Efficient Hydrogen Evolution Reaction with Bulk and Nanostructured Mitrofanovite Pt3Te4
Abstract
:1. Introduction
2. Materials and Methods
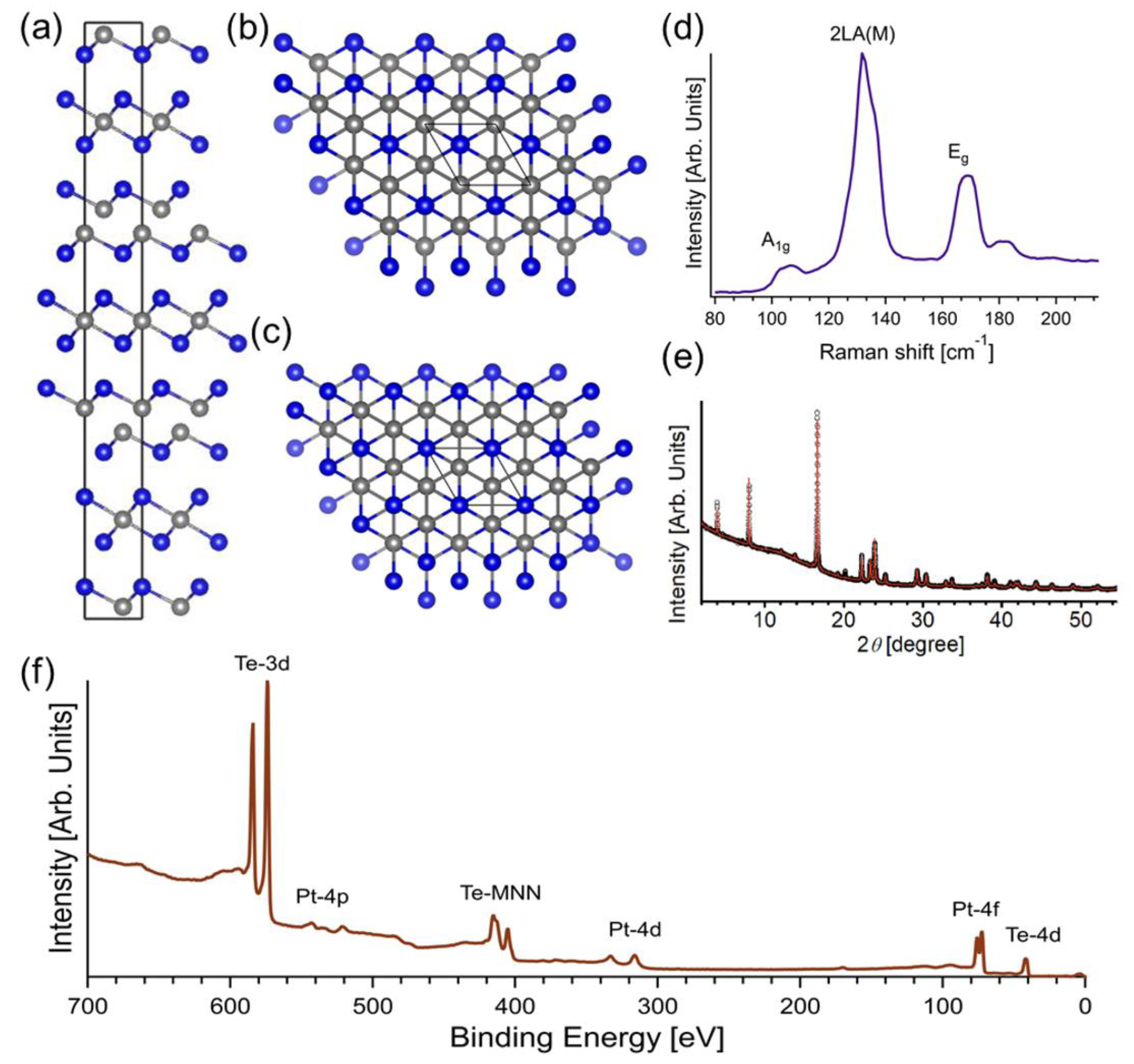
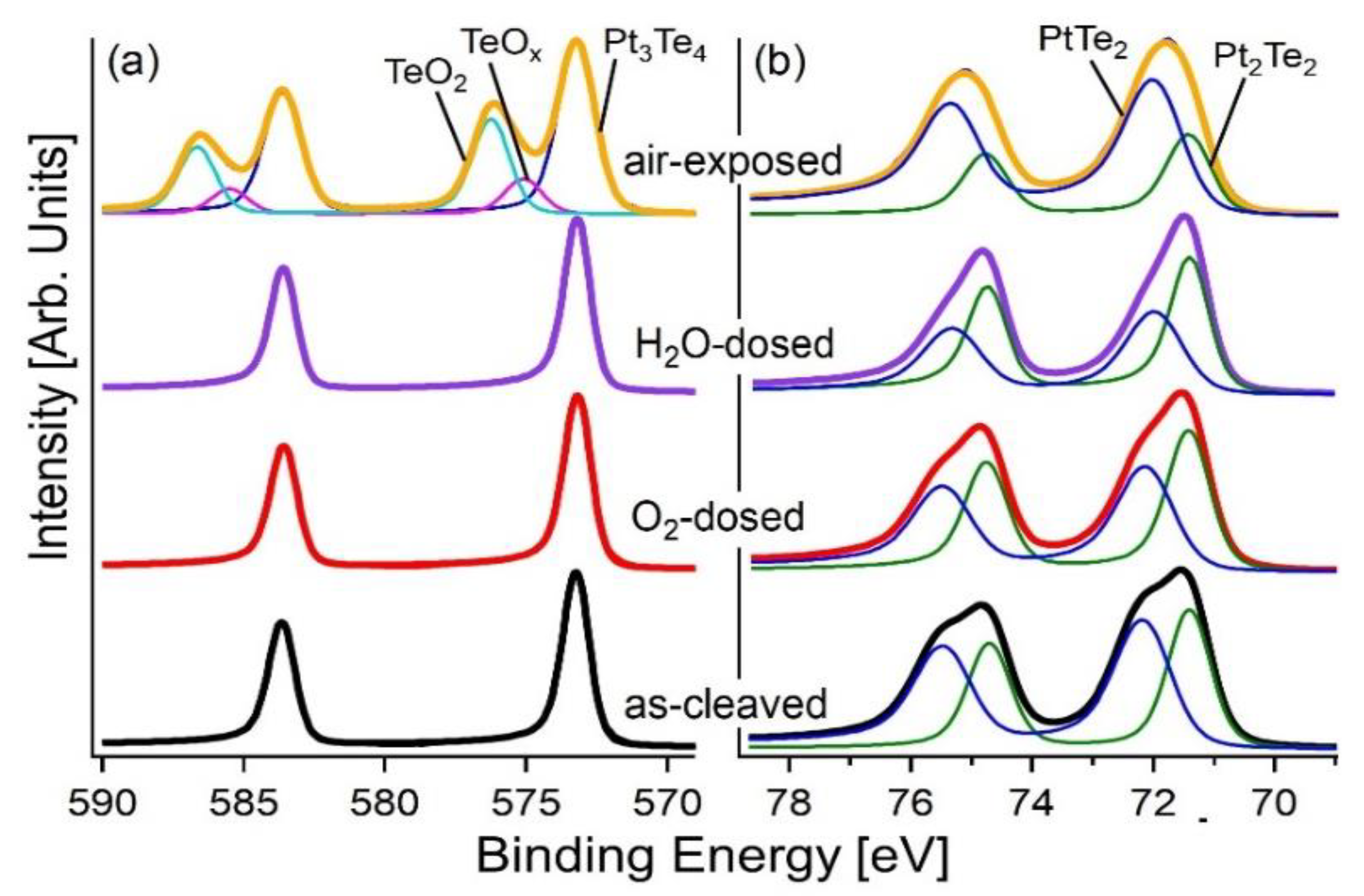
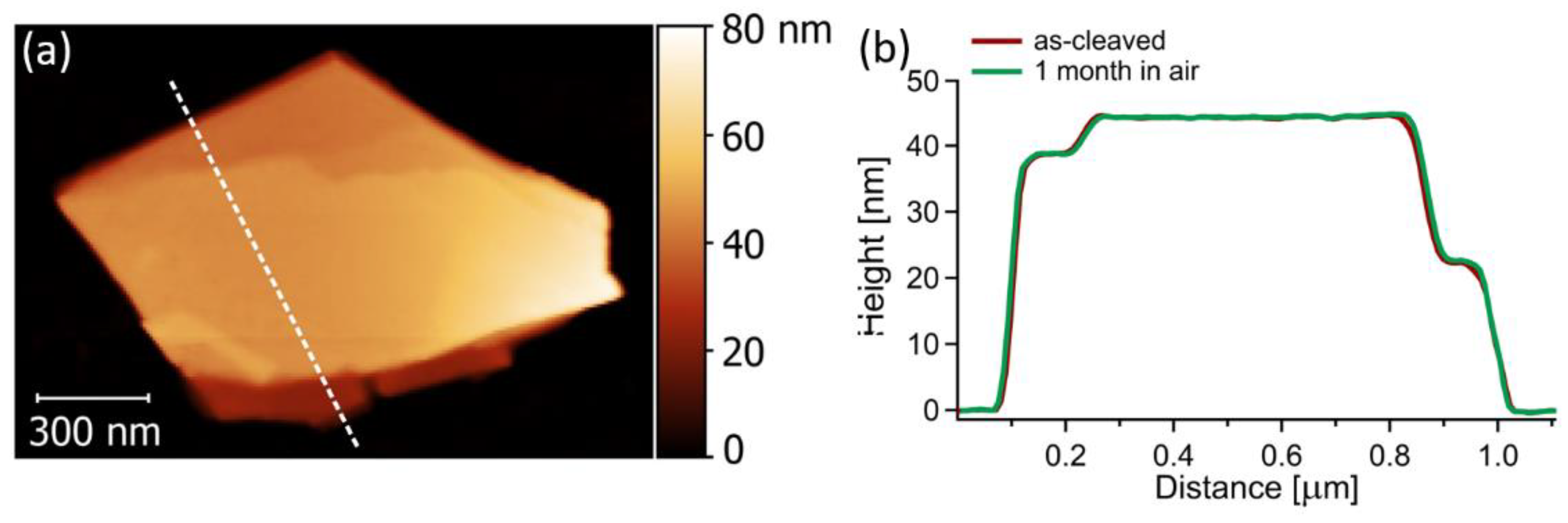
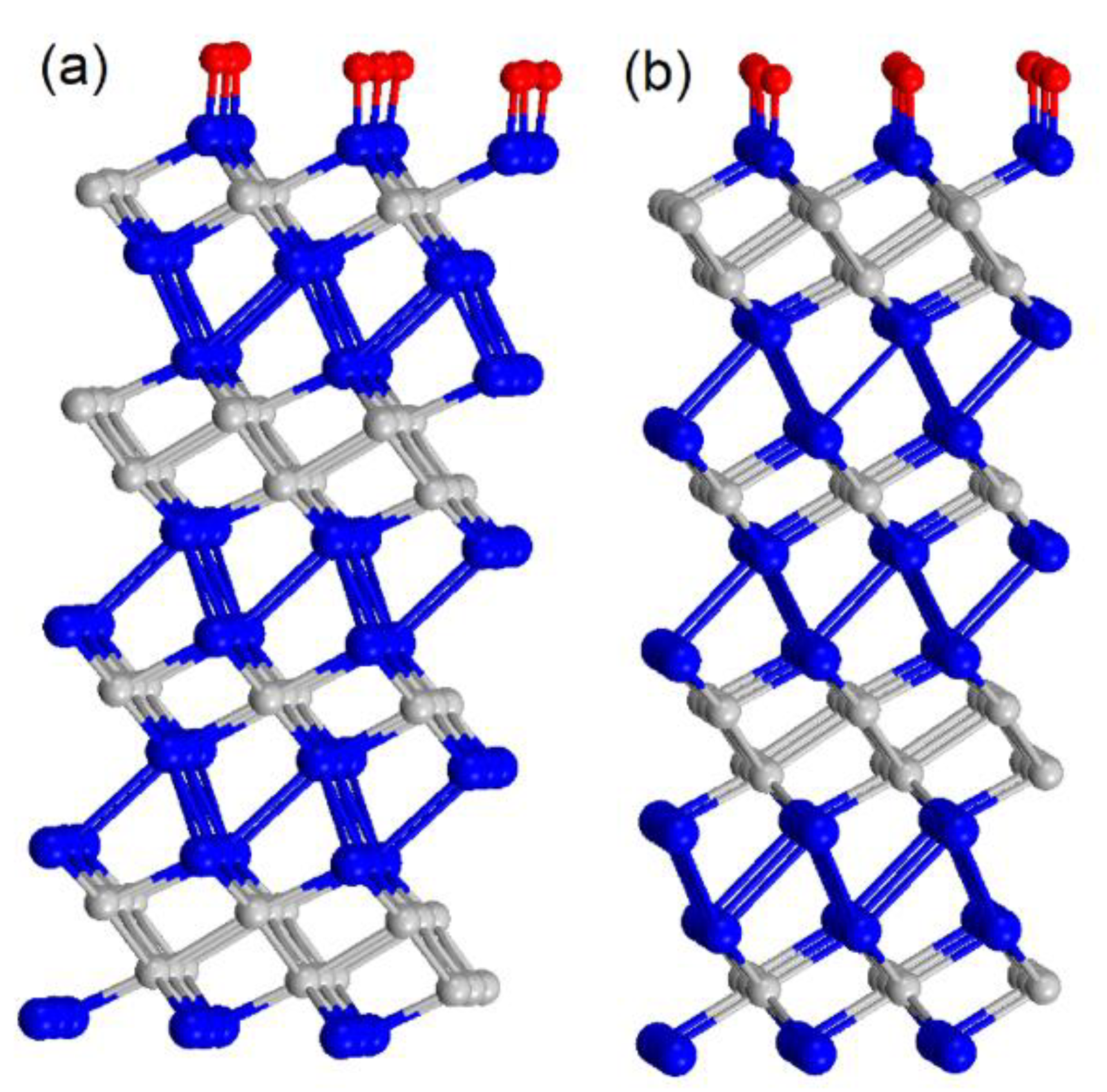
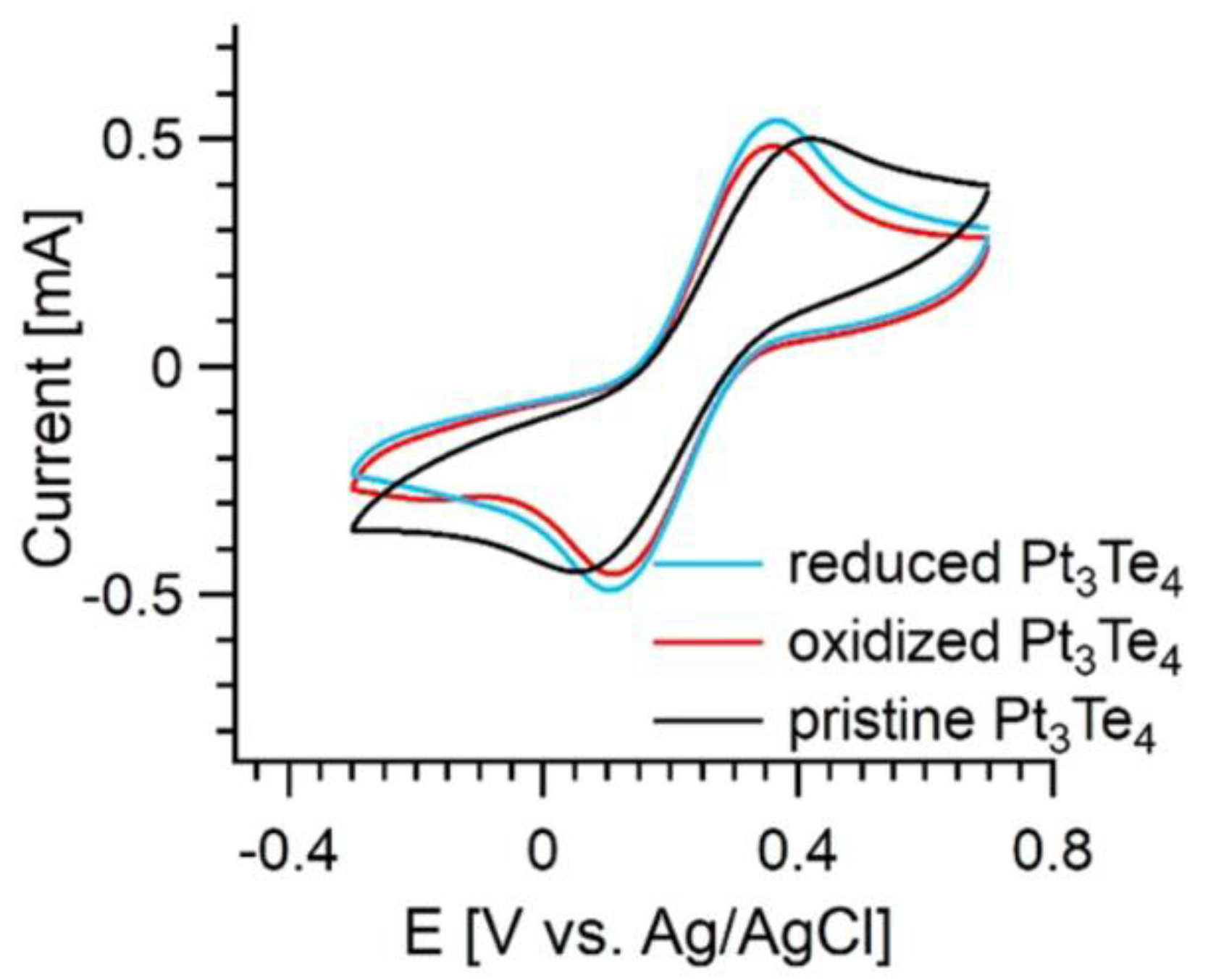
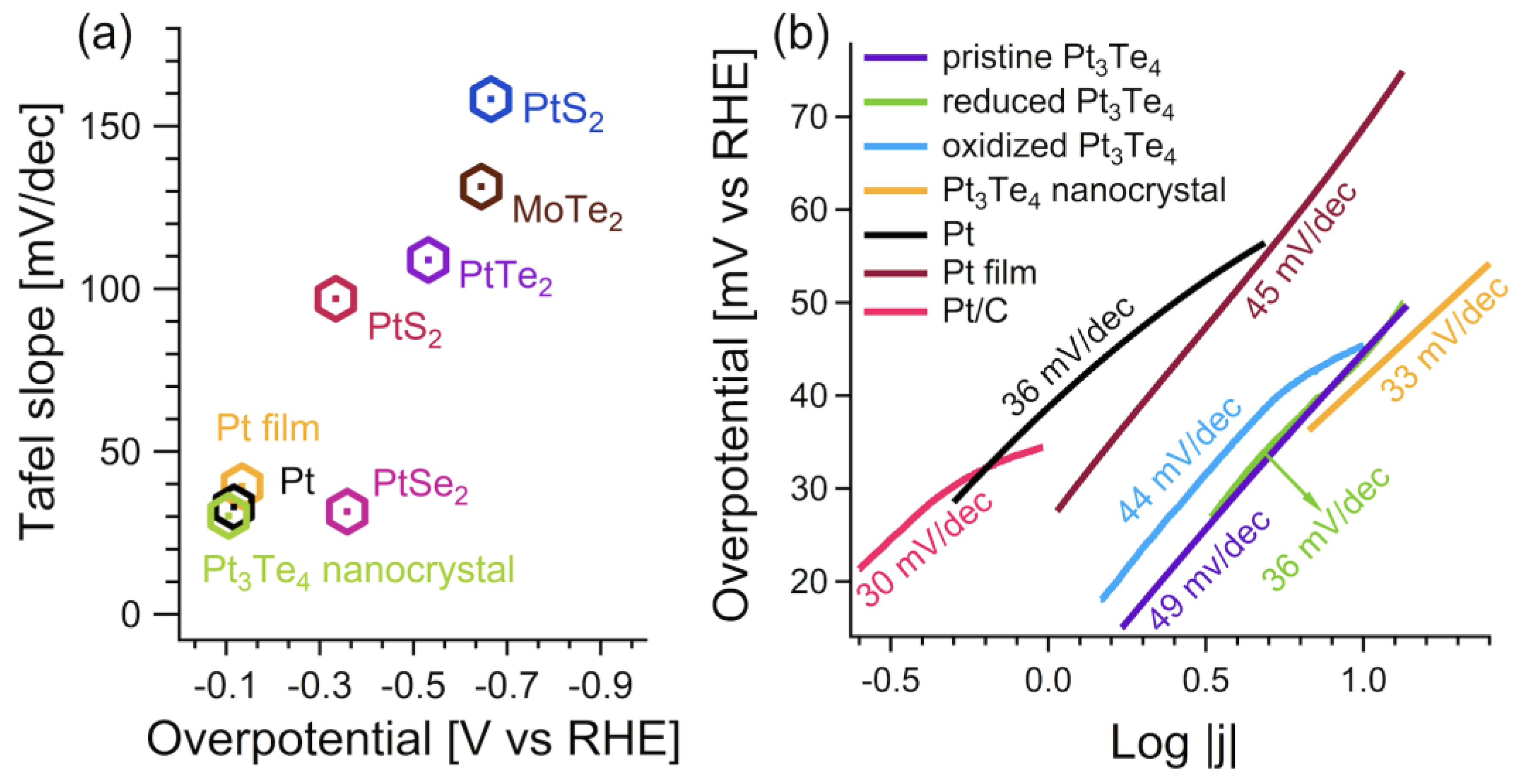
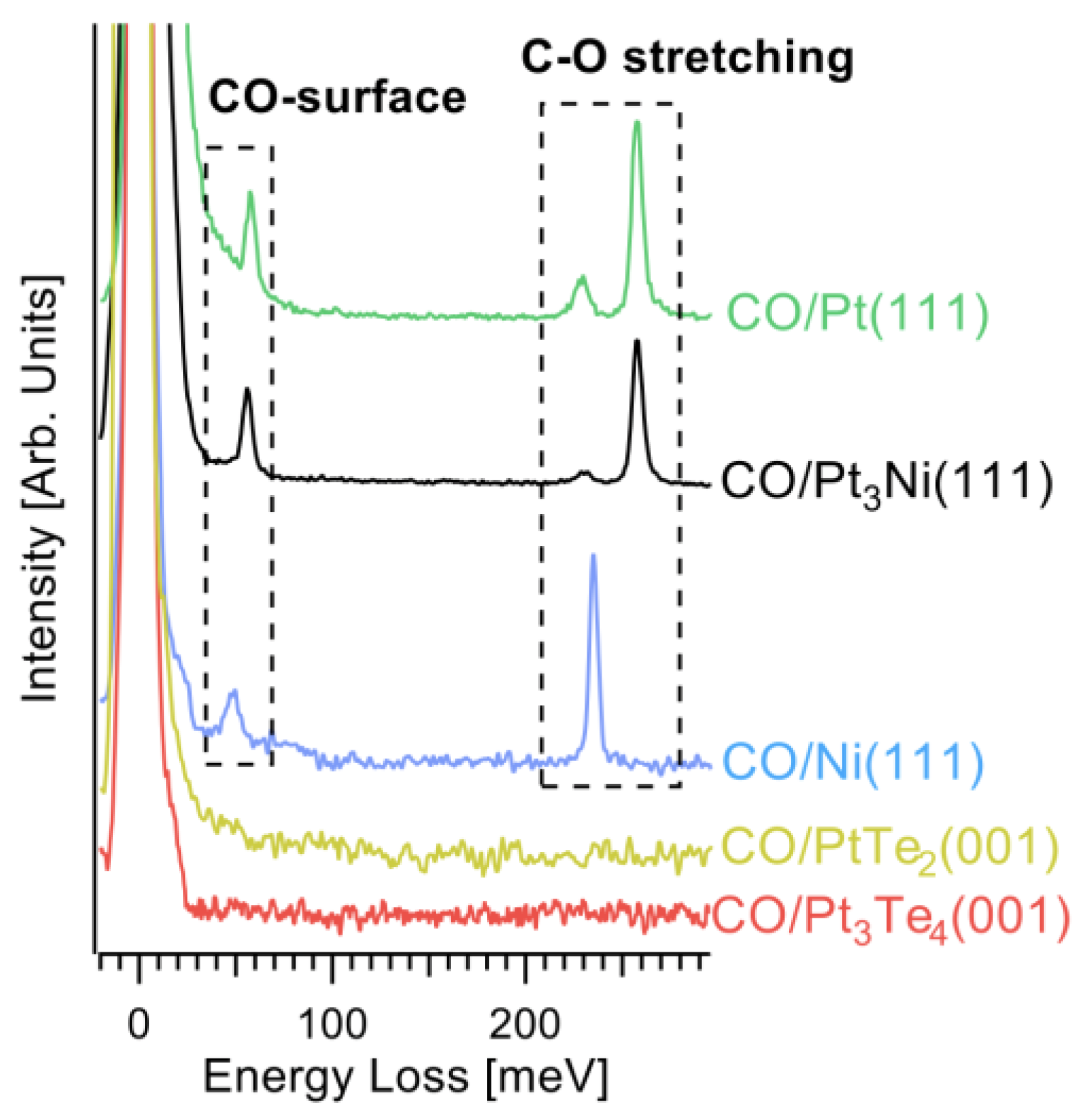
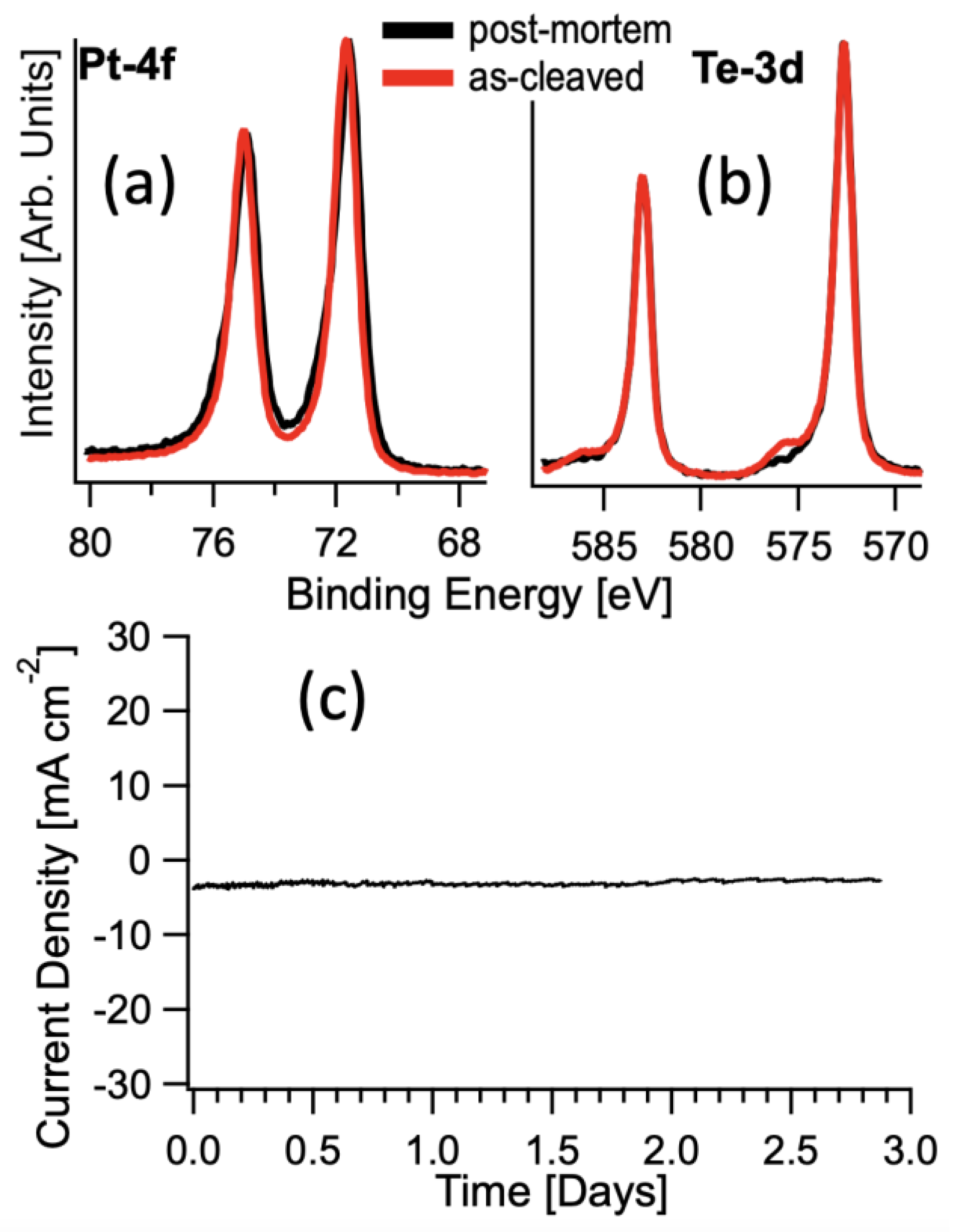
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, Y.; Khan, Z.A.; Alvarez-Alvarado, M.S.; Zhang, Y.; Huang, Z.; Imran, M. A critical review of sustainable energy policies for the promotion of renewable energy sources. Sustainability 2020, 12, 5078. [Google Scholar] [CrossRef]
- Al-Shetwi, A.Q.; Hannan, M.; Jern, K.P.; Mansur, M.; Mahlia, T. Grid-connected renewable energy sources: Review of the recent integration requirements and control methods. J. Clean. Prod. 2020, 253, 119831. [Google Scholar] [CrossRef]
- Kim, C.; Lee, S.; Kim, S.H.; Park, J.; Kim, S.; Kwon, S.H.; Bae, J.S.; Park, Y.S.; Kim, Y. Cobalt–iron–phosphate hydrogen evolution reaction electrocatalyst for solar-driven alkaline seawater electrolyzer. Nanomaterials 2021, 11, 2989. [Google Scholar] [CrossRef] [PubMed]
- Juodkazytė, J.; Juodkazis, K.; Juodkazis, S. Atoms vs. Ions: Intermediates in Reversible Electrochemical Hydrogen Evolution Reaction. Catalysts 2021, 11, 1135. [Google Scholar] [CrossRef]
