Abstract
Porous organometallic nanomaterials are a new class of materials based on a three-dimensional structure. They have excellent applications in different fields, but their applications in gas storage and separation have not been fully developed. CO2 adsorption storage and hydrocarbon separation has been a challenging industrial problem. Several typical molecular adsorbents have been used to study the separation, but the problems of long-term stability, high selectivity and synthetic complexity of these adsorbents remain to be solved. Here, we have designed and synthesized tetrahedral metal supramolecular nanocage with custom cavities based on the unique rigid structure of triptycene derivatives. Using the unique discrete porous structure of tetrahedral metal nanocages, the gas adsorption and separation performance of the metal supramolecular nanocage was investigated. By analyzing the adsorption and desorption isotherms and the multi-component competitive adsorption curves, we noticed that the tetrahedral supramolecular nanocages had good CO2 storage capacity and good separation capacity for C2H2/CO2 and C2H2/N2. All these indicate that porous organic metal nanomaterials are expected to be a new energy saving separation material.
1. Introduction
A variety of supramolecules with attractive structures and flexible properties have been successfully generated by self-assembly strategy [1,2,3,4]. Metal–organic supramolecular cages (MOCs) are discrete molecules constructed by coordination-driven self-assembly of organic connectomes and metal ions or clusters [5,6]. They has become one of the research hotspots in the past two decades. Based on their unique multifunctional topology, metal–organic supramolecular cages have been used in many fields, including catalysis [7,8,9,10], molecular recognition [11,12,13,14,15], biological simulation [16,17,18,19], guest isolation [20,21], reactive species stability [22,23], drug delivery [24,25], membrane transport [26,27], etc. However, MOCs have not been fully developed as adsorbents, especially for hydrocarbon storage and separation.
In recent years, the excessive carbon dioxide produced by the widespread use of fossil fuels has become the main culprit of the greenhouse effect. Acetylene as a basic feedstock for various industrial materials is also a key fuel [28], mainly produced through the cracking of hydrocarbons or partial combustion of methane [29,30], of which carbon dioxide is the main impurity. Up to date, the commonly used C2H2/CO2 separation technology usually relies on low temperature rectification or liquid absorbent, which has high cost and low efficiency [31]. Therefore, there is a great demand for the development of alternative and energy-saving separation technologies [32,33]. Currently, non-thermally driven approaches such as adsorption or membrane separation have received a lot of attention due to their lower energy penalties. However, the adsorption separation performance depends largely on the nature of the adsorbent. In the past two decades, many microporous materials (MPM) for gas storage and separation have been studied, such as polymer membranes [34], inorganic porous materials [35,36], graphene [37], metal–organic frameworks (MOFs) [38,39], covalent organic frameworks (COFs) [40,41], hydrogen-bonded organic frameworks (HOFs) [42,43], etc. However, they lack long-term stability [33,44], and only a minority of them have high selectivity [45,46,47]; thus, it is necessary to develop new molecular and supramolecular adsorbents with good stability and high selectivity. It is known that many discrete porous metal–organic nanocages (MOCs) have topological diversity to ensures higher BET surface area and larger adsorption capacity [48], adjustable size cavities to improve selectivity, and their easy preparation characteristics [49]. Given this, they are expected to be the next generation of adsorbents for energy efficient separation.
Tetrahedral metal supramolecular nanocage molecular adsorbents with 3D structures have shown excellent performance in many discrete porous metal organic nanocages (MOCs), such as the separation of methane, ethane and ethylene [50]. These kinds of supramolecular nanocage have attracted much attention due to their unique customizable cavities, high porosity and easy preparation. In this work, we purposefully designed and constructed tetrahedral nanocage Cage 1 with triptycene derivatives as faces and metal cations as vertices by coordination driven collaborative self-assembly. Based on the unique three-dimensional rigid structure of triptycene derivatives and their unique electron-rich cavities, which are beneficial to improve the gas adsorption capacity and selectivity, the CO2 storage capacity and hydrocarbon separation ability of Cage 1 were tested. Not surprisingly, the gas adsorption experiments showed that the adsorption capacities of Cage 1 for CO2, O2, N2 and C2H2 at 298 K and 1 bar, respectively, were 5.1711 cm3g−1, 0.4806 cm3g−1, 0.5684 cm3g−1 and 10.8463 cm3g−1. Cage1 has obvious differential adsorption capacity for different gases and high adsorption capacity for CO2. This makes Cage 1 possible as a new nano adsorbent for CO2 storage and hydrocarbon gas separation.
2. Materials and Methods
2.1. General Information
All the chemical reagents were purchased from commercial sources and directly used without further purification. Reactions were monitored by Thin Layer Chromatography (TLC) using UV light (254/365 nm). Products were purified by column chromatography, which was carried out on 200–300 mesh of silica gel. NMR spectra data were recorded on a 600 MHz Bruker NMR spectrometer, Germany. The UV-Vis experiments were conducted on dual-beam UV-Vis spectrophotometer (TU-1901), PERSEE, Beijing, China. The fluorescent experiments were recorded on Horiba-FluoroMax-4 spectrofluorometer, HORIBA, Edison, NJ, USA. The high-pressure fluorescence spectra under hydrostatic conditions were measured using a fluorescence microscope (IX71, Olympus, 50, NA = 0.5, Japan) equipped with a spectrometer (Jobin Yvon iHR320, USA). The light source was a mercury lamp with an excitation wavelength of 365 nm.
ESI-MS was recorded with a Waters Synapt G2 -Si mass spectrometer, USA; using solutions of 0.01 mg sample in 1 mL of CHCl3/CH3OH (1:3, v/v) for ligands or 0.5 mg sample in 1 mL of CH3CN/CH3OH (3:1, v/v) for supramolecules. High-resolution electrospray ionization mass spectrometry (HR-ESI MS) experiments were performed with a Water Q-Tof Micro MS/MS high resolution mass spectrometer in ESI mode.
