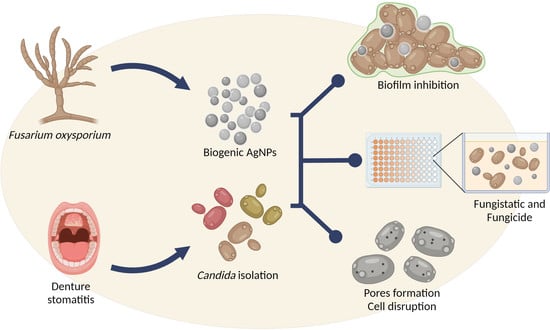
Activity of Fusarium oxysporum-Based Silver Nanoparticles on Candida spp. Oral Isolates
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains, Media and Commercial Antifungals
2.2. Synthesis of Silver Nanoparticles
2.3. Fungal Samples and Ethical Aspects
2.4. Broth Microdilution Assay
2.5. Biofilm Formation Interference Assay
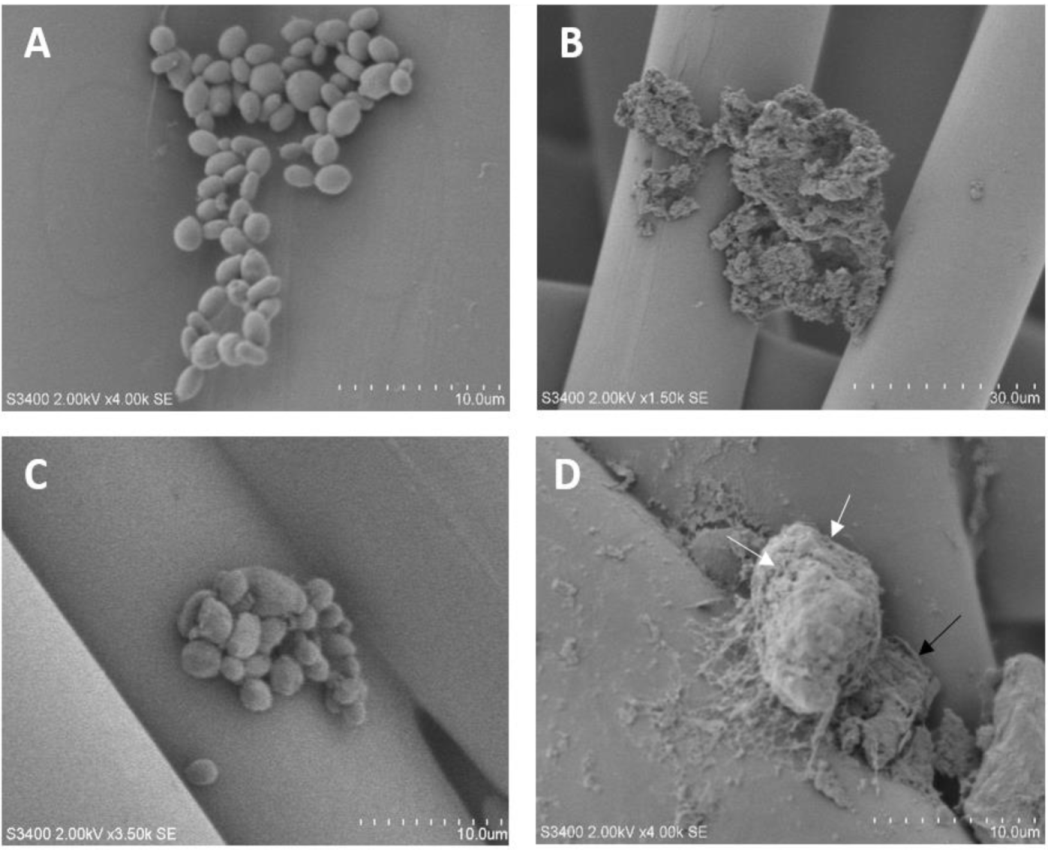
2.6. Scanning Electron Microscopy
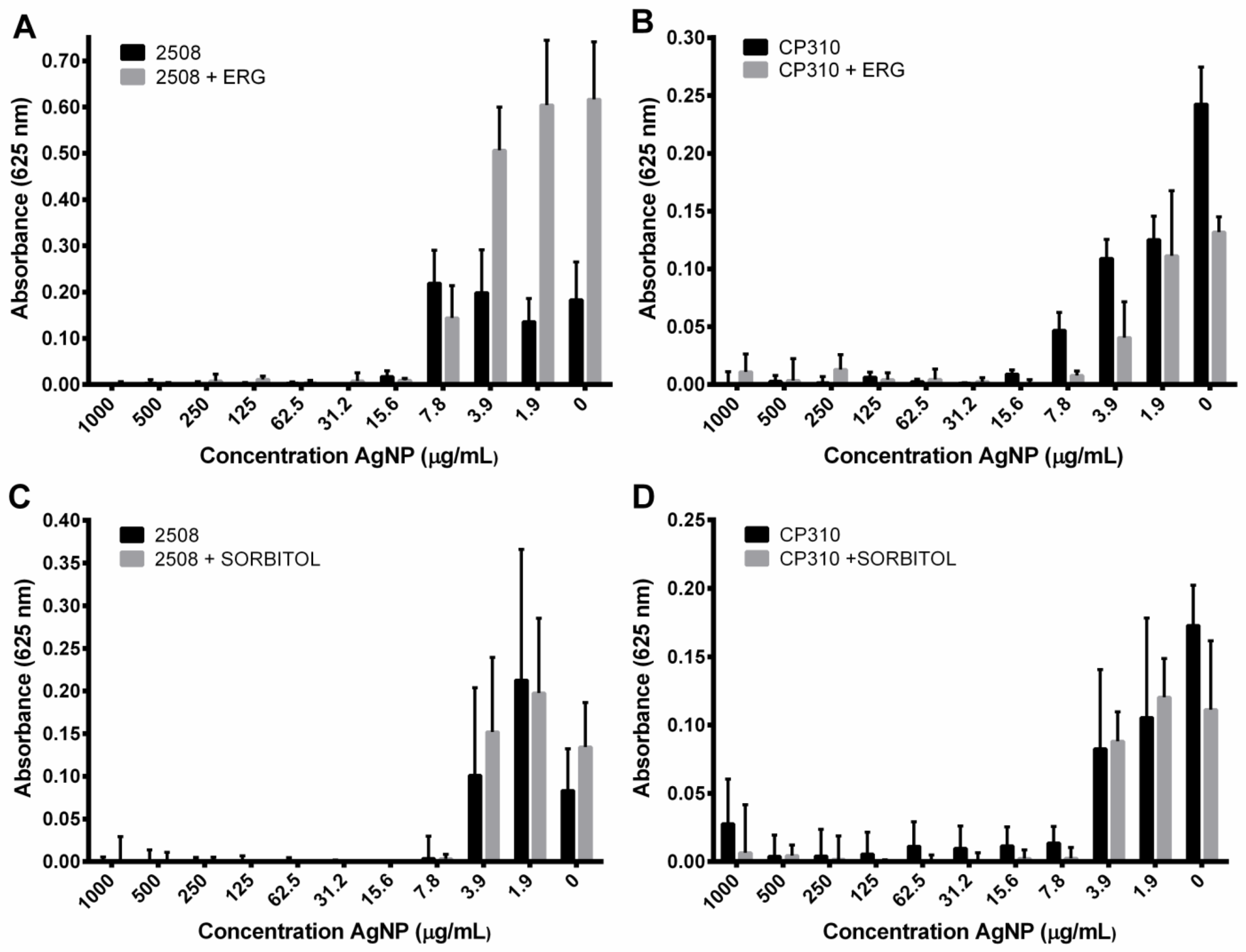
2.7. Exogenous Ergosterol and Sorbitol Supplementation Assays
2.8. Statistical Analysis
3. Results
3.1. Identification of the Candida Species Isolated from Denture Stomatitis Cases
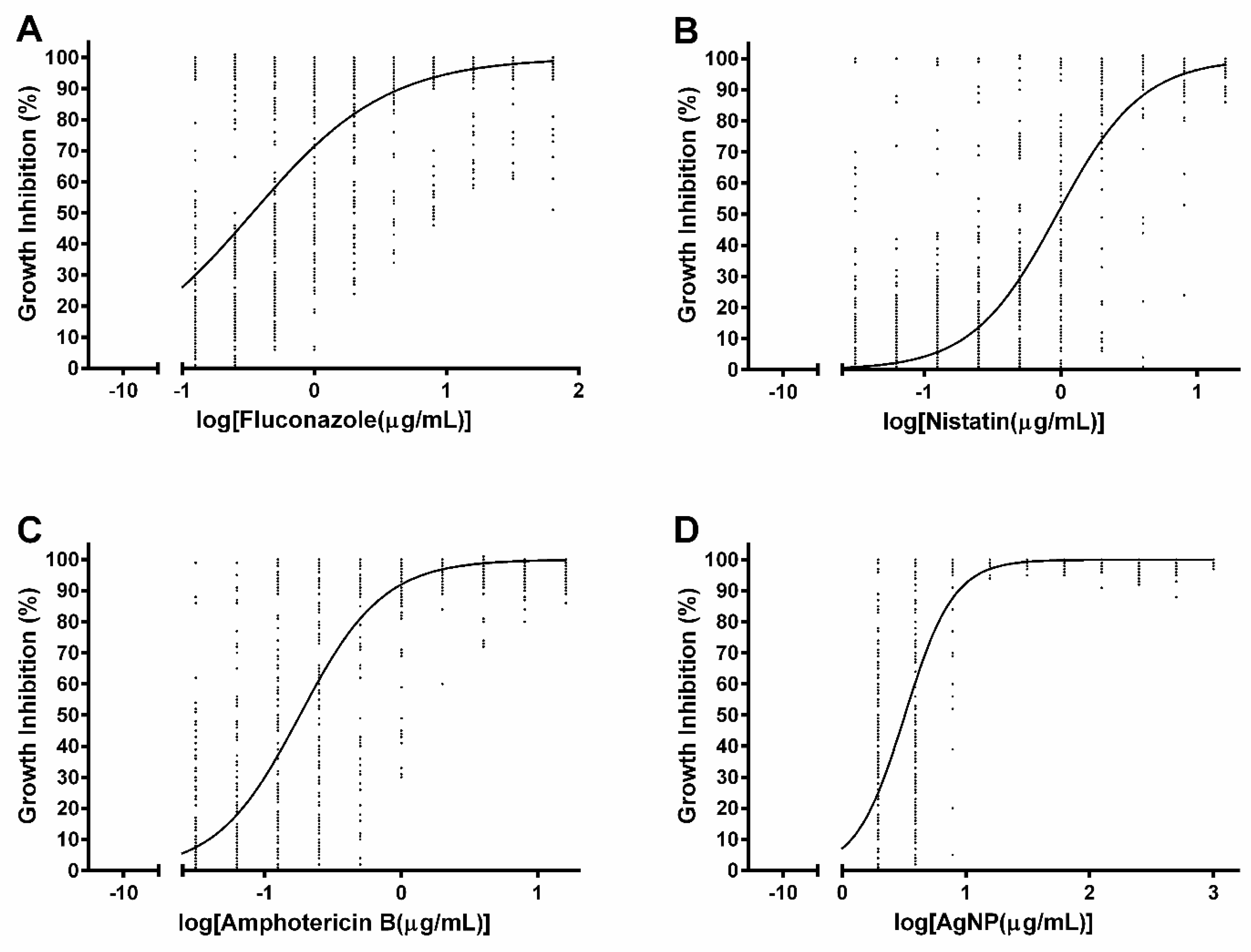
3.2. Susceptibility to Commercial Antifungal Drugs
3.3. Susceptibility to Silver Nanoparticles
3.4. Interference Effect of AgNPs in the Biofilm Formation
3.5. Scanning Electron Microscopy of C. albicans and C. parapsilosis
3.6. Ergosterol and Sorbitol Supplementation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gendreau, L.; Loewy, Z.G. Epidemiology and Etiology of Denture Stomatitis. J. Prosthodont. 2011, 20, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Savastano, C.; de Oliveira Silva, E.; Gonçalves, L.L.; Nery, J.M.; Silva, N.C.; Dias, A.L.T. Candida glabrata among Candida spp. from environmental health practitioners of a Brazilian Hospital. Braz. J. Microbiol. 2016, 47, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Castro, T.L.; Coutinho, H.D.M.; Gedeon, C.C.; Santos, J.M.; Santana, W.J.; Souza, L.B.S. Mecanismos de resistência da Candida sp. a anti-fúngicos. Infarma Ciências Farm 2013, 18, 30–35. [Google Scholar]