- Evangelista, A.J.; Ivanchenko, M.; Jing, H. Efficient near-infrared-activated photocatalytic hydrogen evolution from ammonia borane with core-shell upconversion-semiconductor hybrid nanostructures. Nanomaterials 2021, 11, 23237. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, A.; Hou, J.; Liu, Q.; Li, H.; Guo, X. Ag–Au core–shell triangular nanoprisms for improving p-g-C3N4 photocatalytic hydrogen production. Nanomaterials 2021, 11, 23347. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.G.; Kim, C.S.; Kim, S.J.; Lee, J.W. Phase-controlled NiO nanoparticles on reduced graphene oxide as electrocatalysts for overall water splitting. Nanomaterials 2021, 11, 23379. [Google Scholar] [CrossRef]
- Sun, L.; Li, Y.; Feng, W. Gas-phase fluorination of g-C3N4 for enhanced photocatalytic hydrogen evolution. Nanomaterials 2022, 12, 10037. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, J.; Ye, Y.; Ma, H.; Liu, B.; Zhang, P.; Xu, B. Facile synthesis of 1T-phase MoS2 nanosheets on n-doped carbon nanotubes towards highly efficient hydrogen evolution. Nanomaterials 2021, 11, 3273. [Google Scholar] [CrossRef]
- Ďurovič, M.; Hnát, J.; Bouzek, K. Electrocatalysts for the hydrogen evolution reaction in alkaline and neutral media. A comparative review. J. Power Sources 2021, 493, 229708. [Google Scholar] [CrossRef]