Thermogravimetric analyses (TGA) were carried out on a Netzsch STA 449 F5 thermal analyzer, Germany; under a nitrogen atmosphere with a heating rate of 10 °C·min−1.
Low-pressure gas adsorption measurements were performed on a Micromeritics Instrument Corporation TriStar II 3020 Version 3.02 surface area analyze, USA; Samples were degassed under dynamic vacuum for 12 h at 60 °C prior to each measurement. N2 isotherms were measured using a liquid nitrogen bath (77 K).
All the gas adsorption–desorption measurements were carried out by using an automatic volumetric adsorption equipment (Micromeritics, ASA 2460), USA; at 298 K. Prior to the measurements, the samples were degassed at 120 °C under dynamic vacuum for 10 h to remove the adsorbed impurities.
2.2. General Experimental Procedures for the Synthesis of L and Cage 1
Synthesis of L:
Compounds 1, 2, 3 and 4 were synthesized according to published procedures [51].
4-aminophenylboronic acid pinacol ester (289.08 mg, 1.32 mmol), compound 4 (196.40 mg, 0.4 mmol), sodium carbonate (127.2 mg, 1.2 mmol) and Pd(PPh3)4 (69.30 mg, 0.06 mmol) were added to a round bottom flask containing DMF/H2O (18 mL/6 mL) under nitrogen atmosphere and reacted at 110 °C for 16 h. Then, the mixture was cooled to room temperature, extracted with ethyl acetate (200 mL × 3). The combined organic phase was washed with brine (200 mL × 3), dried over Na2SO4 and the solvent was evaporated. The crude product was purified on a silica gel column using petroleum and ethyl acetate (1:1) as the eluent to obtain pale yellow solid L (84.35 mg, 40%). 1H NMR (600 MHz, CDCl3) δ 7.57 (s, 3H), 7.40 (d, J = 7.6 Hz, 3H), 7.31 (d, J = 8.3 Hz, 6H), 7.14 (d, J = 7.6 Hz, 3H), 6.68 (d, J = 8.3 Hz, 6H), 5.48 (d, J = 9.8 Hz, 2H), 3.61 (s, 6H); 13C NMR (151 MHz, CDCl3) δ 145.8, 145.7, 145.6, 143.3, 143.3, 138.7, 138.6, 131.8, 128.1, 123.8, 123.8, 123.3, 123.3, 122.1, 122.0, 115.3, 54.1, 53.6. HR-ESI MS (m/z) calcd for C38H29N3+ [M+H]+: 528.2434; found: 528.2433.
Synthesis of Cage 1:
Zn(NTf2)2 (9.38 mg, 15.00 µmol, 1.5 equiv), L (5.23 mg, 10.00 µmol, 1 equiv) and pyridyl aldehyde (3.43 mg, 32.00 µmol, 3.2 equiv) were added to a reaction tube, then 1.5 mL acetonitrile was added. The whole reaction mixture was reacted at room temperature for 8 h. Ethyl ether (30 mL) was added into the reaction mixture. The yellow solid was collected by centrifugation and dried under reduced pressure, Cage 1 was obtained by quantitative yield. ESI-MS (m/z): Calcd. [C224H144N24Zn4–8NTf2−]8+ for: 430.13, found: 430.14. Calcd. [C226H145F6N25O4S2Zn4–7NTf2−]7+ for: 531.56, found: 531.56. Calcd. [C228H146F12N26O8S4Zn4–6NTf2−]6+ for: 666.80, found: 666.79. Calcd. [C230H147F18N27O12S6Zn4–5NTf2−]5+ for: 856.35, found: 856.30. Calcd. [C232H148F24N28O16S8Zn4–4NTf2−]4+ for: 1140.41, found: 1140.54. Calcd. [C234H149F30N29O20S10Zn4–3NTf2−]3+ for: 1613.86, found: 1613.80.
3. Results and Discussion
Triptycene-based L was obtained in four steps from commercially-available starting materials (Scheme 1). According to procedures previously reported, using triptycene as the starting material. At first, triptycene was treated with nitric acid under reflux conditions to give 2 in 64% yield. Next, the compound 2 derivatives were reduced by Raney-Ni in the presence of hydrazine hydrate to yield the 3 in quantitative yield. Then, compound 3 diazotized with NaNO2 was added under reflux conditions of HBr and CuBr to give 4 in 67% yield. Finally, under the conditions of 110 °C reaction temperature, tetrakis(triphenylphosphine)palladium as catalyst, sodium carbonate as base, DMF and H2O as the reaction solvent, the Suzuki reaction with 4-amnophenylboronic acid pinacol ester was carried out to obtain L with 40% yield.
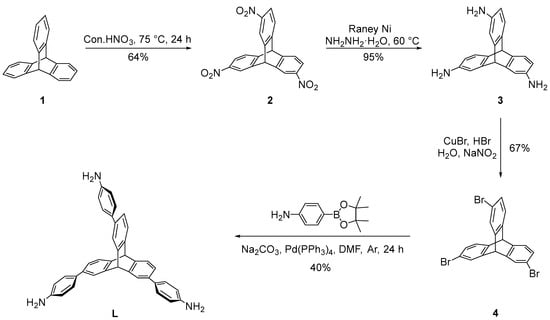
Scheme 1.
Synthesis of L.
With the composition of L, the reaction of L (4 equiv) with Zinc bis(trifluoromethylsulfonyl)imide (4 equiv) and 2-formylpyridine (12 equiv) in acetonitrile at room temperature for 8 h, affording desired tetrahedral Cage 1 as a yellow solid in a quantitative yield (Figure 1a).
