Fabrication of Bi2MoO6 Nanosheets/TiO2 Nanorod Arrays Heterostructures for Enhanced Photocatalytic Performance under Visible-Light Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of TiO2 Nanorod Arrays
2.3. Synthesis of the Bi2MoO6/TiO2 Composites
2.4. Characterization
2.5. Photocatalytic Activity
2.6. Assessment of PEC Performance
3. Results and Discussion
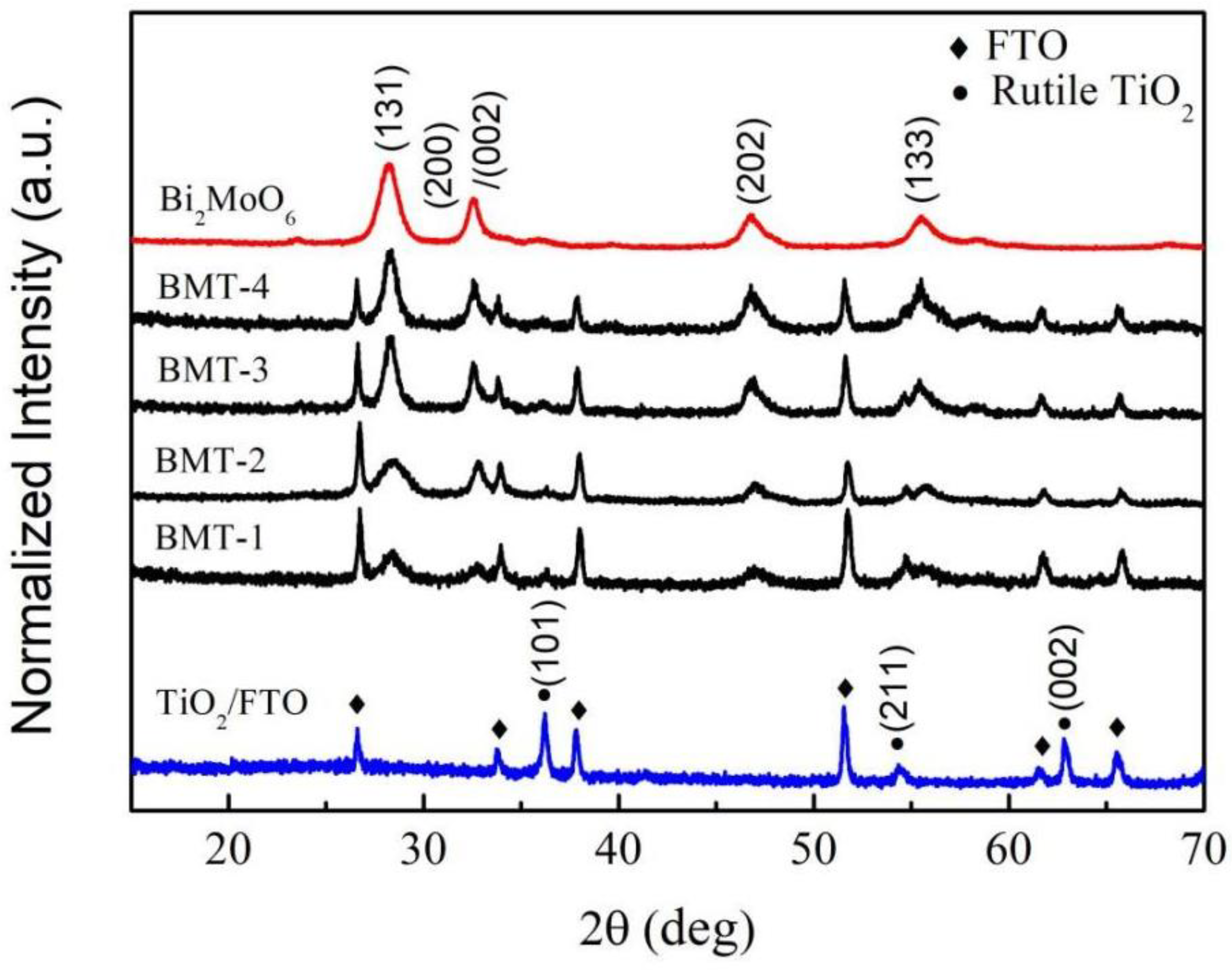
3.1. X-ray Diffraction (XRD)
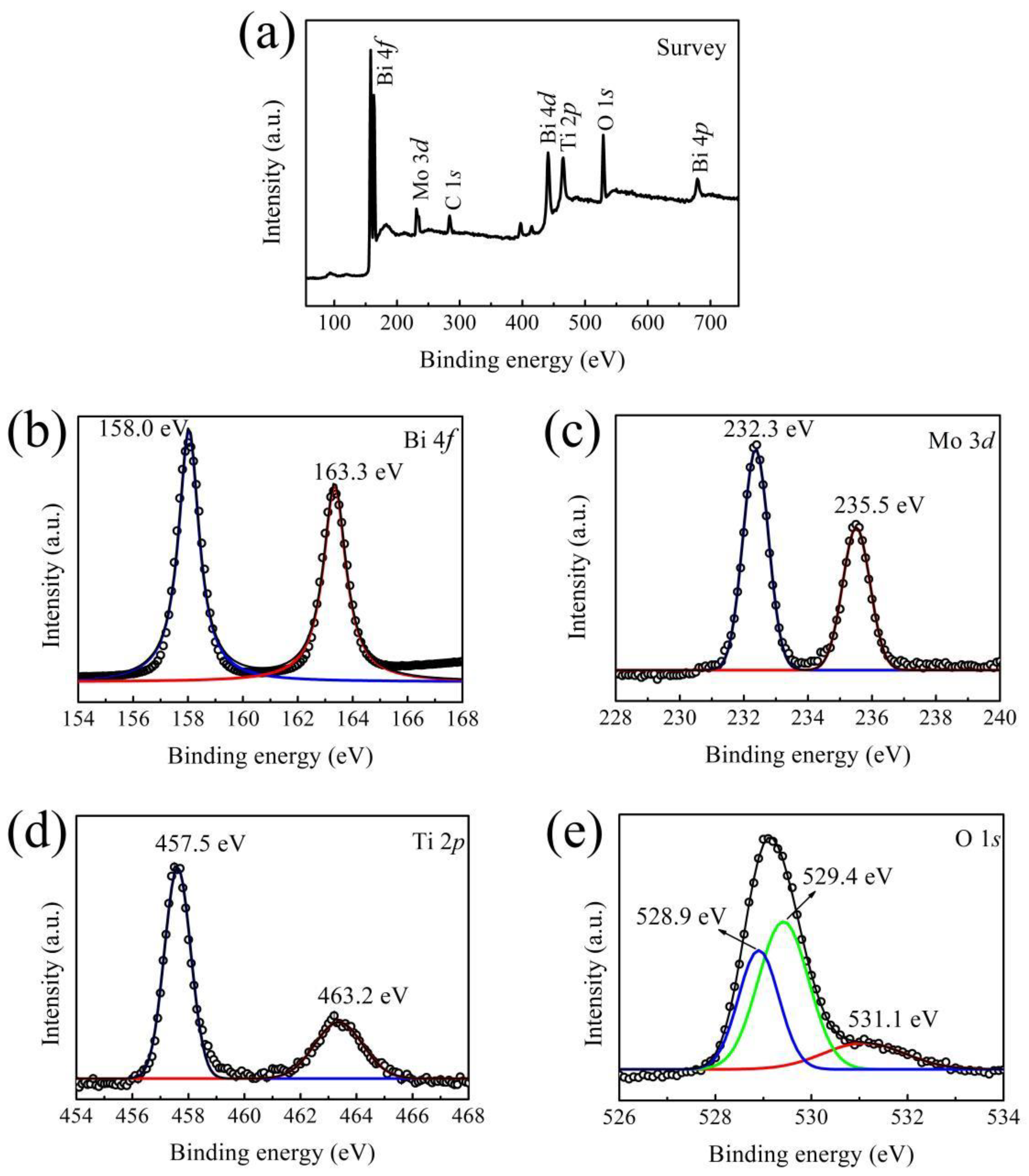
3.2. X-ray Photoelectron Spectroscopy (XPS)
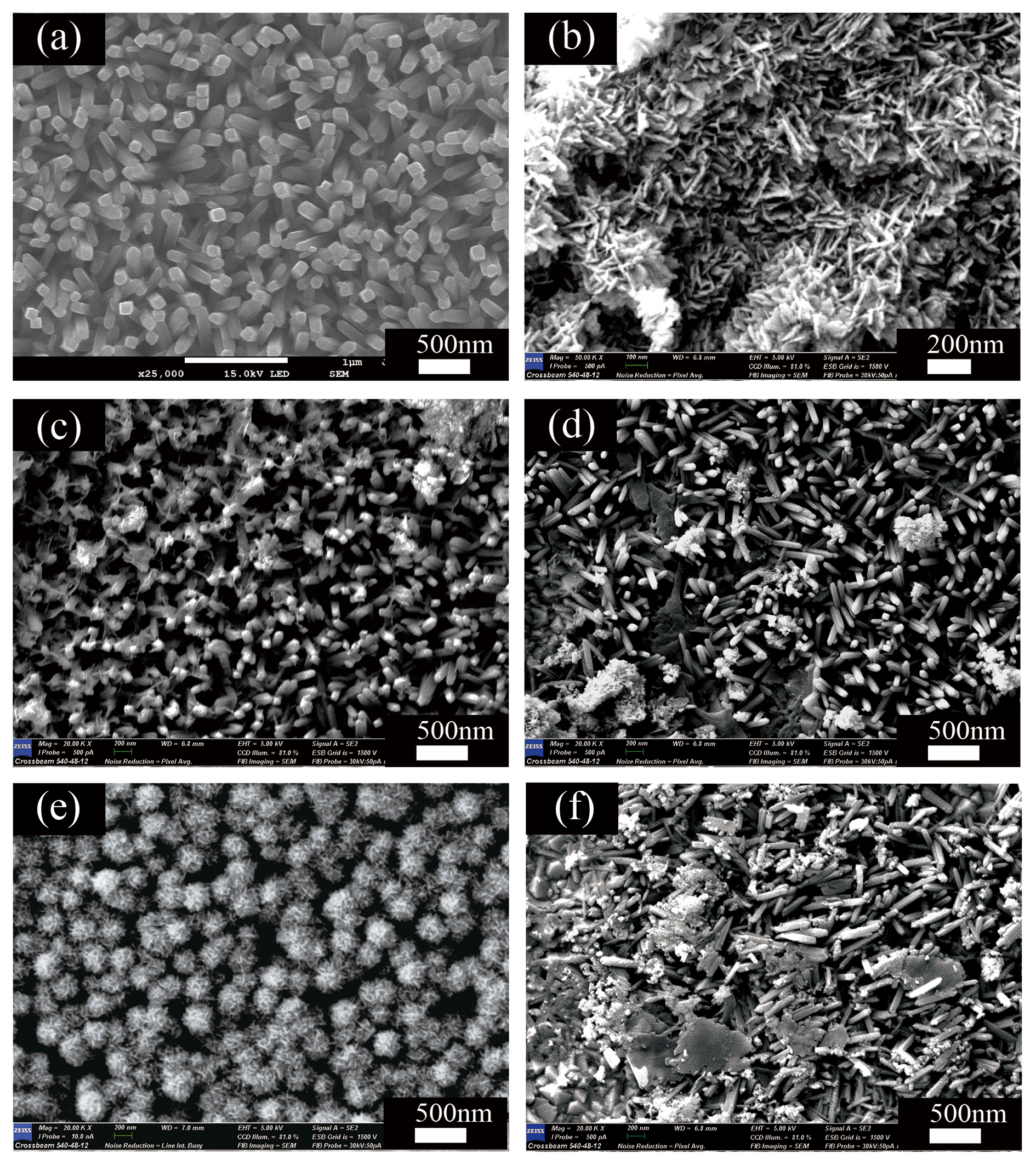
3.3. Morphologies
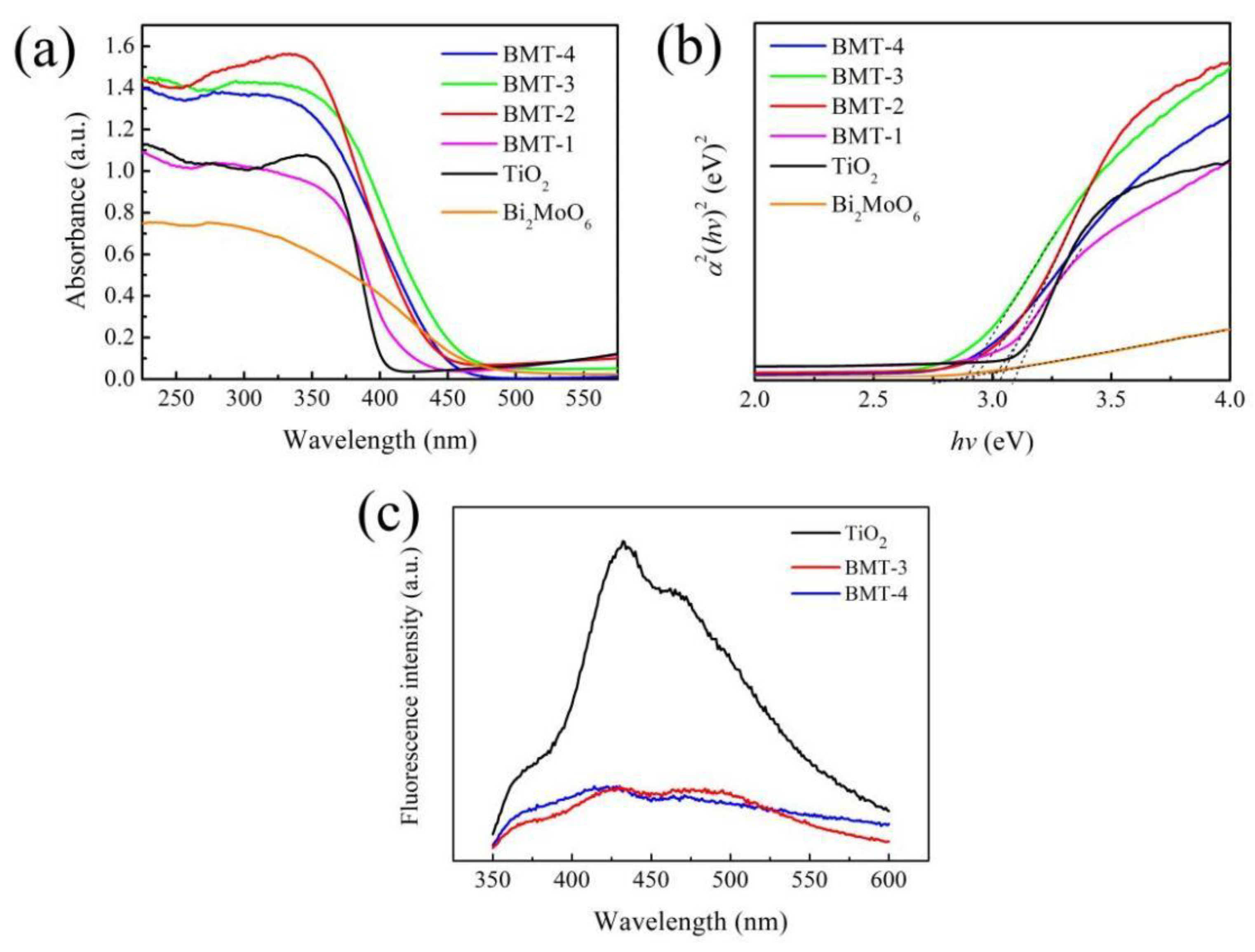
3.4. Optical Properties
3.5. Photocatalytic Properties
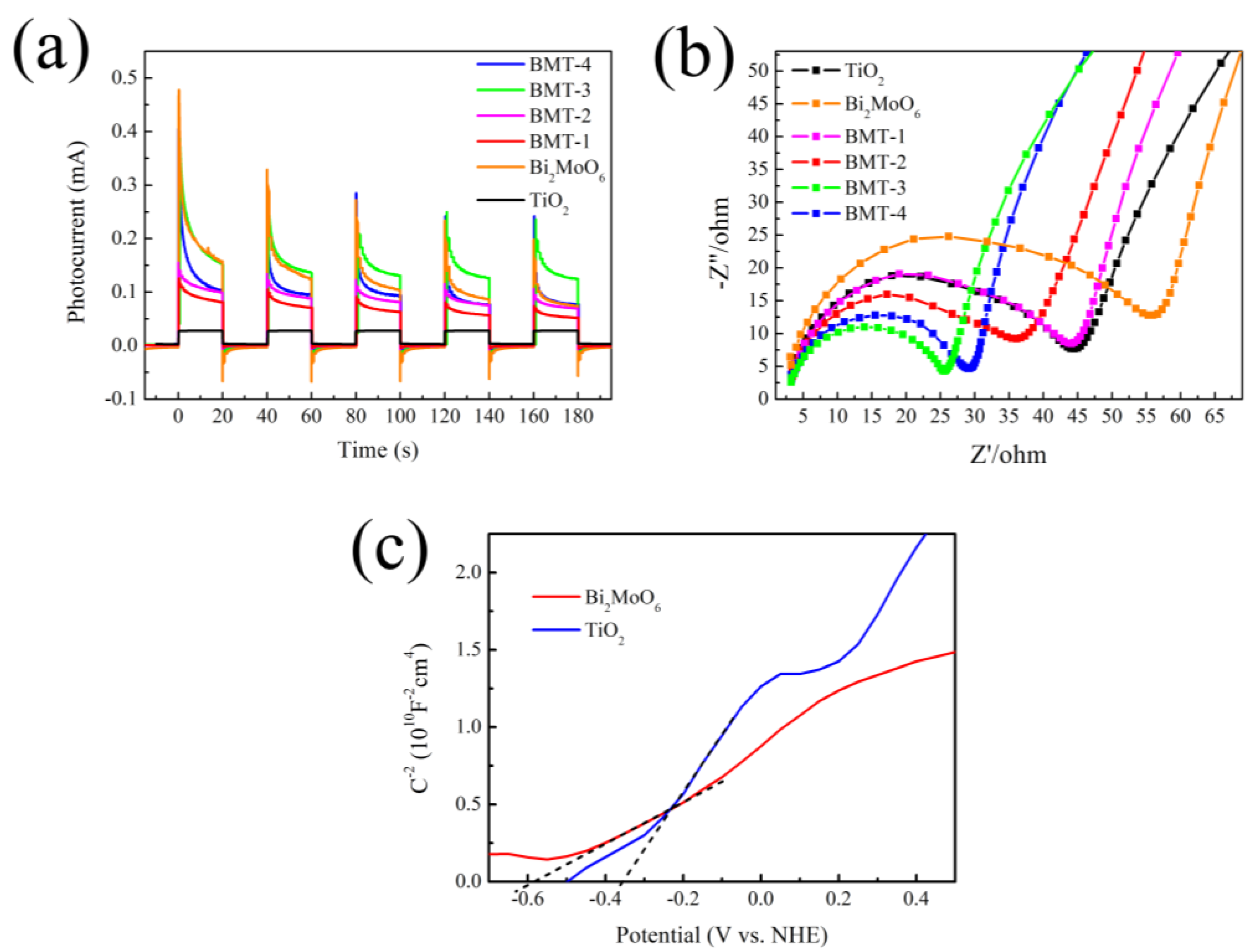
3.6. PEC Analysis
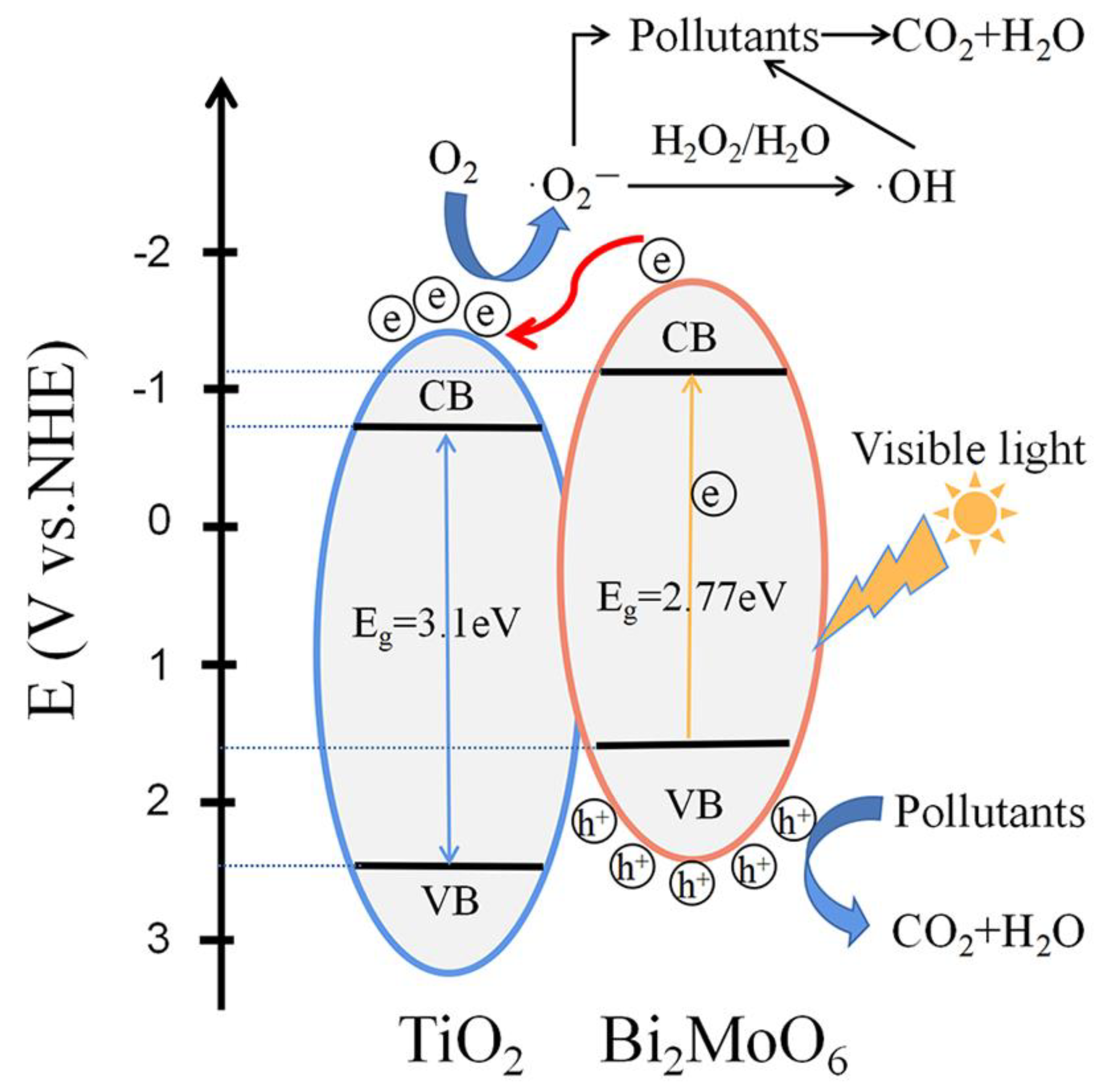
3.7. Energy Band Alignment and Photocatalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Schrauzer, G.N.; Guth, T.D. Photolysis of water and photoreduction of nitrogen on titanium dioxide. J. Am. Chem. Soc. 1977, 99, 7189–7193. [Google Scholar] [CrossRef]
- Kraeutler, B.; Bard, A.J. Heterogeneous photocatalytic preparation of supported catalysts. Photodeposition of platinum on titanium dioxide powder and other substrates. J. Am. Chem. Soc. 1978, 100, 4317–4318. [Google Scholar] [CrossRef]
- Fujishima, A.; Hashimoto, K.; Watanabe, T. TiO2 Photocatalysis: Fundamentals and Applications; BKC, Inc.: Tokyo, Japan, 1999. [Google Scholar]
- Tang, H.; Prasad, K.; Sanjinbs, R.; Schmid, P.E.; Levy, F. Electrical and optical properties of TiO2 anatase thin films. J. Appl. Phys. 1994, 75, 2042–2047. [Google Scholar] [CrossRef]
- Yadav, H.M.; Kim, J.S.; Pawar, S.H. Developments in photocatalytic antibacterial activity of nano TiO2: A review. Korean J. Chem. Eng. 2016, 33, 1989–1998. [Google Scholar] [CrossRef]
