Recent Advances in Synthesis and Application of Metal Oxide Nanostructures in Chemical Sensors and Biosensors
Abstract
1. Introduction
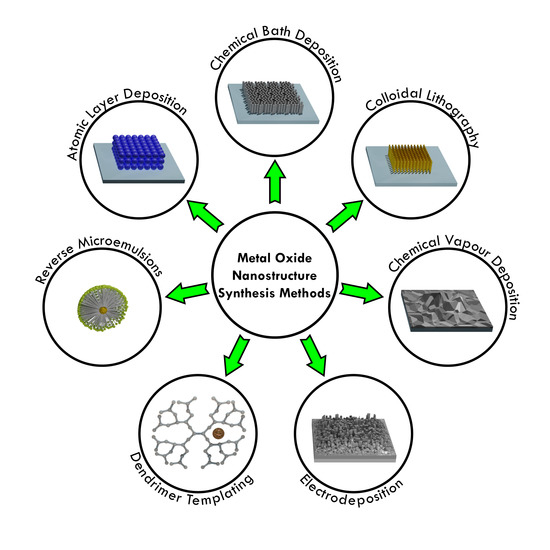
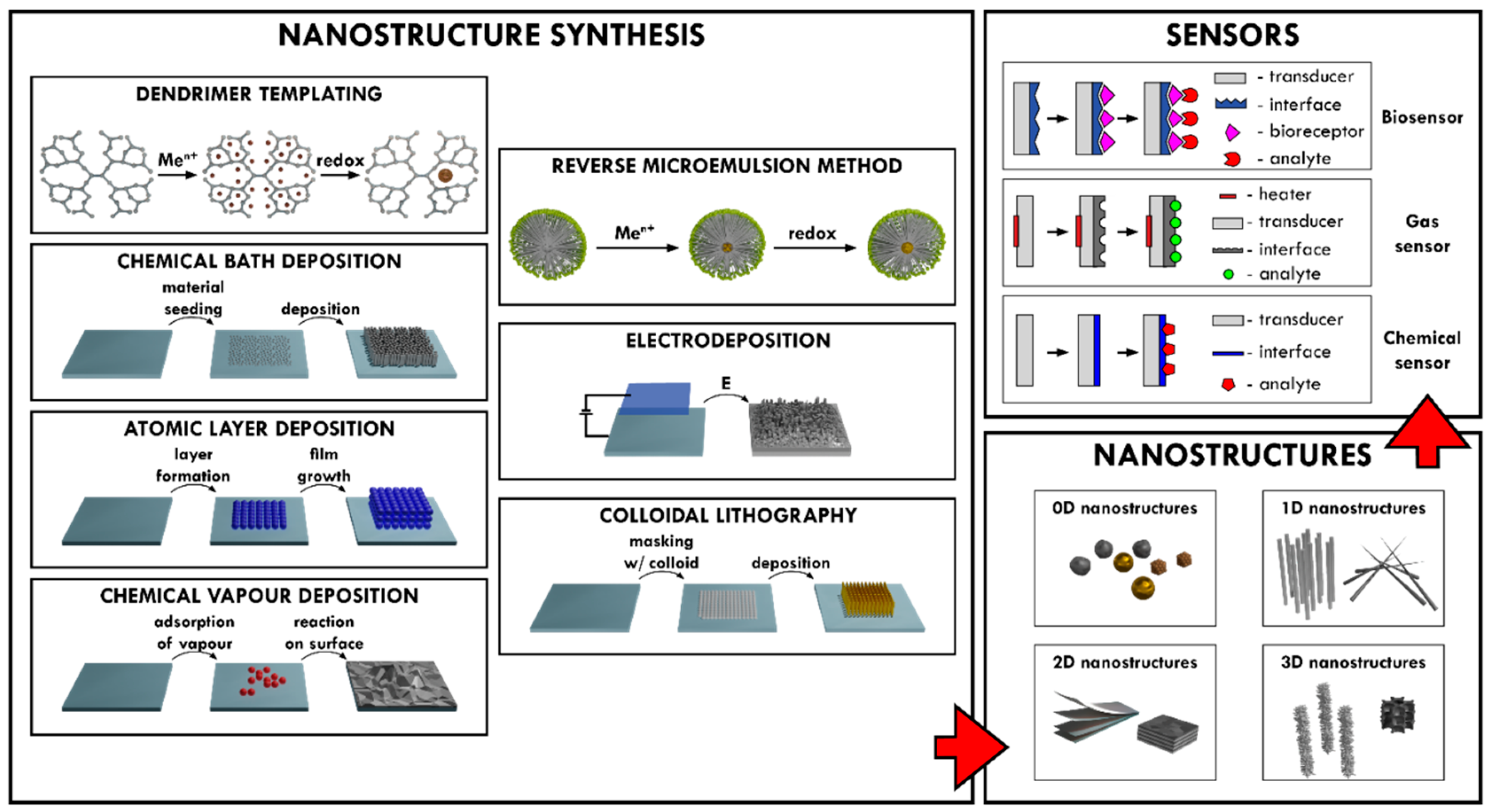
2. Nanostructure Synthesis Methods
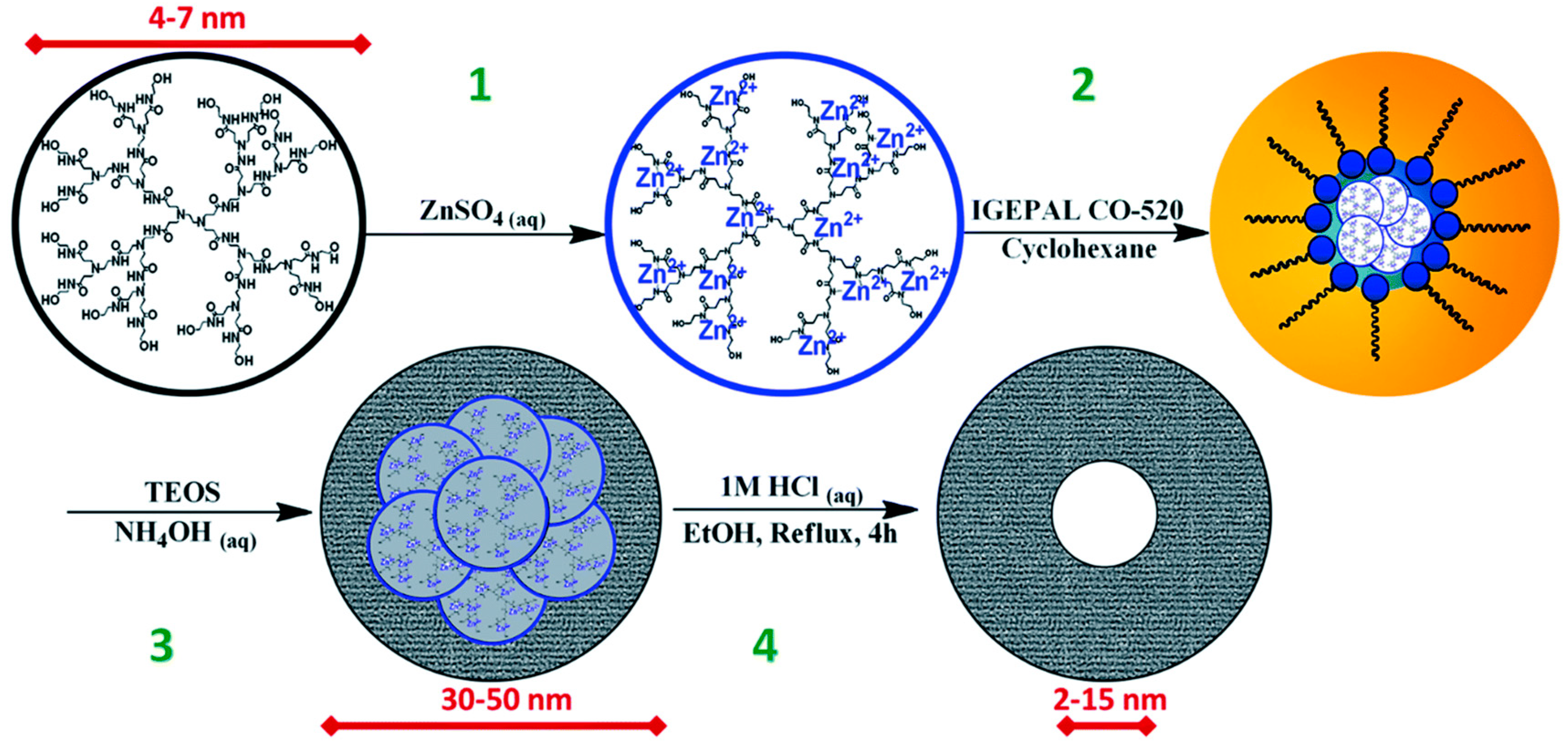
2.1. Reverse Microemulsion Method for the Synthesis of Nanostructures
2.2. Dendrimer Templating for Nanostructure Formation
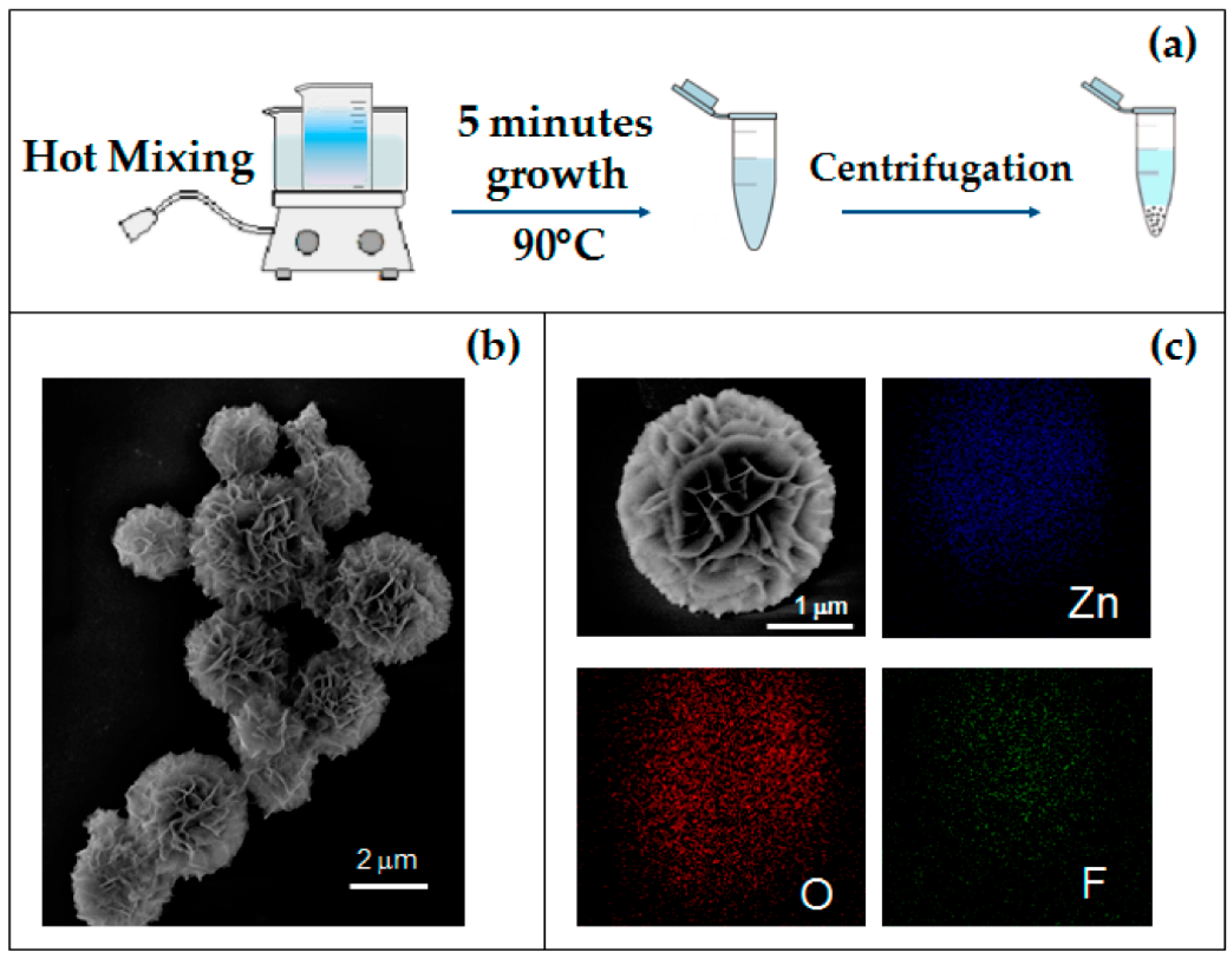
2.3. Chemical Bath Deposition (CBD) for Nanostructure Synthesis
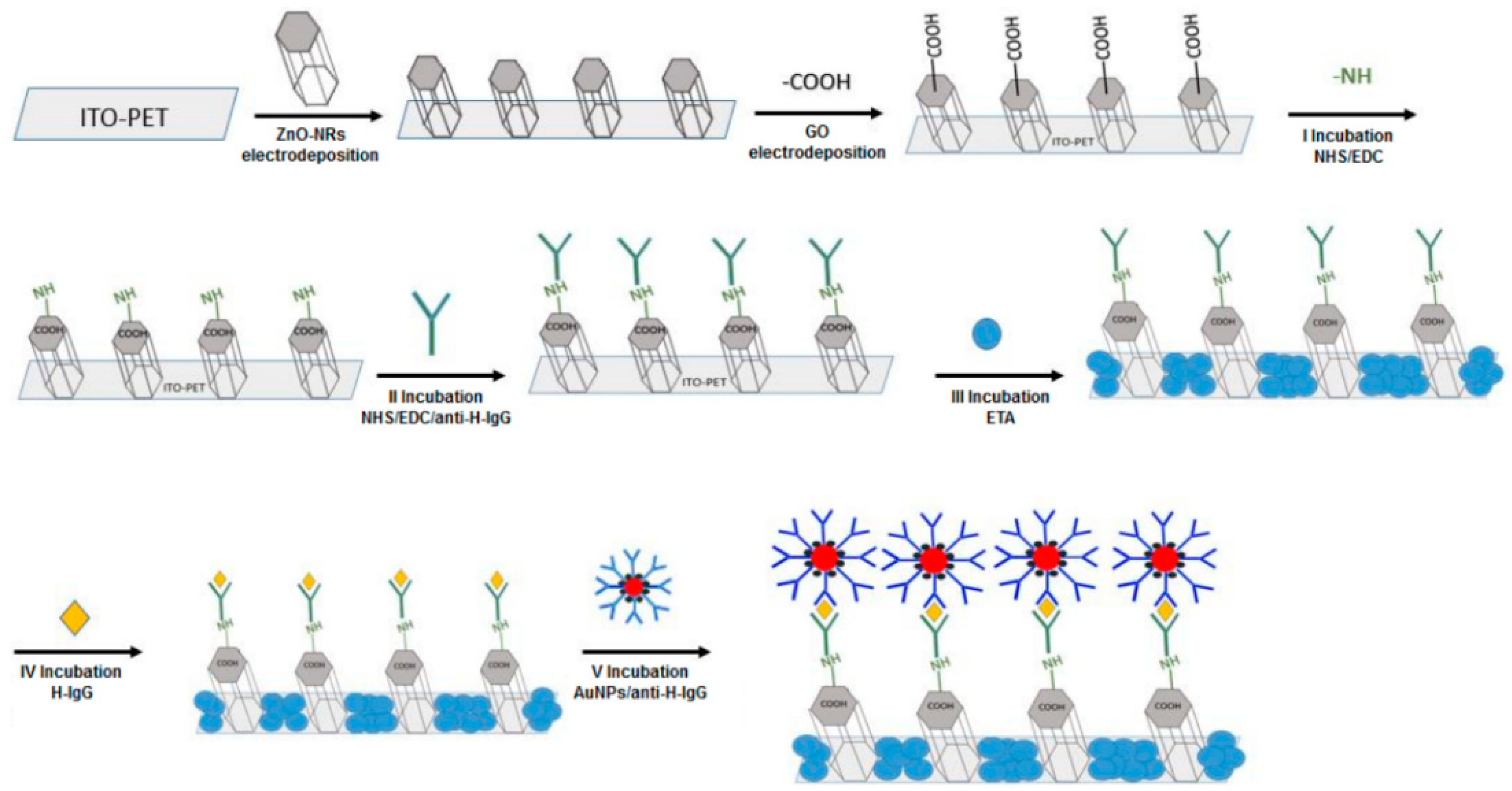
2.4. Electrodeposition for the Formation of Nanostructures
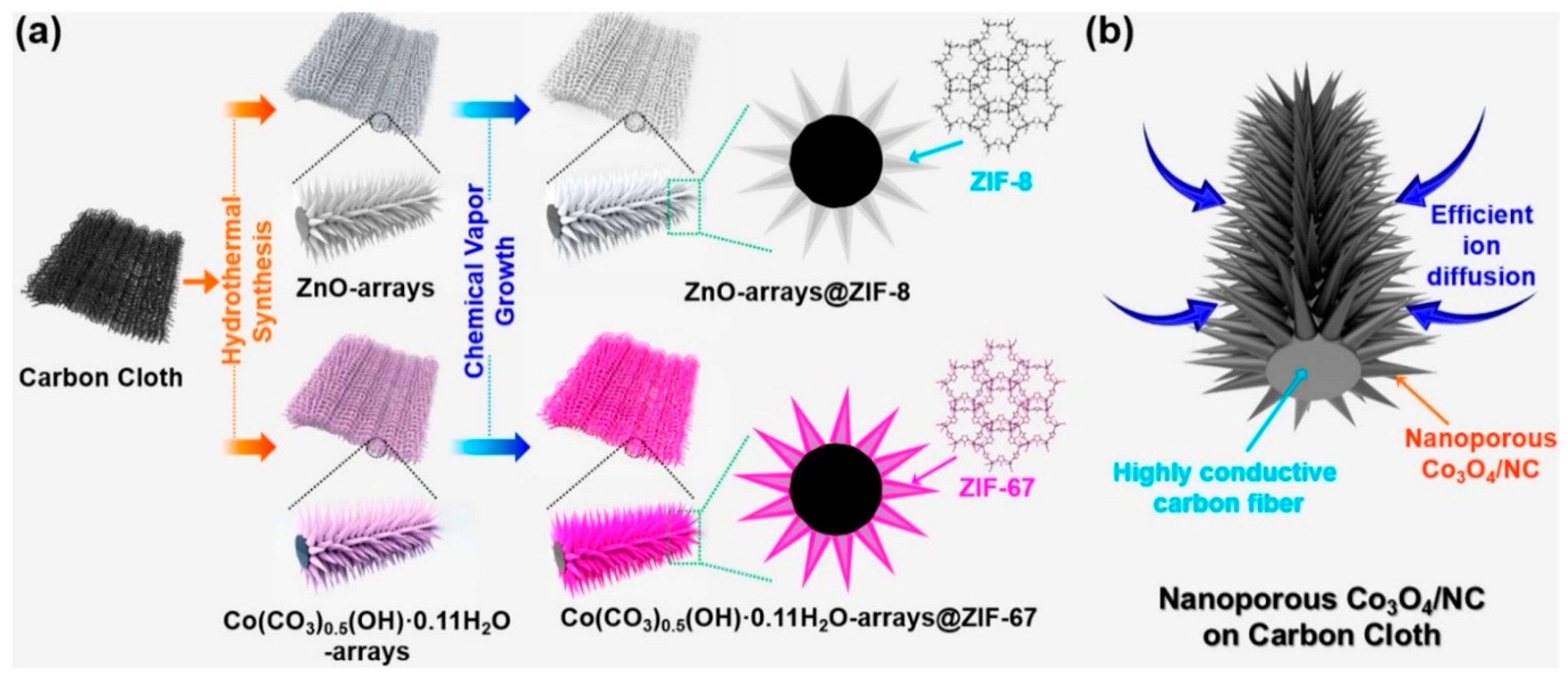
2.5. Chemical Vapor Deposition (CVD) for Synthesis of Nanostructures
2.6. Atomic Layer Deposition (ALD) Method for the Formation of Nanostructures
3. Application of Metal Oxide Nanostructures for Chemical Sensing and Biosensing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balevicius, Z.; Paulauskas, A.; Plikusiene, I.; Mikoliunaite, L.; Bechelany, M.; Popov, A.; Ramanavicius, A.; Ramanaviciene, A. Towards the application of Al2O3/ZnO nanolaminates in immunosensors: Total internal reflection spectroscopic ellipsometry based evaluation of BSA immobilization. J. Mater. Chem. C 2018, 6, 8778–8783. [Google Scholar] [CrossRef]
- Maciulis, V.; Malinovskis, U.; Erts, D.; Ramanavicius, A.; Ramanaviciene, A.; Balevicius, S.; Juciute, S.; Plikusiene, I. Porous aluminium oxide coating for the development of spectroscopic ellipsometry based biosensor: Evaluation of human serum albumin adsorption. Coatings 2020, 10, 1018. [Google Scholar] [CrossRef]
- Plikusiene, I.; Maciulis, V.; Graniel, O.; Bechelany, M.; Balevicius, S.; Vertelis, V.; Balevicius, Z.; Popov, A.; Ramanavicius, A.; Ramanaviciene, A. Total internal reflection ellipsometry for kinetics-based assessment of bovine serum albumin immobilization on ZnO nanowires. J. Mater. Chem. C 2021, 9, 1345–1352. [Google Scholar] [CrossRef]
- Filipovic, L.; Selberherr, S. Application of Two-Dimensional Materials towards CMOS-Integrated Gas Sensors. Nanomaterials 2022, 12, 3651. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, D.; Wang, H.; Huang, W.; Hu, L.; Tang, Y.; Guo, Z.; Ouyang, Z.; Zhang, H. Recent advances in two-dimensional-material-based sensing technology toward health and environmental monitoring applications. Nanoscale 2020, 12, 3535–3559. [Google Scholar] [CrossRef]
- Ponzoni, A.; Baratto, C.; Cattabiani, N.; Falasconi, M.; Galstyan, V.; Nunez-Carmona, E.; Rigoni, F.; Sberveglieri, V.; Zambotti, G.; Zappa, D. Metal oxide gas sensors, a survey of selectivity issues addressed at the SENSOR lab, Brescia (Italy). Sensors 2017, 17, 714. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, J.; Wang, W.; Sun, Y.; Li, P.; Hu, J.; Chen, L.; Gong, W. Synthesis and electrochemical properties of Co3O4-rGO/CNTs composites towards highly sensitive nitrite detection. Appl. Surf. Sci. 2019, 485, 274–282. [Google Scholar] [CrossRef]
- Popov, A.; Lisyte, V.; Kausaite-Minkstimiene, A.; Bernotiene, E.; Ramanaviciene, A. Experimental Evaluation of Quantum Dots and Antibodies Conjugation by Surface Plasmon Resonance Spectroscopy. Int. J. Mol. Sci. 2022, 23, 12626. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
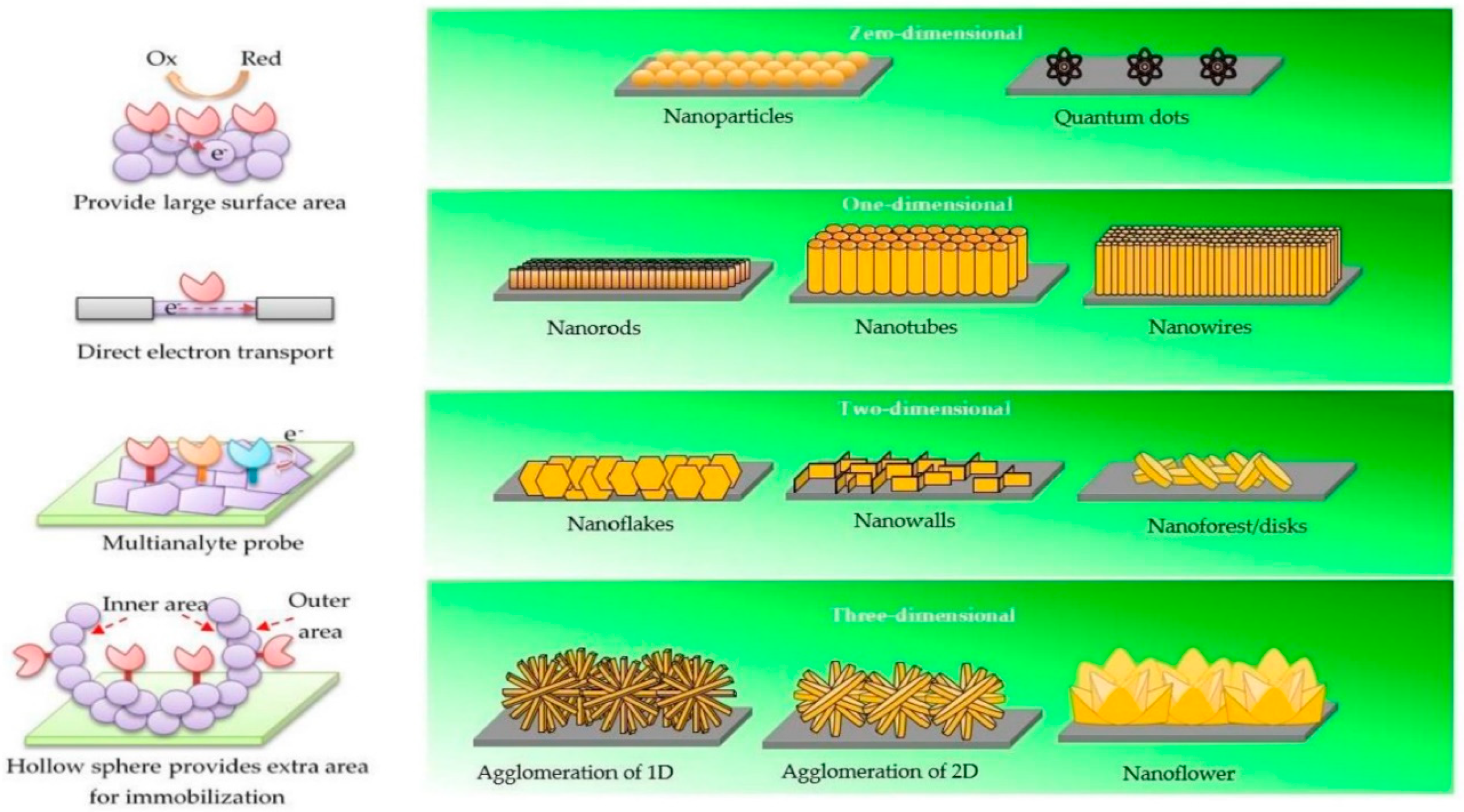
- Pokropivny, V.V.; Skorokhod, V.V. Classification of nanostructures by dimensionality and concept of surface forms engineering in nanomaterial science. Mater. Sci. Eng. C 2007, 27, 990–993. [Google Scholar] [CrossRef]
- Cook, A.W.; Hayton, T.W. Case Studies in Nanocluster Synthesis and Characterization: Challenges and Opportunities. Acc. Chem. Res. 2018, 51, 2456–2464. [Google Scholar] [CrossRef]
- Li, Z.; Wang, W.; Yin, Y. Colloidal Assembly and Active Tuning of Coupled Plasmonic Nanospheres. Trends Chem. 2020, 2, 593–608. [Google Scholar] [CrossRef]
- García de Arquer, F.P.; Talapin, D.V.; Klimov, V.I.; Arakawa, Y.; Bayer, M.; Sargent, E.H. Semiconductor quantum dots: Technological progress and future challenges. Science 2021, 373, eaaz8541. [Google Scholar] [CrossRef]
- Pacholski, C.; Kornowski, A.; Weller, H. Self-assembly of ZnO: From nanodots to nanorods. Angew. Chem.—Int. Ed. 2002, 41, 1188–1191. [Google Scholar] [CrossRef]
