Superhydrophobic and Electrochemical Performance of CF2-Modified g-C3N4/Graphene Composite Film Deposited by PECVD
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plasma Deposition of Graphene, g-C3N4/Graphene and CF2 Modified g-C3N4/Graphene Films
2.3. Characterization Method
2.4. Contact Angle Measurements
2.5. Electrochemical Analysis
2.6. Contact Angle Measurements
3. Results and Discussions
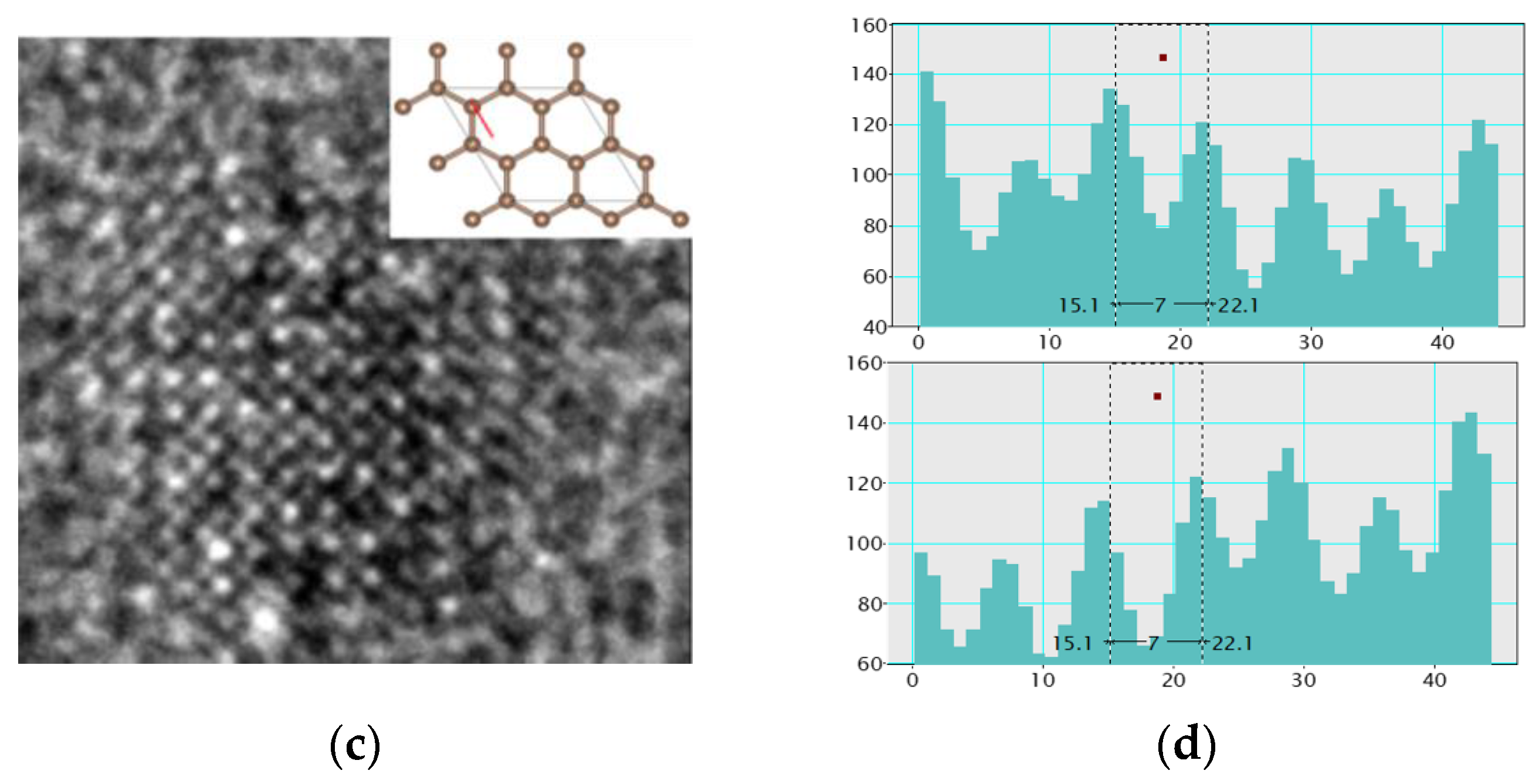
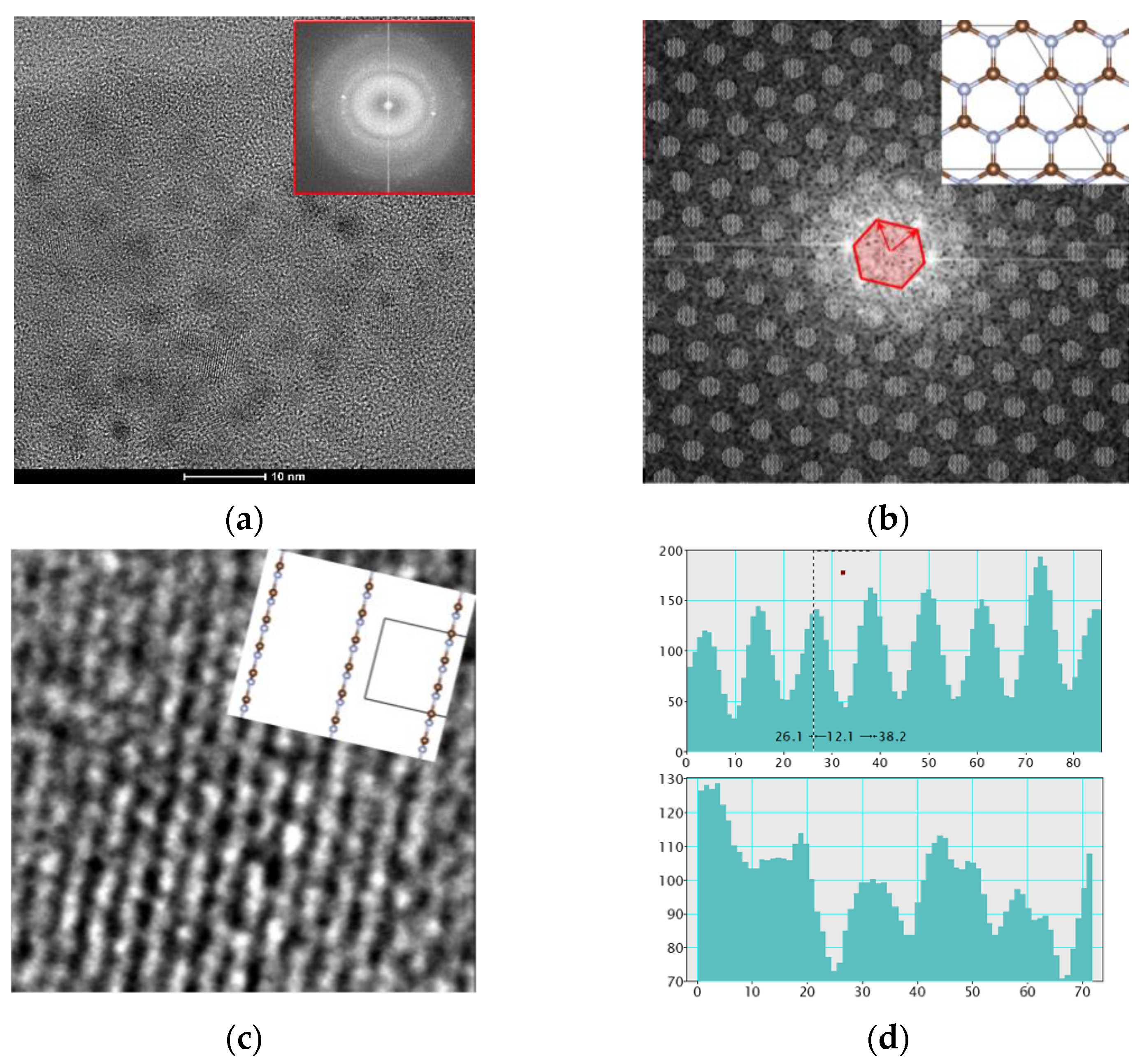
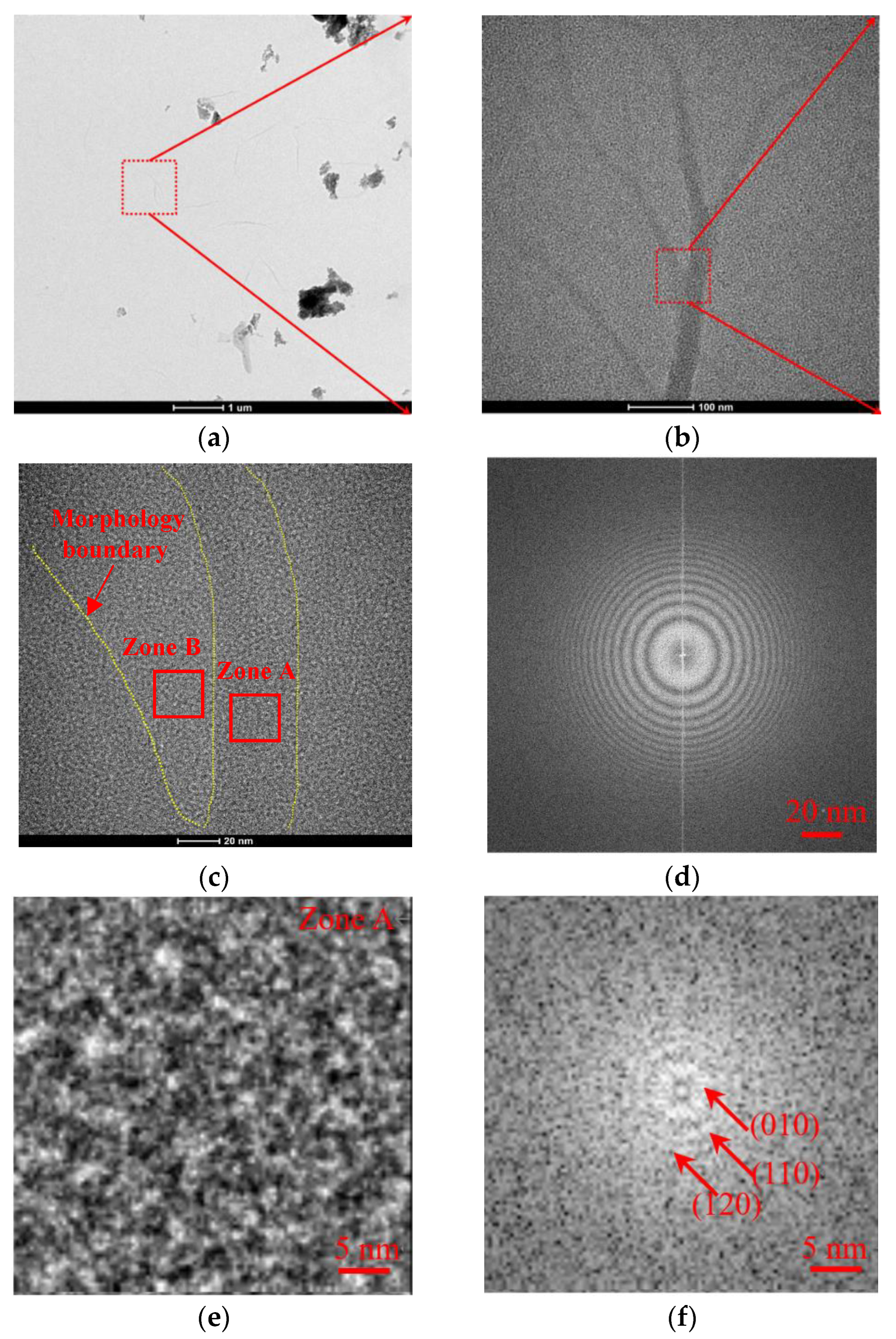
3.1. TEM Analysis on the Film Nanostructures
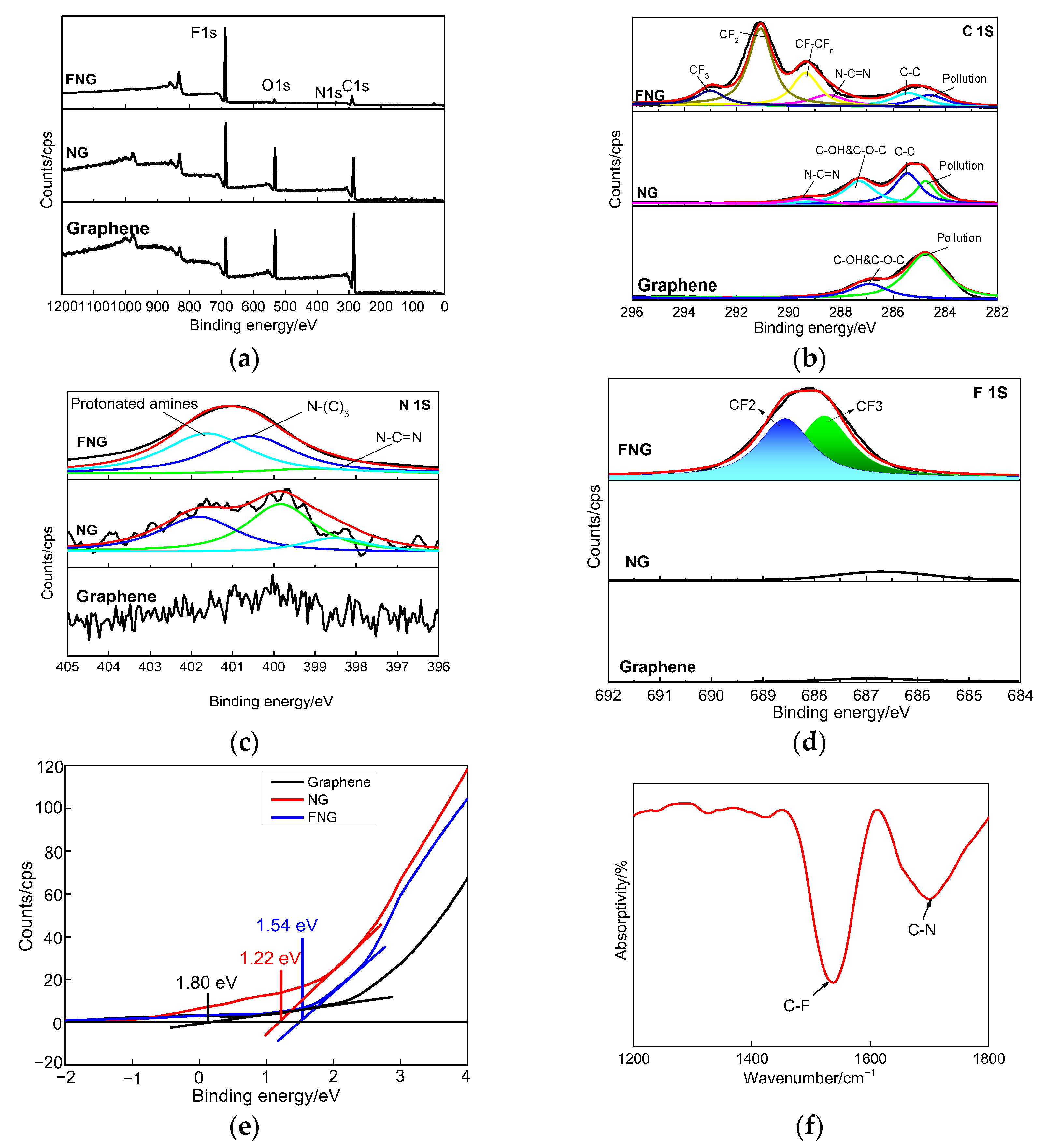
3.2. Chemical Structures by XPS and FTIR Analysis
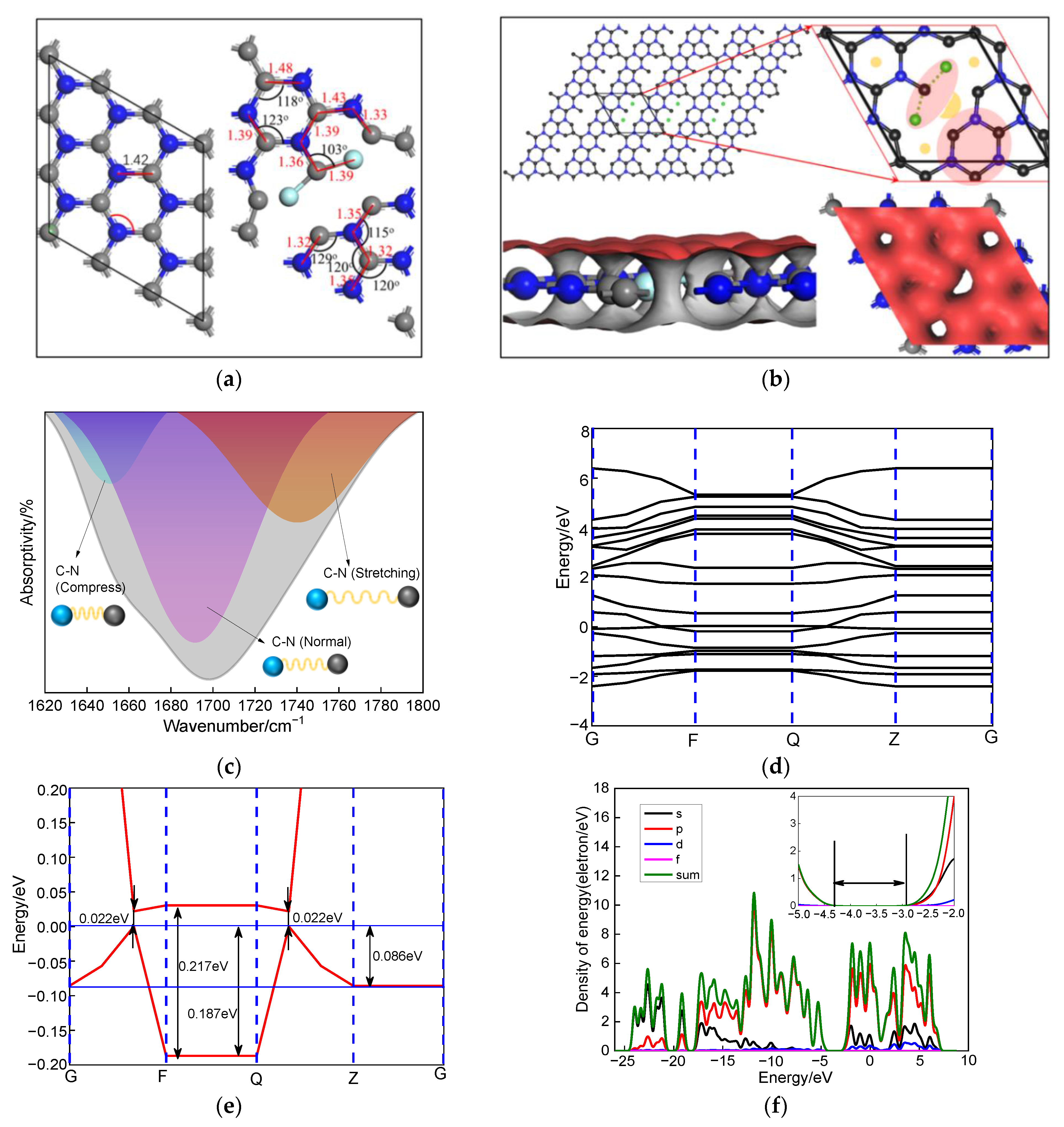
3.3. DFT Calculation
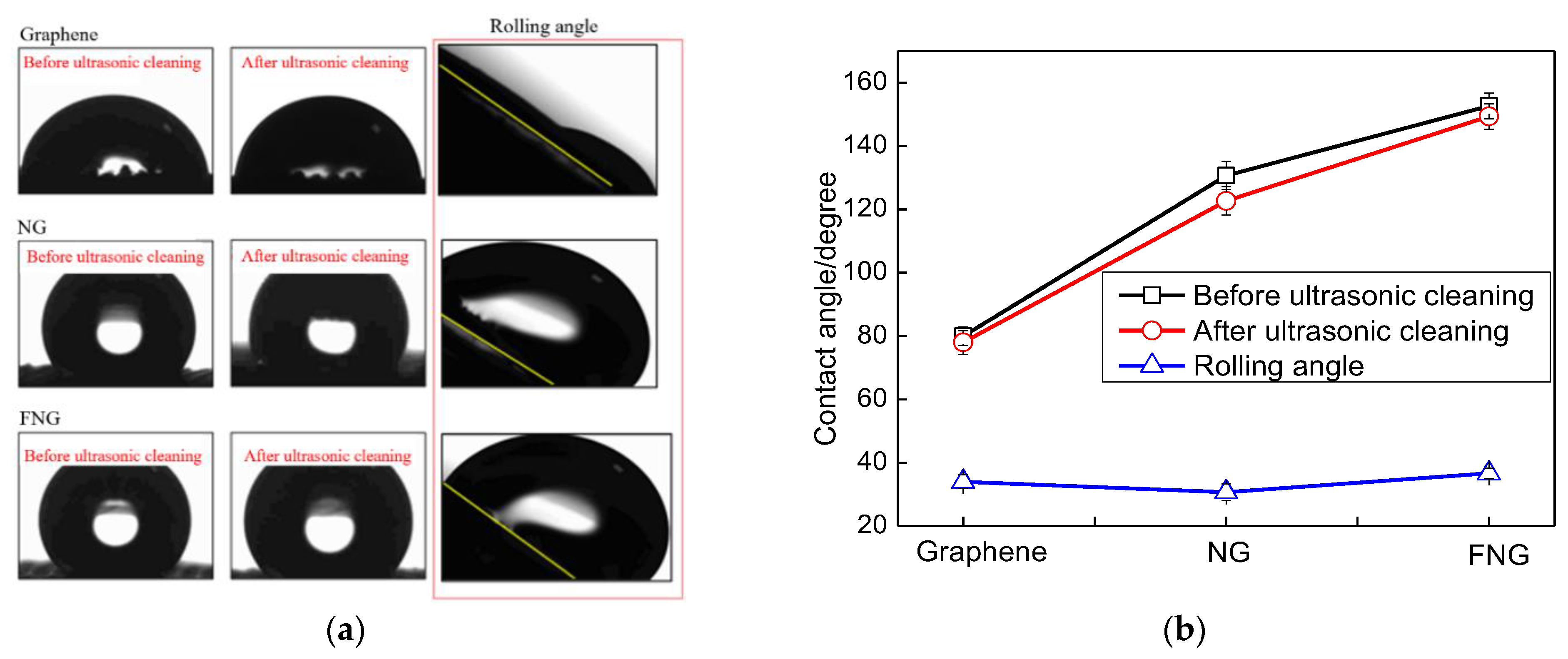
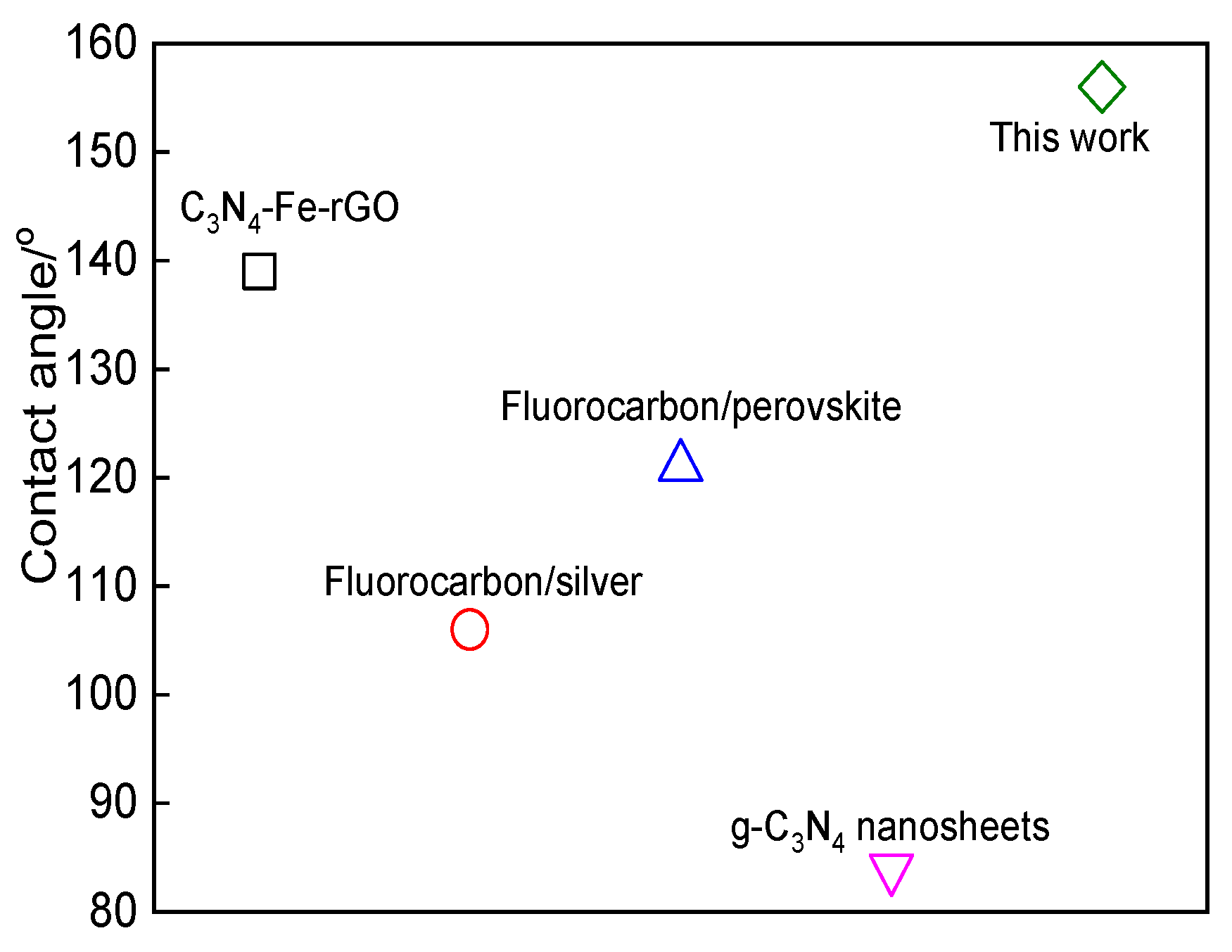
3.4. Superhydrophobic Performance
3.5. Electrochemical Performance
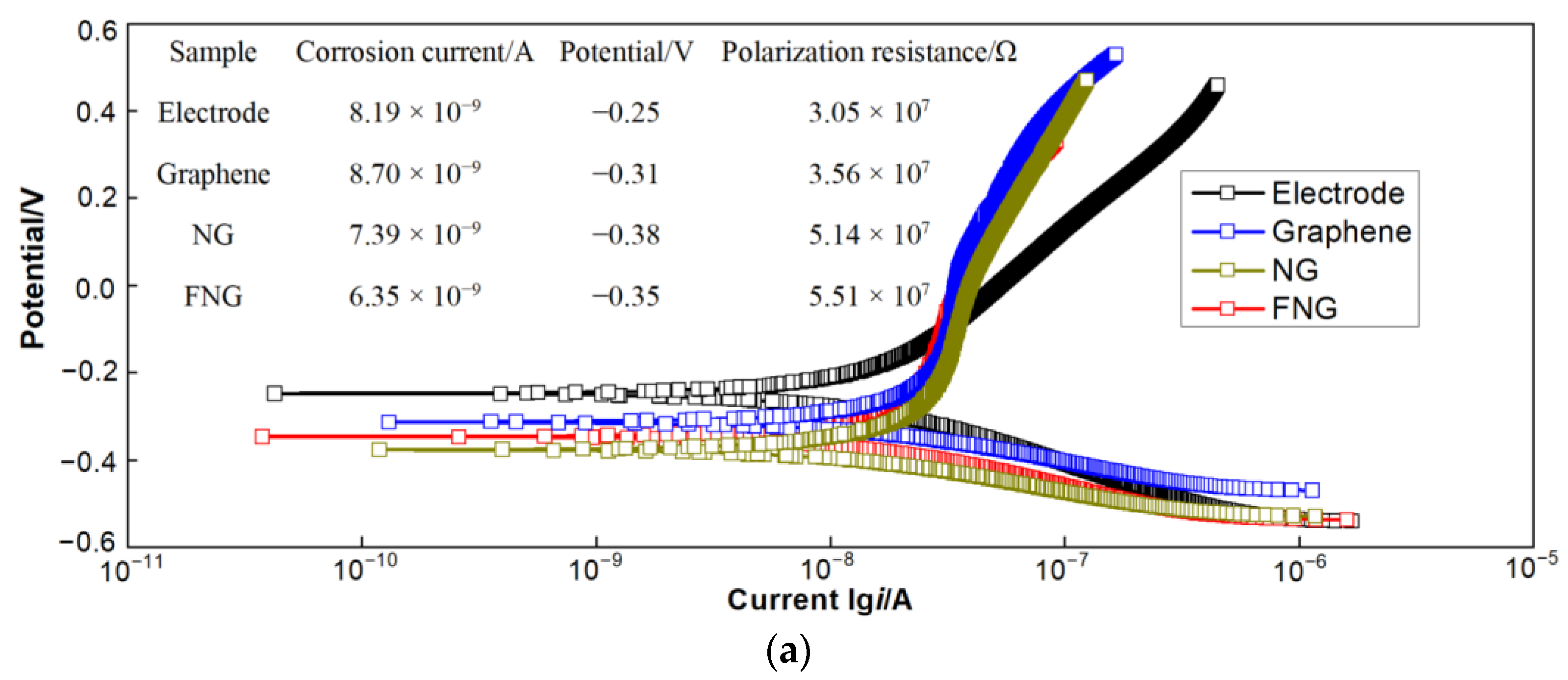
3.5.1. Polarization Curve Analysis
3.5.2. Electrochemical Impedance Spectroscopy Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Maiyalagan, T.; Wang, X. Review on recent progress in nitrogen-doped graphene: Synthesis, characterization, and its potential Applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Liu, B.; Yu, Y.J.; Yan, Z.D.; Cai, P.G.; Gao, F.; Tang, C.J.; Gu, P.; Liu, Z.Q.; Chen, J. The light absorption enhancement in graphene monolayer resulting from the diffraction coupling of surface plasmon polariton resonance. Nanomaterials 2022, 12, 216. [Google Scholar] [CrossRef] [PubMed]
- Stathis, A.; Bouza, Z.; Papadakis, L.; Couris, S. Tailoring the nonlinear optical response of some graphene derivatives by ultraviolet (UV) irradiation. Nanomaterials 2022, 12, 152. [Google Scholar] [CrossRef]
- Li, H.N.; Zhang, H.M.; Huang, K.K.; Liang, D.; Zhao, D.D.; Jiang, Z.Y. Effect of ball milling speed on the quality of Al2O3 stripped graphene in a wet milling medium. Ceram. Int. 2022, 48, 17171–17177. [Google Scholar] [CrossRef]
- Wen, C.K.; Zhong, Y.L.; Solomun, T.T.; Chih, Y.C.; Cheng, Y.Y.; Kuo, J.C.; Jia, C.L. Epitaxial growth and characterization of GaN thin films on graphene/sapphire substrate by embedding a hybrid-AlN buffer layer. Appl. Surf. Sci. 2019, 494, 644–650. [Google Scholar]
- Lu, J.; Liu, X.C.; Zhang, H.; Fu, M.H.; Zheng, H.; Chen, Q.Y. Electrocatalytic activity of nano-flowered yavapaiite anchored on magnetic graphite oxide for nitrate selective reduction. Chem. Eng. J. 2022, 433, 134586. [Google Scholar] [CrossRef]