- Sahare, S.; Veldurthi, N.; Singh, R.; Swarnkar, A.K.; Bhave, T. Enhancing the efficiency of flexible dye-sensitized solar cells utilizing natural dye extracted from Azadirachta indica. Mater. Res. Express 2015, 2, 105903. [Google Scholar] [CrossRef]
- Grabowska, E.; Reszczyńska, J.; Zaleska, A. Mechanism of phenol photodegradation in the presence of pure and modified-TiO2: A review. Water Res. 2012, 46, 5453–5471. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Jaafar, J.; Zain, M.F.M.; Minggu, L.J.; Kassim, M.B.; Salehmin, M.N.I.; Rosmie, M.S.; Salleh, W.N.W.; Othman, M.H.D. Concurrent growth, structural and photocatalytic properties of hybridized C, N co-doped TiO2 mixed phase over g-C3N4 nanostructured. Scr. Mater. 2018, 142, 143–147. [Google Scholar] [CrossRef]
- Saber, N.B.; Mezni, A.; Alrooqi, A.; Altalhi, T. Fabrication of efficient Au@TiO2/rGO heterojunction nanocomposite: Boosted photocatalytic activity under ultraviolet and visible light irradiation. J. Mater. Res. Technol. 2021, 12, 2238–2246. [Google Scholar] [CrossRef]
- Ismael, M. A review and recent advances in solar-hydrogen energy conversion based on photocatalytic water splitting over doped-TiO2 nanoparticles. Sol. Energy 2020, 211, 522–546. [Google Scholar] [CrossRef]
- Pham, V.V.; Phat, B.D.; Huy, T.H.; Cao, M.T.; Le, V.H. Photoreduction route for Cu2O/TiO2 nanotubes junction for enhanced photocatalytic activity. RSC Adv. 2018, 8, 12420–12427. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Mao, Z.P.; Zhou, D.; Hu, Z.L.; Tu, Y.F.; Tian, Y.; Zheng, G. Fabrication of Ni-doped PbTiO3-coated TiO2 nanorod arrays for improved photoelectrochemical performance. J. Nanomater. 2019, 11, 2019. [Google Scholar] [CrossRef]