- Xu, S.; Lei, Y. Template-Assisted Fabrication of Nanostructured Arrays for Sensing Applications. Chempluschem 2018, 83, 741–755. [Google Scholar] [CrossRef]
- Chaaya, A.A.; Bechelany, M.; Balme, S.; Miele, P. ZnO 1D nanostructures designed by combining atomic layer deposition and electrospinning for UV sensor applications. J. Mater. Chem. A 2014, 2, 20650–20658. [Google Scholar] [CrossRef]
- Wu, C.C.; Pan, T.M.; Wu, C.S.; Yen, L.C.; Chuang, C.K.; Pang, S.T.; Yang, Y.S.; Ko, F.H. Label-free detection of prostate specific antigen using a silicon nanobelt field-effect transistor. Int. J. Electrochem. Sci. 2012, 7, 4432–4442. [Google Scholar]
- Ackermann, J.; Metternich, J.T.; Herbertz, S.; Kruss, S. Biosensing with Fluorescent Carbon Nanotubes. Angew. Chem.—Int. Ed. 2022, 61, e202112372. [Google Scholar] [CrossRef]
- Aykaç, A.; Gergeroglu, H.; Beşli, B.; Akkaş, E.Ö.; Yavaş, A.; Güler, S.; Güneş, F.; Erol, M. An Overview on Recent Progress of Metal Oxide/Graphene/CNTs-Based Nanobiosensors. Nanoscale Res. Lett. 2021, 16, 65. [Google Scholar] [CrossRef]
- Huo, D.; Kim, M.J.; Lyu, Z.; Shi, Y.; Wiley, B.J.; Xia, Y. One-Dimensional Metal Nanostructures: From Colloidal Syntheses to Applications. Chem. Rev. 2019, 119, 8972–9073. [Google Scholar] [CrossRef]
- Javed, M.S.; Khan, A.J.; Asim, S.; Shah, S.S.A.; Najam, T.; Siyal, S.H.; Tahir, M.F.; Zhao, Z.; Mai, W. Insights to pseudocapacitive charge storage of binary metal-oxide nanobelts decorated activated carbon cloth for highly-flexible hybrid-supercapacitors. J. Energy Storage 2020, 31, 101602. [Google Scholar] [CrossRef]
- Kwak, D.; Wang, M.; Koski, K.J.; Zhang, L.; Sokol, H.; Maric, R.; Lei, Y. Molybdenum Trioxide (α-MoO3) Nanoribbons for Ultrasensitive Ammonia (NH3) Gas Detection: Integrated Experimental and Density Functional Theory Simulation Studies. ACS Appl. Mater. Interfaces 2019, 11, 10697–10706. [Google Scholar] [CrossRef] [PubMed]
- Samykano, M. Progress in one-dimensional nanostructures. Mater. Charact. 2021, 179, 111373. [Google Scholar] [CrossRef]
- Heard, C.J.; Čejka, J.; Opanasenko, M.; Nachtigall, P.; Centi, G.; Perathoner, S. 2D Oxide Nanomaterials to Address the Energy Transition and Catalysis. Adv. Mater. 2019, 31, e1801712. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, L.; Tan, W.C.; Feng, X.; Chen, L.; Huang, X.; Ang, K.W. 2D Photovoltaic Devices: Progress and Prospects. Small Methods 2018, 2, 1700294. [Google Scholar] [CrossRef]
- Kumar, S.; Pavelyev, V.; Mishra, P.; Tripathi, N.; Sharma, P.; Calle, F. A review on 2D transition metal di-chalcogenides and metal oxide nanostructures based NO2 gas sensors. Mater. Sci. Semicond. Process. 2020, 107, 104865. [Google Scholar] [CrossRef]
- Napi, M.L.M.; Sultan, S.M.; Ismail, R.; How, K.W.; Ahmad, M.K. Electrochemical-Based Biosensors on Different Zinc Oxide Nanostructures: A Review. Materials 2019, 12, 2985. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Cheng, H.; Liu, J.; Shao, M.; Wei, M.; Evans, D.G.; Zhang, H.; Duan, X. Confined Synthesis of 2D Nanostructured Materials toward Electrocatalysis. Adv. Energy Mater. 2020, 10, 1900486. [Google Scholar] [CrossRef]
- Jang, J.M.; Kim, S.D.; Choi, H.M.; Kim, J.Y.; Jung, W.G. Morphology change of self-assembled ZnO 3D nanostructures with different pH in the simple hydrothermal process. Mater. Chem. Phys. 2009, 113, 389–394. [Google Scholar] [CrossRef]
- Chiu, H.Y.; Wi-Afedzi, T.; Liu, Y.T.; Ghanbari, F.; Lin, K.Y.A. Cobalt Oxides with Various 3D Nanostructured Morphologies for Catalytic Reduction of 4-Nitrophenol: A Comparative Study. J. Water Process Eng. 2020, 37, 101379. [Google Scholar] [CrossRef]
- Yang, Z.; Lin, Y.; Jiao, F.; Li, J.; Wang, W.; Gong, Y.; Jing, X. Morphology engineering of 3D nanostructure MMNS as bifunctional electrocatalysts towards high-efficient overall water splitting. Appl. Surf. Sci. 2020, 502, 144147. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Tiwari, R.N.; Kim, K.S. Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Prog. Mater. Sci. 2012, 57, 724–803. [Google Scholar] [CrossRef]
- Joshi, R.K.; Schneider, J.J. Assembly of one dimensional inorganic nanostructures into functional 2D and 3D architectures. Synthesis, arrangement and functionality. Chem. Soc. Rev. 2012, 41, 5285–5312. [Google Scholar] [CrossRef]