- Douglas, L. Candida biofilms and their role in infection. Trends Microbiol. 2003, 11, 30–36. [Google Scholar] [CrossRef]
- Pereira, R.; Fontenelle, R.O.D.S.; de Brito, E.H.S.; de Morais, S.M. Biofilm of Candida albicans: Formation, regulation and resistance. J. Appl. Microbiol. 2021, 131, 11–22. [Google Scholar] [CrossRef]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef]
- Perlin, D.S. Echinocandin Resistance in Candida. Clin. Infect. Dis. 2015, 61, S612–S617. [Google Scholar] [CrossRef]
- Halbus, A.F.; Horozov, T.S.; Paunov, V.N. Colloid particle formulations for antimicrobial applications. Adv. Colloid Interface Sci. 2017, 249, 134–148. [Google Scholar] [CrossRef]
- El-Batal, A.I.; Gharib, F.A.E.-L.; Ghazi, S.M.; Hegazi, A.Z.; El Hafz, A.G.M.A. Physiological Responses of Two Varieties of Common Bean (Phaseolus Vulgaris L.) to Foliar Application of Silver Nanoparticles. Nanomater. Nanotechnol. 2016, 6, 13. [Google Scholar] [CrossRef]
- Durán, N.; Marcato, P.D.; De Conti, R.; Alves, O.L.; Costa, F.T.M.; Brocchi, M. Potential use of silver nanoparticles on pathogenic bacteria, their toxicity and possible mechanisms of action. J. Braz. Chem. Soc. 2010, 21, 949–959. [Google Scholar] [CrossRef]
- Ballottin, D.; Fulaz, S.; Cabrini, F.; Tsukamoto, J.; Durán, N.; Alves, O.L.; Tasic, L. Antimicrobial textiles: Biogenic silver nanoparticles against Candida and Xanthomonas. Mater. Sci. Eng. C 2017, 75, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, A.M.; Girilal, M.; Rahman, M.S.; Venkatesan, R.; Kalaichelvan, P. Biosynthesis of silver and gold nanoparticles using thermophilic bacterium Geobacillus stearothermophilus. Process Biochem. 2011, 46, 1958–1962. [Google Scholar] [CrossRef]