- Fu, Y.; Mao, Z.P.; Zhou, D.; Hu, Z.L.; Zheng, G. Preparation of BiFeO3-overcoated TiO2 nanorod arrays for the enhanced visible-light activity. Mater. Res. Express 2019, 6, 1050c6. [Google Scholar] [CrossRef]
- Liu, X.; Gu, S.; Zhao, Y.; Zhou, G.; Li, W. BiVO4, Bi2WO6 and Bi2MoO6 photocatalysis: A brief review. J. Mater. Sci. Technol. 2020, 56, 45–68. [Google Scholar] [CrossRef]
- Chai, S.Y.; Kim, Y.J.; Jung, M.H.; Chakraborty, A.K.; Jung, D.; Lee, W.I. Heterojunctioned BiOCl/Bi2O3, a new visible light photocatalyst. J. Catal. 2009, 262, 144–149. [Google Scholar] [CrossRef]
- Xiang, Z.; Wang, Y.; Zhang, D.; Ju, P. BiOI/BiVO4 p-n heterojunction with enhanced photocatalytic activity under visible-light irradiation. J. Ind. Eng. Chem. 2016, 40, 83–92. [Google Scholar] [CrossRef]
- Wei, W.; Dai, Y.; Huang, B. First-principles characterization of Bi-based photocatalysts: Bi12TiO20, Bi2Ti2O7, and Bi4Ti3O12. J. Phys. Chem. C 2009, 113, 5658–5663. [Google Scholar] [CrossRef]
- Yu, H.; Jiang, L.; Wang, H.; Huang, B.; Zeng, G. Photocatalysis: Modulation of Bi2MoO6-based materials for photocatalytic water splitting and environmental application: A critical review. Small 2019, 15, 1901008. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Liu, Q.; Zhang, J.; Zhang, Y.; Geng, M.; Yu, J. Enhanced visible-light-driven photocatalytic activity by 0D/2D phase heterojunction of quantum dots/nanosheets on bismuth molybdates. J. Phys. Chem. C. 2018, 122, 3738–3747. [Google Scholar] [CrossRef]
