Tying Covalent Organic Frameworks through Alkene Metathesis and Supported Platinum as Efficient Catalysts for Hydrosilylation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Characterization and Measurements
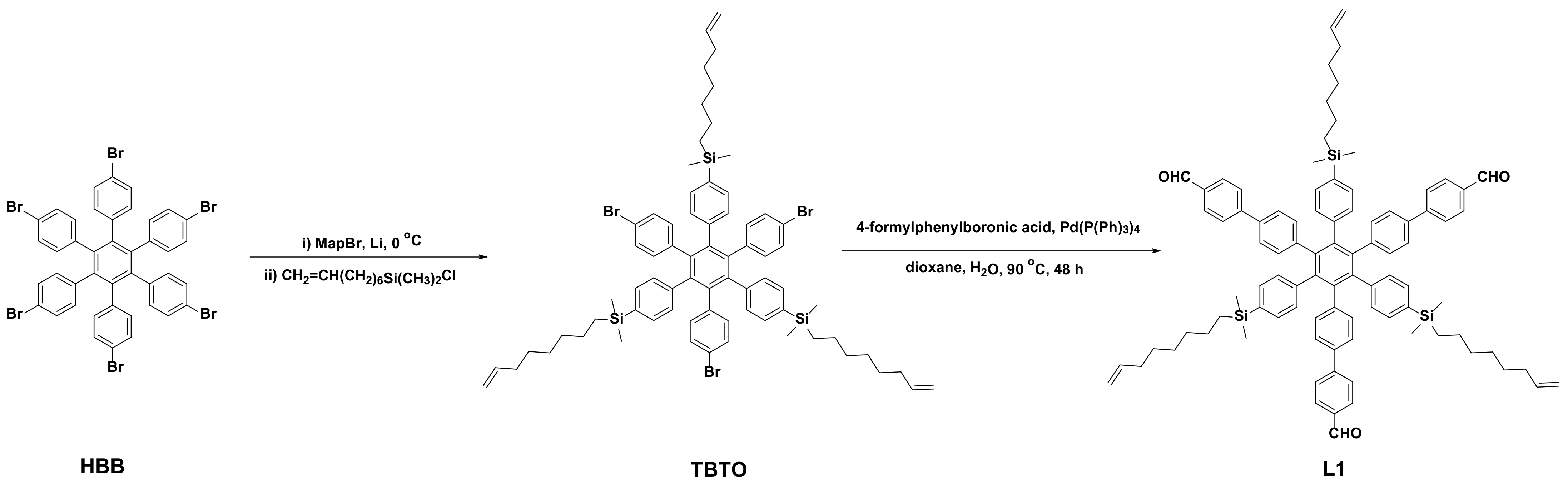
2.3. Synthesis of Compound L1
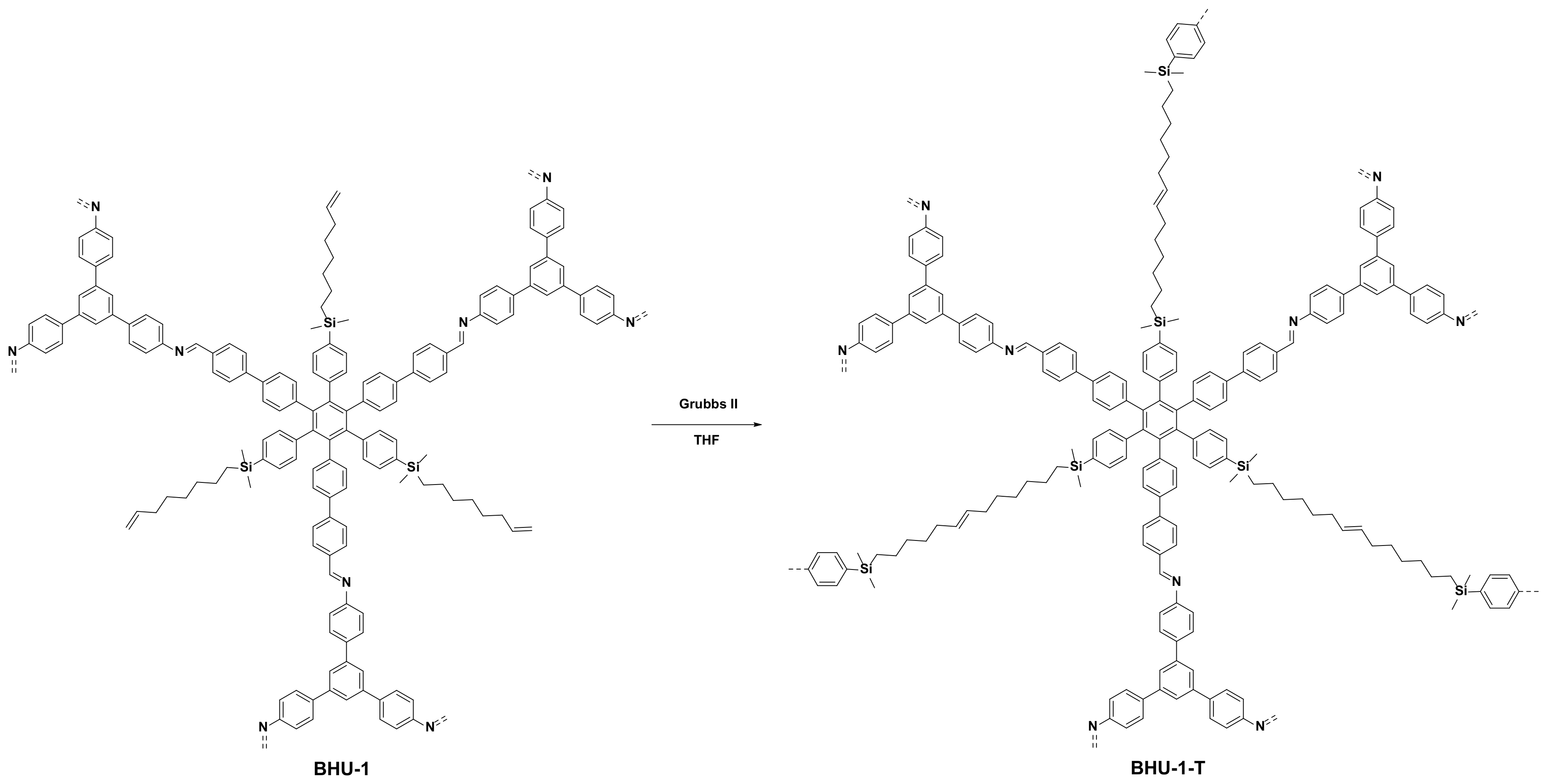
2.4. Synthesis of BHU-1
2.5. Synthesis of BHU-1-T
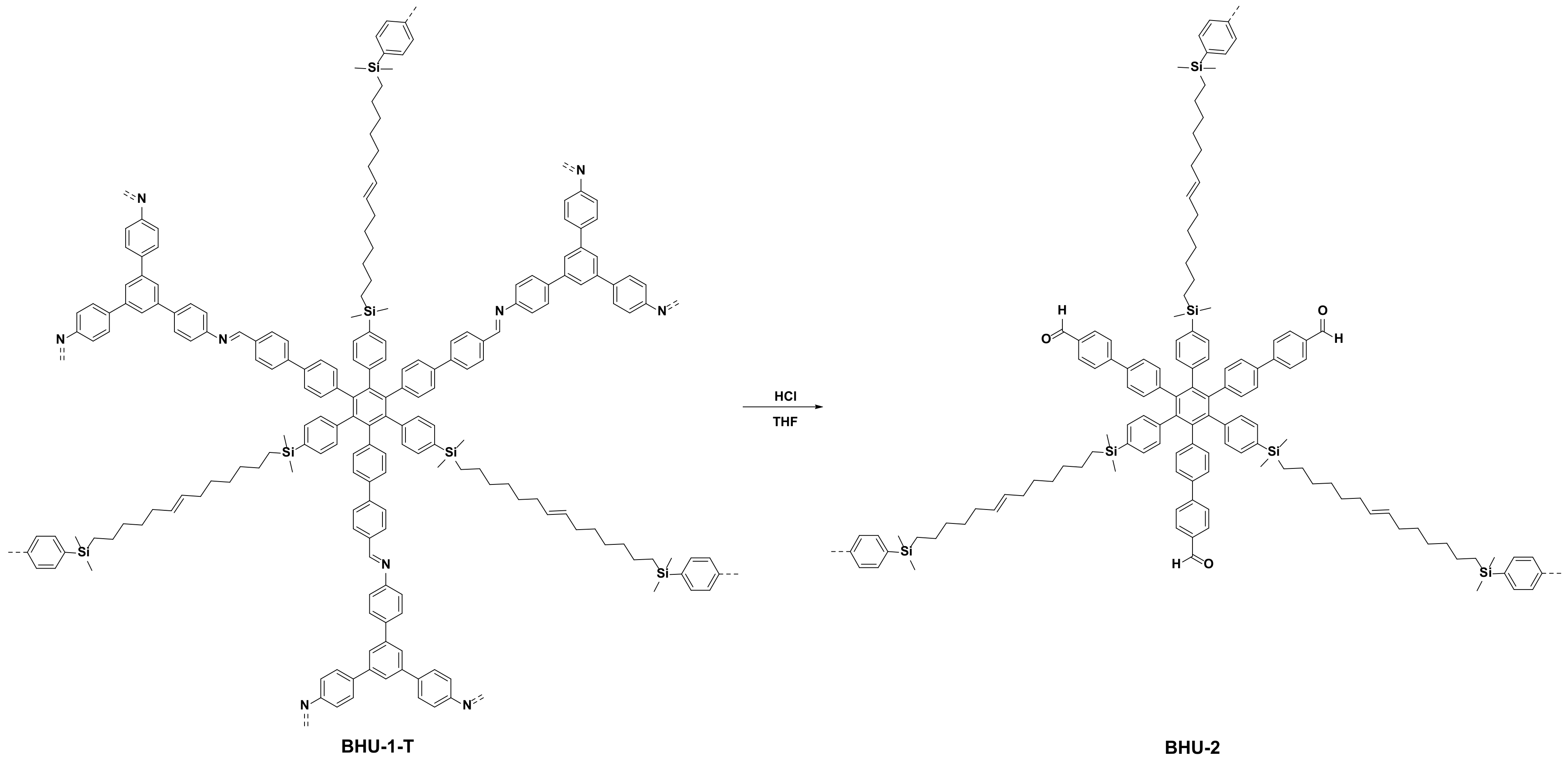
2.6. Synthesis of BHU-2
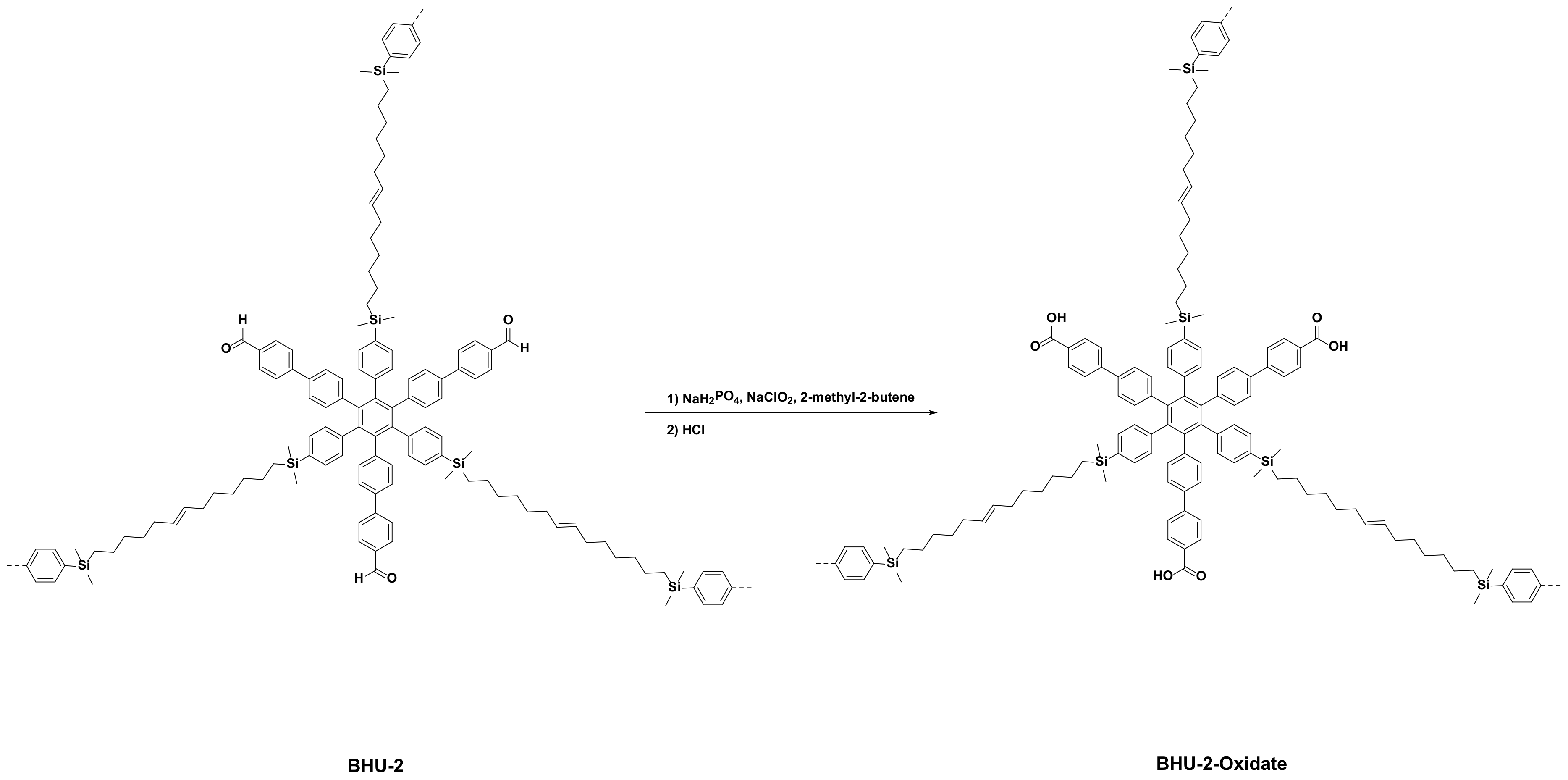
2.7. Synthesis of BHU-2-Oxidate
2.8. Synthesis of Pt@BHU-2 or Pt@BHU-2-Oxidate
2.9. Catalytic Activity Test
3. Results
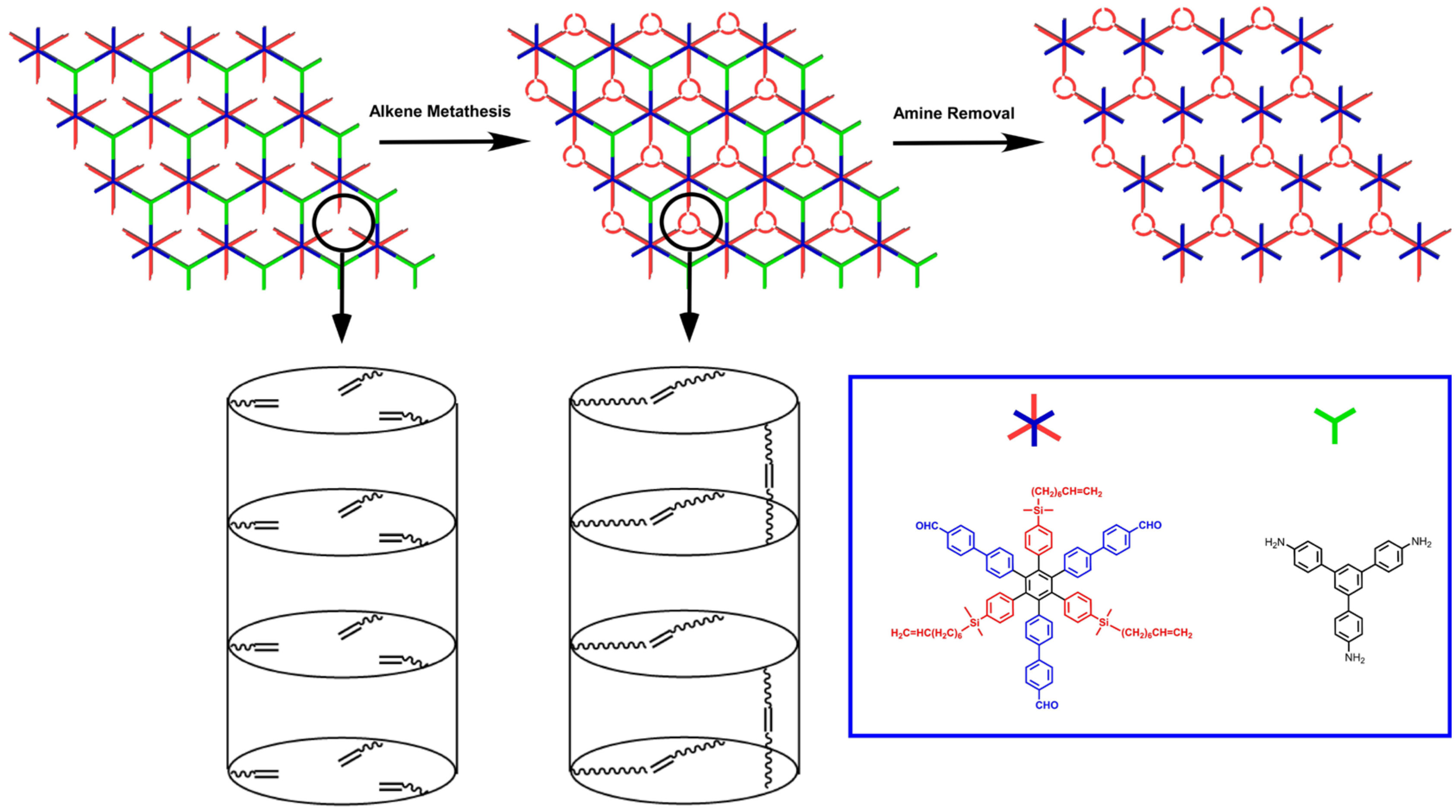
3.1. Overall Design
3.2. Synthesis of C3 Symmetrical Aldehyde Precursor L1
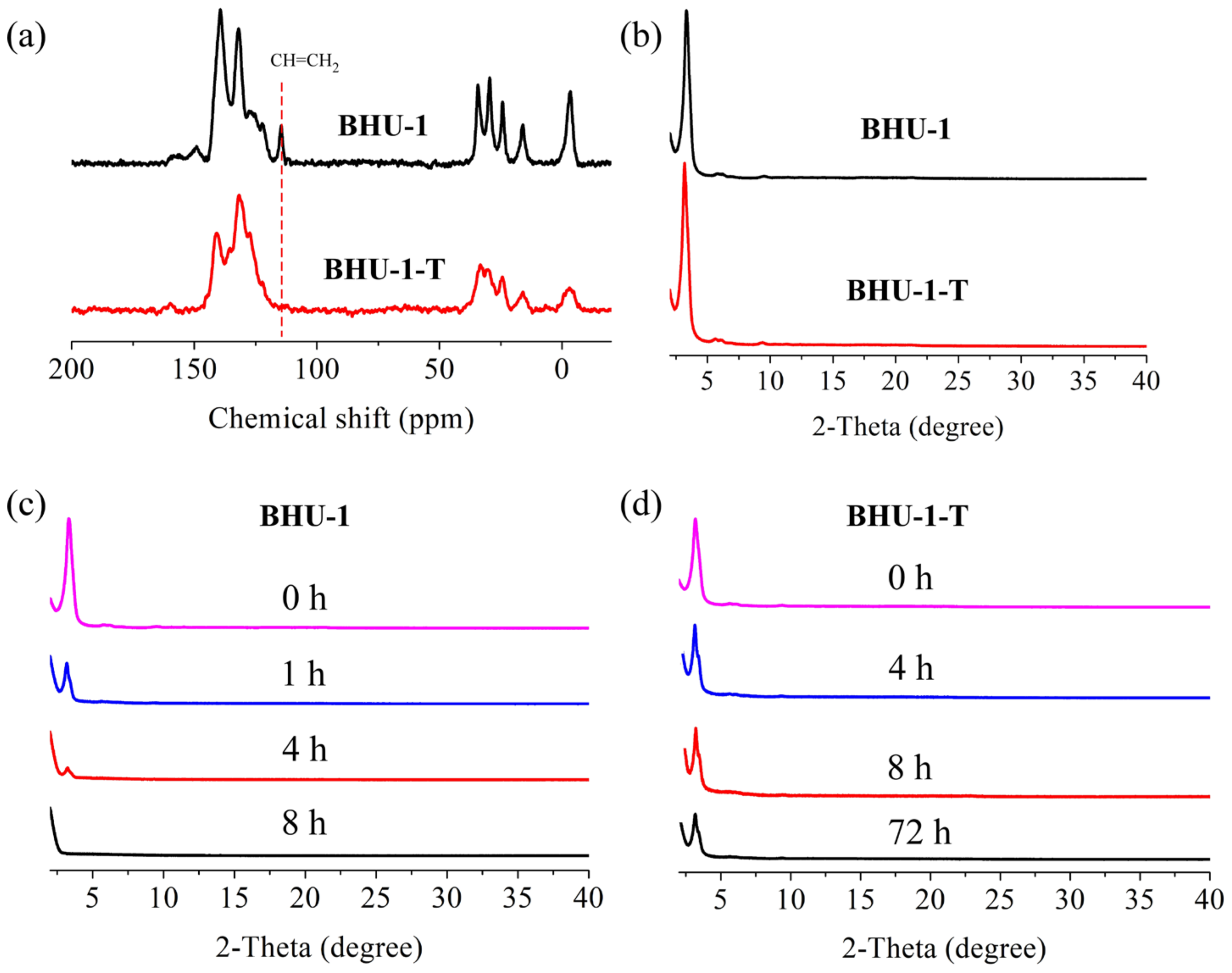
3.3. Preparation and Characterization of BHU-1
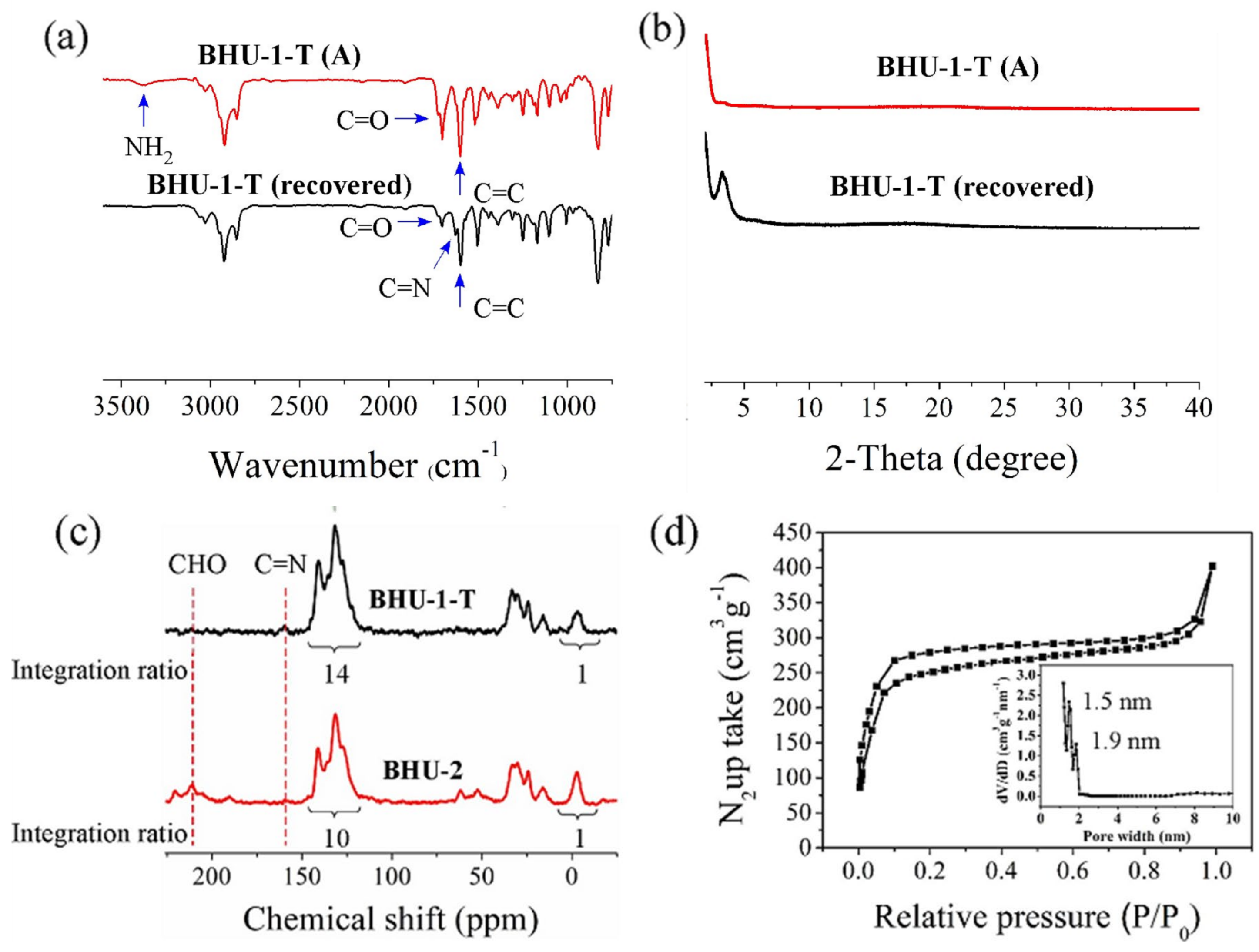
3.4. Tying of BHU-1
3.5. Hydrolysis of BHU-1-T (BHU-2)
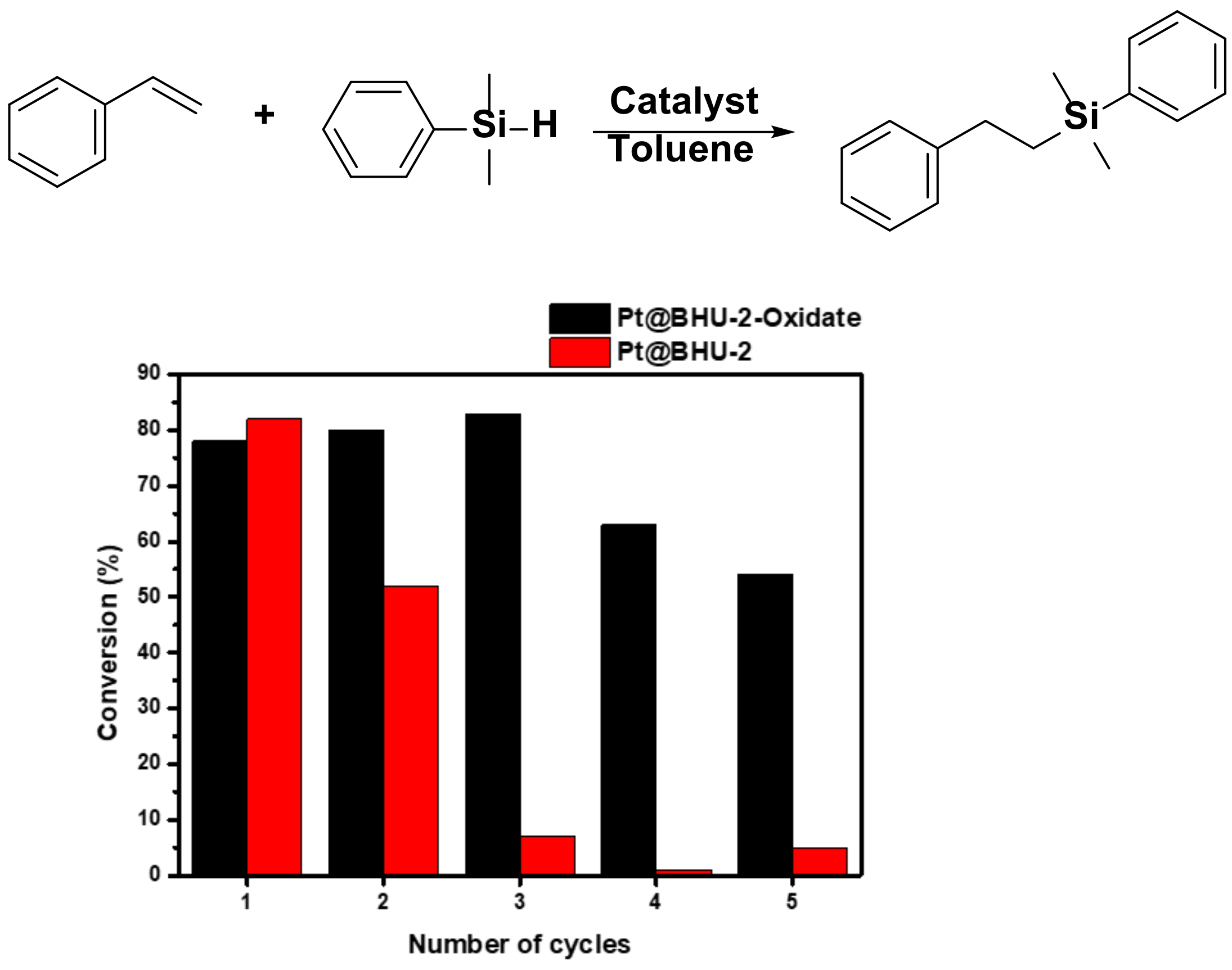
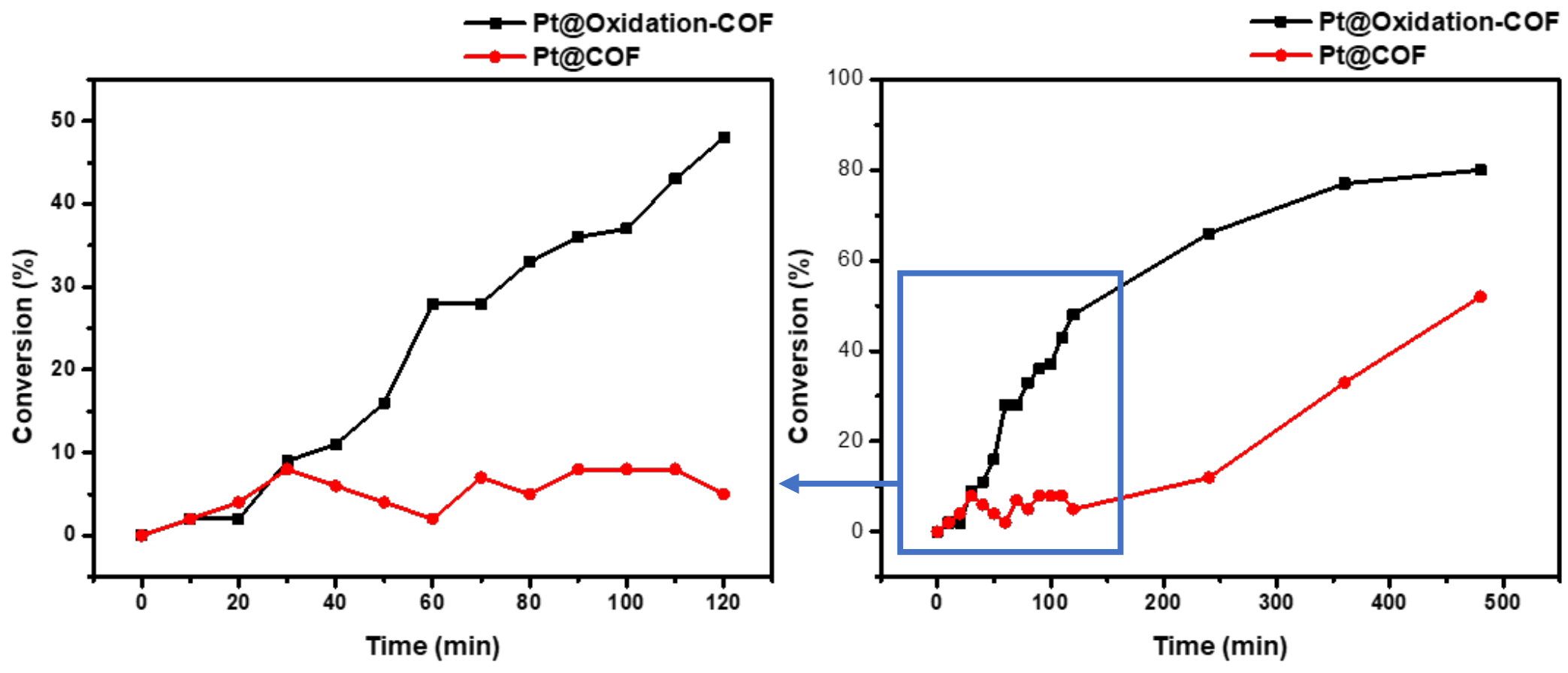
3.6. Hydrosilylation of Styrene Catalyzed by Pt@BHU-2 or Pt@BHU-2-Oxidate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, Y.; Huang, Y.; Zhu, Y.; Chen, B.; Wang, L.; Lai, Z.; Zhang, Z.; Zhao, M.; Tan, C.; Yang, N.; et al. Ultrathin two-dimensional covalent organic framework nanosheets: Preparation and application in highly sensitive and selective DNA detection. J. Am. Chem. Soc. 2017, 139, 8698–8704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, C.; Zhao, D.; Chen, W.; Li, H.; Zhang, S.; Liang, C. Covalent Organic Framework-Functionalized Magnetic CuFe2O4/Ag Nanoparticles for the Reduction of 4-Nitrophenol. Nanomaterials 2020, 10, 426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, T.; Jiang, W.; Li, Y.; Xu, Y.; Zhao, M.; Deng, M.; Wang, Y. Facile regulation of porous N-doped carbon-based catalysts from covalent organic frameworks nanospheres for highly-efficient oxygen reduction reaction. Carbon 2021, 180, 92–100. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Liu, H. A Novel Fluorescence and SPE Adsorption Nanomaterials of Molecularly Imprinted Polymers Based on Quantum Dot-Grafted Covalent Organic Frameworks for the High Selectivity and Sensitivity Detection of Ferulic Acid. Nanomaterials 2019, 9, 305. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; Zhou, S.; Strømme, M.; Xu, C. Redox active covalent organic framework-based conductive nanofibers for flexible energy storage device. Carbon 2021, 171, 248–256. [Google Scholar] [CrossRef]
- Huang, N.; Chen, X.; Krishna, R.; Jiang, D. Two-dimensional covalent organic frameworks for carbon dioxide capture through channel-wall functionalization. Angew. Chem. 2015, 127, 3029–3033. [Google Scholar] [CrossRef] [Green Version]
- Tong, M.; Lan, Y.; Yang, Q.; Zhong, C. Exploring the structure-property relationships of covalent organic frameworks for noble gas separations. Chem. Eng. Sci. 2017, 168, 456–464. [Google Scholar] [CrossRef]
- Rodríguez-San-Miguel, D.; Zamora, F. Processing of covalent organic frameworks: An ingredient for a material to succeed. Chem. Soc. Rev. 2019, 48, 4375–4386. [Google Scholar] [CrossRef]
- Meng, Z.; Mirica, K.A. Covalent organic frameworks as multifunctional materials for chemical detection. Chem. Soc. Rev. 2021, 50, 13498–13558. [Google Scholar] [CrossRef]