- Fernandez, C.; Sokolonski, A.; Fonseca, M.; Stanisic, D.; Araújo, D.; Azevedo, V.; Portela, R.; Tasic, L. Applications of Silver Nanoparticles in Dentistry: Advances and Technological Innovation. Int. J. Mol. Sci. 2021, 22, 2485. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, V.; Cascione, M.; Toma, C.C.; Albanese, G.; De Giorgi, M.L.; Corsalini, M.; Rinaldi, R. Silver Nanoparticles Addition in Poly(Methyl Methacrylate) Dental Matrix: Topographic and Antimycotic Studies. Int. J. Mol. Sci. 2019, 20, 4691. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.Z.; Khan, S.B. Antimicrobial efficacy of silver nanoparticles against Candida albicans: A systematic review protocol. PLoS ONE 2021, 16, e0245811. [Google Scholar] [CrossRef] [PubMed]
- Telles, I.S.F.; Prado, M.D.; Simão, R.A. Nanopartículas e aplicações endodônticas: Uma revisão da literatura. Rev. Bras. Odontol. 2017, 74, 167. [Google Scholar] [CrossRef]
- Durán, N.; Durán, M.; de Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 789–799. [Google Scholar] [CrossRef]
- Guilger Casagrande, M.; De Lima, R. Synthesis of Silver Nanoparticles Mediated by Fungi: A Review. Front. Bioeng. Biotechnol. 2019, 7, 287. [Google Scholar] [CrossRef]
- Mare, A.D.; Man, A.; Ciurea, C.N.; Toma, F.; Cighir, A.; Mareș, M.; Berța, L.; Tanase, C. Silver Nanoparticles Biosynthesized with Spruce Bark Extract—A Molecular Aggregate with Antifungal Activity against Candida Species. Antibiotics 2021, 10, 1261. [Google Scholar] [CrossRef]
