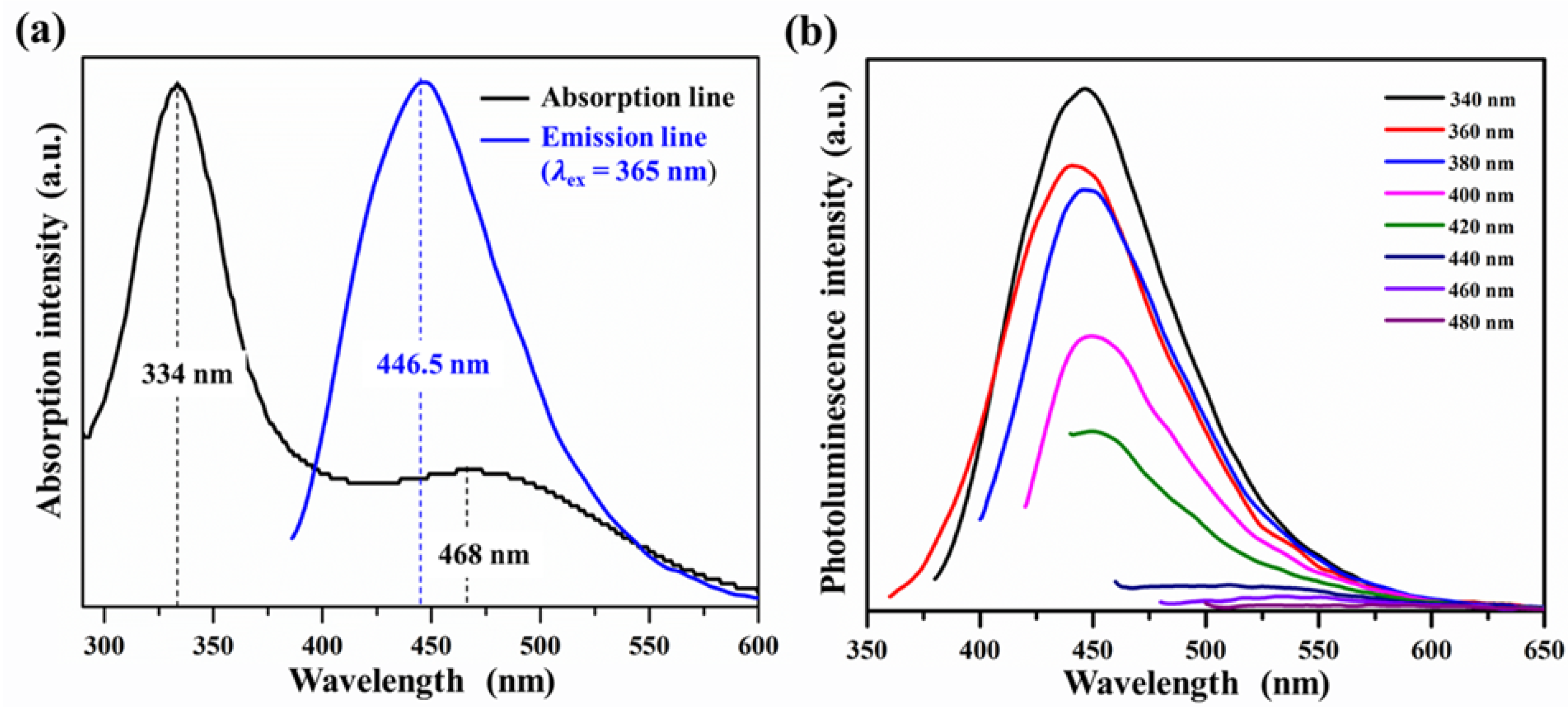
3.1. Characterization of GQDs
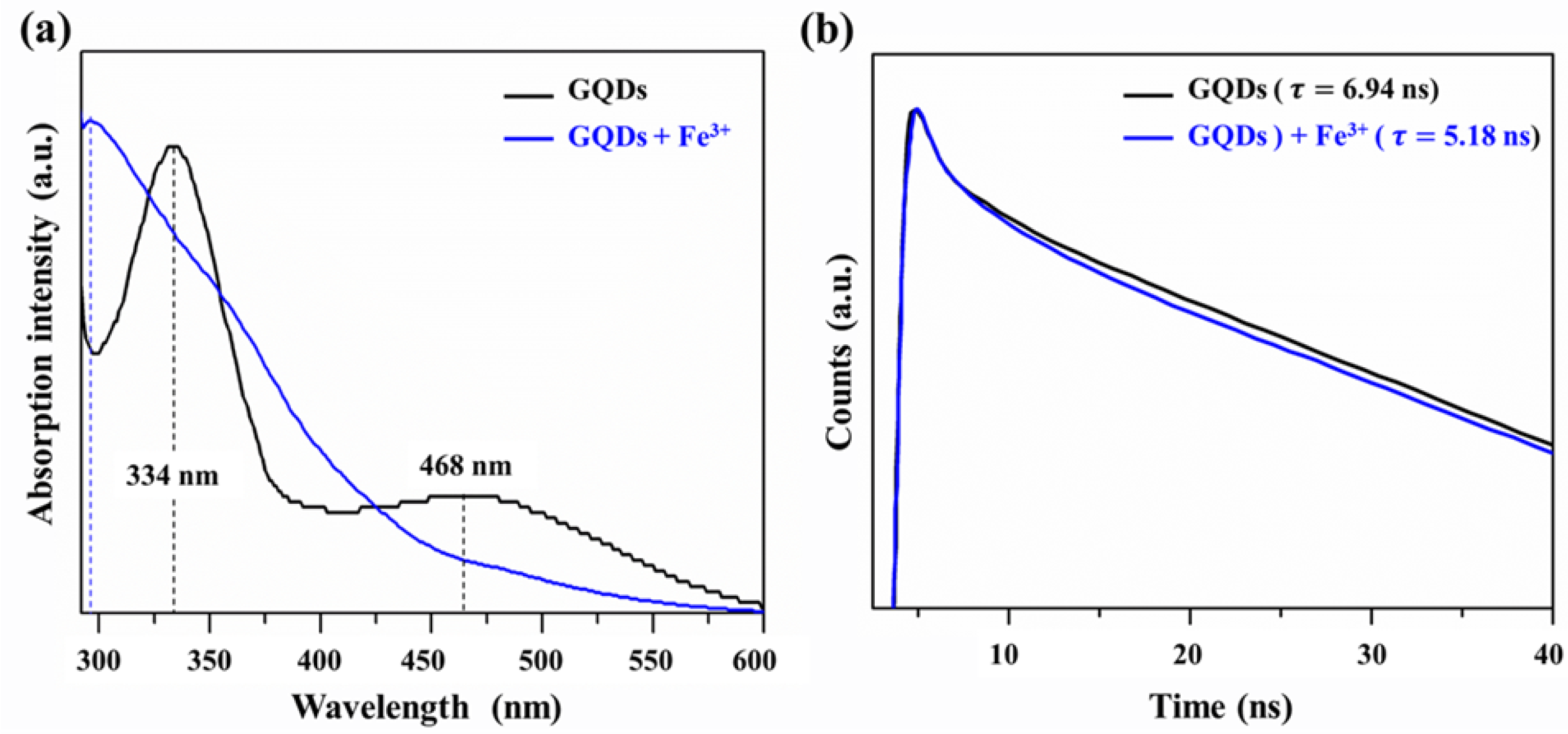
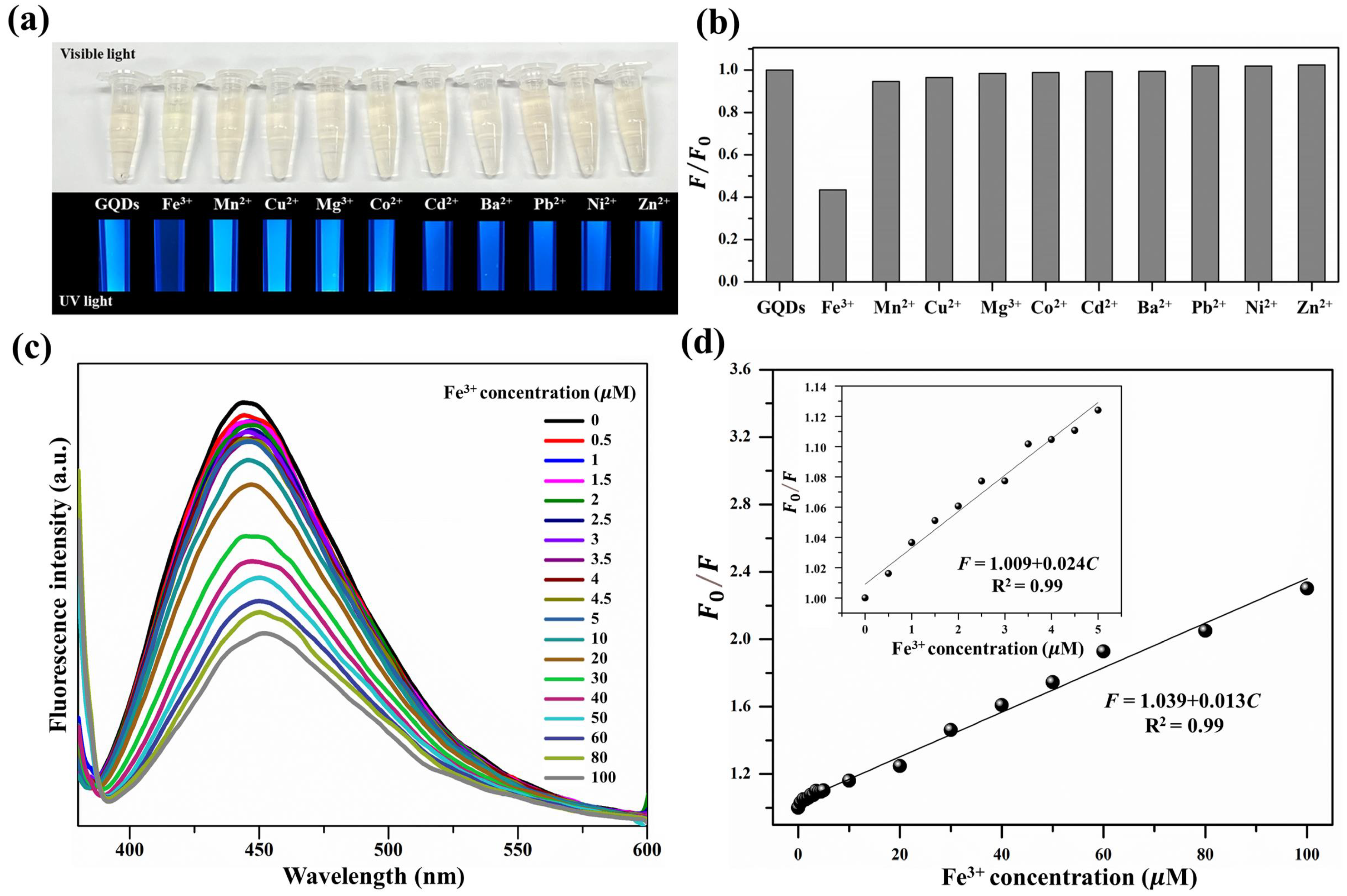
3.2. Detection of Fe in Water Samples
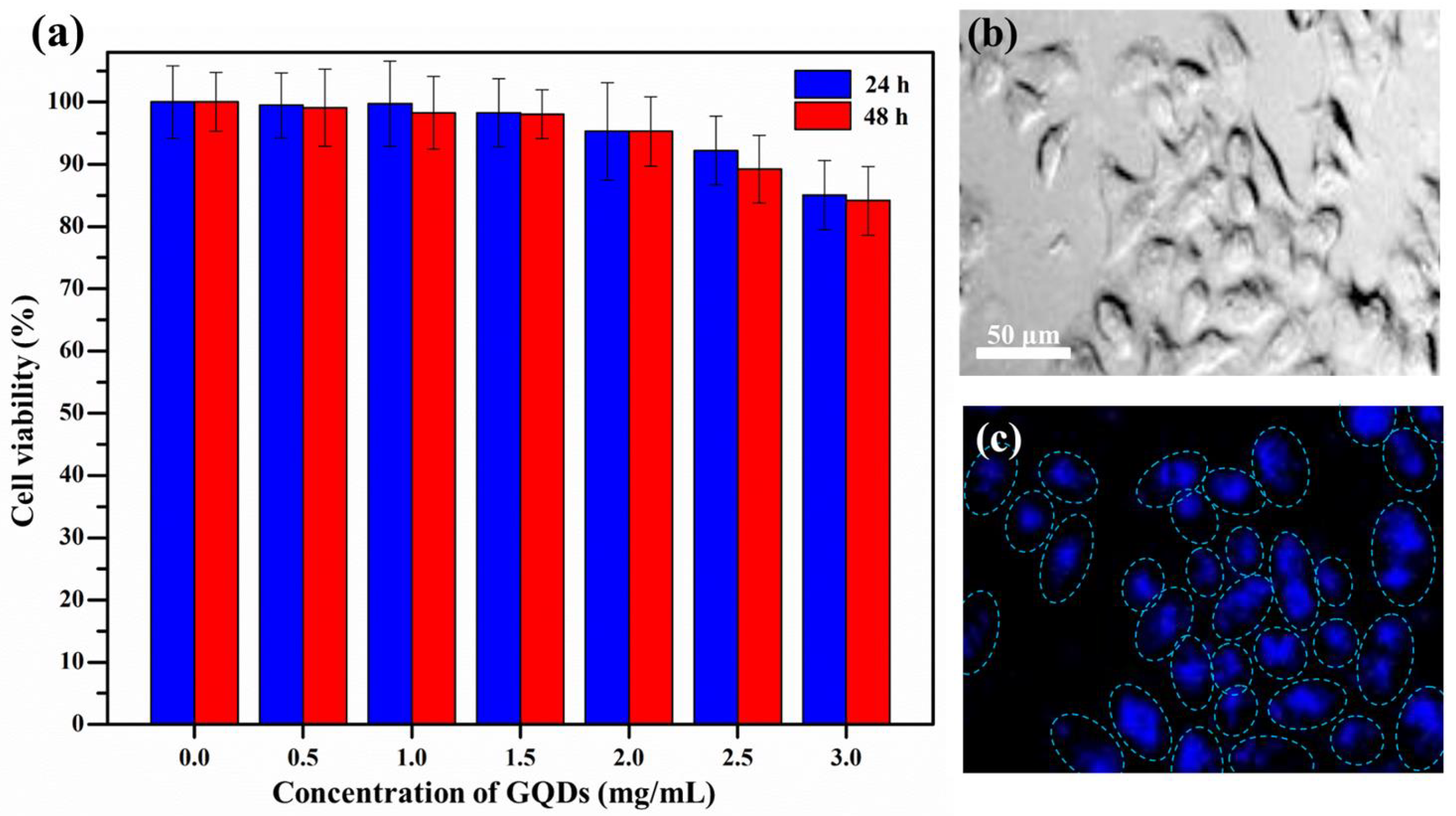
3.3. Cytotoxicity Test and Cellular Bioimaging
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Reagents and Instruments
| Nanocarbon Type | Source | Synthesis Technique | Quantum Yield (%) | LOD of Fe (M) | Reference |
|---|---|---|---|---|---|
| P-CQDs | Pine wood | Hydrothermal | 4.69 | 0.36 | [22] |
| GQDs | Nickel form (Growth substrate) + Ethanol (Carbon source) | CVD—Electronical cutting | 10 | 7.22 | [48] |
| RBD-GQDs | Rhodamine B + Ethanol + EDA | Reflux condensation | 43 | 0.02 | [49] |
| S-GQDs | Graphite + NaPTS | Electrolysis | 10.6 | 0.004 | [50] |
| DA-GQDs | CA + EDC + NHS | Pyrolysis | 10.2 | 0.007 | [51] |
| GQDs | CA | Pyrolysis | 27.4 | 1.60 | [52] |
| CDs | Onion waste | Hydrothermal | 28 | 0.31 | [53] |
| CDs | Mango peel | Pyrolysis | 8.5 | 1.20 | [54] |
| N-CDs | Poa Tratensis | Hydrothermal | 7 | 1.40 | [55] |
| N-GQDs | Citric acid + ammonia | Hydrothermal | 30.7 | - | [56] |
| GQDs | Watermelon rind waste | Hydrothermal | 37 | 0.28 | Our work |
| Sample | (ns) | (ns) | (ns) | (%) | (%) | (%) | (ns) |
|---|---|---|---|---|---|---|---|
| GQDs | 2.12 | 5.98 | 9.69 | 28.99 | 49.93 | 21.73 | 6.94 |
| GQDs + Fe | 1.78 | 3.55 | 7.22 | 25.28 | 46.18 | 28.54 | 5.18 |
References
- Fan, H.; Zhang, M.; Bhandari, B.; Yang, C.H. Food waste as a carbon source in carbon quantum dots technology and their applications in food safety detection. Trends Food Sci. Technol. 2020, 95, 86–96. [Google Scholar] [CrossRef]
- Gao, A.; Tian, Z.; Wang, Z.; Wennersten, R.; Sun, Q. Comparison between the technologies for food waste treatment. Energy Procedia 2017, 105, 3915–3921. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Pu, Y.; Cheng, W.; Lin, S.; Shao, Z.; Liao, X. Selective, sensitive and label-free detection of Fe3+ ion in tap water using highly fluorescent graphene quantum dots. J. Fluoresc. 2019, 29, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Song, L.; Han, Y.; Wang, Y.; Tang, X.; Cui, G.; Xu, Z. Effects of Fe3+ on Acute Toxicity and Regeneration of Planarian (Dugesia japonica) at Different Temperatures. BioMed Res. Int. 2019, 2019, 8591631. [Google Scholar] [CrossRef] [Green Version]