- Young, C.; Wang, J.; Kim, J.; Sugahara, Y.; Henzie, J.; Yamauchi, Y. Controlled Chemical Vapor Deposition for Synthesis of Nanowire Arrays of Metal-Organic Frameworks and Their Thermal Conversion to Carbon/Metal Oxide Hybrid Materials. Chem. Mater. 2018, 30, 3379–3386. [Google Scholar] [CrossRef]
- Altintas Yildirim, O. Efficient vapor-liquid-solid synthesis of copper doped zinc oxide (Cu:ZnO) nanonails with highly homogeneous dopant distribution. Mater. Sci. Semicond. Process. 2019, 101, 238–246. [Google Scholar] [CrossRef]
- Pei, Y.; Hu, M.; Xia, Y.; Huang, W.; Li, Z.; Chen, S. Electrochemical preparation of Pt nanoparticles modified nanoporous gold electrode with highly rough surface for efficient determination of hydrazine. Sens. Actuators B Chem. 2020, 304, 127416. [Google Scholar] [CrossRef]
- Dondapati, J.S.; Govindhan, M.; Chen, A. Direct growth of three-dimensional nanoflower-like structures from flat metal surfaces. Chem. Commun. 2022, 1, 11127–11130. [Google Scholar] [CrossRef]
- Slomkowski, S.; Alemán, J.V.; Gilbert, R.G.; Hess, M.; Horie, K.; Jones, R.G.; Kubisa, P.; Meisel, I.; Mormann, W.; Penczek, S.; et al. Terminology of polymers and polymerization processes in dispersed systems (IUPAC recommendations 2011). Pure Appl. Chem. 2011, 83, 2229–2259. [Google Scholar] [CrossRef]
- Ye, Z.; Tan, M.; Wang, G.; Yuan, J. Preparation, characterization and application of fluorescent terbium complex-doped zirconia nanoparticles. J. Fluoresc. 2005, 15, 499–505. [Google Scholar] [CrossRef]
- Shlapa, Y.; Sarnatskaya, V.; Timashkov, I.; Yushko, L.; Antal, I.; Gerashchenko, B.; Nychyporenko, I.; Belous, A.; Nikolaev, V.; Timko, M. Synthesis of CeO2 nanoparticles by precipitation in reversal microemulsions and their physical–chemical and biological properties. Appl. Phys. A Mater. Sci. Process. 2019, 125, 1–10. [Google Scholar] [CrossRef]
- Malik, M.A.; Wani, M.Y.; Hashim, M.A. Microemulsion method: A novel route to synthesize organic and inorganic nanomaterials. 1st Nano Update. Arab. J. Chem. 2012, 5, 397–417. [Google Scholar] [CrossRef]
- Du, Y.; Wang, W.; Li, X.; Zhao, J.; Ma, J.; Liu, Y.; Lu, G. Preparation of NiO nanoparticles in microemulsion and its gas sensing performance. Mater. Lett. 2012, 68, 168–170. [Google Scholar] [CrossRef]
- Liang, S.; Li, J.; Wang, F.; Qin, J.; Lai, X.; Jiang, X. Highly sensitive acetone gas sensor based on ultrafine α-Fe2O3 nanoparticles. Sens. Actuators B Chem. 2017, 238, 923–927. [Google Scholar] [CrossRef]
- Ali, H.S.; Alghamdi, A.S.; Murtaza, G.; Arif, H.S.; Naeem, W.; Farid, G.; Sharif, S.; Ashiq, M.G.B.; Shabbir, S.A. Facile microemulsion synthesis of vanadium-doped ZnO nanoparticles to analyze the compositional, optical, and electronic properties. Materials 2019, 12, 821. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, D.J.; Alayoglu, S.; Kostecki, R.; Ahmed, M. Synthesis of microporous silica nanoparticles to study water phase transitions by vibrational spectroscopy. Nanoscale Adv. 2019, 1, 4878–4887. [Google Scholar] [CrossRef]
- Li, B.; You, N.; Liang, Y.; Zhang, Q.; Zhang, W.; Chen, M.; Pang, X. Organic Templates for Inorganic Nanocrystal Growth. Energy Environ. Mater. 2019, 2, 38–54. [Google Scholar] [CrossRef]
- Yamamoto, K.; Imaoka, T.; Tanabe, M.; Kambe, T. New Horizon of Nanoparticle and Cluster Catalysis with Dendrimers. Chem. Rev. 2020, 120, 1397–1437. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Imae, T. Synthesis of dendrimer-protected TiO2 nanoparticles and photodegradation of organic molecules in an aqueous nanoparticle suspension. J. Colloid Interface Sci. 2005, 285, 158–162. [Google Scholar] [CrossRef]
- Vijayalakshmi, R.V.; Kuppan, R.; Kumar, P.P. Investigation on the impact of different stabilizing agents on structural, optical properties of Ag@SnO2 core-shell nanoparticles and its biological applications. J. Mol. Liq. 2020, 307, 112951. [Google Scholar] [CrossRef]
- Hodes, G. Semiconductor and ceramic nanoparticle films deposited by chemical bath deposition. Phys. Chem. Chem. Phys. 2007, 9, 2181–2196. [Google Scholar] [CrossRef]
- Thamaraiselvan, C.; Carmiel, Y.; Eliad, G.; Sukenik, C.N.; Semiat, R.; Dosoretz, C.G. Modification of a polypropylene feed spacer with metal oxide-thin film by chemical bath deposition for biofouling control in membrane filtration. J. Memb. Sci. 2019, 573, 511–519. [Google Scholar] [CrossRef]