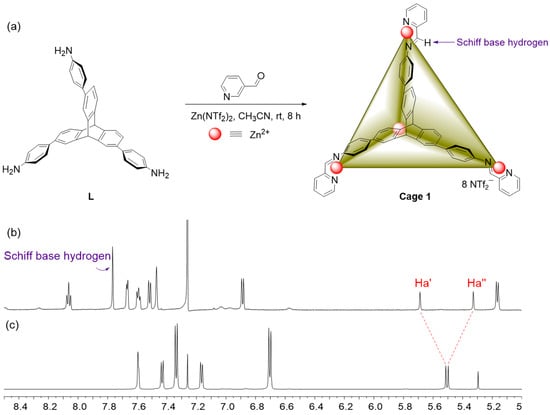
Figure 1.
(a) Coordination-driven self-assembly and geometric structure of Cage 1 (ZnII4L4). 1H NMR spectra (600 MHZ, CD3CN/CDCl3, 298 K) of Cage 1 (b) and L (c).
As shown in Figure 1b,c, 1H NMR spectrum of Cage 1 was thoroughly different from its assembled precursors, implying the formation of a mew substance. Depending on previous literature reports, we supposed that the Cage 1 should be a regular tetrahedral nanostructure and simultaneously has a certain size cavity. Fortunately, the aforementioned speculation was also demonstrated by the 1H NMR and 13C. Through 1H NMR contrast of Cage 1 and L (Figure 1), the resonances of the protons on the center of triptycene of ligand A were equally divided into two sets of different signal peaks after the formation of Cage 1. One set (Ha′) appeared at lower chemical shift and the other set (Ha′′) at higher field shift as a result of the strong shielding effect of the Cage 1. The regular splitting of the group of protons shows that half of the two hydrogens in the center of triptycene were located outside the cage and the other half of them were encapsulated inside the cavity of Cage 1. In the same breath, the hydrogen on the pyridine formaldehyde also disappeared with the synthesis of the cage, which proves the formation of the tetrahedral metal supramolecular nanocage.
Electrospray ionization mass spectrometry (ESI-MS) date demonstrated that Cage 1 are substantially stable in solution. Furthermore, the molecular weight of Cage 1 was accurately determined and the tetrahedral structure of Cage 1 was confirmed. (See the Supporting Information for details). As shown in Figure 2a, the ESI-MS of Cage 1 successively revealed a set of dominant peaks with persistent charge states ranging from. 3+ to 8+ with the sequential loss of NTf2− (Figure S5 see the Supporting Information for details). Experimental isotope patterns for each peak matched faultless with simulated isotope patterns, indicative of the formation of a supramolecular ensemble with the proposed stoichiometry. After deconvolution, the average molar masses of self-assembled Cage 1 were 5672 Da, respectively, matching faultless with its expected chemical composition [(C56H38N6)4Zn4(NTf2)8]. In addition, ion mobility profiles in Figure 2b shows that Cage 1 is a single compound, ruling out the possibilities of forming other undesired complexes and isomers.
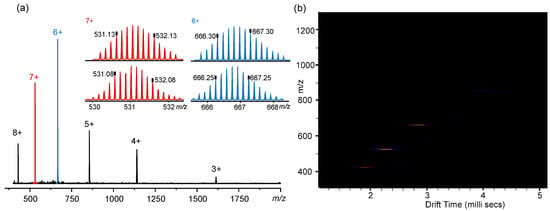
Figure 2.
(a) ESI-MS spectra of Cage 1. (b) ion mobility profiles of Cage 1.
Numerous efforts have been made to obtain complete data for single crystal X-ray analysis were unsuccessful. Therefore, we constructed the simulated molecular model of Cage 1 (Figure 3 and Figure S7 see the Supporting Information for details). Simulated molecular models show that the supramolecular nanostructure of Cage 1 perfectly matches the designed tetrahedron. The two protons at the center of the triptycene are divided into two groups, with one group pointing inside the cavity and the other group pointing outside. The distance between two adjacent zinc ions in the tetrahedron is 17.4 Å as measured by the Materials Studio software.
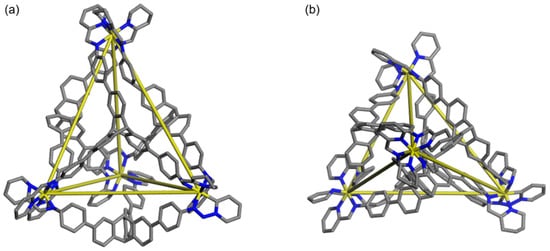
Figure 3.
Energy-minimized molecular model of Cage 1. (Zn, yellow; N, blue; C, gray). Hydrogen atoms and counter anions are omitted for clarity: (a) side view and (b) top view.
Triptycene has a unique three-dimensional rigid structure, the included angle between the three benzene rings is 120 degrees, forming three open negative electron cavities. Consequently, after L generates Cage 1 through self-assembly, its internal cavity is electronegative, which is more conducive to encapsulating positively charged molecules. Taking into consideration the unique structure of Cage 1, we envisaged this space to be suitable for the binding of small hydrocarbon gases via van der Waals interactions and can be resolved by the independent cavity size of the Cage 1. Hence, its gas adsorption behavior was studied. First, Cage 1 was obtained as a powder following precipitation of Cage 1 from acetonitrile by adding diethyl ether. The porous features of Cage 1 prompted us to examine their stability and gas uptake behavior. Thermogravimetric analysis (TGA) of Cage 1 shows a 4% weight loss in the temperature range of 0–100 °C under N2, corresponding to the loss of water molecules and the framework is stable up to ca. 400 °C (Figure S8 see the Supporting Information for details). Cage 1 was thus activated by heating at 120 °C under vacuum (<0.013 mBr) for 10 h to remove solvent. Powder X-ray diffraction (PXRD) of Cage 1 revealed no distinct sharp peaks, consistent with the presence of amorphous material (Figure S7 see the Supporting Information for details).