- Tian, G.; Chen, Y.; Zhai, R.; Zhou, J.; Zhou, W.; Wang, R.; Pan, K.; Tiana, C.; Fu, H. Hierarchical flake-like Bi2MoO6/TiO2 bilayer films for visible-light-induced self-cleaning applications. J. Mater. Chem. A 2013, 1, 6961–6968. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Sun, Z.; Pan, L. Novel Bi2MoO6/TiO2 heterostructure microspheres for degradation of benzene series compound under visible light irradiation. J. Colloid Interface Sci. 2016, 463, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Shao, C.; Mu, J.; Zhang, Z.; Guo, Z.; Zhang, P.; Liu, Y. One-dimensional Bi2MoO6/TiO2 hierarchical heterostructures with enhanced photocatalytic activity. CrystEngComm 2012, 14, 605–612. [Google Scholar] [CrossRef]
- Li, L.; Salvador, P.A.; Rohrer, G.S. Photocatalysts with internal electric fields. Nanoscale 2014, 6, 24–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Basu, S. Highly reusable visible light active hierarchical porous WO3/SiO2 monolith in centimeter length scale for enhanced photocatalytic degradation of toxic pollutants. Sep. Purif. Technol. 2020, 231, 115916. [Google Scholar] [CrossRef]
- Lindgren, T.; Wang, H.; Beermann, N.; Vayssieres, L.; Hagfeldt, A.; Lindquist, S.-E. Aqueous photoelectrochemistry of hematite nanorod array. Sol. Energy Mater. Sol. Cells 2002, 71, 231–243. [Google Scholar] [CrossRef]
- Zhang, D.S.; Downing, J.A.; Knorr, F.J.; McHale, J.L. Nanostructured semiconductor composites for solar cells. J. Chem. B 2006, 110, 32–36. [Google Scholar]
- Ge, M.; Cao, C.; Huang, J.; Li, S.; Lai, Y. A review of one-dimensional TiO2 nanostructured materials for environmental and energy applications. J. Mater. Chem. A 2016, 4, 6772–6801. [Google Scholar] [CrossRef]
- Shuang, S.; Zheng, X.; Zhang, Z. Review: Nanostructured TiO2 for enhanced photocatalytic property by glancing angle deposition method. J. Harbin Inst. Technol. 2017, 24, 1–11. [Google Scholar]