- Duan, T.B.; Li, H.; Leifer, K. Electron-beam-induced fluorination cycle for long-term preservation of graphene under ambient conditions. Nanomaterials 2022, 12, 383. [Google Scholar] [CrossRef]
- Ushio, S.; Yoshii, A.; Tamai, N.; Ohtani, N.; Kaneko, T. Wide-range temperature dependence of epitaxial graphene growth on 4H-SiC (0 0 0 −1): A study of ridge structures formation dynamics associated with temperature. J. Cryst. Growth 2011, 318, 590–594. [Google Scholar] [CrossRef]
- Aixart, J.; Díaz, F.; Llorca, J.; Llompart, J.R. Increasing reaction time in Hummers’ method towards well exfoliated graphene oxide of low oxidation degree. Ceram. Int. 2021, 47, 22130–22137. [Google Scholar] [CrossRef]
- Shen, Y.; Boffa, V.; Corazzari, I.; Qiao, A.; Tao, H.; Yue, Y. Revealing hidden endotherm of hummers’ graphene oxide during low-temperature thermal reduction. Carbon 2018, 138, 337–347. [Google Scholar] [CrossRef]
- Bo, Z.; Yang, Y.; Chen, J.; Yu, K.; Yan, J.; Cen, K. Plasma-enhanced chemical vapor deposition synthesis of vertically oriented graphene nanosheets. Nanoscale 2013, 5, 5180–5204. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.A.; Ermolaev, G.A.; Voronin, K.A.; Romanov, R.I.; Tselikov, G.I.; Yakubovsky, D.I.; Doroshina, N.V.; Nemtsov, A.B.; Solovey, V.R.; Voronov, A.A.; et al. Optical constants of chemical vapor deposited graphene for photonic applications. Nanomaterials 2021, 11, 1230. [Google Scholar] [CrossRef] [PubMed]
- Burdanova, M.G.; Kharlamova, M.V.; Kramberger, C.; Nikitin, M.P. Applications of pristine and functionalized carbon nanotubes, graphene, and graphene nanoribbons in biomedicine. Nanomaterials 2021, 11, 3020. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liang, Y.H.; An, W.J.; Hu, J.S.; Zhu, Y.F.; Cui, W.Q. Removal of chromium (VI) by a self-regenerating and metal free g-C3N4/graphene hydrogel system via the synergy of adsorption and photo-catalysis under visible light. Appl. Catal. B-Environ. 2017, 219, 53–62. [Google Scholar] [CrossRef]
- Li, J.J.; Zhang, Y.M.; Zhang, X.H.; Han, J.C.; Wang, Y.; Gu, L.; Zhang, Z.H.; Wang, X.J.; Jian, J.K.; Xu, P.; et al. Direct transformation from graphitic C3N4 to nitrogen-doped graphene: An efficient metal-free electrocatalyst for oxygen reduction reaction. ACS Appl. Mater. Interfaces 2015, 7, 19626–19634. [Google Scholar] [CrossRef]
- Han, Q.; Cheng, Z.H.; Gao, J.; Zhao, Y.; Zhang, Z.P.; Dai, L.M.; Qu, L.T. Mesh-on-mesh graphitic-C3N4 @graphene for highly efficient hydrogen evolution. Adv. Funct. Mater. 2017, 27, 1606352. [Google Scholar] [CrossRef]