By analyzing the N2 adsorption and desorption curve of the Cage 1 at 77 K, we can obtain the information of its aperture structure. As can be seen from Figure 4, the N2 isotherm of Cage 1 shows a reversible type-II curve with point B at low P/P0. It can also be seen from Figure 4a that Cage 1 adsorption is lower at 77 K and lower pressure, and the adsorption capacity of N2 increases with the increasing of the pressure. When P/P0 is approach 1, the adsorption value reaches the maximum, the maximum adsorption capacity of N2 by Cage 1 is 71.85397 cm3g−1 at 77 K and 1 bar, and its BET surface area is 25.3955 m2g−1 (Figure S9 see the Supporting Information for details). To sum up, these data suggest that both micropores and mesoporous exist in Cage 1, and this is consistent with the regular tetrahedral nanopore structure of Cage 1.
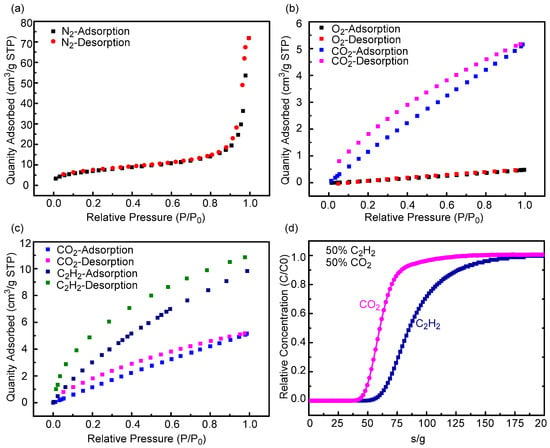
Figure 4.
(a) N2 adsorption and desorption isotherms of Cage 1 at 298 K. (b) CO2 and O2 adsorption and desorption isotherms of Cage 1 at 298 K. (c) C2H2 and CO2 adsorption and desorption isotherms of Cage 1 at 298 K. (d) Transient breakthrough simulation for separation of equimolar C2H2/CO2 mixture using Cage 1 at 298 K, with a partial pressure of 50 KPa for each.
Furthermore, we also examined the gas adsorption behaviors of Cage 1 for CO2 and O2. According to the isothermal curve of CO2 and O2 of Cage 1 at 298 K as shown in Figure 4b, it can be procured that the adsorption and desorption of CO2 are positively correlated with the pressure. It maximizes at P/P0 = 1, the amount of adsorption and desorption is 5.1711 cm3g−1. The adsorption capacity and desorption capacity of O2 are similar to CO2, and also positively correlated with pressure. When P/P0 = 1, the maximum adsorption capacity is 0.4806 cm3g−1. The adsorption behavior of Cage 1 for CO2 and O2 is physical adsorption. Hence, the different adsorption capacity of Cage 1 for CO2 and O2 is mainly due to the different molecular weight and structure of CO2 and O2. We believe that the size of O2 is relatively small and unable match the cavity size of Cage1; hence, it cannot be adsorbed by the cavity, while CO2 can be adsorbed by the Cage cavity due to its larger linear spatial structure and relatively high matching degree with the Cage cavity. Beyond that, the hysteresis loop in the adsorption and desorption curve shown in the Figure 4b may be caused by capillary condensation hysteresis, which is mainly affected by the aperture and structure of the cage itself, and has nothing to do with the adsorbent.
Acetylene (C2H2) is an essential chemical feedstock for modern petrochemical products such as acrylic acid derivatives, vinyl chloride, plastics and rubber. Acetylene is mainly produced via natural gas combustion or hydrocarbon. However, CO2 as an impurity always exists in the production process of acetylene, due to their highly similar molecular size, shape, boiling points (boiling points: C2H2, 189.3 K; CO2, 194.7 K; molecular shapes and size: C2H2, 3.3 × 3.3 × 5.7 Å3; CO2, 3.2 × 3.3 × 5.4 Å3) and almost identical kinetic diameters of ~ 3.3 Å3 [52,53]. Hence, how to efficiently separate acetylene and carbon dioxide has been a problem that unable be ignored in industrial production of acetylene. The isothermal curve of Cage 1 to C2H2 of desorption in Figure 4c was analyzed. We found that the adsorption capacity of the Cage 1 to C2H2 was similar to that of CO2; all were positively correlated with pressure. When P/P0 = 1, the maximum adsorption capacity is 10.8463 cm3g−1. By comparison, the Cage 1 adsorbed about twice as much to C2H2 as it did to CO2. Hence, we think that Cage 1 might have the property of separating C2H2 and CO2. To further evaluate the actual separation performance of the C2H2/CO2 mixture in Cage 1, laboratory scale fixed-bed breakthrough experiments were carried out under condition of C2H2/CO2 (50/50) gas mixture. As Figure 4d shows that Cage 1 achieves effective separation of C2H2/CO2 within a certain range. It can be observed from Figure 4d that CO2 was first eluted and then rapidly approached a pure grade without detectable C2H2, then C2H2 is gradually eluted. The only fly in the ointment is that C2H2 stays in the packed column for a short time, which leads to the inefficient separation of C2H2/CO2 in the Cage 1. This is related to the structure of the Cage 1 itself and the size of the aperture. In addition, the loading level of Cage 1 samples in the packed column is low, so that the saturation absorption value of C2H2 is quickly reached, which also has a non-negligible impact on the separation efficiency.