- Figueroa, J.A.L.; Stiner, C.A.; Radzyukevich, T.L.; Heiny, J.A. Metal ion transport quantified by ICP-MS in intact cells. Sci. Rep. 2016, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Lázár, K. Redistribution of iron ions in porous ferrisilicates during redox treatments. Pure Appl. Chem. 2017, 89, 471–479. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhang, C.; Wang, S.; Liu, Q.; Feng, H.; Ma, X.; Guo, J. Electrochemical microfluidics techniques for heavy metal ion detection. Analyst 2018, 143, 4230–4246. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, Y.; Zhang, Y.; Lin, T.; Zeng, G.; Zhang, S.; Wang, Y.; He, W.; Zhang, M.; Long, H. An efficient adsorbent: Simultaneous activated and magnetic ZnO doped biochar derived from camphor leaves for ciprofloxacin adsorption. Bioresour. Technol. 2019, 288, 121511. [Google Scholar] [CrossRef]
- Liu, M.; Li, T.; Zhang, C.; Zheng, Y.; Wu, C.; Zhang, J.; Zhang, K.; Zhang, Z. Fluorescent carbon dots embedded in mesoporous silica nanospheres: A simple platform for Cr (VI) detection in environmental water. J. Hazard. Mater. 2021, 415, 125699. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Dadashzadeh, A.; Moghassemi, S.; Ashrafizadeh, M.; Dehshahri, A.; Pardakhty, A.; Sassan, H.; Sohrevardi, S.M.; Mandegary, A. Shedding light on gene therapy: Carbon dots for the minimally invasive image-guided delivery of plasmids and noncoding RNAs-A review. J. Adv. Res. 2019, 18, 81–93. [Google Scholar] [CrossRef]
- Nair, A.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Natural carbon-based quantum dots and their applications in drug delivery: A review. Biomed. Pharmacother. 2020, 132, 110834. [Google Scholar] [CrossRef]
- Rasal, A.S.; Yadav, S.; Yadav, A.; Kashale, A.A.; Manjunatha, S.T.; Altaee, A.; Chang, J.Y. Carbon quantum dots for energy applications: A review. ACS Appl. Nano Mater. 2021, 4, 6515–6541. [Google Scholar] [CrossRef]