- Dong, M.M.; He, C.; Zhang, W.X. A tunable and sizable bandgap of a g-C3N4/ graphene/g-C3N4 sandwich heterostructure: A van der Waals density functional study. J. Mater. Chem. C 2017, 5, 3830–3837. [Google Scholar] [CrossRef]
- Zhang, S.L.; Hang, N.T.; Zhang, Z.J.; Yue, H.Y.; Yang, W.C. Preparation of g-C3N4/graphene composite for detecting NO2 at room temperature. Nanomaterials 2017, 7, 12. [Google Scholar] [CrossRef]
- Rao, X.; Du, L.; Zhao, J.J.; Tan, X.D.; Fang, Y.X.; Xu, L.Q.; Zhang, Y.P. Hybrid TiO2/AgNPs/g-C3N4 nanocomposite coatings on TC4 titanium alloy for enhanced synergistic antibacterial effect under full spectrum light. J. Mater. Sci. Technol. 2022, 118, 35–43. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Wang, T.Y.; Zhang, L.; Che, S.Y.; Liu, H.; Liu, S.X.; Wang, C.Y.; Su, D.W.; Teng, Z.Y. Highly efficient Ag2O/Na-g-C3N4 heterojunction for photocatalytic desulfurization of thiophene in fuel under ambient air conditions. Catal. B-Environ. 2022, 316, 121614. [Google Scholar] [CrossRef]
- Chen, K.; Chai, Z.G.; Li, C.; Shi, L.R.; Liu, M.X.; Xie, Q.; Zhang, Y.F.; Xu, D.S.; Manivannan, A.; Liu, Z.F. Catalyst-free growth of three-dimensional graphene flakes and graphene/g-C3N4 composite for hydrocarbon oxidation. ACS Nano 2016, 10, 3665–3673. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Chen, F.Y.; Zhu, Z.Q.; Tang, Y.B.; Shu, K.K.; Shi, W.L. Vacancy-modified g-C3N4 nanosheets via one-step thermal polymerization of thiosemicarbazide precursor for visible-light-driven photocatalytic activity. Mater. Chem. Phys. 2022, 275, 125192. [Google Scholar] [CrossRef]
- Zhao, J.J.; Shang, B.; Zhai, J. N-doped graphene as an efficient metal-free electrocatalyst for indirect nitrate reduction reaction. Nanomaterials 2021, 11, 2418. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, Q.; Xu, Q.H.; Li, M.; Tang, N.J.; Du, Y.W. Synthesis and photoluminescence of F and N co-doped reduced graphene oxide. Carbon 2013, 61, 436–440. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, H.Y.; Wang, L.; Bai, L.G.; Yang, C.L.; Zhu, T. Porous graphene oxide functionalized by covalent organic framework for the application in adsorption and electrochemical: The effect of C-F bonds to structure. Microchem. J. 2021, 170, 106710. [Google Scholar] [CrossRef]
- Kaleli, H.; Demirta, S.; Uysal, V.; Karnis, L.; Stylianakis, M.M.; Anastasiadis, S.H.; Kim, D.E. Tribological performance investigation of a commercial engine oil incorporating reduced graphene oxide as additive. Nanomaterials 2021, 11, 386. [Google Scholar] [CrossRef]