The calcium carbide process is a common process to produce acetylene. When calcium carbide is added, nitrogen replacement must be carried out on the feeding storage hopper of acetylene generator to prevent explosion. Then, a N2 /C2H2 mixture will be discharged in this process. This leads to waste of N2 and C2H2 and pollution of the environment. Hence, it is of great significance to effectively separate and recover the emissions N2 and C2H2. According to the analysis of the isotherms of the Cage 1 for C2H2 and N2 at 298 K in Figure 5a, the adsorption capacity of the Cage 1 for acetylene and nitrogen all increases with the increase in pressure. When P/P0 = 1, the adsorption capacity of the Cage 1 for N2 reaches a maximum of 0.5684 cm3g−1. By comparison, it was found that the adsorption capacity of Cage 1 for acetylene was about 20 times that of Cage 1 for nitrogen. Therefore, in order to further prove that Cage 1 demonstrates good separation performance for C2H2 and N2, experiments were carried out under dynamic instantaneous breakthrough conditions to further evaluate the actual separation performance of Cage 1 in C2H2/N2 gas mixture. In these experiments, the gas mixture with 2 mL/min was the total flow through the packed tower. According to the C2H2/N2 (50/50) penetration curve in Figure 5b, Cage 1 can efficiently separate the C2H2/N2 mixture. As shown in the figure, N2 was eluted first because Cage 1 has a small adsorption capacity for N2. At this time, pure Nitrogen productivities, calculated as an area under the breakthrough curves, for the equimolar gas mixtures were 7.6 mL (STP) g−1 (0.3 mol kg−1) for N2/C2H2. (Figure S14 see the Supporting Information for details). Then C2H2 is retained in the packed column for a long time until the saturation adsorption capacity is broken. Therefore, the purpose of efficient separation of acetylene and nitrogen is achieved.
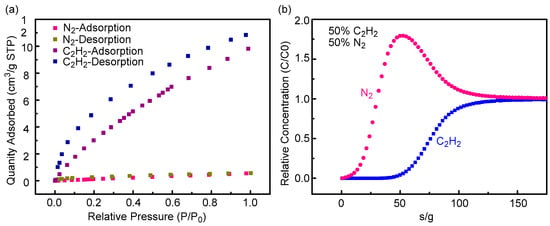
Figure 5.
(a) C2H2 and N2 adsorption and desorption isotherms of Cage 1 at 298 K. (b) Transient breakthrough simulation for separation of equimolar C2H2/N2 mixture using Cage 1 at 298 K, with a partial pressure of 50 KPa for each.
4. Conclusions
In conclusion, we have successfully constructed triptycene-functionalized supramolecular metal–organic tetrahedral cages through coordination-driven synergetic self-assembly, which were thoroughly characterized by NMR and ESI-MS. Considering the unique three-dimensional rigid structure and high porosity of triptycene derivatives, we investigated the gas adsorption properties of the Cage 1. Through a large amount of data, we found some interesting, but not surprising, phenomenon. Cage 1 has a good adsorption ability for some hydrocarbon gas molecules, such as C2H2, leading also to a high adsorption ability of the main culprit of greenhouse effects, CO2; however, on small molecules, such as O2 and N2, it has almost no adsorption capacity, which is beneficial to the separation of CO2 and O2. The remarkable thing is that Cage 1 also has good separation performance for C2H2/CO2 and C2H2/N2. It has a good application prospect in the industrial production and separation of acetylene. Unlike some complex synthesis methods of porous coordination polymers (PCP) or metal-organic frameworks (MOFs) previously reported, Cage 1 is easier to obtain, can be prepared in gram scale, and has good stability at room temperature. This study provides a new way to solve the difficult problem of CO2 storage and the difficult separation of C2H2/CO2 and C2H2/N2 in industrial production, and also provides a possibility to develop a new porous metal–organic nanocage adsorbents that can be prepared and used in an industrial scale, which has broad application prospects.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano12244402/s1. Figure S1: 1H NMR (600 MHZ, CDCl3) spectrum of L.; Figure S2: 13C NMR (151 MHZ, CDCl3) spectrum of L.; Figure S3: 13C NMR (151 MHZ, CDCl3/CD3CN 1:1) spectrum of L.; Figure S4: 1H NMR (151 MHZ, CDCl3/CD3CN 1:1) spectrum of Cage 1.; Figure S5: (a) and (b) ESI-MS spectrum of Cage 1.; Theoretical (top) and experimental (bottom) isotope patterns for different charge states observed from Cage 1 (NTf2− as the counterion).; Figure S6: Energy-minimized molecular model of Cage 1. (Zn, yellow; N, blue; C, gray). Hydrogens, counter anions are omitted for clarity.; Figure S7: Experimental PXRD pattern of Cage 1.; Figure S8: TGA plot of solid Cage 1 under N2.; Figure S9: N2 sorption isotherms of activated Cage 1 measured at 77 K (upper) and plot of the linear region of the BET equation for Cage 1 (bottom).; Figure S10: CO2 adsorption/desorption isotherms of activated Cage 1 at 298 K.; Figure S11: O2 adsorption/desorption isotherms of activated Cage 1 at 298 K.; Figure S12: C2H2 adsorption/desorption isotherms of activated Cage 1 at 298 K.; Figure S13: N2 adsorption/desorption isotherms of activated Cage 1 at 298 K.