- Breen, M.L.; Woodward IV, J.T.; Schwartz, D.K.; Apblett, A.W. Direct Evidence for an Ion-by-Ion Deposition Mechanism in Solution Growth of CdS Thin Films. Chem. Mater. 1998, 10, 710–717. [Google Scholar] [CrossRef]
- Lu, Y.; Meng, X.; Yi, G.; Jia, J. In situ growth of CuS thin films on functionalized self-assembled monolayers using chemical bath deposition. J. Colloid Interface Sci. 2011, 356, 726–733. [Google Scholar] [CrossRef]
- Collins, R.J.; Shin, H.; DeGuire, M.R.; Heuer, A.H.; Sukenik, C.N. Low temperature deposition of patterned TiO2 thin films using photopatterned self-assembled monolayers. Appl. Phys. Lett. 1996, 69, 860–862. [Google Scholar] [CrossRef]
- Masuda, Y.; Saito, N.; Hoffmann, R.; De Guire, M.R.; Koumoto, K. Nano/micro-patterning of anatase TiO2 thin film from an aqueous solution by site-selective elimination method. Sci. Technol. Adv. Mater. 2003, 4, 461–467. [Google Scholar] [CrossRef]
- Strano, V.; Greco, M.G.; Ciliberto, E.; Mirabella, S. Zno microflowers grown by chemical bath deposition: A low-cost approach for massive production of functional nanostructures. Chemosensors 2019, 7, 62. [Google Scholar] [CrossRef]
- Chua, W.H.; Yaacob, M.H.; Tan, C.Y.; Ong, B.H. Chemical bath deposition of h-MoO3 on optical fibre as room-temperature ammonia gas sensor. Ceram. Int. 2021, 47, 32828–32836. [Google Scholar] [CrossRef]
- Vessalli, B.A.; Zito, C.A.; Perfecto, T.M.; Volanti, D.P.; Mazon, T. ZnO nanorods/graphene oxide sheets prepared by chemical bath deposition for volatile organic compounds detection. J. Alloys Compd. 2017, 696, 996–1003. [Google Scholar] [CrossRef]
- Husham, M.; Hamidon, M.N.; Paiman, S.; Abuelsamen, A.A.; Farhat, O.F.; Al-Dulaimi, A.A. Synthesis of ZnO nanorods by microwave-assisted chemical-bath deposition for highly sensitive self-powered UV detection application. Sens. Actuators A Phys. 2017, 263, 166–173. [Google Scholar] [CrossRef]
- Wu, T.; Wang, Z.; Tian, M.; Miao, J.; Zhang, H.; Sun, J. UV excitation NO2 gas sensor sensitized by ZnO quantum dots at room temperature. Sens. Actuators B Chem. 2018, 259, 526–531. [Google Scholar] [CrossRef]
- Wang, H.; Dai, M.; Li, Y.; Bai, J.; Liu, Y.; Li, Y.; Wang, C.; Liu, F.; Lu, G. The influence of different ZnO nanostructures on NO2 sensing performance. Sens. Actuators B Chem. 2021, 329, 129145. [Google Scholar] [CrossRef]
- Yuliarto, B.; Ramadhani, M.F.; Septiani, N.L.W.; Hamam, K.A. Enhancement of SO2 gas sensing performance using ZnO nanorod thin films: The role of deposition time. J. Mater. Sci. 2017, 52, 4543–4554. [Google Scholar] [CrossRef]
- Ananthoju, B.; Biroju, R.K.; Theis, W.; Dryfe, R.A.W. Controlled Electrodeposition of Gold on Graphene: Maximization of the Defect-Enhanced Raman Scattering Response. Small 2019, 15, 1901555. [Google Scholar] [CrossRef] [PubMed]
- Ojo, A.A.; Dharmadasa, I.M. Electroplating of semiconductor materials for applications in large area electronics: A review. Coatings 2018, 8, 262. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, L.; Liu, H.; Li, W. Effects of preparing conditions on the nanostructures electrodeposited from the Zn(NO3)2 electrolyte containing KCl. Thin Solid Films 2013, 534, 205–213. [Google Scholar] [CrossRef]
- Mollarasouli, F.; Majidi, M.R.; Asadpour-Zeynali, K. Enhanced activity for non-enzymatic glucose biosensor by facile electro-deposition of cauliflower-like NiWO4 nanostructures. J. Taiwan Inst. Chem. Eng. 2021, 118, 301–308. [Google Scholar] [CrossRef]
- Patella, B.; Moukri, N.; Regalbuto, G.; Cipollina, C.; Pace, E.; Di Vincenzo, S.; Aiello, G.; O’Riordan, A.; Inguanta, R. Electrochemical Synthesis of Zinc Oxide Nanostructures on Flexible Substrate and Application as an Electrochemical Immunoglobulin-G Immunosensor. Materials 2022, 15, 713. [Google Scholar] [CrossRef]
- Sun, L.; Yuan, G.; Gao, L.; Yang, J.; Chhowalla, M.; Gharahcheshmeh, M.H.; Gleason, K.K.; Choi, Y.S.; Hong, B.H.; Liu, Z. Chemical vapour deposition. Nat. Rev. Methods Prim. 2021, 1, 5. [Google Scholar] [CrossRef]
- Chen, K.; Shi, L.; Zhang, Y.; Liu, Z. Scalable chemical-vapour-deposition growth of three-dimensional graphene materials towards energy-related applications. Chem. Soc. Rev. 2018, 47, 3018–3036. [Google Scholar] [CrossRef]
- Gleason, K.K. Nanoscale control by chemically vapour-deposited polymers. Nat. Rev. Phys. 2020, 2, 347–364. [Google Scholar] [CrossRef]