- Sagbas, S.; Sahiner, N. Carbon dots: Preparation, properties, and application. In Nanocarbon and Its Composites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 651–676. [Google Scholar]
- Wang, H.J.; Zhang, J.; Liu, Y.H.; Luo, T.Y.; He, X.; Yu, X.Q. Hyaluronic acid-based carbon dots for efficient gene delivery and cell imaging. RSC Adv. 2017, 7, 15613–15624. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.R.; Kumar, S.; Ortega, G.A.; Srinivasan, S.; Rajabzadeh, A.R. Target specific aptamer-induced self-assembly of fluorescent graphene quantum dots on palladium nanoparticles for sensitive detection of tetracycline in raw milk. Food Chem. 2021, 346, 128893. [Google Scholar] [CrossRef]
- Saravanan, A.; Maruthapandi, M.; Das, P.; Ganguly, S.; Margel, S.; Luong, J.H.; Gedanken, A. Applications of N-doped carbon dots as antimicrobial agents, antibiotic carriers, and selective fluorescent probes for nitro explosives. ACS Appl. Bio Mater. 2020, 3, 8023–8031. [Google Scholar] [CrossRef] [PubMed]
- El-Hnayn, R.; Canabady-Rochelle, L.; Desmarets, C.; Balan, L.; Rinnert, H.; Joubert, O.; Medjahdi, G.; Ben Ouada, H.; Schneider, R. One-step synthesis of diamine-functionalized graphene quantum dots from graphene oxide and their chelating and antioxidant activities. Nanomaterials 2020, 10, 104. [Google Scholar] [CrossRef] [Green Version]
- Gu, D.; Hong, L.; Zhang, L.; Liu, H.; Shang, S. Nitrogen and sulfur co-doped highly luminescent carbon dots for sensitive detection of Cd (II) ions and living cell imaging applications. J. Photochem. Photobiol. B Biol. 2018, 186, 144–151. [Google Scholar] [CrossRef]
- Jana, J.; Lee, H.J.; Chung, J.S.; Kim, M.H.; Hur, S.H. Blue emitting nitrogen-doped carbon dots as a fluorescent probe for nitrite ion sensing and cell-imaging. Anal. Chim. Acta 2019, 1079, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Yuan, H.; Zhang, M.; Jiang, P.; Liu, M.; Xu, D.; Guo, X.; Wu, Y. A 3D porous fluorescent hydrogel based on amino-modified carbon dots with excellent sorption and sensing abilities for environmentally hazardous Cr (VI). J. Hazard. Mater. 2021, 401, 123432. [Google Scholar] [CrossRef]