- Su, C.Y.; Yang, C.Y.; Jhang, B.W.; Hsieh, Y.L.; Sin, Y.Y.; Huan, C.C. Pool boiling heat transfer enhanced by fluorinated graphene as atomic layered modifiers. ACS Appl. Mater. Interfaces 2020, 12, 10233–10239. [Google Scholar]
- Feng, Q.; Zheng, Y.P.; Li, J.X.; Jiang, L.Q.; Lin, Y.D.; Ye, Q.Y.; Chen, L.Z.; Huang, Z.G. Observation of ferromagnetic ordering by fragmenting fluorine clusters in highly fluorinated graphene. Carbon 2018, 132, 691–697. [Google Scholar] [CrossRef]
- Wallace, A.R.M.; Colombo, L.; Kim, J. Partially fluorinated graphene: Structural and electrical characterization. ACS Appl. Mater. Interfaces 2016, 8, 5002–5008. [Google Scholar]
- Jayasinghe, R.; Thapa, A.K.; Dharmasena, R.R.; Nguyen, T.Q.; Pradhan, B.K.; Paudel, H.S.; Jasinski, J.B.; Sherehiy, A.; Yoshio, M.; Sumanasekera, G.U. Optimization of multi-walled carbon nanotube based CFx electrodes for improved primary and secondary battery performances. J. Power Sources 2014, 253, 404–411. [Google Scholar] [CrossRef]
- Akiki, G.; Suchet, D.; Daineka, D.; Filonovich, S.; Bulkin, P.; Johnson, E.V. Area selective deposition of silicon by plasma enhanced chemical vapor deposition using a fluorinated precursor. Appl. Surf. Sci. 2020, 531, 147305. [Google Scholar] [CrossRef]
- Yi, K.Y.; Liu, D.H.; Chen, X.S.; Yang, J.; Wei, D.P.; Liu, Y.Q.; Wei, D.C. Plasma-enhanced chemical vapor deposition of two-dimensional materials for applications. Accounts Chem. Res. 2021, 54, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.C.; Chang, Y.C.; Tsai, H.C.; Woon, W.Y. Nucleation and growth dynamics of graphene grown through low power capacitive coupled radio frequency plasma enhanced chemical vapor deposition. Carbon 2019, 154, 420–427. [Google Scholar] [CrossRef]
- Chang, Y.C.; Yen, C.C.; Tsai, H.C.; Chen, T.C.; Yang, C.M.; Chen, C.H.; Woon, W.Y. Characteristics of graphene grown through low power capacitive coupled radio frequency plasma enhanced chemical vapor deposition. Carbon 2020, 159, 570–578. [Google Scholar] [CrossRef]
- Ho, K.I.; Liao, J.H.; Huang, C.H.; Hsu, C.L.; Zhang, W.J.; Lu, A.Y.; Li, L.J.; Lai, C.S.; Su, C.Y. One-step formation of a single atomic-layer transistor by the selective fluorination of a graphene film. Small 2014, 10, 989–997. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, K.; Yao, L.; Qiu, Y.; Wang, S. Hierarchically porous sheath-core graphene based fiber-shaped supercapacitors with high energy density. J. Mater. Chem. A 2018, 6, 896–907. [Google Scholar] [CrossRef]