- Ansari, M.; Kalam, A.; Al-Sehemi, A.; Alomary, M.; AlYahya, S.; Aziz, M.; Srivastava, S.; Alghamdi, S.; Akhtar, S.; Almalki, H.; et al. Counteraction of Biofilm Formation and Antimicrobial Potential of Terminalia catappa Functionalized Silver Nanoparticles against Candida albicans and Multidrug-Resistant Gram-Negative and Gram-Positive Bacteria. Antibiotics 2021, 10, 725. [Google Scholar] [CrossRef]
- Alves, O.L.; De Moraes, A.C.M.; Simões, M.B.; Fonseca, L.C.; Nascimento, R.O.D.; Holtz, R.D.; De Faria, A.F. Nanomaterials. In Nanotoxicology Mater. Methodol. Assessments, 1st ed.; Durán, N., Guterres, S.S., Alves, O.L., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2014; p. 411. [Google Scholar]
- Radhakrishnan, V.S.; Mudiam, M.K.R.; Kumar, M.; Dwivedi, S.P.; Singh, S.P.; Prasad, T. Silver nanoparticles induced alterations in multiple cellular targets, which are critical for drug susceptibilities and pathogenicity in fungal pathogen (Candida albicans). Int. J. Nanomed. 2018, 13, 2647–2663. [Google Scholar] [CrossRef] [PubMed]
- Stanisic, D.; Fregonesi, N.L.; Barros, C.H.N.; Pontes, J.G.M.; Fulaz, S.; Menezes, U.J.; Nicoleti, J.L.; Castro, T.L.P.; Seyffert, N.; Azevedo, V.; et al. NMR insights on nano silver post-surgical treatment of superficial caseous lymphadenitis in small ruminants. RSC Adv. 2018, 8, 40778–40786. [Google Scholar] [CrossRef]
- ANVISA—Agência Nacional de Vigilância Sanitária. Manual de Microbiologia Clínica Para o Controle de Infecção em Serviços de Saúde, 2004. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/manual_microbiologia_completo.pdf (accessed on 16 January 2022).