- Wang, R.; Jiao, L.; Zhou, X.; Guo, Z.; Bian, H.; Dai, H. Highly fluorescent graphene quantum dots from biorefinery waste for tri-channel sensitive detection of Fe3+ ions. J. Hazard. Mater. 2021, 412, 125096. [Google Scholar] [CrossRef]
- Zhao, S.; Song, X.; Chai, X.; Zhao, P.; He, H.; Liu, Z. Green production of fluorescent carbon quantum dots based on pine wood and its application in the detection of Fe3+. J. Clean. Prod. 2020, 263, 121561. [Google Scholar] [CrossRef]
- Zhou, J.; Sheng, Z.; Han, H.; Zou, M.; Li, C. Facile synthesis of fluorescent carbon dots using watermelon peel as a carbon source. Mater. Lett. 2012, 66, 222–224. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Margel, S.; Gedanken, A. Tailor made magnetic nanolights: Fabrication to cancer theranostics applications. Nanoscale Adv. 2021, 3, 6762–6796. [Google Scholar] [CrossRef]
- Gedda, G.; Lee, C.Y.; Lin, Y.C.; Wu, H.F. Green synthesis of carbon dots from prawn shells for highly selective and sensitive detection of copper ions. Sens. Actuators B Chem. 2016, 224, 396–403. [Google Scholar] [CrossRef]
- Guan, Y.; Yu, H.Y.; Abdalkarim, S.Y.H.; Wang, C.; Tang, F.; Marek, J.; Chen, W.L.; Militky, J.; Yao, J.M. Green one-step synthesis of ZnO/cellulose nanocrystal hybrids with modulated morphologies and superfast absorption of cationic dyes. Int. J. Biol. Macromol. 2019, 132, 51–62. [Google Scholar] [CrossRef]
- Lu, W.; Qin, X.; Liu, S.; Chang, G.; Zhang, Y.; Luo, Y.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Economical, green synthesis of fluorescent carbon nanoparticles and their use as probes for sensitive and selective detection of mercury (II) ions. Anal. Chem. 2012, 84, 5351–5357. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Tripathi, K.M.; Singh, N.; Choudhary, S.; Gupta, R.K. Green synthesis of carbon quantum dots from lemon peel waste: Applications in sensing and photocatalysis. RSC Adv. 2016, 6, 72423–72432. [Google Scholar] [CrossRef]
- Xue, M.; Zhan, Z.; Zou, M.; Zhang, L.; Zhao, S. Green synthesis of stable and biocompatible fluorescent carbon dots from peanut shells for multicolor living cell imaging. New J. Chem. 2016, 40, 1698–1703. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Z.; Zhang, W.; Wang, W.; Chang, J.; Kai, J. Fluorescent carbon dots as nanoprobe for determination of lidocaine hydrochloride. Sens. Actuators B Chem. 2018, 262, 928–937. [Google Scholar] [CrossRef]