- Dai, Z.R.; Lian, J.J.; Sun, Y.S.; Li, L.; Zhang, H.; Hu, N.; Ding, D.X. Fabrication of g-C3N4/Sn3O4/Ni electrode for highly efficient photoelectrocatalytic reduction of U(VI). Chem. Eng. J. 2022, 433, 133766. [Google Scholar] [CrossRef]
- Yuan, X.; Qu, S.L.; Huang, X.Y.; Xue, X.G.; Yuan, C.L.; Wang, S.W.; Wei, L.; Cai, P. Design of core-shelled g-C3N4@ZIF-8 photocatalyst with enhanced tetracycline adsorption for boosting photocatalytic degradation. Chem. Eng. J. 2021, 416, 129148. [Google Scholar] [CrossRef]
- Wang, M.; Jin, C.Y.; Kang, J.; Liu, J.Y.; Tang, Y.W.; Li, Z.L.; Li, S.Y. CuO/g-C3N4 2D/2D heterojunction photocatalysts as efficient peroxymonosulfate activators under visible light for oxytetracycline degradation: Characterization, efficiency and mechanism. Chem. Eng. J. 2021, 416, 128118. [Google Scholar] [CrossRef]
- Li, Y.; Yin, Q.; Zeng, Y.S.; Liu, Z. Hollow spherical biomass derived-carbon dotted with SnS2/g-C3N4 Z-scheme heterojunction for efficient CO2 photoreduction into CO. Chem. Eng. J. 2022, 438, 135652. [Google Scholar] [CrossRef]
- Sosulin, I.S.; Feldman, V.I. Spectroscopy and radiation-induced chemistry of an atmospherically relevant CH2F2…H2O complex: Evidence for the formation of CF2…H2O complex as revealed by FTIR matrix isolation and ab initio study. Chemosphere 2022, 291, 132967. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Dai, H.L.; Tan, C.Q.; Pan, Q.Y.; Hu, F.P.; Peng, X.M. Photo-Fenton degradation of tetracycline over Z-scheme Fe-g-C3N4/Bi2WO6 heterojunctions: Mechanism insight, degradation pathways and DFT calculation. Appl. Catal. B-Environ. 2022, 310, 121326. [Google Scholar] [CrossRef]
- Chakraborty, B.; Mane, P.; Vaidyanathan, A. Hydrogen storage in scandium decorated triazine based g-C3N4: Insights from DFT simulations. Int. J. Hydrogen Energ. 2022, 47, 41878–41890. [Google Scholar] [CrossRef]
- Roongcharoen, T.; Impeng, S.; Chitpakdee, C.; Rungrotmongkol, T.; Jitwatanasirikul, T.; Jungsuttiwong, S.; Namuangruk, S. Intrinsic property and catalytic performance of single and double metal atoms incorporated g-C3N4 for O2 activation: A DFT insight. Appl. Surf. Sci. 2021, 541, 148671. [Google Scholar] [CrossRef]
- Jakkapan, S. Effects of doping a boron atom in g-C3N4 on the catalytic activity of deposited Pd atom for the dehydrogenation of formic acid: A DFT study. Appl. Surf. Sci. 2022, 599, 153950. [Google Scholar]