- Zhao, X.; Pachfule, P.; Thomas, A. Covalent organic frameworks (COFs) for electrochemical applications. Chem. Soc. Rev. 2021, 50, 6871–6913. [Google Scholar] [CrossRef]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent Organic Frameworks: Design, Synthesis, and Functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef] [PubMed]
- Kandambeth, S.; Mallick, A.; Lukose, B.; Mane, M.V.; Heine, T.; Banerjee, R. Construction of Crystalline 2D Covalent Organic Frameworks with Remarkable Chemical (Acid/Base) Stability via a Combined Reversible and Irreversible Route. J. Am. Chem. Soc. 2012, 134, 19524–19527. [Google Scholar] [CrossRef] [PubMed]
- Halder, A.; Karak, S.; Addicoat, M.; Bera, S.; Chakraborty, A.; Kunjattu, S.H.; Pachfule, P.; Heine, T.; Banerjee, R. Ultrastable Imine-Based Covalent Organic Frameworks for Sulfuric Acid Recovery: An Effect of Interlayer Hydrogen Bonding. Angew.Chem.Int. Ed. 2018, 57, 5797–5802. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, C.; Bi, S.; Wang, W.; He, Y.; Wu, D.; Liang, Q.; Wang, X.; Zhang, F. Vinylene-Linked Covalent Organic Frameworks (COFs) with Symmetry-Tuned Polarity and Photocatalytic Activity. Angew.Chem.Int.Ed. 2020, 59, 23845–23853. [Google Scholar] [CrossRef] [PubMed]
- Biswal, B.P.; Chandra, S.; Kandambeth, S.; Lukose, B.; Heine, T.; Banerjee, R. Mechanochemical synthesis of chemically stable isoreticular covalent organic frameworks. J. Am. Chem. Soc. 2013, 134, 5328–5331. [Google Scholar] [CrossRef]
- Rao, M.R.; Fang, Y.; Feyter, S.D.; Perepichka, D.F. Conjugated covalent organic frameworks via michael addition-elimination. J. Am. Chem. Soc. 2017, 139, 2421–2427. [Google Scholar] [CrossRef]
- Wei, P.; Qi, M.; Wang, Z.; Ding, S.; Yu, W.; Liu, Q.; Wang, L.; Wang, H.; An, W.; Wang, W. Benzoxazole-linked ultrastable covalent organic frameworks for photocatalysis. J. Am. Chem. Soc. 2018, 140, 4623–4631. [Google Scholar] [CrossRef]