- Lee, J.H.; Luyima, D.; Lee, C.H.; Park, S.J.; Oh, T.K. Efficiencies of unconventional bulking agents in composting food waste in Korea. Appl. Biol. Chem. 2020, 63, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, C.; Dutta, S.; Saxena, V.K. A review on biosorptive removal of dyes and heavy metals from wastewater using watermelon rind as biosorbent. Environ. Adv. 2020, 2, 100007. [Google Scholar] [CrossRef]
- Xu, D.; Fu, N.; Xie, Y.; Wang, Y.; Xie, R.; Yang, H.; Sun, W.; Liu, X.; Han, A. Easy formation of nitrogen-doped carbon dots towards Hg2+ fluorescent measurement and multicolor intracellular imaging. Mater. Chem. Phys. 2021, 266, 124547. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, L.; Zhang, S.; Yang, Y.; Chen, X.; Zhang, M. Fluorescent carbon nanoparticles for the fluorescent detection of metal ions. Biosens. Bioelectron. 2015, 63, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, L.; Dong, X.; Shi, Z.; Jin, J. Chemically tailoring graphene oxides into fluorescent nanosheets for Fe3+ ion detection. Carbon 2012, 50, 2147–2154. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for cell viability assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- Ahn, J.; Song, Y.; Kwon, J.E.; Lee, S.H.; Park, K.S.; Kim, S.; Woo, J.; Kim, H. Food waste-driven N-doped carbon dots: Applications for Fe3+ sensing and cell imaging. Mater. Sci. Eng. C 2019, 102, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Gao, W.; Gupta, B.K.; Liu, Z.; Romero-Aburto, R.; Ge, L.; Song, L.; Alemany, L.B.; Zhan, X.; Gao, G.; et al. Graphene quantum dots derived from carbon fibers. Nano Lett. 2012, 12, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Song, N.; Zhang, Y.K.; Zhong, S.X.; Wang, A.J.; Chen, J. Green preparation of carbon dots by Jinhua bergamot for sensitive and selective fluorescent detection of Hg2+ and Fe3+. Sens. Actuators B Chem. 2015, 214, 29–35. [Google Scholar] [CrossRef]