Author Contributions
Conceptualization, L.S. and X.-Q.H.; methodology, X.J. and H.J.; validation, X.H. and Y.C.; formal analysis, X.J., H.J. and X.H.; investigation, L.S. and K.S., writing—original draft preparation, L.S. and X.J.; writing—review and editing, L.S.; supervision, L.S., M.-P.S., and X.-Q.H.; project administration, L.S.; funding acquisition, L.S. and X.-Q.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (nos. 22101267, 21803059, U1904212, and U2004191), the Natural Science Foundation of Henan Province (202300410477) and China Postdoctoral Science Foundation (No. 2021M692905).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Lifschitz, A.M.; Rosen, M.S.; McGuirk, C.M.; Mirkin, C.A. Allosteric Supramolecular Coordination Constructs. J. Am. Chem. Soc. 2015, 137, 7252–7261. [Google Scholar] [CrossRef] [PubMed]
- McConnell, A.J.; Wood, C.S.; Neelakandan, P.P.; Nitschke, J.R. Stimuli-Responsive Metal-Ligand Assemblies. Chem. Rev. 2015, 115, 7729–7793. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.L.; De, S.; Pramanik, S.; Schmittel, M. Orthogonality in discrete self-assembly-Survey of current concepts. Chem. Soc. Rev. 2013, 42, 6860–6909. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Y.X.; Yang, H.B. Supramolecular Transformations Within Discrete Coordination-Driven Supramolecular Architectures. Chem. Soc. Rev. 2016, 45, 2656–2693. [Google Scholar] [CrossRef] [PubMed]
- Pilgrim, B.S.; Champness, N.R. Metal-Organic Frameworks and Metal-Organic Cages—A Perspective. ChemPlusChem 2020, 85, 1842–1856. [Google Scholar] [CrossRef]
- Rizzuto, F.J.; von Krbek, L.K.S.; Nitschke, J.R. Strategies for Binding Multiple Guests in Metal-Organic Cages. Nat. Rev. Chem. 2019, 3, 204–222. [Google Scholar] [CrossRef]
- Cullen, W.; Misuraca, M.C.; Hunter, C.A.; Williams, N.H.; Ward, M.D. Highly Efficient Catalysis of the Kemp Elimination in the Cavity of a Cubic Coordination Cage. Nat. Chem. 2016, 8, 231–236. [Google Scholar] [CrossRef]
- Holloway, L.R.; Bogie, P.M.; Lyon, Y.; Ngai, C.; Miller, T.F.; Julian, R.R.; Hooley, R.J. Tandem Reactivity of a Self-Assembled Cage Catalyst with Endohedral Acid Groups. J. Am. Chem. Soc. 2018, 140, 8078–8081. [Google Scholar] [CrossRef]
- Marcos, V.; Stephens, A.J.; Jaramillo-Garcia, J.; Nussbaumer, A.L.; Woltering, S.L.; Valero, A.; Lemonnier, J.F.; Vitorica-Yrezabal, I.J.; Leigh, D.A. Allosteric initiation and Regulation of Catalysis with a Molecular Knot. Science 2016, 352, 1555–1559. [Google Scholar] [CrossRef]
- Oldacre, A.N.; Friedman, A.E.; Cook, T.R. A Self-Assembled Cofacial Cobalt Porphyrin Prism for Oxygen Reduction Catalysis. J. Am. Chem. Soc. 2017, 139, 1424–1427. [Google Scholar] [CrossRef]
- Burke, M.J.; Nichol, G.S.; Lusby, P.J. Orthogonal Selection and Fixing of Coordination Self-Assembly Pathways for Robust Metallo-organic Ensemble Construction. J. Am. Chem. Soc. 2016, 138, 9308–9315. [Google Scholar] [CrossRef]
- Kurihara, K.; Yazaki, K.; Akita, M.; Yoshizawa, M. A Switchable Open/closed Polyaromatic Macrocycle that Shows Reversible Binding of Long Hydrophilic Molecules. Angew. Chem. Int. Ed. 2017, 56, 11360–11364. [Google Scholar] [CrossRef]
- Li, R.J.; Holstein, J.J.; Hiller, W.G.; Andréasson, J.; Clever, G.H. Mechanistic Interplay between Light Switching and Guest Binding in Photochromic [Pd2Dithienylethene4] Coordination Cages. J. Am. Chem. Soc. 2019, 141, 2097–2103. [Google Scholar] [CrossRef]
- Samanta, D.; Mukherjee, P.S. Sunlight-Induced Covalent Marriage of Two Triply Interlocked Pd6 Cages and Their Facile Thermal Separation. J. Am. Chem. Soc. 2014, 136, 17006–17009. [Google Scholar] [CrossRef]
- Cullen, W.; Takezawa, H.; Fujita, M. Demethylenation of Cyclopropanes via Photoinduced Guest-to-Host Electron Transfer in an M6L4 Cage. Angew. Chem. Int. Ed. 2019, 58, 9171–9173. [Google Scholar] [CrossRef]
- Wiester, M.J.; Ulmann, P.A.; Mirkin, C.A. Enzyme Mimics Based Upon Supramolecular Coordination Chemistry. Angew. Chem. Int. Ed. 2011, 50, 114–137. [Google Scholar] [CrossRef]
- Mondal, M.; Hirsch, A.K.H. Dynamic Combinatorial Chemistry: A Tool to Facilitate the Identification of Inhibitors for Protein Targets. Chem. Soc. Rev. 2015, 44, 2455–2488. [Google Scholar] [CrossRef]
- Zamora-Olivares, D.; Kaoud, T.S.; Dalby, K.N.; Anslyn, E.V. In-Situ Generation of Differential Sensors that Fingerprint Kinases and the Cellular Response to Their Expression. J. Am. Chem. Soc. 2013, 135, 14814–14820. [Google Scholar] [CrossRef]