- Ren, Y.H.; Han, Q.Z.; Yang, J.; Zhao, Y.H.; Xie, Y.B.; Wen, H.; Jiang, Z.T. A promising catalytic solution of NO reduction by CO using g-C3N4/TiO2: A DFT study. J. Colloid Interf. Sci. 2022, 610, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.D.; Zhang, T.L.; Zheng, C.C. Study of foam drainage agent based on g-C3N4 nanosheets reinforced stabilization. Colloids Surf. A Physicochem. Eng. Asp. 2023, 657, 130607. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Wu, Y.H.; Zhao, W.J.; Pu, J.B.; Yang, D.H.; Xue, Q.J. Large-area preparation of defect-repaired fluorocarbon polymer coatings on graphene for long-term corrosion resistance. Prog. Org. Coat. 2019, 134, 234–243. [Google Scholar] [CrossRef]
- Cho, E.; Young, Y.K.; Dong, S.H.; Jae, H.L.; Park, J.S.; Seo, J.; Lee, S.J. Highly efficient and stable flexible perovskite solar cells enabled by using plasma-polymerized-fluorocarbon antireflection layer. Nano Energy 2021, 82, 105737. [Google Scholar] [CrossRef]
- Zoghi, A.M.; Allahyari, S. Multifunctional magnetic C3N4-rGO adsorbent with high hydrophobicity and simulated solar light-driven photocatalytic activity for oil spill removal. Sol. Energy 2022, 237, 320–332. [Google Scholar] [CrossRef]
- Yu, X.H.; Xie, J.; Liu, Q.Q.; Dong, H.L.; Li, Y.Y. The origin of enhanced photocatalytic activity in g-C3N4/TiO2 heterostructure revealed by DFT calculations. J. Colloid Interf. Sci. 2021, 593, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.C.; Li, K.M.; Hu, J. Immersion corrosion behavior, electrochemical performance and corrosion mechanism of subsonic flame sprayed FeCoCrMoSi amorphous coating in 3.5% NaCl solution. Int. J. Hydrog. Energ. 2022, 47, 6911–6923. [Google Scholar] [CrossRef]
- Liu, J.J.; Xiong, C.B.; Jiang, S.J.; Wu, X.; Song, S.Q. Efficient evolution of reactive oxygen species over the coordinated π-delocalization g-C3N4 with favorable charge transfer for sustainable pollutant elimination. Appl. Catal. B-Environ. 2019, 249, 282–291. [Google Scholar] [CrossRef]
- Arbi, H.M.; Yadav, A.A.; Kumar, Y.A.; Moniruzzaman, M.; Alzahmi, S.; Obaidat, I.M. Polypyrrole-Assisted Ag Doping Strategy to Boost Co(OH)2 Nanosheets on Ni Foam as a Novel Electrode for High-Performance Hybrid Supercapacitors. Nanomaterials 2022, 12, 3982. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.H.; Ke, K.H.; Lin, E.Z.; Qin, N.; Wu, J.; Huang, R.; Bao, D.H. Piezoelectric polarization modulated novel Bi2WO6/g-C3N4/ZnO Z-scheme heterojunctions with g-C3N4 intermediate layer for efficient piezo-photocatalytic decomposition of harmful organic pollutants. J. Colloid Interf. Sci. 2022, 607, 1589–1602. [Google Scholar] [CrossRef]
- Kong, W.C.; Yu, Z.; Hu, J. Nanostructures and electrochemical performances of Cr0.4Al0.4N0.2 medium-entropy and Cr0.9N0.1 low–entropy ceramic coatings by DFT calculation. Corros. Sci. 2022, 204, 110375. [Google Scholar]