- Cheng, X.; Zhang, Y.; Bi, Y. Spatial dual-electric fields for highly enhanced the solar water splitting of TiO2 nanotube arrays. Nano Energy 2019, 57, 542–548. [Google Scholar] [CrossRef]
- Nazim, M.; Khan, A.A.P.; Asiri, A.M.; Kim, J.H. Exploring rapid photocatalytic degradation of organic pollutants with porous CuO nanosheets: Synthesis, dye removal, and kinetic studies at room temperature. ACS Omega 2021, 6, 2601–2612. [Google Scholar] [CrossRef]
- Cani, D.; Waal, J.C.; Pescarmona, P.P. Highly-accessible, doped TiO2 nanoparticles embedded at the surface of SiO2 as photocatalysts for the degradation of pollutants under visible and UV radiation. Appl. Catal. A Gen. 2021, 621, 118179. [Google Scholar] [CrossRef]
- D’Angelo, D.; Filice, S.; Scarangella, A.; Iannazzo, D.; Compagnini, G.; Scalese, S. Bi2O3/Nexar® polymer nanocomposite membranes for azo dyes removal by UV–vis or visible light irradiation. Catal. Today 2019, 321–322, 158–163. [Google Scholar] [CrossRef]
- Li, Z.Q.; Chen, X.T.; Xue, Z.L. Bi2MoO6 microstructures: Controllable synthesis, growth mechanism, and visible-light-driven photocatalytic activities. CrystEngComm 2013, 15, 498–508. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, T.; Zhao, X.; Zhu, Y. Controllable synthesis of Bi2MoO6 and effect of morphology and variation in local structure on photocatalytic activities. Appl. Catal. B Environ. 2010, 98, 138–146. [Google Scholar] [CrossRef]
- Li, N.; Gao, H.; Wang, X.; Zhao, S.; Da, L.; Yang, G.; Gao, X.; Fan, H.; Gao, Y.; Ge, L. Novel indirect Z-scheme g-C3N4/Bi2MoO6/Bi hollow microsphere heterojunctions with SPR-promoted visible absorption and highly enhanced photocatalytic performance. Chin. J. Catal. 2020, 41, 426–434. [Google Scholar] [CrossRef]