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). M27-S3: Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Third Informational Supplement, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). M27-S4: Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Third Informational Supplement, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Lohse, M.B.; Gulati, M.; Arevalo, A.V.; Fishburn, A.; Johnson, A.D.; Nobile, C.J. Assessment and Optimizations of Candida albicans In Vitro Biofilm Assays. Antimicrob. Agents Chemother. 2017, 61, e02749-16. [Google Scholar] [CrossRef]
- Souto Maior, L.F.; Maciel, P.P.; Ferreira, V.Y.N.; de Lima Gouveia Dantas, C.; de Lima, J.M.; Castellano, L.R.C. Antifungal activity and Shore A hardness of a tissue conditioner incorporated with terpinen-4-ol and cinnamaldehyde. Clin. Oral Investig. 2019, 23, 2837–2848. [Google Scholar] [CrossRef] [PubMed]
- Kalil, M.A.; Santos, L.M.; Barral, T.D.; Rodrigues, D.M.; Pereira, N.P.; Sá, M.D.C.A.; Umsza-Guez, M.A.; Machado, B.A.S.; Meyer, R.; Portela, R.W. Brazilian Green Propolis as a Therapeutic Agent for the Post-surgical Treatment of Caseous Lymphadenitis in Sheep. Front. Vet. Sci. 2019, 6, 399. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Cadena, K.; Marcos-Arias, C.; Mateo, E.; Aguirre, J.M.; Quindós, G.; Eraso, E. Prevalence and antifungal susceptibility profiles of Candida glabrata, Candida parapsilosis and their close-related species in oral candidiasis. Arch. Oral Biol. 2018, 95, 100–107. [Google Scholar] [CrossRef]
- Sullivan, D.; Haynes, K.A.; Bennett, D.E.; Coleman, D.; Westerneng, T.J.; Haynes, K.A.; Bennett, D.E.; Coleman, D.C. Candida dubliniensis sp. nov.: Phenotypic and molecular characterization of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology 1995, 141, 1507–1521. [Google Scholar] [CrossRef]
- Gauch, L.M.R.; Pedrosa, S.S.; Silveira-Gomes, F.; Esteves, R.A.; Marques-Da-Silva, S.H. Isolation of Candida spp. from denture-related stomatitis in Pará, Brazil. Braz. J. Microbiol. 2018, 49, 148–151. [Google Scholar] [CrossRef]
- Siqueira, A.B.S.; Rodriguez, L.R.N.D.A.; Santos, R.K.B.; Marinho, R.R.B.; Abreu, S.; Peixoto, R.F.; Gurgel, B.C.D.V. Antifungal activity of propolis against Candidaspecies isolated from cases of chronic periodontitis. Braz. Oral Res. 2015, 29, 1–6. [Google Scholar] [CrossRef]
- Loreto, S.; Scheid, L.A.; Nogueira, C.W.; Zeni, G.; Santurio, J.M.; Alves, S.H. Candida dubliniensis: Epidemiology and Phenotypic Methods for Identification. Mycopathologia 2010, 169, 431–443. [Google Scholar] [CrossRef]
- Liu, S.; Hou, Y.; Chen, X.; Gao, Y.; Li, H.; Sun, S. Combination of fluconazole with non-antifungal agents: A promising approach to cope with resistant Candida albicans infections and insight into new antifungal agent discovery. Int. J. Antimicrob. Agents 2014, 43, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Romera, D.; Aguilera-Correa, J.J.; Gadea, I.; Viñuela-Sandoval, L.; García-Rodríguez, J.; Esteban, J. Candida auris: A comparison between planktonic and biofilm susceptibility to antifungal drugs. J. Med. Microbiol. 2019, 68, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.H.; Mueller, P.R.; Szeszs, M.W.; Levin, A.S.; Melhem, M.S.C. Five-year evaluation of bloodstream yeast infections in a tertiary hospital: The predominance of non-C. albicans Candidaspecies. Med. Mycol. 2010, 48, 839–842. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sanguinetti, M.; Posteraro, B.; Lass-Flörl, C. Antifungal drug resistance among Candidaspecies: Mechanisms and clinical impact. Mycoses 2015, 58, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Krishnasamy, L.; Krishnakumar, S.; Kumaramanickavel, G.; Saikumar, C. Molecular Mechanisms of Antifungal Drug Resistance in Candida Species. J. Clin. Diagn. Res. 2018, 12, 9. [Google Scholar] [CrossRef]