- Van Tam, T.; Choi, W.M. One-pot synthesis of highly fluorescent amino-functionalized graphene quantum dots for effective detection of copper ions. Curr. Appl. Phys. 2018, 18, 1255–1260. [Google Scholar]
- Rodwihok, C.; Wongratanaphisan, D.; Thi Ngo, Y.L.; Khandelwal, M.; Hur, S.H.; Chung, J.S. Effect of GO additive in ZnO/rGO nanocomposites with enhanced photosensitivity and photocatalytic activity. Nanomaterials 2019, 9, 1441. [Google Scholar] [CrossRef] [Green Version]
- Khose, R.V.; Bangde, P.; Bondarde, M.P.; Dhumal, P.S.; Bhakare, M.A.; Chakraborty, G.; Ray, A.K.; Dandekar, P.; Some, S. Waste derived approach towards wealthy fluorescent N-doped graphene quantum dots for cell imaging and H2O2 sensing applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 266, 120453. [Google Scholar] [CrossRef]
- Ngo, Y.L.T.; Choi, W.M.; Chung, J.S.; Hur, S.H. Highly biocompatible phenylboronic acid-functionalized graphitic carbon nitride quantum dots for the selective glucose sensor. Sens. Actuators B Chem. 2019, 282, 36–44. [Google Scholar] [CrossRef]
- Wang, L.; Jana, J.; Chung, J.S.; Hur, S.H. High quantum yield aminophenylboronic acid-functionalized N-doped carbon dots for highly selective hypochlorite ion detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 260, 119895. [Google Scholar] [CrossRef]
- Van Tam, T.; Hong, S.H.; Choi, W.M. Facile synthesis of cysteine–functionalized graphene quantum dots for a fluorescence probe for mercury ions. RSC Adv. 2015, 5, 97598–97603. [Google Scholar]
- Issa, M.A.; Abidin, Z.Z.; Sobri, S.; Rashid, S.A.; Mahdi, M.A.; Ibrahim, N.A. Fluorescent recognition of Fe3+ in acidic environment by enhanced-quantum yield N-doped carbon dots: Optimization of variables using central composite design. Sci. Rep. 2020, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Zhu, S.; Maharjan, S.; Hao, Z.; Song, Y.; Zhao, X.; Jiang, Y.; Yang, B.; Lu, L. Elucidating the endocytosis, intracellular trafficking, and exocytosis of carbon dots in neural cells. RSC Adv. 2014, 4, 62086–62095. [Google Scholar] [CrossRef]
- Ananthanarayanan, A.; Wang, X.; Routh, P.; Sana, B.; Lim, S.; Kim, D.H.; Lim, K.H.; Li, J.; Chen, P. Facile synthesis of graphene quantum dots from 3D graphene and their application for Fe3+ sensing. Adv. Funct. Mater. 2014, 24, 3021–3026. [Google Scholar] [CrossRef]