- Saeed, M.; Alshammari, Y.; Majeed, S.A.; Al-Nasrallah, E. Chemical Vapour Deposition of Graphene—Synthesis, Characterisation, and Applications: A Review. Molecules 2020, 25, 3856. [Google Scholar] [CrossRef]
- Choy, K.L. Chemical vapour deposition of coatings. Prog. Mater. Sci. 2003, 48, 57–170. [Google Scholar] [CrossRef]
- Wu, C.; Guo, D.Y.; Zhang, L.Y.; Li, P.G.; Zhang, F.B.; Tan, C.K.; Wang, S.L.; Liu, A.P.; Wu, F.M.; Tang, W.H. Systematic investigation of the growth kinetics of β-Ga2O3 epilayer by plasma enhanced chemical vapor deposition. Appl. Phys. Lett. 2020, 116, 072102. [Google Scholar] [CrossRef]
- Hulkko, J.G.; Böőr, K.; Qiu, R.; Bäcke, O.; Boman, M.; Halvarsson, M.; Lindahl, E. Kinetics of the low-pressure chemical vapor deposited tungsten nitride process using tungsten hexafluoride and ammonia precursors. J. Vac. Sci. Technol. A 2021, 39, 063403. [Google Scholar] [CrossRef]
- Xu, W.; Li, S.; Zhou, S.; Lee, J.K.; Wang, S.; Sarwat, S.G.; Wang, X.; Bhaskaran, H.; Pasta, M.; Warner, J.H. Large Dendritic Monolayer MoS2 Grown by Atmospheric Pressure Chemical Vapor Deposition for Electrocatalysis. ACS Appl. Mater. Interfaces 2018, 10, 4630–4639. [Google Scholar] [CrossRef]
- Barreca, D.; Bekermann, D.; Comini, E.; Devi, A.; Fischer, R.A.; Gasparotto, A.; MacCato, C.; Sberveglieri, G.; Tondello, E. 1D ZnO nano-assemblies by Plasma-CVD as chemical sensors for flammable and toxic gases. Sens. Actuators B Chem. 2010, 149, 1–7. [Google Scholar] [CrossRef]
- Zhang, J.B.; Li, X.N.; Bai, S.L.; Luo, R.X.; Chen, A.F.; Lin, Y. High-yield synthesis of SnO2 nanobelts by water-assisted chemical vapor deposition for sensor applications. Mater. Res. Bull. 2012, 47, 3277–3282. [Google Scholar] [CrossRef]
- Wu, Y.; Li, G.; Hong, Y.; Zhao, X.; Reyes, P.I.; Lu, Y. Rapid and dynamic detection of antimicrobial treatment response using spectral amplitude modulation in MZO nanostructure-modified quartz crystal microbalance. J. Microbiol. Methods 2020, 178, 106071. [Google Scholar] [CrossRef]
- Barreca, D.; Bekermann, D.; Comini, E.; Devi, A.; Fischer, R.A.; Gasparotto, A.; Gavagnin, M.; MacCato, C.; Sada, C.; Sberveglieri, G.; et al. Plasma enhanced-CVD of undoped and fluorine-doped Co3O4 nanosystems for novel gas sensors. Sens. Actuators B Chem. 2011, 160, 79–86. [Google Scholar] [CrossRef]
- Oviroh, P.O.; Akbarzadeh, R.; Pan, D.; Coetzee, R.A.M.; Jen, T.C. New development of atomic layer deposition: Processes, methods and applications. Sci. Technol. Adv. Mater. 2019, 20, 465–496. [Google Scholar] [CrossRef]
- Hausmann, D.; Becker, J.; Wang, S.; Gordon, R.G. Rapid vapor deposition of highly conformal silica nanolaminates. Science (80-.) 2002, 298, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Marichy, C.; Journet, C. Atomic layer deposition of stable 2D materials. 2D Mater. 2019, 6, 012001. [Google Scholar] [CrossRef]
- Makhlouf, H.; Karam, C.; Lamouchi, A.; Tingry, S.; Miele, P.; Habchi, R.; Chtourou, R.; Bechelany, M. Analysis of ultraviolet photo-response of ZnO nanostructures prepared by electrodeposition and atomic layer deposition. Appl. Surf. Sci. 2018, 444, 253–259. [Google Scholar] [CrossRef]
- Song, G.-T.; Wang, Y.; Qi, Y.; Li, W.-M.; Zhang, L.-X. Fabrication of titanium nitride nanoparticles onto carbon nanotubes by atomic layer deposition for utilization as Pt electrocatalyst supports. Rare Met. 2020, 39, 784–791. [Google Scholar] [CrossRef]
- Cho, S.; Ahn, C.; Park, J.; Jeon, S. 3D nanostructured N-doped TiO2 photocatalysts with enhanced visible absorption. Nanoscale 2018, 10, 9747–9751. [Google Scholar] [CrossRef]
- Hazarika, A.; Fedin, I.; Hong, L.; Guo, J.; Srivastava, V.; Cho, W.; Coropceanu, I.; Portner, J.; Diroll, B.T.; Philbin, J.P.; et al. Colloidal Atomic Layer Deposition with Stationary Reactant Phases Enables Precise Synthesis of “digital” II-VI Nano-heterostructures with Exquisite Control of Confinement and Strain. J. Am. Chem. Soc. 2019, 141, 13487–13496. [Google Scholar] [CrossRef]
- Xu, X.; Wang, G.; Wan, G.; Shi, S.; Hao, C.; Tang, Y.; Wang, G. Magnetic Ni/graphene connected with conductive carbon nano-onions or nanotubes by atomic layer deposition for lightweight and low-frequency microwave absorption. Chem. Eng. J. 2020, 382, 122980. [Google Scholar] [CrossRef]