- Mia, M.S.; Yao, P.; Zhu, X.; Lei, X.; Chen, G. Degradation of textile dyes from aqueous solution using tea-polyphenol/Fe loaded waste silk fabrics as Fenton-like catalysts. RSC Adv. 2021, 11, 8290–8305. [Google Scholar] [CrossRef]
- Li, H.; Zhang, T.; Pan, C.; Pu, C.; Hu, Y.; Hu, X.; Liu, E.; Fan, J. Self-assembled Bi2MoO6/TiO2 nanofiber heterojunction film with enhanced photocatalytic activities. Appl. Surf. Sci. 2017, 391, 303–310. [Google Scholar] [CrossRef]
- Cai, K.; Lv, S.; Song, L.; Chen, L.; He, J.; Chen, P.; Au, C.; Yin, S. Facile preparation of ultrathin Bi2MoO6 nanosheets for photocatalytic oxidation of toluene to benzaldehyde under visible light irradiation. J. Solid State Chem. 2019, 269, 145–150. [Google Scholar] [CrossRef]
- Yunarti, R.T.; Safitri, T.N.; Dimonti, L.C.C.; Aulia, G.; Khalil, M.; Ridwan, M. Facile synthesis of composite between titania nanoparticles with highly exposed (001) facet and coconut shell-derived graphene oxide for photodegradation of methylene blue. J. Phys. Chem. Solids 2022, 160, 110357. [Google Scholar] [CrossRef]
- Dinh, C.T.; Yen, H.; Kleitz, F.; Do, T.O. Three-dimensional ordered assembly of thin-shell Au/TiO2 hollow nanospheres for enhanced visible-light-driven photocatalysis. Angew. Chem. Int. Ed. Engl. 2014, 53, 6618–6623. [Google Scholar] [CrossRef]
- Li, Q.; Li, L.; Long, X.; Tu, Y.; Ling, L.; Gu, J.; Hou, L.; Xu, Y.; Liu, N.; Li, Z. Rational design of MIL-88A(Fe)/Bi2WO6 heterojunctions as an efficient photocatalyst for organic pollutant degradation under visible light irradiation. Opt. Mater. 2021, 118, 111260. [Google Scholar] [CrossRef]