- Guo, R.; Zhou, S.; Li, Y.; Li, X.; Fan, L.; Voelcker, N.H. Rhodamine-functionalized graphene quantum dots for detection of Fe3+ in cancer stem cells. ACS Appl. Mater. Interfaces 2015, 7, 23958–23966. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, Y.; Cao, J.; Zhu, J.; Fan, L.; Li, X. Sulfur-doped graphene quantum dots as a novel fluorescent probe for highly selective and sensitive detection of Fe3+. Anal. Chem. 2014, 86, 10201–10207. [Google Scholar] [CrossRef]
- Dutta Chowdhury, A.; Doong, R.A. Highly sensitive and selective detection of nanomolar ferric ions using dopamine functionalized graphene quantum dots. ACS Appl. Mater. Interfaces 2016, 8, 21002–21010. [Google Scholar] [CrossRef]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew. Chem. 2013, 125, 4045–4049. [Google Scholar] [CrossRef]
- Bandi, R.; Gangapuram, B.R.; Dadigala, R.; Eslavath, R.; Singh, S.S.; Guttena, V. Facile and green synthesis of fluorescent carbon dots from onion waste and their potential applications as sensor and multicolour imaging agents. RSC Adv. 2016, 6, 28633–28639. [Google Scholar] [CrossRef]
- Jiao, X.Y.; Li, L.S.; Qin, S.; Zhang, Y.; Huang, K.; Xu, L. The synthesis of fluorescent carbon dots from mango peel and their multiple applications. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 577, 306–314. [Google Scholar] [CrossRef]
- Krishnaiah, P.; Atchudan, R.; Perumal, S.; Salama, E.S.; Lee, Y.R.; Jeon, B.H. Utilization of waste biomass of Poa pratensis for green synthesis of n-doped carbon dots and its application in detection of Mn2+ and Fe3+. Chemosphere 2022, 286, 131764. [Google Scholar] [CrossRef] [PubMed]
- Van Tam, T.; Trung, N.B.; Kim, H.R.; Chung, J.S.; Choi, W.M. One-pot synthesis of N-doped graphene quantum dots as a fluorescent sensing platform for Fe3+ ions detection. Sens. Actuators B Chem. 2014, 202, 568–573. [Google Scholar]

| Sample | Integrated Emission Intensity | Absorbance at 365 nm | Refractive Index of Solvent | Quantum Yield at 365 nm |
|---|---|---|---|---|
| Quinine sulfate | 30,110 (I) | 1.59 (A) | 1.33 (n) | 0.54 (Q) |