- Pu, Z.; Amiinu, I.S.; Cheng, R.; Wang, P.; Zhang, C.; Mu, S.; Zhao, W.; Su, F.; Zhang, G.; Liao, S. Single-atom catalysts for electrochemical hydrogen evolution reaction: Recent advances and future perspectives. Nano-Micro Lett. 2020, 12, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Boukhvalov, D.W.; Edla, R.; Cupolillo, A.; Fabio, V.; Sankar, R.; Zhu, Y.; Mao, Z.; Hu, J.; Torelli, P.; Chiarello, G.; et al. Surface Instability and Chemical Reactivity of ZrSiS and ZrSiSe Nodal-Line Semimetals. Adv. Funct. Mater. 2019, 29, 1900438. [Google Scholar] [CrossRef]
- Gao, J.; Cupolillo, A.; Nappini, S.; Bondino, F.; Edla, R.; Fabio, V.; Sankar, R.; Zhang, Y.W.; Chiarello, G.; Politano, A. Surface Reconstruction, Oxidation Mechanism, and Stability of Cd3As2. Adv. Funct. Mater. 2019, 29, 1900965. [Google Scholar] [CrossRef]
- Politano, A.; Chiarello, G.; Kuo, C.-N.; Lue, C.S.; Edla, R.; Torelli, P.; Pellegrini, V.; Boukhvalov, D.W. Tailoring the Surface Chemical Reactivity of Transition-Metal Dichalcogenide PtTe2 Crystals. Adv. Funct. Mater. 2018, 28, 1706504. [Google Scholar] [CrossRef]
- Politano, A.; Chiarello, G.; Li, Z.; Fabio, V.; Wang, L.; Guo, L.; Chen, X.; Boukhvalov, D.W. Toward the effective exploitation of topological phases of matter in catalysis: Chemical reactions at the surfaces of NbAs and TaAs Weyl semimetals. Adv. Funct. Mater. 2018, 28, 1800511. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, Z.; Cao, L.; Chen, Y.; Zhu, E.; Lin, Z.; Li, M.; Yan, A.; Zettl, A.; Wang, Y.M.; et al. High-performance transition metal–doped Pt3Ni octahedra for oxygen reduction reaction. Science 2015, 348, 1230–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subbotin, V.V.; Vymazalová, A.; Laufek, F.; Savchenko, Y.E.; Stanley, C.J.; Gabov, D.A.; Plášil, J. Mitrofanovite, Pt3Te4, a new mineral from the East Chuarvy deposit, Fedorovo-Pana intrusion, Kola Peninsula, Russia. Mineral. Mag. 2019, 83, 523–530. [Google Scholar] [CrossRef]
- Fujii, J.; Ghosh, B.; Vobornik, I.; Bari Sarkar, A.; Mondal, D.; Kuo, C.N.; Bocquet, F.C.; Zhang, L.; Boukhvalov, D.W.; Lue, C.S.; et al. Mitrofanovite Pt3Te4: A Topological Metal with Termination-Dependent Surface Band Structure and Strong Spin Polarization. ACS Nano 2021, 15, 14786–14793. [Google Scholar] [CrossRef] [PubMed]
- Boukhvalov, D.W.; Cheng, J.; D’Olimpio, G.; Bocquet, F.C.; Kuo, C.-N.; Sarkar, A.B.; Ghosh, B.; Vobornik, I.; Fujii, J.; Hsu, K.; et al. Unveiling the Mechanisms Ruling the Efficient Hydrogen Evolution Reaction with Mitrofanovite Pt3Te4. J. Phys. Chem. Lett. 2021, 12, 8627–8636. [Google Scholar] [CrossRef]
- Bae, D.; Park, K.; Kwon, H.; Won, D.; Ling, N.; Baik, H.; Yang, J.; Park, H.J.; Cho, J.; Yang, H.; et al. Mitrofanovite, Layered Platinum Telluride, for Active Hydrogen Evolution. ACS Appl. Mater. Interfaces 2021, 13, 2437–2446. [Google Scholar] [CrossRef]