- Singh, J.A.; Thissen, N.F.W.; Kim, W.H.; Johnson, H.; Kessels, W.M.M.; Bol, A.A.; Bent, S.F.; MacKus, A.J.M. Area-Selective Atomic Layer Deposition of Metal Oxides on Noble Metals through Catalytic Oxygen Activation. Chem. Mater. 2018, 30, 663–670. [Google Scholar] [CrossRef]
- Lee, K.; Losego, M.D.; Kim, D.H.; Parsons, G.N. High performance photocatalytic metal oxide synthetic bi-component nanosheets formed by atomic layer deposition. Mater. Horizons 2014, 1, 419–423. [Google Scholar] [CrossRef]
- Cremers, V.; Puurunen, R.L.; Dendooven, J. Conformality in atomic layer deposition: Current status overview of analysis and modelling. Appl. Phys. Rev. 2019, 6, 021302. [Google Scholar] [CrossRef]
- Chaaya, A.A.; Viter, R.; Baleviciute, I.; Bechelany, M.; Ramanavicius, A.; Gertnere, Z.; Erts, D.; Smyntyna, V.; Miele, P. Tuning optical properties of Al2O3/ZnO nanolaminates synthesized by atomic layer deposition. J. Phys. Chem. C 2014, 118, 3811–3819. [Google Scholar] [CrossRef]
- Baitimirova, M.; Viter, R.; Andzane, J.; Van Der Lee, A.; Voiry, D.; Iatsunskyi, I.; Coy, E.; Mikoliunaite, L.; Tumenas, S.; Zaleski, K.; et al. Tuning of Structural and Optical Properties of Graphene/ZnO Nanolaminates. J. Phys. Chem. C 2016, 120, 23716–23725. [Google Scholar] [CrossRef]
- Iatsunskyi, I.; Coy, E.; Viter, R.; Nowaczyk, G.; Jancelewicz, M.; Baleviciute, I.; Załeski, K.; Jurga, S. Study on Structural, Mechanical, and Optical Properties of Al2O3-TiO2 Nanolaminates Prepared by Atomic Layer Deposition. J. Phys. Chem. C 2015, 119, 20591–20599. [Google Scholar] [CrossRef]
- Iatsunskyi, I.; Pavlenko, M.; Viter, R.; Jancelewicz, M.; Nowaczyk, G.; Baleviciute, I.; Załęski, K.; Jurga, S.; Ramanavicius, A.; Smyntyna, V. Tailoring the Structural, Optical, and Photoluminescence Properties of Porous Silicon/TiO2 Nanostructures. J. Phys. Chem. C 2015, 119, 7164–7171. [Google Scholar] [CrossRef]
- Lou, C.; Yang, C.; Zheng, W.; Liu, X.; Zhang, J. Atomic layer deposition of ZnO on SnO2 nanospheres for enhanced formaldehyde detection. Sens. Actuators B Chem. 2021, 329, 129218. [Google Scholar] [CrossRef]
- Clark, L.C.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef]
- Şerban, I.; Enesca, A. Metal Oxides-Based Semiconductors for Biosensors Applications. Front. Chem. 2020, 8, 1–8. [Google Scholar] [CrossRef]
- Zhou, X.; Pu, H.; Sun, D.-W. DNA functionalized metal and metal oxide nanoparticles: Principles and recent advances in food safety detection. Crit. Rev. Food Sci. Nutr. 2021, 61, 2277–2296. [Google Scholar] [CrossRef]
- Perumal, V.; Hashim, U. Advances in biosensors: Principle, architecture and applications. J. Appl. Biomed. 2014, 12, 1–15. [Google Scholar] [CrossRef]
- Faria, A.M.; Mazon, T. Early diagnosis of Zika infection using a ZnO nanostructures-based rapid electrochemical biosensor. Talanta 2019, 203, 153–160. [Google Scholar] [CrossRef]
- Mukundan, A.; Feng, S.W.; Weng, Y.H.; Tsao, Y.M.; Artemkina, S.B.; Fedorov, V.E.; Lin, Y.S.; Huang, Y.C.; Wang, H.C. Optical and Material Characteristics of MoS2/Cu2O Sensor for Detection of Lung Cancer Cell Types in Hydroplegia. Int. J. Mol. Sci. 2022, 23, 4745. [Google Scholar] [CrossRef] [PubMed]
- Ramanaviciene, A.; Popov, A.; Baliunaite, E.; Brasiunas, B.; Kausaite-Minkstimiene, A.; Tamer, U.; Kirdaite, G.; Bernotiene, E.; Mobasheri, A. Magneto-Immunoassay for the Detection and Quantification of Human Growth Hormone. Biosensors 2022, 12, 65. [Google Scholar] [CrossRef] [PubMed]
- Kausaite-Minkstimiene, A.; Popov, A.; Ramanaviciene, A. Surface Plasmon Resonance Immunosensor with Antibody-Functionalized Magnetoplasmonic Nanoparticles for Ultrasensitive Quantification of the CD5 Biomarker. ACS Appl. Mater. Interfaces 2022, 14, 20720–20728. [Google Scholar] [CrossRef] [PubMed]
- Rui, Q.; Komori, K.; Tian, Y.; Liu, H.; Luo, Y.; Sakai, Y. Electrochemical biosensor for the detection of H2O2 from living cancer cells based on ZnO nanosheets. Anal. Chim. Acta 2010, 670, 57–62. [Google Scholar] [CrossRef] [PubMed]
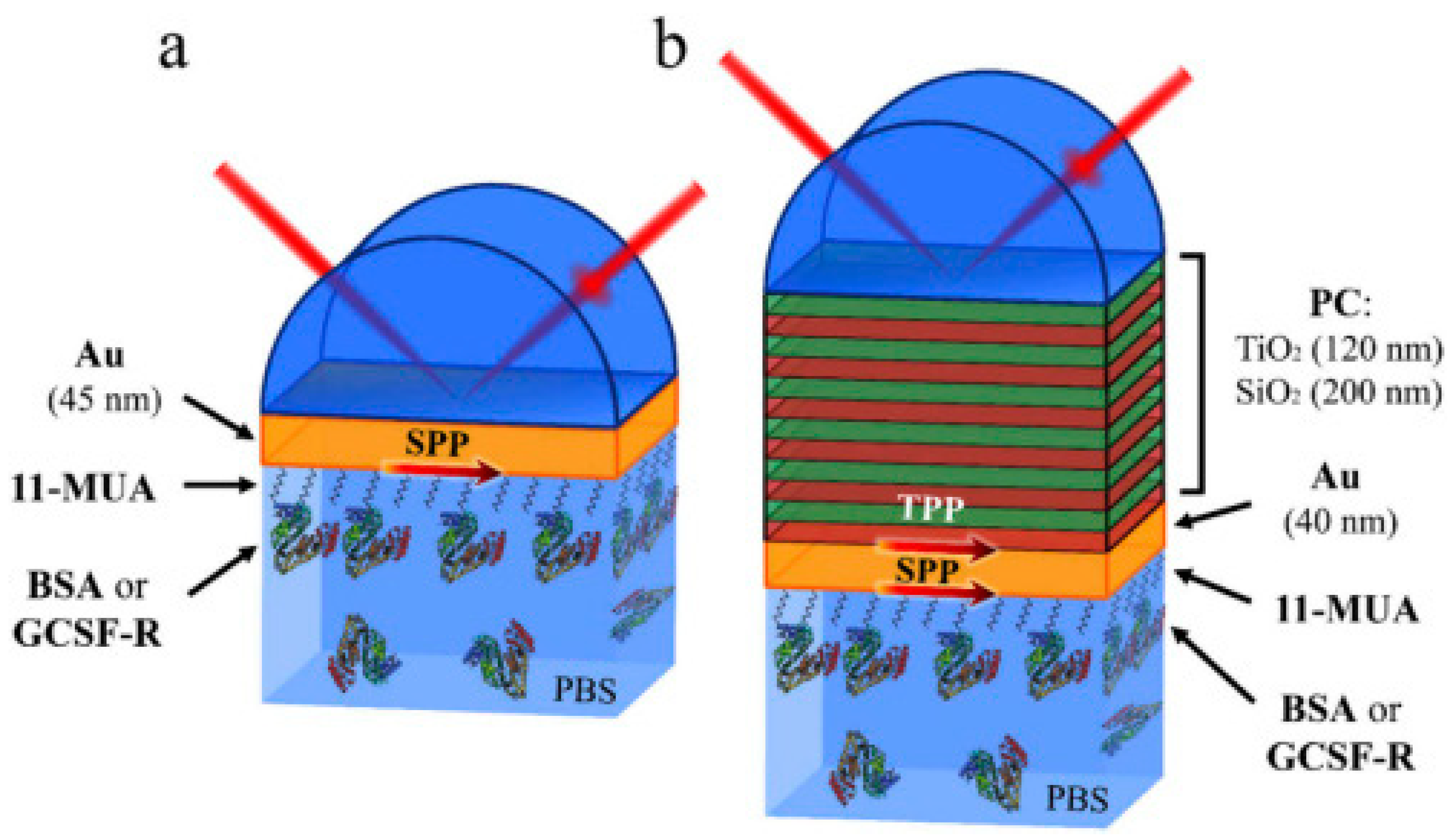
- Buzavaite-Verteliene, E.; Plikusiene, I.; Tolenis, T.; Valavicius, A.; Anulyte, J.; Ramanavicius, A.; Balevicius, Z. Hybrid Tamm-surface plasmon polariton mode for highly sensitive detection of protein interactions. Opt. Express 2020, 28, 29033. [Google Scholar] [CrossRef]