- Yang, G.; Zheng, W.; Tao, G.; Wu, L.; Zhou, Q.F.; Kochovski, Z.; Ji, T.; Chen, H.; Li, X.; Lu, Y.; et al. Diversiform and Transformable Glyco-Nanostructures Constructed from Amphiphilic Supramolecular Metallocarbohydrates through Hierarchical Self-Assembly: The Balance between Metallacycles and Saccharides. ACS Nano 2019, 13, 13474–13485. [Google Scholar] [CrossRef]
- García-Simón, C.; Garcia-Borràs, M.; Gómez, L.; Parella, T.; Osuna, S.; Juanhuix, J.; Imaz, I.; Maspoch, D.; Costas, M.; Ribas, X. Sponge-like Molecular Cage for Purification of Fullerenes. Nat. Commun. 2014, 5, 5557. [Google Scholar] [CrossRef]
- Howlader, P.; Mondal, S.; Ahmed, S.; Mukherjee, P.S. Guest-Induced Enantioselective Self-Assembly of a Pd6 Homochiral Octahedral Cage with a C3-Symmetric Pyridyl Donor. J. Am. Chem. Soc. 2020, 142, 20968–20972. [Google Scholar] [CrossRef] [PubMed]
- Sawada, T.; Fujita, M. A Single Watson-Crick G·C Base Pair in Water: Aqueous Hydrogen Bonds in Hydrophobic Cavities. J. Am. Chem. Soc. 2010, 132, 7194–7201. [Google Scholar] [CrossRef] [PubMed]
- Sawada, T.; Yoshizawa, M.; Sato, S.; Fujita, M. Minimal Nucleotide Duplex Formation in Water through Enclathration in Self-Assembled Hosts. Nat. Chem. 2009, 1, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.K.; Moncelet, D.; Briken, V.; Isaacs, L. Metal-Organic Polyhedron Capped with Cucurbit[8]uril Delivers Doxorubicin to Cancer Cells. J. Am. Chem. Soc. 2016, 138, 14488–14496. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.K.; Quigley, J.; Vinciguerra, B.; Briken, V.; Isaacs, L. Cucurbit[7]uril Enables Multi-Stimuli-Responsive Release from the Self-Assembled Hydrophobic Phase of a Metal Organic Polyhedron. J. Am. Chem. Soc. 2017, 139, 9066–9074. [Google Scholar] [CrossRef] [PubMed]
- Haynes, C.J.E.; Zhu, J.; Chimerel, C.; Hernández-Ainsa, S.; Riddell, I.A.; Ronson, T.K.; Keyser, U.F.; Nitschke, J.R. Blockable Zn10L15 Ion Channels through Subcomponent Self-Assembly. Angew. Chem. Int. Ed. 2017, 56, 15388–15392. [Google Scholar] [CrossRef]
- Kawano, R.; Horike, N.; Hijikata, Y.; Kondo, M.; Carné-Sánchez, A.; Larpent, P.; Ikemura, S.; Osaki, T.; Kamiya, K.; Kitagawa, S.; et al. Metal-Organic Cuboctahedra for Synthetic Ion Channels with Multiple Conductance States. Chem 2017, 2, 393–403. [Google Scholar] [CrossRef]
- Zhang, Z.; Peh, S.B.; Wang, Y.; Kang, C.; Fan, W.; Zhao, D. Efficient Trapping of Trace Acetylene from Ethylene in an Ultramicroporous Metal-Organic Framework: Synergistic Effect of High-Density Open Metal and Electronegative Sites. Angew. Chem. Int. Ed. 2020, 59, 18927–18932. [Google Scholar] [CrossRef]
- Fan, W.; Wang, X.; Liu, X.; Xu, B.; Zhang, X.; Wang, W.; Wang, X.; Wang, Y.; Dai, F.; Yuan, D.; et al. Regulating C2H2 and CO2 Storage and Separation through Pore Environment Modification in a Microporous Ni-MOF. ACS Sustain. Chem. Eng. 2019, 7, 2134–2140. [Google Scholar] [CrossRef]
- Lin, R.B.; Li, L.; Wu, H.; Arman, H.; Li, B.; Lin, R.G.; Zhou, W.; Chen, B. Optimized Separation of Acetylene from Carbon Dioxide and Ethylene in a Microporous Material. J. Am. Chem. Soc. 2017, 139, 8022–8028. [Google Scholar] [CrossRef]
- Chen, K.J.; Madden, D.G.; Mukherjee, S.; Pham, T.; Forrest, K.A.; Kumar, A.; Space, B.; Kong, J.; Zhang, Q.Y.; Zaworotko, M.J. Synergistic Ssorbent Separation for One-Step Ethylene Purification from a Four-Component Mixture. Science 2019, 366, 241–246. [Google Scholar] [CrossRef]
- Chu, S.; Cui, Y.; Liu, N. The Path Towards Sustainable Energy. Nat. Mater. 2017, 16, 16–22. [Google Scholar] [CrossRef]
- Kuznicki, S.M.; Bell, V.A.; Nair, S.; Hillhouse, H.W.; Jacubinas, R.M.; Braunbarth, C.M.; Toby, B.H.; Tsapatsis, M. A Titanosilicate Molecular Sieve with Adjustable Pores for Size-Selective Adsorption of Molecules. Nature 2001, 412, 720–724. [Google Scholar] [CrossRef]
- Isfahani, A.P.; Sadeghi, M.; Dehaghani, A.H.S.; Aravand, M.A. Enhancement of the Gas Separation Properties of Polyurethane Membrane by Epoxy Nanoparticles. J. Ind. Eng. Chem. 2016, 44, 67–72. [Google Scholar] [CrossRef]
- Humayun, M.; Qu, Y.; Raziq, F.; Yan, R.; Li, Z.; Zhang, X.; Jing, L. Exceptional Visible-Light Activities of TiO2-Coupled N-Doped Porous Perovskite LaFeO3 for 2,4-Dichlorophenol Decomposition and CO2 Conversion. Environ. Sci. Technol. 2016, 50, 13600–13610. [Google Scholar] [CrossRef]