- Kumar, Y.A.; Das, H.T.; Guddeti, P.R.; Nallapureddy, R.R.; Pallavolu, M.R.; Alzahmi, S.; Obaidat, I.M. Self-Supported Co3O4@Mo-Co3O4 Needle-like Nanosheet Heterostructured Architectures of Battery-Type Electrodes for High-Performance Asymmetric Supercapacitors. Nanomaterials 2022, 12, 2330. [Google Scholar] [CrossRef]
- Ren, Y.C.; Gong, T.; Tan, S.L.; Chen, M.; Zhou, F.; Lin, Y.J.; Yang, L.; Peng, Q. Photocatalytic activities of g-C3N4, Bi3NbO7 and g-C3N4/Bi3NbO7 in photocatalytic reduction of Cr(Ⅵ). J. Alloy. Compd. 2022, 902, 163752. [Google Scholar] [CrossRef]
- Kumar, Y.A.; Kumar, K.D.; Kim, H. Reagents assisted ZnCo2O4 nanomaterial for supercapacitor application. Electrochim. Acta 2020, 330, 135261. [Google Scholar] [CrossRef]
- Sun, M.L.; Zhou, P.; Peng, J.L.; He, C.S.; Du, Y.; Pan, Z.C.; Su, S.J.; Dong, F.; Liu, Y.; Lai, B. Insights into peroxymonosulfate activation under visible Light: Sc2O3@C3N4 mediated photoexcited electron transfer. Chem. Eng. J. 2022, 435, 134836. [Google Scholar] [CrossRef]
- Wang, H.Y.; Xie, A.J.; Li, S.J.; Wang, J.J.; Chen, K.X.; Su, Z.L.; Song, N.N.; Luo, S.P. Three-dimensional g-C3N4/MWNTs/GO hybrid electrode as electrochemical sensor for simultaneous determination of ascorbic acid, dopamine and uric acid. Anal. Chim. Acta 2022, 1211, 339907. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.A.; Sambasivam, S.; Hirac, S.A.; Zeb, K.; Uddine, W.; Krishna, T.N.V.; Kumar, K.D.; Obaidat, I.M.; Kim, H. Boosting the energy density of highly efficient flexible hybrid supercapacitors via selective integration of hierarchical nanostructured energy materials. Electrochim. Acta 2020, 364, 137318. [Google Scholar] [CrossRef]
- Mola, B.A.; Pallavolu, M.R.; Al-Asbahi, B.A.; Noh, Y.; Jilcha, S.K.; Kumar, Y.A. Design and construction of hierarchical MnFe2Ce4@MnNiCe4 nanosheets on Ni foam as an advanced electrode for battery-type supercapacitor applications. J. Energy Storage 2022, 51, 104542. [Google Scholar] [CrossRef]
- Harandi, M.H.; Nooshabadi, M.S.; Darabi, R. Simultaneous determination of citalopram and selegiline using an efficient electrochemical sensor based on ZIF-8 decorated with RGO and g-C3N4 in real samples. Anal. Chim. Acta 2022, 1203, 339662. [Google Scholar] [CrossRef]




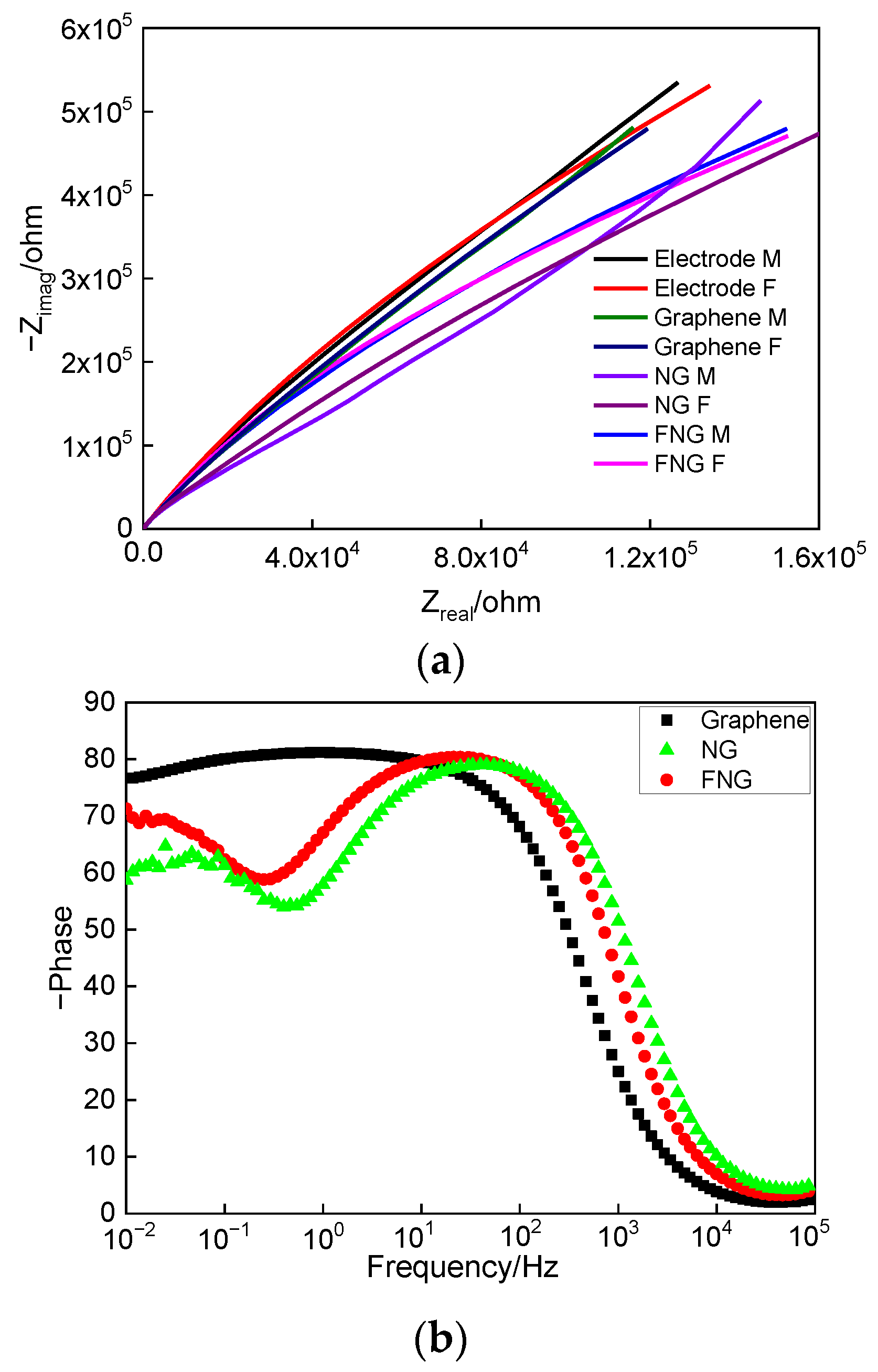
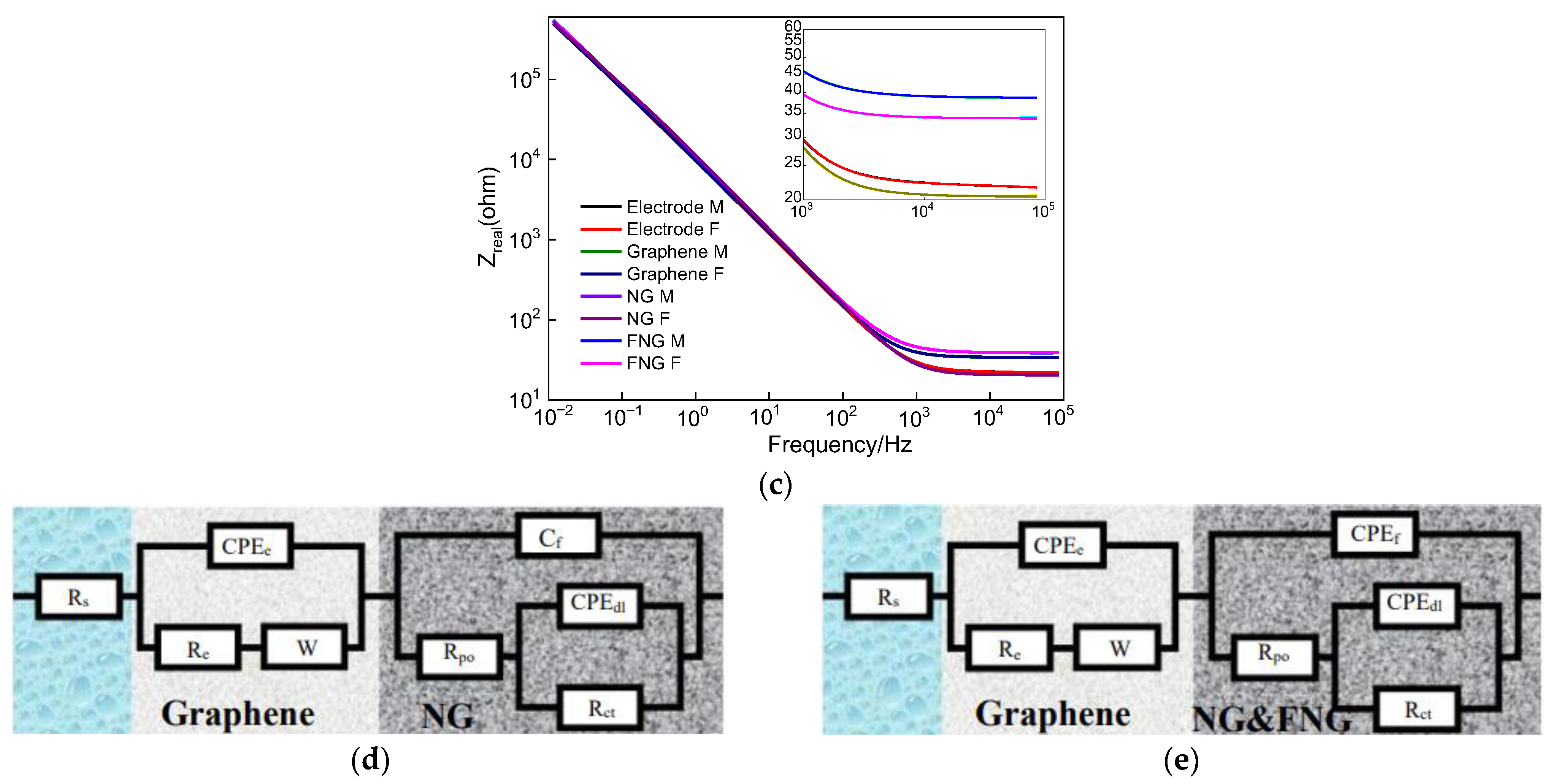
| Parameter | Electrode | Graphene | NG | FNG |
|---|---|---|---|---|
| Solution resistance (Rs)/Ω | 3.861 × 10 | 3.382 × 10 | 6.131 | 1.022 |