- Lemos, J.D.A.; Costa, C.R.; De Araújo, C.R.; E Souza, L.K.H.; Silva, M.D.R.R. Susceptibility testing of Candida albicans isolated from oropharyngeal mucosa of HIV+ patients to fluconazole, amphotericin B and Caspofungin: Killing kinetics of caspofungin and amphotericin B against fluconazole resistant and susceptible isolates. Braz. J. Microbiol. 2009, 40, 163–169. [Google Scholar] [CrossRef]
- Radhakrishnan, V.S.; Dwivedi, S.P.; Siddiqui, M.H.; Prasad, T. In vitro studies on oxidative stress-independent, Ag nanoparticles-induced cell toxicity of Candida albicans, an opportunistic pathogen. Int. J. Nanomed. 2018, 13, 91–96. [Google Scholar] [CrossRef][Green Version]
- Jalal, M.; Ansari, M.A.; Alzohairy, M.A.; Ali, S.G.; Khan, H.M.; Almatroudi, A.; Raees, K. Biosynthesis of Silver Nanoparticles from Oropharyngeal Candida glabrata Isolates and Their Antimicrobial Activity against Clinical Strains of Bacteria and Fungi. Nanomaterials 2018, 8, 586. [Google Scholar] [CrossRef]
- Rodrigues, A.G.; Ping, L.Y.; Marcato, P.D.; Alves, O.L.; Silva, M.C.P.; Ruiz, R.D.C.; Melo, I.S.; Tasic, L.; De Souza, A.O. Biogenic antimicrobial silver nanoparticles produced by fungi. Appl. Microbiol. Biotechnol. 2012, 97, 775–782. [Google Scholar] [CrossRef]
- Longhi, C.; Santos, J.P.; Morey, A.T.; Marcato, P.D.; Durán, N.; Pinge-Filho, P.; Nakazato, G.; Yamada-Ogatta, S.F.; Yamauchi, L.M. Combination of fluconazole with silver nanoparticles produced by Fusarium oxysporumimproves antifungal effect against planktonic cells and biofilm of drug-resistantCandida albicans. Med. Mycol. 2015, 54, 428–432. [Google Scholar] [CrossRef]
- Santos, L.M.; Stanisic, D.; Menezes, U.J.; Mendonça, M.A.; Barral, T.; Seyffert, N.; Azevedo, V.; Durán, N.; Meyer, R.; Tasic, L.; et al. Biogenic Silver Nanoparticles as a Post-surgical Treatment for Corynebacterium pseudotuberculosis Infection in Small Ruminants. Front. Microbiol. 2019, 10, 824. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-J.; Sung, W.S.; Suh, B.K.; Moon, S.-K.; Choi, J.-S.; Kim, J.G.; Lee, D.G. Antifungal activity and mode of action of silver nano-particles on Candida albicans. BioMetals 2009, 22, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kim, Y.J.; Singh, H.; Wang, C.; Hwang, K.H.; Farh, M.E.A.; Yang, D.C. Biosynthesis, characterization, and antimicrobial applications of silver nanoparticles. Int. J. Nanomed. 2015, 10, 2567–2577. [Google Scholar]
- Ahmad, A.; Mukherjee, P.; Senapati, S.; Mandal, D.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B Biointerfaces 2003, 28, 313–318. [Google Scholar] [CrossRef]
- Kumar, S.A.; Abyaneh, M.K.; Gosavi, S.W.; Kulkarni, S.K.; Pasricha, R.; Ahmad, A.; Khan, M.I. Nitrate reductase-mediated synthesis of silver nanoparticles from AgNO3. Biotechnol. Lett. 2007, 29, 439–445. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Babu, R.; Venkataraman, D.; Bilal, M.; Gurunathan, S. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf. B 2008, 65, 150–153. [Google Scholar] [CrossRef]
- Żarowska, B.; Koźlecki, T.; Piegza, M.; Jaros-Koźlecka, K.; Robak, M. New Look on Antifungal Activity of Silver Nanoparticles (AgNPs). Pol. J. Microbiol. 2019, 68, 515–525. [Google Scholar] [CrossRef]