- Chia, X.; Sofer, Z.; Luxa, J.; Pumera, M. Layered Noble Metal Dichalcogenides: Tailoring Electrochemical and Catalytic Properties. ACS Appl. Mater. Interfaces 2017, 9, 25587–25599. [Google Scholar] [CrossRef] [PubMed]
- Chia, X.; Adriano, A.; Lazar, P.; Sofer, Z.; Luxa, J.; Pumera, M. Layered Platinum Dichalcogenides (PtS2, PtSe2, and PtTe2) Electrocatalysis: Monotonic Dependence on the Chalcogen Size. Adv. Funct. Mater. 2016, 26, 4306–4318. [Google Scholar] [CrossRef]
- Wang, Y.; Szokolova, K.; Nasir, M.Z.M.; Sofer, Z.; Pumera, M. Layered Crystalline and Amorphous Platinum Disulfide (PtS2): Contrasting Electrochemistry. Chem. Eur. J. 2019, 25, 7330–7338. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Zhao, T.; Ping, X.; Zheng, H.; Xing, L.; Liu, X.; Zheng, J.; Sun, L.; Gu, L.; Tao, C.; et al. Unveiling the Layer-Dependent Catalytic Activity of PtSe2 Atomic Crystals for the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2019, 58, 6977–6981. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Liu, Y.; Hu, Z.; Lu, W.; Mak, C.H.; Zeng, L.; Zhao, J.; Li, Y.; Yan, F.; Tsang, Y.H.; et al. Tunable active edge sites in PtSe2 films towards hydrogen evolution reaction. Nano Energy 2017, 42, 26–33. [Google Scholar] [CrossRef]