- Ohlin, L.; Berezovsky, V.; Öberg, S.; Farzaneh, A.; Holmgren, A.; Grahn, M. Effect of Water on the Adsorption of Methane and Carbon Dioxide in Zeolite Na-ZSM-5 Studied Using in Situ ATR-FTIR Spectroscopy. J. Phys. Chem. 2016, 120, 29144–29152. [Google Scholar] [CrossRef]
- Kaur, G.; Gupta, S.; Dharamvir, K. Theoretical Investigation of Adsorption of Gas Molecules on Li Metal Adsorbed at H-site of Graphene: A Search for Graphene based Gas Sensors. Surf. Interfaces 2017, 8, 83–90. [Google Scholar] [CrossRef]
- Liu, L.; Yao, Z.; Ye, Y.; Yang, Y.; Lin, Q.; Zhang, Z.; O’Keeffe, M.; Xiang, S. Integrating the Pillared-Layer Strategy and Pore-Space Partition Method to Construct Multicomponent MOFs for C2H2/CO2 Separation. J. Am. Chem. Soc. 2020, 142, 9258–9266. [Google Scholar] [CrossRef]
- Yang, S.; Ramirez-Cuesta, A.J.; Newby, R.; Garcia-Sakai, V.; Manuel, P.; Callear, S.K.; Campbell, S.I.; Tang, C.C.; Schröder, M. Supramolecular Binding and Separation of Hydrocarbons within A Functionalized Porous Metal-Organic Framework. Nat. Chem. 2015, 7, 121–129. [Google Scholar] [CrossRef]
- Jiang, L.; Tian, Y.; Sun, T.; Zhu, Y.; Ren, H.; Zou, X.; Ma, Y.; Meihaus, K.R.; Long, J.R.; Zhu, G. A Crystalline Polyimide Porous Organic Framework for Selective Adsorption of Acetylene over Ethylene. J. Am. Chem. Soc. 2018, 140, 15724–15730. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, P.; Li, M.; Zhang, P.; Li, J.; Liu, J.; Ma, Y.; Ren, H.; Zhu, G. Construction of a Stable Crystalline Polyimide Porous Organic Framework for C2H2/C2H4 and CO2/N2 Separation. Chem. Eur. J. 2019, 25, 9045–9051. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Xie, D.; Chang, G.; Wu, H.; Li, L.; Zhou, W.; Wang, H.; Zhang, Z.; Xing, H.; Yang, Q.; et al. Fine Tuning and Specific Binding Sites with a Porous Hydrogen-Bonded Metal-Complex Framework for Gas Selective Separations. J. Am. Chem. Soc. 2018, 140, 4596–4603. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, L.; Gong, L.; Krishna, R.; Gao, Z.; Tao, Y.; Yin, W.; Xu, Z.; Luo, F. Constructing Redox-Active Microporous Hydrogen-Bonded Organic Framework by Imide-Functionalization: Photochromism, Electrochromism, and Selective Adsorption of C2H2 over CO2. Chem. Eng. J. 2020, 383, 123117. [Google Scholar] [CrossRef]
- Morris, R.E.; Brammer, L. Coordination Change, Lability and Hemilability in Metal-Organic Frameworks. Chem. Soc. Rev. 2017, 46, 5444–5462. [Google Scholar] [CrossRef] [PubMed]
- Bavykina, A.; Gascon, J. An efficient nanosieve. Nat. Mater. 2018, 17, 1057–1058. [Google Scholar] [CrossRef]
- Cadiau, A.; Adil, K.; Bhatt, P.M.; Belmabkhout, Y.; Eddaoudi, M. A Metal-Organic Framework-Based Splitter for Separating Propylene from Propane. Science 2016, 353, 137–140. [Google Scholar] [CrossRef]
- Lin, R.B.; Li, L.; Zhou, H.L.; Wu, H.; He, C.; Li, S.; Krishna, R.; Li, J.; Zhou, W.; Chen, B. Molecular Sieving of Ethylene from Ethane Using a Rigid Metal-Organic Framework. Nat. Mater. 2018, 17, 1128–1133. [Google Scholar] [CrossRef]
- Fan, W.; Peh, S.B.; Zhang, Z.; Yuan, H.; Yang, Z.; Wang, Y.; Chai, K.; Sun, D.; Zhao, D. Tetrazole-Functionalized Zirconium Metal-Organic Cages for Efficient C2H2/C2H4 and C2H2/CO2 Separations. Angew. Chem. Int. Ed. 2021, 60, 17338–17343. [Google Scholar] [CrossRef]
- Hua, B.; Ding, Y.; Alimi, L.O.; Moosa, B.; Zhang, G.; Baslyman, W.S.; Sessler, J.; Khashab, N.M. Tuning the Porosity of Triangular Supramolecular Adsorbents for Superior Haloalkane Isomer Separations. Chem. Sci. 2021, 12, 12286–12291. [Google Scholar] [CrossRef]
- Zhu, J.L.; Zhang, D.; Ronson, T.K.; Wang, W.; Xu, L.; Yang, H.B.; Nitschke, J.R. A Cavity-Tailored Metal-Organic Cage Entraps Gases Selectively in Solution and the Amorphous Solid State. Angew. Chem. Int. Ed. 2021, 60, 11789–11792. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, C.F. Synthesis and Structure of 2,6,14- and 2,7,14-Trisubstituted Triptycene Derivatives. J. Org. Chem. 2006, 71, 6626–6629. [Google Scholar] [CrossRef]
- Reid, C.R.; Thomas, K.M. Adsorption Kinetics and Size Exclusion Properties of Probe Molecules for the Selective Porosity in a Carbon Molecular Sieve Used for Air Separation. J. Phys. Chem. B 2001, 105, 10619–10629. [Google Scholar] [CrossRef]
- Webster, C.E.; Drago, R.S.; Zerner, M.C. Molecular Dimensions for Adsorptives. J. Am. Chem. Soc. 1998, 120, 5509–5516. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).