- Ali, H.; Guler, A.C.; Masar, M.; Urbanek, P.; Urbanek, M.; Skoda, D.; Suly, P.; Machovsky, M.; Galusek, D.; Kuritka, I. Solid-state synthesis of direct Z-scheme Cu2O/WO3 nanocomposites with enhanced visible-light photocatalytic performance. Catalysts 2021, 11, 293. [Google Scholar] [CrossRef]
- Tian, J.; Hao, P.; Wei, N.; Cui, H.; Liu, H. 3D Bi2MoO6 nanosheet/TiO2 nanobelt heterostructure: Enhanced photocatalytic activities and photoelectochemistry performance. ACS Catal. 2015, 5, 4530–4536. [Google Scholar] [CrossRef]
- Yus, J.; Ferrari, B.; Sanchez-Herencia, A.J.; Gonzalez, Z. Understanding the effects of different microstructural contributions in the electrochemical response of Nickel-based semiconductor electrodes with 3D hierarchical networks shapes. Electrochim. Acta 2020, 335, 135629. [Google Scholar] [CrossRef]
- Zhao, K.; Zhao, S.; Gao, C.; Qi, J.; Yin, H.; Wei, D.; Mideksa, M.F.; Wang, X.; Gao, Y.; Tang, Z.; et al. Metallic Cobalt–Carbon Composite as Recyclable and Robust Magnetic Photocatalyst for Efficient CO2 Reduction. Small 2018, 14, 1800762. [Google Scholar] [CrossRef]
- Singh, S.; Sangle, A.L.; Wu, T.; Khare, N.; Macmanus-Driscoll, J.L. Growth of doped SrTiO3 ferroelectric nanoporous thin films and tuning of photoelectrochemical properties with switchable ferroelectric polarization. ACS Appl. Mater. Interfaces 2019, 11, 45683–45691. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, J.; Zhao, K.; Cui, D.; Liu, P.; Yin, H.; Al-Mamun, M.; Lowe, S.E.; Zhang, W.; Zhong, Y.L.; et al. Ru(bpy)32+-sensitized {001} facets LiCoO2 nanosheets catalyzed CO2 reduction reaction with 100% carbonaceous products. Nano Res. 2021, 15, 1061–1068. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, H.; Zhang, X.; Yang, W.; Cao, Y.; Yang, P. BiOI/Bi2O2CO3 two-dimensional heteronanostructures with boosting charge carrier separation behavior and enhanced visiblelight photocatalytic performance. J. Phys. Chem. C 2020, 124, 20294–20308. [Google Scholar] [CrossRef]
- Hu, J.R.T.; Gong, Q.; Wang, Q.; Sun, B.; Gao, T.; Cao, P.; Zhou, G. Spherical Bi2WO6/Bi2S3/MoS2 n-p heterojunction with excellent visible-light photocatalytic reduction Cr(VI) activity. Nanomaterials 2020, 10, 1813. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, D.; Du, R.; Hu, Z.; Gao, S.; Tu, Y.; Fu, Y.; Zheng, G.; Zhou, Y. Fabrication of Bi2MoO6 Nanosheets/TiO2 Nanorod Arrays Heterostructures for Enhanced Photocatalytic Performance under Visible-Light Irradiation. Nanomaterials 2022, 12, 574. https://doi.org/10.3390/nano12030574
Zhou D, Du R, Hu Z, Gao S, Tu Y, Fu Y, Zheng G, Zhou Y. Fabrication of Bi2MoO6 Nanosheets/TiO2 Nanorod Arrays Heterostructures for Enhanced Photocatalytic Performance under Visible-Light Irradiation. Nanomaterials. 2022; 12(3):574. https://doi.org/10.3390/nano12030574
Chicago/Turabian StyleZhou, Di, Rui Du, Zhenglong Hu, Shu Gao, Yafang Tu, Yunfei Fu, Guang Zheng, and Youhua Zhou. 2022. "Fabrication of Bi2MoO6 Nanosheets/TiO2 Nanorod Arrays Heterostructures for Enhanced Photocatalytic Performance under Visible-Light Irradiation" Nanomaterials 12, no. 3: 574. https://doi.org/10.3390/nano12030574
APA StyleZhou, D., Du, R., Hu, Z., Gao, S., Tu, Y., Fu, Y., Zheng, G., & Zhou, Y. (2022). Fabrication of Bi2MoO6 Nanosheets/TiO2 Nanorod Arrays Heterostructures for Enhanced Photocatalytic Performance under Visible-Light Irradiation. Nanomaterials, 12(3), 574. https://doi.org/10.3390/nano12030574