- Plikusienė, I.; Bužavaitė-Vertelienė, E.; Mačiulis, V.; Valavičius, A.; Ramanavičienė, A.; Balevičius, Z. Application of tamm plasmon polaritons and cavity modes for biosensing in the combined spectroscopic ellipsometry and quartz crystal microbalance method. Biosensors 2021, 11, 501. [Google Scholar] [CrossRef]
- Pal, A.K.; Pagal, S.; Prashanth, K.; Chandra, G.K.; Umapathy, S.; Mohan, D.B. Ag/ZnO/Au 3D hybrid structured reusable SERS substrate as highly sensitive platform for DNA detection. Sens. Actuators B Chem. 2019, 279, 157–169. [Google Scholar] [CrossRef]
- Kim, D.-K.; Kim, D.M.; Yoo, S.M.; Lee, S.Y. Controllable gold-capped nanoporous anodic alumina chip for label-free, specific detection of bacterial cells. RSC Adv. 2017, 7, 18815–18820. [Google Scholar] [CrossRef]
- Plikusiene, I.; Balevicius, Z.; Ramanaviciene, A.; Talbot, J.; Mickiene, G.; Balevicius, S.; Stirke, A.; Tereshchenko, A.; Tamosaitis, L.; Zvirblis, G.; et al. Evaluation of affinity sensor response kinetics towards dimeric ligands linked with spacers of different rigidity: Immobilized recombinant granulocyte colony-stimulating factor based synthetic receptor binding with genetically engineered dimeric analyte d. Biosens. Bioelectron. 2020, 156, 112112. [Google Scholar] [CrossRef]
- Balevicius, Z.; Talbot, J.; Tamosaitis, L.; Plikusiene, I.; Stirke, A.; Mickiene, G.; Balevicius, S.; Paulauskas, A.; Ramanavicius, A. Modelling of immunosensor response: The evaluation of binding kinetics between an immobilized receptor and structurally-different genetically engineered ligands. Sens. Actuators B Chem. 2019, 297, 126770. [Google Scholar] [CrossRef]
- Plikusiene, I.; Maciulis, V.; Ramanaviciene, A.; Balevicius, Z.; Buzavaite-Verteliene, E.; Ciplys, E.; Slibinskas, R.; Simanavicius, M.; Zvirbliene, A.; Ramanavicius, A. Evaluation of kinetics and thermodynamics of interaction between immobilized SARS-CoV-2 nucleoprotein and specific antibodies by total internal reflection ellipsometry. J. Colloid Interface Sci. 2021, 594, 195–203. [Google Scholar] [CrossRef]
- Plikusiene, I.; Maciulis, V.; Juciute, S.; Maciuleviciene, R.; Balevicius, S.; Ramanavicius, A.; Ramanaviciene, A. Investigation and Comparison of Specific Antibodies’ Affinity Interaction with SARS-CoV-2 Wild-Type, B.1.1.7, and B.1.351 Spike Protein by Total Internal Reflection Ellipsometry. Biosensors 2022, 12, 351. [Google Scholar] [CrossRef]
- Stetter, J.R.; Penrose, W.R. Understanding Chemical Sensors and Chemical Sensor Arrays (Electronic Noses): Past, Present, and Future. Sens. Update 2002, 10, 189–229. [Google Scholar] [CrossRef]
- Yang, J.; Cho, M.; Lee, Y. Synthesis of hierarchical Ni(OH)2 hollow nanorod via chemical bath deposition and its glucose sensing performance. Sens. Actuators B Chem. 2016, 222, 674–681. [Google Scholar] [CrossRef]
- Kim, E.B.; Seo, H.K. Highly sensitive formaldehyde detection using well-aligned zinc oxide nanosheets synthesized by chemical bath deposition technique. Materials 2019, 12, 250. [Google Scholar] [CrossRef]
- Kaur, N.; Raj, P.; Singh, A.; Singh, N.; Kim, D.Y. A facile route to ionic liquids-functionalized ZnO nanorods for the fluorometric sensing of thiabendazole drug. J. Mol. Liq. 2018, 261, 137–145. [Google Scholar] [CrossRef]
- Shariati, R.; Rezaei, B.; Jamei, H.R.; Ensafi, A.A. Application of coated green source carbon dots with silica molecularly imprinted polymers as a fluorescence probe for selective and sensitive determination of phenobarbital. Talanta 2019, 194, 143–149. [Google Scholar] [CrossRef]
- Shetti, N.P.; Malode, S.J.; Nayak, D.S.; Aminabhavi, T.M.; Reddy, K.R. Nanostructured silver doped TiO2/CNTs hybrid as an efficient electrochemical sensor for detection of anti-inflammatory drug, cetirizine. Microchem. J. 2019, 150, 104124. [Google Scholar] [CrossRef]
- Yadav, M.; Dhanda, M.; Arora, R.; Jagdish, R.; Singh, G.; Lata, S. Titania (TiO2)/silica (SiO2) nanospheres or NSs amalgamated on a pencil graphite electrode to sense l-ascorbic acid electrochemically and augmented NSs for antimicrobial behaviour. New J. Chem. 2022, 46, 12783–12796. [Google Scholar] [CrossRef]
- Xiao, W.; Ding, L.; He, J.; Huang, J. Preparation of lucigenin-doped silica nanoparticles and their application in fiber optic chloride ion sensor. Opt. Mater. (Amst.) 2019, 98, 109467. [Google Scholar] [CrossRef]