- Wang, P.L.; Ding, S.Y.; Zhang, Z.C.; Wang, Z.P.; Wang, W. Constructing Robust Covalent Organic Frameworks via Multicomponent Reaction. J. Am. Chem. Soc. 2019, 141, 18004–18008. [Google Scholar] [CrossRef]
- Pan, G.; Hu, C.; Hong, S.; Li, H.; Yu, D.; Cui, C.; Li, Q.; Liang, N.; Jiang, Y.; Zheng, L.; et al. Biomimetic caged platinum catalyst for hydrosilylation reaction with high site selectivity. Nat. Commun. 2021, 12, 64. [Google Scholar] [CrossRef]
- Kojima, T.; Hiraoka, S. Selective alternate derivatization of the hexaphenylbenzene framework through a thermodynamically controlled halogen dance. Org. Lett. 2014, 16, 1024–1027. [Google Scholar] [CrossRef]
- Kojima, T.; Hiraoka, S.C. Mesityllithium and p-(dimethylamino) phenyllithium for the selective alternate trilithiation of the hexaphenylbenzene framework. Chem. Commun. 2014, 50, 10420–10423. [Google Scholar] [CrossRef] [PubMed]
- Maly, K.E.; Gagnon, E.; Maris, T.; Wuest, J. Engineering hydrogen-bonded molecular crystals built from derivatives of hexaphenylbenzene and related compounds. J. Am. Chem. Soc. 2007, 129, 4306–4322. [Google Scholar] [CrossRef] [PubMed]
- Dalapati, S.; Addicoat, M.; Jin, S.; Sakurai, T.; Gao, J.; Xu, H.; Irle, S.; Seki, S.; Jiang, D. Rational design of crystalline supermicroporous covalent organic frameworks with triangular topologies. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, F.; Teske, J.; Elser, I.; Dyballa, M.; Frey, W.; Kraus, H.; Rybka, J.; Tallarek, U.; Buchmeiser, M.R. Olefin Metathesis in Confined Geometries: A Biomimetic Approach toward Selective Macrocyclization. J. Am. Chem. Soc. 2019, 141, 19014–19022. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Ma, Z.; Wang, Y.; Wang, Y.; Fang, L. Cheminform abstract: Hoveyda-grubbs′ catalyst confined in the nanocages of sba-1: Enhanced recyclability for olefin metathesis. Chem. Commun. 2010, 46, 8659–8661. [Google Scholar] [CrossRef]
- Han, X.; Xia, Q.; Huang, J.; Liu, Y.; Tan, C.; Cui, Y. Chiral Covalent Organic Frameworks with High Chemical Stability for Heterogeneous Asymmetric Catalysis. J. Am. Chem. Soc. 2017, 139, 8693–8697. [Google Scholar] [CrossRef]
- Wu, X.; Wang, B.; Yang, Z.; Chen, L. Novel imine-linked covalent organic frameworks: Preparation, characterization and application. J. Mater. Chem. A. 2019, 7, 5650–5655. [Google Scholar] [CrossRef]
- Gao, H.; Ding, L.; Bai, H.; Liu, A.; Li, S.; Li, L. Pitch-based hyper-cross-linked polymers with high performance for gas adsorption. J. Mater. Chem. A. 2016, 4, 16490–16498. [Google Scholar] [CrossRef]
- Stegbauer, L.; Schwinghammer, K.; Lotsch, B. A hydrazone-based covalent organic framework for photocatalytic hydrogen production. Chem. Sci. 2014, 5, 2789–2793. [Google Scholar] [CrossRef] [Green Version]
- Alahakoon, S.B.; Thompson, C.M.; Nguyen, A.X.; Occhialini, G.; McCandless, G.T.; Smaldone, R.A. An azine-linked hexaphenylbenzene based covalent organic framework. Chem. Commun. 2016, 52, 2843–2845. [Google Scholar] [CrossRef]
- Lohse, M.S.; Bein, T. Covalent Organic Frameworks: Structures, Synthesis, and Applications. Adv. Funct. Mater. 2018, 28, 1705553–1705623. [Google Scholar] [CrossRef] [Green Version]