- Rosli, N.F.; Mayorga-Martinez, C.C.; Latiff, N.M.; Rohaizad, N.; Sofer, Z.; Fisher, A.C.; Pumera, M. Layered PtTe2 Matches Electrocatalytic Performance of Pt/C for Oxygen Reduction Reaction with Significantly Lower Toxicity. ACS Sustain. Chem. Eng. 2018, 6, 7432–7441. [Google Scholar] [CrossRef]
- Seok, J.; Lee, J.-H.; Cho, S.; Ji, B.; Kim, H.W.; Kwon, M.; Kim, D.; Kim, Y.-M.; Oh, S.H.; Kim, S.W.; et al. Active hydrogen evolution through lattice distortion in metallic MoTe2. 2D Mater. 2017, 4, 25061. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Mohmad, A.R.; Wang, Y.; Fullon, R.; Song, X.; Zhao, F.; Bozkurt, I.; Augustin, M.; Santos, E.J.G.; Shin, H.S.; et al. Ultrahigh-current-density niobium disulfide catalysts for hydrogen evolution. Nat. Mater. 2019, 18, 1309–1314. [Google Scholar] [CrossRef]
- Mishra, I.K.; Zhou, H.; Sun, J.; Qin, F.; Dahal, K.; Bao, J.; Chen, S.; Ren, Z. Hierarchical CoP/Ni5P4/CoP microsheet arrays as a robust pH-universal electrocatalyst for efficient hydrogen generation. Energy Environ. Sci. 2018, 11, 2246–2252. [Google Scholar] [CrossRef]
- Yu, F.; Zhou, H.; Huang, Y.; Sun, J.; Qin, F.; Bao, J.; Goddard, W.A.; Chen, S.; Ren, Z. High-performance bifunctional porous non-noble metal phosphide catalyst for overall water splitting. Nat. Commun. 2018, 9, 2551. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Yu, G.; Chen, W.; Liu, Y.; Li, G.-D.; Zhu, P.; Tao, Q.; Li, Q.; Liu, J.; Shen, X.; et al. Highly Active, Nonprecious Electrocatalyst Comprising Borophene Subunits for the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2017, 139, 12370–12373. [Google Scholar] [CrossRef] [PubMed]