| GQDs | 7593 (I) | 0.59 (A) | 1.33 (n) | 0.37 (Q) |
| Sample | Standard Fe Concentration (M) | Detected Fe Concentration (M) | Recovery Percentage of Fe (%) | Relative Standard Deviation (%) |
|---|---|---|---|---|
| Tap water | 80 | 77.63 | 97.04 | 0.68 |
| 78.51 | 98.14 | |||
| 77.55 | 96.94 | |||
| 50 | 47.83 | 95.66 | 0.98 | |
| 48.02 | 96.04 | |||
| 48.72 | 97.45 | |||
| 10 | 9.55 | 95.47 | 1.12 | |
| 9.67 | 96.74 | |||
| 9.76 | 97.63 | |||
| Drinking water | 80 | 78.66 | 97.04 | 0.68 |
| 77.58 | 96.98 | |||
| 78.03 | 97.54 | |||
| 50 | 48.93 | 97.86 | 0.34 | |
| 49.06 | 98.12 | |||
| 48.73 | 97.46 | |||
| 10 | 9.72 | 97.23 | 0.50 | |
| 9.74 | 97.36 | |||
| 9.57 | 95.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodwihok, C.; Tam, T.V.; Choi, W.M.; Suwannakaew, M.; Woo, S.W.; Wongratanaphisan, D.; Kim, H.S. Preparation and Characterization of Photoluminescent Graphene Quantum Dots from Watermelon Rind Waste for the Detection of Ferric Ions and Cellular Bio-Imaging Applications. Nanomaterials 2022, 12, 702. https://doi.org/10.3390/nano12040702
Rodwihok C, Tam TV, Choi WM, Suwannakaew M, Woo SW, Wongratanaphisan D, Kim HS. Preparation and Characterization of Photoluminescent Graphene Quantum Dots from Watermelon Rind Waste for the Detection of Ferric Ions and Cellular Bio-Imaging Applications. Nanomaterials. 2022; 12(4):702. https://doi.org/10.3390/nano12040702
Chicago/Turabian StyleRodwihok, Chatchai, Tran Van Tam, Won Mook Choi, Mayulee Suwannakaew, Sang Woon Woo, Duangmanee Wongratanaphisan, and Han S. Kim. 2022. "Preparation and Characterization of Photoluminescent Graphene Quantum Dots from Watermelon Rind Waste for the Detection of Ferric Ions and Cellular Bio-Imaging Applications" Nanomaterials 12, no. 4: 702. https://doi.org/10.3390/nano12040702
APA StyleRodwihok, C., Tam, T. V., Choi, W. M., Suwannakaew, M., Woo, S. W., Wongratanaphisan, D., & Kim, H. S. (2022). Preparation and Characterization of Photoluminescent Graphene Quantum Dots from Watermelon Rind Waste for the Detection of Ferric Ions and Cellular Bio-Imaging Applications. Nanomaterials, 12(4), 702. https://doi.org/10.3390/nano12040702