- Kong, W.; Jia, W.; Wang, R.; Gong, Y.; Wang, C.; Wu, P.; Guo, J. Amorphous-to-crystalline transformation toward controllable synthesis of fibrous covalent organic frameworks enabling promotion of proton transport. Chem. Commun. 2019, 55, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Namuangruk, S.; Kong, W.; Kungwan, N.; Guo, J.; Wang, C. Manipulation of amorphous-to-crystalline transformation: Towards the construction of covalent organic framework hybrid microspheres with NIR photothermal conversion ability. Angew. Chem. Int. Ed. 2016, 55, 13979–13984. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Heasman, P.; Ben, T.; Qiu, S. Porous organic materials: Strategic design and structure-function correlation. Chem. Rev. 2017, 117, 1515–1563. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhao, W.; Wang, S.; Liang, Y.; Yao, Z.-J. Access to Functionalized E-Allylsilanes and E-Alkenylsilanes through Visible-Light-Driven Radical Hydrosilylation of Mono- and Disubstituted Allenes. Org. Lett. 2019, 21, 9836–9840. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, E.E.; Chen, G.; Li, J.-L.; Liu, P.; Wang, H. Radical Hydroboration and Hydrosilylation of gem-Difluoroalkenes: Synthesis of α-Difluorinated Alkylborons and Alkylsilanes. Org. Lett. 2019, 21, 8454–8458. [Google Scholar] [CrossRef]
- Asensio, J.M.; Bouzouita, D.; Leeuwen, P.W.N.M.V.; Chaudret, B. σ-H–H, σ-C–H, and σ-Si–H Bond Activation Catalyzed by Metal Nanoparticles. Chem. Rev. 2020, 120, 1042–1084. [Google Scholar] [CrossRef]
- Sun, J.; Deng, L. Cobalt Complex-Catalyzed Hydrosilylation of Alkenes and Alkynes. ACS Catal. 2016, 6, 290–300. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Pandarus, V.; Béland, F. Platinum-Based Heterogeneously Catalyzed Hydrosilylation. Eur. J. Org. Chem. 2013, 28, 6227–6235. [Google Scholar] [CrossRef]