- Politano, A.; Chiarello, G. Vibrational investigation of catalyst surfaces: Change of the adsorption site of CO molecules upon coadsorption. J. Phys. Chem. C 2011, 115, 13541–13553. [Google Scholar] [CrossRef]
- Politano, A.; Formoso, V.; Agostino, R.G.; Colavita, E.; Chiarello, G. Influence of CO adsorption on the alkali-substrate bond studied by high-resolution electron energy loss spectroscopy. Phys. Rev. B 2007, 76, 233403. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Barone, V.; Casarin, M.; Forrer, D.; Pavone, M.; Sambi, M.; Vittadini, A. Role and effective treatment of dispersive forces in materials: Polyethylene and graphite crystals as test cases. J. Comput. Chem. 2009, 30, 934–939. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Greeley, J.; Jaramillo, T.F.; Bonde, J.; Chorkendorff, I.; Nørskov, J.K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat. Mater. 2006, 5, 909–913. [Google Scholar] [CrossRef]
- Tang, Y.; Allen, B.L.; Kauffman, D.R.; Star, A. Electrocatalytic activity of nitrogen-doped carbon nanotube cups. J. Am. Chem. Soc. 2009, 131, 13200–13201. [Google Scholar] [CrossRef]
| Adsorbent | SurfaceTermination | Site | ΔHads [kJ/mol] | ΔGads [kJ/mol] | ΔHdec [kJ/mol] |
|---|---|---|---|---|---|
| O2 | PtTe2 | on-top | −42.7 | −31.2 | −51.8 |