- Alam, M.M.; Rahman, M.M.; Uddin, M.T.; Asiri, A.M.; Chani, M.T.S.; Islam, M.A. Development of L-glutamic acid biosensor with ternary ZnO/NiO/Al2O3 nanoparticles. J. Lumin. 2020, 227, 117528. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ahmed, J.; Asiri, A.M.; Alamry, K.A. Fabrication of a hydrazine chemical sensor based on facile synthesis of doped NZO nanostructure materials. New J. Chem. 2020, 44, 13018–13029. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, M.; Wang, X.; Sun, Y.; Li, P.; Zhang, W.; Lian, K.; Chen, L.; Chen, Y. Synthesis and characterization of flower-like MoO3/In2O3 microstructures for highly sensitive ethanol detection. RSC Adv. 2017, 7, 23478–23485. [Google Scholar] [CrossRef]
| Nanostructure | Method of Synthesis | Application | Reported Sensing Properties | Ref. |
|---|---|---|---|---|
| ZnO nanostructures | Chemical bath deposition | Electrochemical immunosensor for ZIKV-NS1 antigen | LOD: 1.00 pg/mL LDR: 0.1–100 ng/mL | [100] |
| MoS2/Cu2O | Chemical vapour deposition, electrodeposition | GSH, GSSG biosensor for indirect cancer cell detection | LDR: 0–50,000 cells | [101] |
| Au(Fe3O4) MNPs | Co-precipitation, wet chemical | Colorimetric hGH sensor | LOD: 0.082 nM LDR: 0.1–5.0 nM | [102] |
| Au(Fe3O4) MNPs | Co-precipitation, wet chemical | SPR CD5 immunosensor | LOD: 8.31 fM | [103] |
| ZnO NRs-rGO | Electrodeposition | Electrochemical IgG detection | LOD: 1.25 ng/mL LDR: 10–1000 ng/mL | [67] |
| ZnO NWLs | Electrodeposition | Electrochemical enzymatic H2O2 sensor for cancer cell detection | LOD: 0.8 μM LDR: 1–1000 μM | [104] |
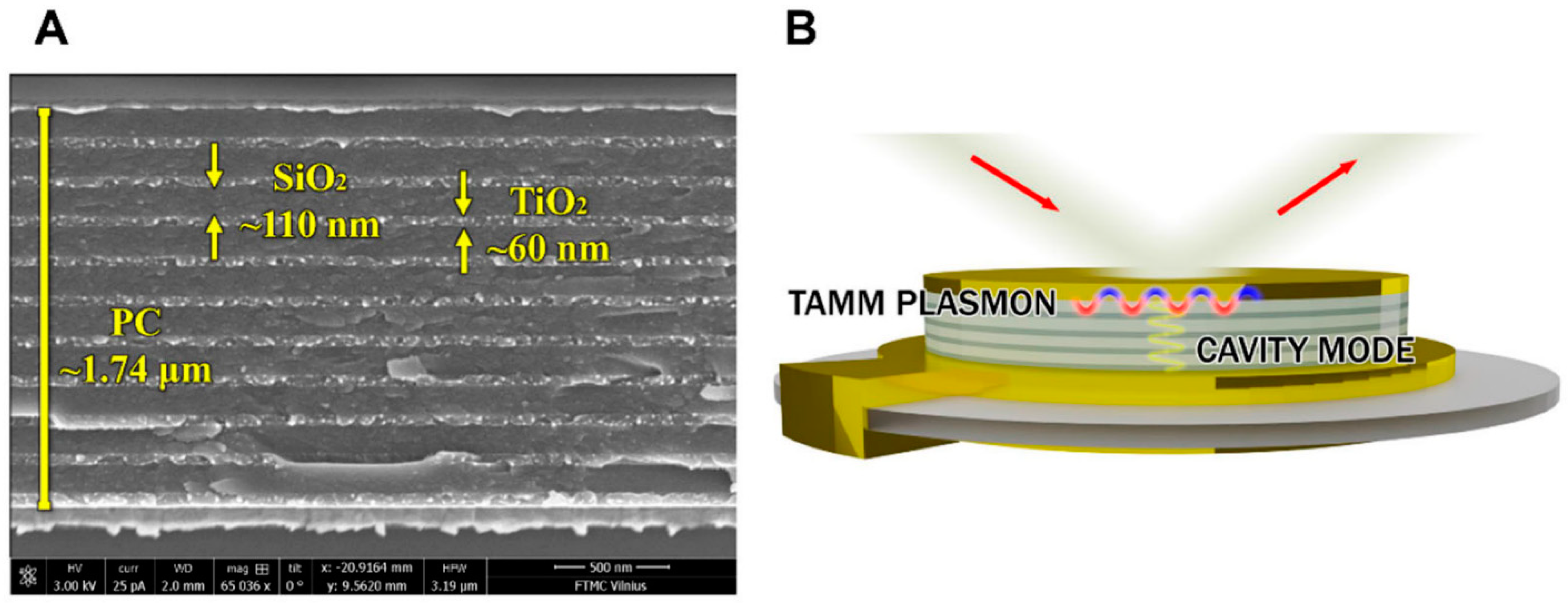
| Au/(TiO2/SiO2) Photonic crystal | Ion beam sputtering | TIRE signal enhancement | 6.4 times more sensitive than SPR | [105] |
| Au/(TiO2/SiO2)/Au | Ion beam sputtering | SE/QCM-D signal enhancement | 23 and 49 times increased SE signal | [106] |
| Ag/ZnO-NRs/Au | Thermal evaporation; hydrothermal; sputtering | SERS sensing platform | LOD (λ-DNA): 10 ng/μL LOD (Rh6G): 10−16 M | [107] |
| Au/PAA | Two-step anodization; thermal evaporation | Interferometric aptamer based bacterial cell sensor | LOD: 20 CFU/mL LDR: 103–109 CFU/mL | [108] |
| Nanostructure | Method of Synthesis | Application | Reported Sensing Properties | Ref. |
|---|---|---|---|---|
| Ni(OH)2 HRs | Chemical bath deposition | Electrochemical glucose sensor | LOD: 0.6 μM LDR: 0.002–3.8 mM | [114] |
| ZnO NSs | Chemical bath deposition | Electrochemical aqueous formaldehyde sensor | LOD: 210 nM | [115] |
| ZnO NRs | Hydrothermal | Fluorescence thiabendazole sensor | LOD: 304 nM LDR: 10–80 μM | [116] |
| SiO2/GSCDs | Hydrothermal, reverse microemulsion | Fluorescence phenobarbital sensor | LOD: 0.1 nM LDR: 0.4–34.5 nM | [117] |
| Ag-TiO2 NPs/MWCNTs | Hydrothermal, powder blending | Electrochemical cetirizine sensor | LOD: 8.76 nM LDR: 0.3–3 μM | [118] |
| TiO2@SiO2 NSs | Sol-gel | Electrochemical ascorbic acid sensor | LOD: 383.3 μM LDR: 50–2500 μM | [119] |
| Lucigenin doped SiO2 NPs | Reverse microemulsion | Fibre optic Cl− sensor | LDR: 0.02–0.06 M | [120] |
| ZnO/NiO/Al2O3 NPs | Wet chemical | Electrochemical L-glutamic acid sensor | LOD: 95.35 pM LDR: 0.1 nM–0.01 mM | [121] |
| Ni-ZnO NPs | Wet chemical | Electrochemical hydrazine sensor | LOD: 1.7 pM LDR: 0.2 nM–0.02 M | [122] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maciulis, V.; Ramanaviciene, A.; Plikusiene, I. Recent Advances in Synthesis and Application of Metal Oxide Nanostructures in Chemical Sensors and Biosensors. Nanomaterials 2022, 12, 4413. https://doi.org/10.3390/nano12244413
Maciulis V, Ramanaviciene A, Plikusiene I. Recent Advances in Synthesis and Application of Metal Oxide Nanostructures in Chemical Sensors and Biosensors. Nanomaterials. 2022; 12(24):4413. https://doi.org/10.3390/nano12244413
Chicago/Turabian StyleMaciulis, Vincentas, Almira Ramanaviciene, and Ieva Plikusiene. 2022. "Recent Advances in Synthesis and Application of Metal Oxide Nanostructures in Chemical Sensors and Biosensors" Nanomaterials 12, no. 24: 4413. https://doi.org/10.3390/nano12244413
APA StyleMaciulis, V., Ramanaviciene, A., & Plikusiene, I. (2022). Recent Advances in Synthesis and Application of Metal Oxide Nanostructures in Chemical Sensors and Biosensors. Nanomaterials, 12(24), 4413. https://doi.org/10.3390/nano12244413