- Jawale, D.V.; Geertsen, V.; Miserque, F.; Berthault, P.; Gravel, E.; Doris, E. Solvent-free hydrosilylation of alkenes and alkynes using recyclable platinum on carbon nanotubes. Green Chem. 2021, 23, 815–820. [Google Scholar] [CrossRef]
- Zai, H.; Zhao, Y.; Chen, S.; Wang, R.; Ge, L.; Chenab, C.; Li, Y. A novel hierachically-nanostructured Pt/SiO2/Fe3O4 catalyst with high activity and recyclability towards hydrosilylation. RSC Adv. 2016, 6, 98520–98527. [Google Scholar] [CrossRef]
- Moorthy, J.N.; Natarajan, R.; Venugopalan, P. Three-Dimensional Four-Connecting Organic Scaffolds with a Twist: Synthesis and Self-Assembly. J. Org. Chem. 2005, 70, 8568–8571. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Peng, J.; Hu, Y.; Li, J.; Qiu, H.; Lai, G. Effect of Aminoaromatic Acids as Additives on the Activity and Selectivity of the Platinum-catalyzed Hydrosilylation of Alkenes. Chin. J. Chem. Eng. 2009, 17, 1038–1042. [Google Scholar] [CrossRef]
- Ding, S.; Deng, S.; Zhang, N.; Yang, L. Carboxylic Acids Promoted Speier’S Catalyst for the Hydrosilylation of Styrene with Triethoxysilane: Activity, Selectivity, and Mechanism. Phosphorus Sulfur Silicon Relat. Elem. 2014, 189, 803–811. [Google Scholar] [CrossRef]
- Ciriminna, R.; Pandarus, V.; Gingras, G.; Béland, F.; Pagliaro, M. Closing the Organosilicon Synthetic Cycle: Efficient Heterogeneous Hydrosilylation of Alkenes over SiliaCat Pt(0). ACS Sustain. Chem. Eng. 2013, 1, 249–253. [Google Scholar] [CrossRef]
- Huo, Y.; Hu, J.; Lin, S.; Ju, X.; Wei, Y.; Huang, Z.; Hu, Y.; Tu, Y. Platinum(II) complexes bearing bulky Schiff base ligands anchored onto mesoporous SBA-15 supports as efficient catalysts for hydrosilylation. Appl. Organometal Chem. 2019, 33, e4874. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, L.; Sterbinsky, G.E.; Mukherjee, D.; Unocic, R.R.; Tait, S.L. Pt-Ligand single-atom catalysts: Tuning activity by oxide support defect density. Catal. Sci. Technol. 2020, 10, 3353–3365. [Google Scholar] [CrossRef]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Yazaydin, A.Ö.; Snurr, R.Q.; OKeeffe, M.; Kim, J.; et al. Ultrahigh porosity in metal-organic frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef] [Green Version]
- Zheng, B.; Bai, J.; Duan, J.; Wojtas, L.; Zaworotko, M. Enhanced CO2 binding affinity of a high-uptake rht-type metal-organic framework decorated with acylamide groups. J. Am. Chem. Soc. 2010, 133, 748–751. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, D.; Li, G.; Liu, Y.; Liu, Y. Tying Covalent Organic Frameworks through Alkene Metathesis and Supported Platinum as Efficient Catalysts for Hydrosilylation. Nanomaterials 2022, 12, 499. https://doi.org/10.3390/nano12030499
Gu D, Li G, Liu Y, Liu Y. Tying Covalent Organic Frameworks through Alkene Metathesis and Supported Platinum as Efficient Catalysts for Hydrosilylation. Nanomaterials. 2022; 12(3):499. https://doi.org/10.3390/nano12030499
Chicago/Turabian StyleGu, Defa, Guangwen Li, Yushan Liu, and Yuzhou Liu. 2022. "Tying Covalent Organic Frameworks through Alkene Metathesis and Supported Platinum as Efficient Catalysts for Hydrosilylation" Nanomaterials 12, no. 3: 499. https://doi.org/10.3390/nano12030499
APA StyleGu, D., Li, G., Liu, Y., & Liu, Y. (2022). Tying Covalent Organic Frameworks through Alkene Metathesis and Supported Platinum as Efficient Catalysts for Hydrosilylation. Nanomaterials, 12(3), 499. https://doi.org/10.3390/nano12030499