- Porenczuk, A.; Grzeczkowicz, A.; Maciejewska, I.; Gołaś, M.; Piskorska, K.; Kolenda, A.; Gozdowski, D.; Kopeć-Swoboda, E.; Olczak-Kowalczyk, D. An initial evaluation of cytotoxicity, genotoxicity and antibacterial effectiveness of a disinfection liquid containing silver nanoparticles alone and combined with a glass-ionomer cement and dentin bonding systems. Adv. Clin. Exp. Med. 2018, 28, 75–83. [Google Scholar] [CrossRef]
- Bocate, K.P.; Reis, G.F.; de Souza, P.C.; Junior, A.G.O.; Durán, N.; Nakazato, G.; Furlaneto, M.C.; de Almeida, R.S.; Panagio, L.A. Antifungal activity of silver nanoparticles and simvastatin against toxigenic species of Aspergillus. Int. J. Food Microbiol. 2019, 291, 79–86. [Google Scholar] [CrossRef]
- Lara, H.H.; Ixtepan-Turrent, L.; Jose Yacaman, M.; Lopez-Ribot, J. Inhibition of Candida auris Biofilm Formation on Medical and Environmental Surfaces by Silver Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 21183–21191. [Google Scholar] [CrossRef]
- Al Aboody, M.S. Silver/silver chloride (Ag/AgCl) nanoparticles synthesized from Azadirachta indica lalex and its antibiofilm activity against fluconazole resistant Candida tropicalis. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2107–2113. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.X.; Lai, Y.X.; Choudhury, M.; Amalraj, F.D. Efficacy of incorporating silver nanoparticles into maxillofacial silicone against Staphylococcus aureus, Candida albicans, and polymicrobial biofilms. J. Prosthet. Dent. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Munoz, R.; Avalos-Borja, M.; Castro-Longoria, E. Ultrastructural Analysis of Candida albicans When Exposed to Silver Nanoparticles. PLoS ONE 2014, 9, e108876. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, N.; Mishra, A.; Alam, H.; Manzoor, N.; Sardar, M. Biosynthesis, Characterization, and Antifungal Activity of the Silver Nanoparticles Against Pathogenic Candida species. BioNanoScience 2015, 5, 65–74. [Google Scholar] [CrossRef]
- Mukhopadhyay, K.; Prasad, T.; Saini, P.; Pucadyil, T.J.; Chattopadhyay, A.; Prasad, R. Membrane Sphingolipid-Ergosterol Interactions Are Important Determinants of Multidrug Resistance in Candida albicans. Antimicrob. Agents Chemother. 2004, 48, 1778–1787. [Google Scholar] [CrossRef] [PubMed]
- Prasad, T.; Saini, P.; Gaur, N.; Vishwakarma, R.A.; Khan, L.A.; Haq, Q.M.R.; Prasad, R. Functional Analysis of Ca IPT1, a Sphingolipid Biosynthetic Gene Involved in Multidrug Resistance and Morphogenesis of Candida albicans. Antimicrob. Agents Chemother. 2005, 49, 3442–3452. [Google Scholar] [CrossRef]
- Frost, D.J.; Brandt, K.D.; Cugier, D.; Goldman, R. A Whole-Cell Candida albicans Assay for the Detection of Inhibitors towards Fungal Cell Wall Synthesis and Assembly. J. Antibiot. 1995, 48, 306–310. [Google Scholar] [CrossRef]

| Sample | PDI | Zeta Potential (mV) |
|---|---|---|
| AgNP Batch 1 | 0.22 ± 0.02 | −29.8 ± 0.1 |
| AgNP Batch 2 | 0.24 ± 0.04 | −31.7 ± 2.8 |
| Strain | Candida Species | Fluconazole (µg/mL) | Ketoconazole (µg/mL) | Nystatin (µg/mL) | Amphotericin B (µg/mL) | Silver Nanoparticles (µg/mL) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC100 | MFC100 | MIC100 | MFC100 | MIC100 | MFC100 | MIC100 | MFC100 | MIC100 | MFC100 | ||
| 2508 | Candida albicans | 0.125 (S) | 0.125 | 0.03125 | 0.03125 | 4 | 4 | 1 | 1 | 15.6 | 125 |
| 2517 | C. albicans | 0.25 (S) | 0.125 | 0.03125 | 0.03125 | 4 | 8 | 1 | 1 | 7.8 | 250 |