| Te vacancy | −33.3 | −22.0 | −69.4 | ||
| Pt2Te2 | on-top | −40.9 | −29.6 | −98.1 | |
| Te vacancy | −35.0 | −23.7 | −163.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Olimpio, G.; Zhang, L.; Kuo, C.-N.; Farias, D.; Ottaviano, L.; Lue, C.S.; Fujii, J.; Vobornik, I.; Agarwal, A.; Torelli, P.; et al. Efficient Hydrogen Evolution Reaction with Bulk and Nanostructured Mitrofanovite Pt3Te4. Nanomaterials 2022, 12, 558. https://doi.org/10.3390/nano12030558
D’Olimpio G, Zhang L, Kuo C-N, Farias D, Ottaviano L, Lue CS, Fujii J, Vobornik I, Agarwal A, Torelli P, et al. Efficient Hydrogen Evolution Reaction with Bulk and Nanostructured Mitrofanovite Pt3Te4. Nanomaterials. 2022; 12(3):558. https://doi.org/10.3390/nano12030558
Chicago/Turabian StyleD’Olimpio, Gianluca, Lixue Zhang, Chia-Nung Kuo, Daniel Farias, Luca Ottaviano, Chin Shan Lue, Jun Fujii, Ivana Vobornik, Amit Agarwal, Piero Torelli, and et al. 2022. "Efficient Hydrogen Evolution Reaction with Bulk and Nanostructured Mitrofanovite Pt3Te4" Nanomaterials 12, no. 3: 558. https://doi.org/10.3390/nano12030558
APA StyleD’Olimpio, G., Zhang, L., Kuo, C.-N., Farias, D., Ottaviano, L., Lue, C. S., Fujii, J., Vobornik, I., Agarwal, A., Torelli, P., Boukhvalov, D. W., & Politano, A. (2022). Efficient Hydrogen Evolution Reaction with Bulk and Nanostructured Mitrofanovite Pt3Te4. Nanomaterials, 12(3), 558. https://doi.org/10.3390/nano12030558