| CPEe, Yo (S × secn) | 1.861 × 10−5 | 2.055 × 10−5 | 1.689 × 10−5 | 2.013 × 10−5 |
| Freq power (n)/0 < n < 1 | 0.905 | 0.925 | 0.946 | 0.922 |
| Electrode resistance Re/Ω | 3.866 × 106 | 6.381 × 105 | 1.766 × 104 | 1.506 × 106 |
| Warburg resistance/Ω | 2.236 × 10−6 | 1.363 × 10−6 | 2.600 × 10−6 | 3.455 × 10−6 |
| CPEf, Yo (S × secn) | 7.691 × 10−9 | 4.238 × 10−8 | ||
| Freq power (n)/0 < n < 1 | 1 | 0.859 | ||
| Capacitance F | 7.758 × 10−5 | |||
| Pore resistance Rpo/Ω | 9.926 × 10 | 1.430 × 10 | 2.99 × 10 | |
| CPEdl, Yo (S × secn) | 0.215 × 10−3 | 0.128 × 10−3 | 0.270 × 10−4 | |
| Freq power (n)/0 < n < 1 | 0.565 | 0.926 | 0.893 | |
| Charge transfer resistance Rct/Ω | 1.809 × 104 | 7.584 × 103 | 1.768 × 103 | |
| χ2 (10−3) | 8.800 × 10−2 | 2.486 × 10−2 | 3.524 × 10−1 | 3.280 × 10−2 |
| Solution resistance (Rs)/Ω | 3.861 × 10 | 3.382 × 10 | 6.131 | 1.022 |
| CPEe, Yo (S × secn) | 1.861 × 10−5 | 2.055 × 10−5 | 1.689 × 10−5 | 2.013 × 10−5 |
| Freq power (n)/0 < n < 1 | 0.905 | 0.925 | 0.946 | 0.922 |
| Electrode resistance Re/Ω | 3.866 × 106 | 6.381 × 105 | 1.766 × 104 | 1.506 × 106 |
| Warburg resistance/Ω | 2.236 × 10−6 | 1.363 × 10−6 | 2.600 × 10−6 | 3.455 × 10−6 |
| CPEf, Yo (S × secn) | 7.691 × 10−9 | 4.238 × 10−8 | ||
| Freq power (n)/0 < n < 1 | 1 | 0.859 | ||
| Capacitance F | 7.758 × 10−5 | |||
| Pore resistance Rpo/Ω | 9.926 × 10 | 1.430 × 10 | 2.99 × 10 | |
| CPEdl, Yo (S × secn) | 0.215 × 10−3 | 0.128 × 10−3 | 0.270 × 10−4 | |
| Freq power (n)/0 < n < 1 | 0.565 | 0.926 | 0.893 | |
| Charge transfer resistance Rct/Ω | 1.809 × 104 | 7.584 × 103 | 1.768 × 103 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Lu, Y.; Zhang, C. Superhydrophobic and Electrochemical Performance of CF2-Modified g-C3N4/Graphene Composite Film Deposited by PECVD. Nanomaterials 2022, 12, 4387. https://doi.org/10.3390/nano12244387
Li D, Lu Y, Zhang C. Superhydrophobic and Electrochemical Performance of CF2-Modified g-C3N4/Graphene Composite Film Deposited by PECVD. Nanomaterials. 2022; 12(24):4387. https://doi.org/10.3390/nano12244387
Chicago/Turabian StyleLi, Dayu, Yuling Lu, and Chao Zhang. 2022. "Superhydrophobic and Electrochemical Performance of CF2-Modified g-C3N4/Graphene Composite Film Deposited by PECVD" Nanomaterials 12, no. 24: 4387. https://doi.org/10.3390/nano12244387
APA StyleLi, D., Lu, Y., & Zhang, C. (2022). Superhydrophobic and Electrochemical Performance of CF2-Modified g-C3N4/Graphene Composite Film Deposited by PECVD. Nanomaterials, 12(24), 4387. https://doi.org/10.3390/nano12244387