| 3703 | C. albicans | 0.125 (S) | 0.125 | 0.03125 | 0.03125 | 4 | 4 | 0.5 | 0.5 | 7.8 | 31.25 |
| 3704 | C. albicans | 0.5 (S) | 0.25 | 0.03125 | 0.0625 | 2 | 4 | 0.5 | 0.5 | 7.8 | 62.5 |
| PAC 06 | C. albicans | 0.25 (S) | 2 | 0.0625 | 0.125 | 4 | 4 | 2 | 2 | 7.8 | 500 |
| PAC 13 | C. albicans | 0.5 (S) | 16 | 0.03125 | >16 | 1 | 4 | 0.5 | 2 | 7.8 | 500 |
| PAC 08 | C. albicans | 1 (S) | 8 | 0.03125 | 0.125 | 2 | 4 | 2 | 2 | 7.8 | 250 |
| PAC 18 | C. albicans | 1 (S) | >64 | 0.03125 | >16 | 16 | 16 | 1 | 2 | 7.8 | 500 |
| PAC 09 | C. albicans | 1 (S) | >64 | 0.0625 | >16 | 2 | 4 | 0.5 | 0.5 | 7.8 | 500 |
| PAC 10 | C. albicans | 1 (S) | >64 | 0.125 | >16 | 2 | 4 | 0.5 | 0.5 | 7.8 | 250 |
| PAC 03 | C. albicans | 1 (S) | >64 | 0.125 | >16 | 2 | 2 | 0.25 | 0.5 | 7.8 | 62.5 |
| PAC 19 | C. albicans | 4 (SDD) | 16 | 0.125 | 1 | 8 | 8 | 0.5 | 0.5 | 7.8 | 500 |
| PAC 20 | C. albicans | 4 (SDD) | >64 | 0.0625 | >16 | 4 | 16 | 0.25 | 0.5 | 7.8 | 500 |
| PAC 16 | C. albicans | 4 (SDD) | >64 | 0.125 | >16 | 2 | 4 | 0.5 | 0.5 | 7.8 | 125 |
| PAC 17 | C. albicans | 16 (R) | >64 | 0.03125 | >16 | 16 | >16 | 4 | 4 | 7.8 | 15.6 |
| PAC 11 | C. albicans. | >64 (R) | >64 | 0.0625 | 8 | 2 | 4 | 0.5 | 0.5 | 7.8 | 125 |
| PAC 14 | C. albicans | >64 (R) | >64 | 8 | >16 | 4 | 4 | 0.5 | 0.5 | 3.9 | 15.6 |
| PAC 12 | C. albicans | >64 (R) | >64 | >16 | >16 | 2 | 2 | 0.5 | 0.5 | 7.8 | 31.25 |
| CG 74 | Candida glabrata | >64 (R) | >64 | >16 | >16 | 2 | 2 | 0.5 | 0.5 | 7.8 | 250 |
| PAC 01 | C. dubliniensis | 32 (SDD) | >64 | 0.03125 | 0.125 | 2 | 4 | 2 | 2 | 15.6 | 500 |
| CT 309 | Candida tropicalis | >64 (R) | >64 | 8 | >16 | 4 | 4 | 0.5 | 0.5 | 7.8 | 15.6 |
| PAC 04 | C. tropicalis | 0.125 (S) | 0.5 | 0.03125 | 0.03125 | 0.5 | 2 | 0.25 | 0.5 | 3.9 | 62.5 |
| PAC 02 | C. tropicalis | 2 (S) | 16 | 0.03125 | 0.125 | 2 | 4 | 2 | 2 | 7.8 | 125 |
| PAC 15 | C. tropicalis | 2 (S) | >64 | 0.03125 | >16 | 1 | 4 | 2 | 2 | 3.9 | 62.5 |
| PAC 05 | C. tropicalis | 8 (R) | >64 | 0.25 | >16 | 2 | 4 | 2 | 4 | 7.8 | 31.25 |
| PAC 07 | C. tropicalis | >64 (R) | >64 | 16 | >16 | 4 | 8 | 1 | 1 | 3.9 | 15.6 |
| CP 310 | Candida parapsilosis | 16 (R) | >64 | 0.125 | >16 | 8 | 16 | 2 | 8 | 7.8 | >1000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca, M.S.; Rodrigues, D.M.; Sokolonski, A.R.; Stanisic, D.; Tomé, L.M.; Góes-Neto, A.; Azevedo, V.; Meyer, R.; Araújo, D.B.; Tasic, L.; et al. Activity of Fusarium oxysporum-Based Silver Nanoparticles on Candida spp. Oral Isolates. Nanomaterials 2022, 12, 501. https://doi.org/10.3390/nano12030501
Fonseca MS, Rodrigues DM, Sokolonski AR, Stanisic D, Tomé LM, Góes-Neto A, Azevedo V, Meyer R, Araújo DB, Tasic L, et al. Activity of Fusarium oxysporum-Based Silver Nanoparticles on Candida spp. Oral Isolates. Nanomaterials. 2022; 12(3):501. https://doi.org/10.3390/nano12030501
Chicago/Turabian StyleFonseca, Maísa Santos, Daniela Méria Rodrigues, Ana Rita Sokolonski, Danijela Stanisic, Luiz Marcelo Tomé, Aristóteles Góes-Neto, Vasco Azevedo, Roberto Meyer, Danilo Barral Araújo, Ljubica Tasic, and et al. 2022. "Activity of Fusarium oxysporum-Based Silver Nanoparticles on Candida spp. Oral Isolates" Nanomaterials 12, no. 3: 501. https://doi.org/10.3390/nano12030501
APA StyleFonseca, M. S., Rodrigues, D. M., Sokolonski, A. R., Stanisic, D., Tomé, L. M., Góes-Neto, A., Azevedo, V., Meyer, R., Araújo, D. B., Tasic, L., & Portela, R. D. (2022). Activity of Fusarium oxysporum-Based Silver Nanoparticles on Candida spp. Oral Isolates. Nanomaterials, 12(3), 501. https://doi.org/10.3390/nano12030501