Highly Efficient Antibacterial Polymer Composites Based on Hydrophobic Riboflavin Carbon Polymerized Dots
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of RF-CPDs and RF-CPDs Composites
2.3. Characterization of the RF-CPDs and RF-CPD Composites
2.4. Chemical Composition of RF-CPDs
2.5. UV-Vis and PL Measurements of RF-CPDs and RF-CPD/PU Composites
2.6. Reactive Oxygen Species Generation of RF-CPDs and RF-CPD/PU Composites
2.6.1. Singlet Oxygen Luminescence at 1270 nm Measurements
2.6.2. EPR Measurements RF-CPDs and RF-CPD/PU Composites
2.7. Photocatalytic Activity of the RF-CPD/PU Composites
2.8. Contact Angle Measurement of the RF-CPD/PU Composites
2.9. Antibacterial Testing of the RF-CPD/PU Composites
2.10. Cytotoxicity Assay of RF-CPD/PU Composites
3. Results
3.1. Solubility of RF-CPDs
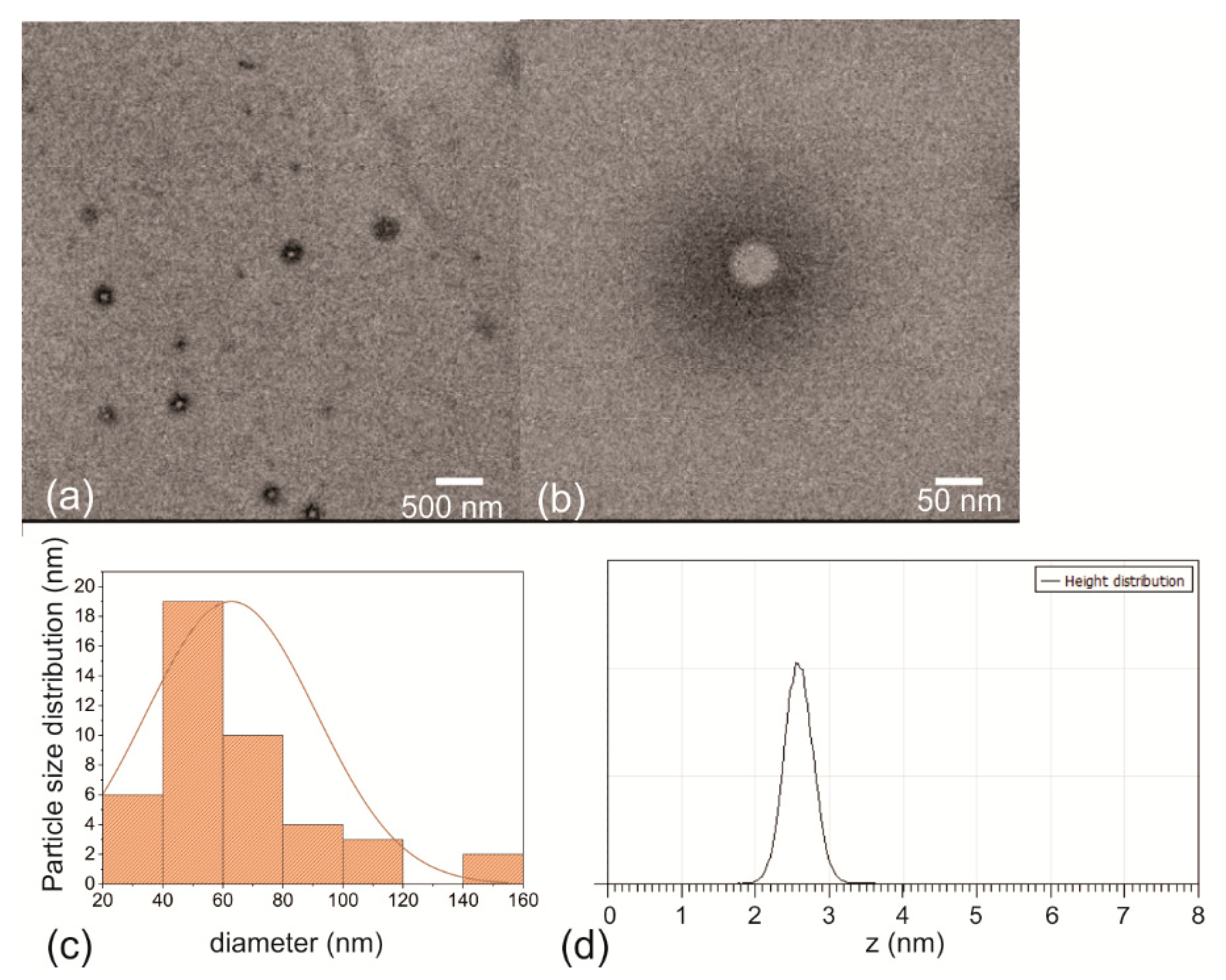
3.2. Surface Morphology of the RF-CPDs and RF-CPD/PU Composites
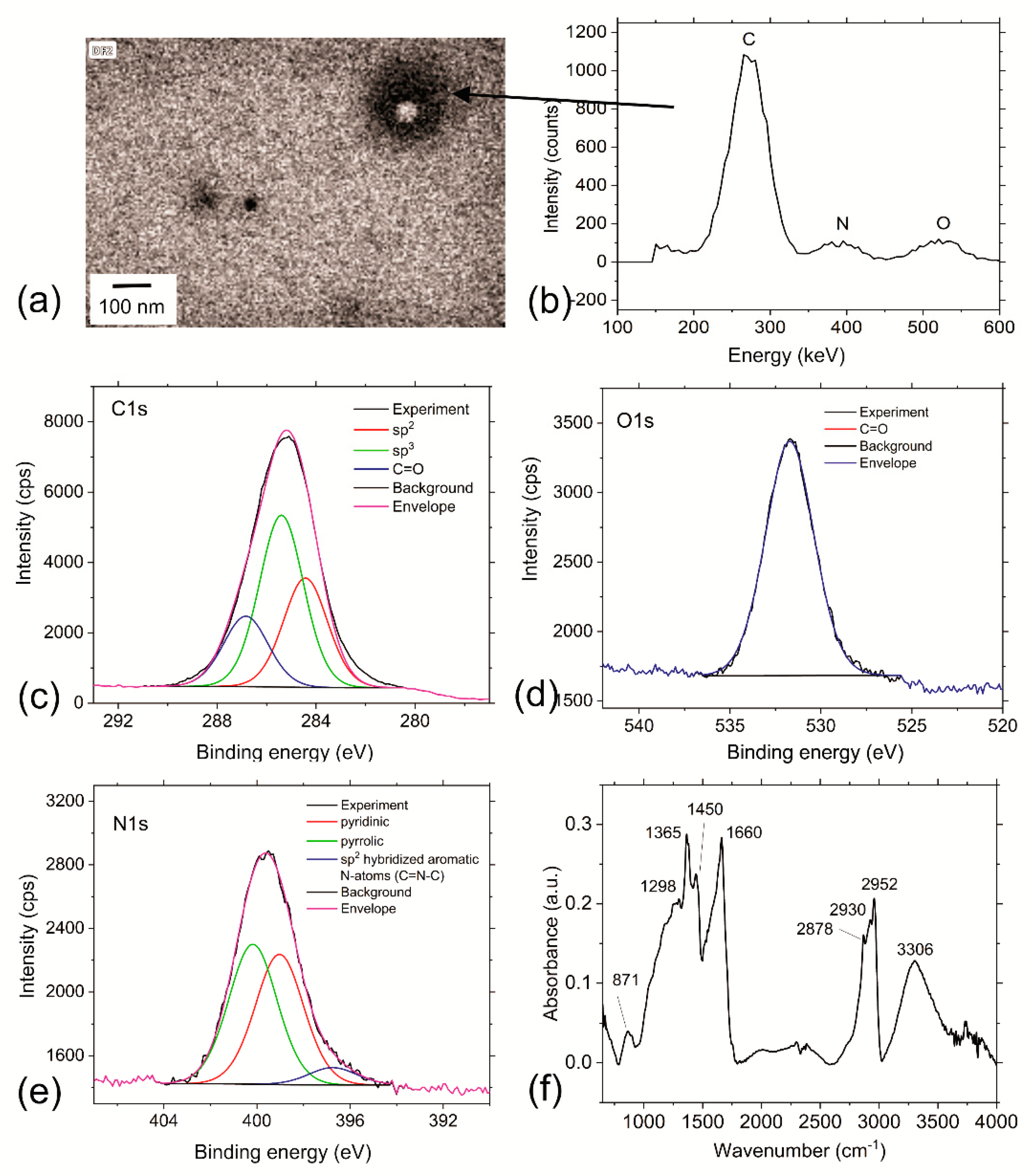
3.3. Chemical Composition of RF-CPDs
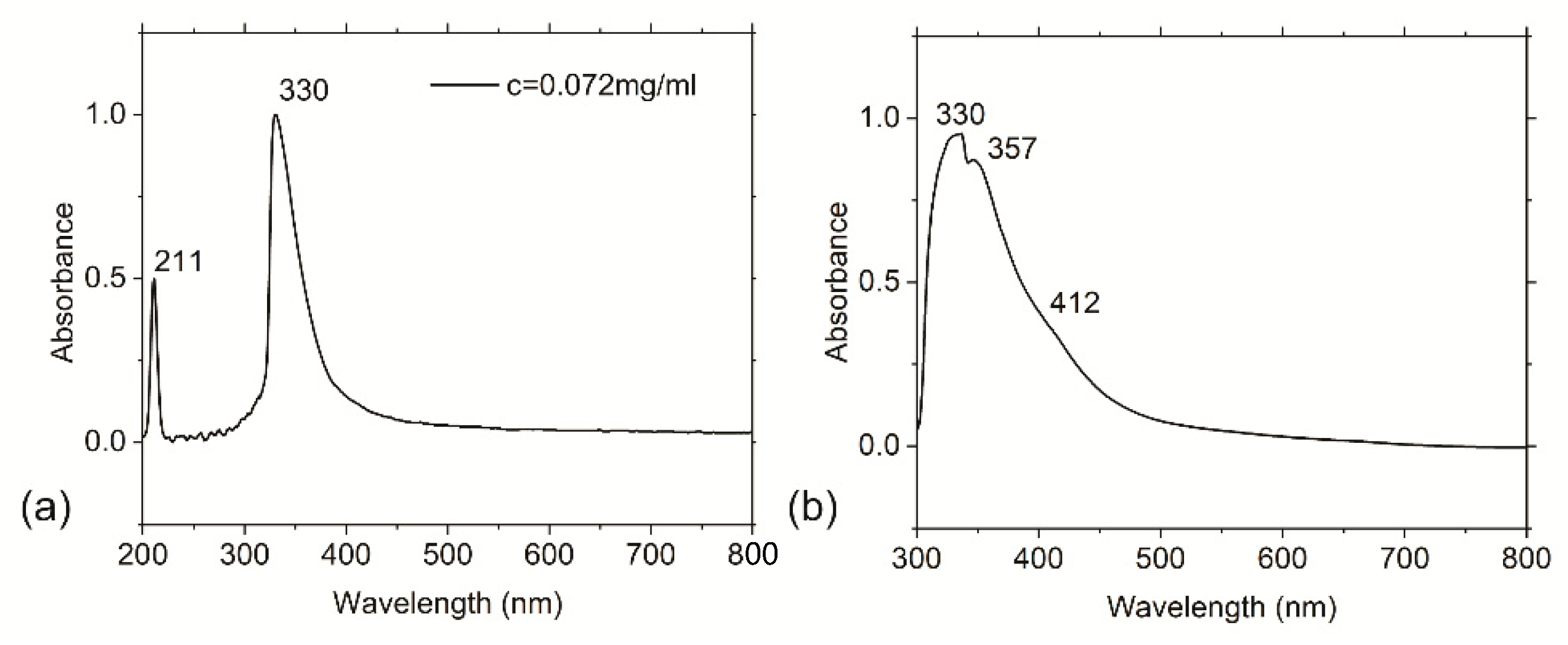
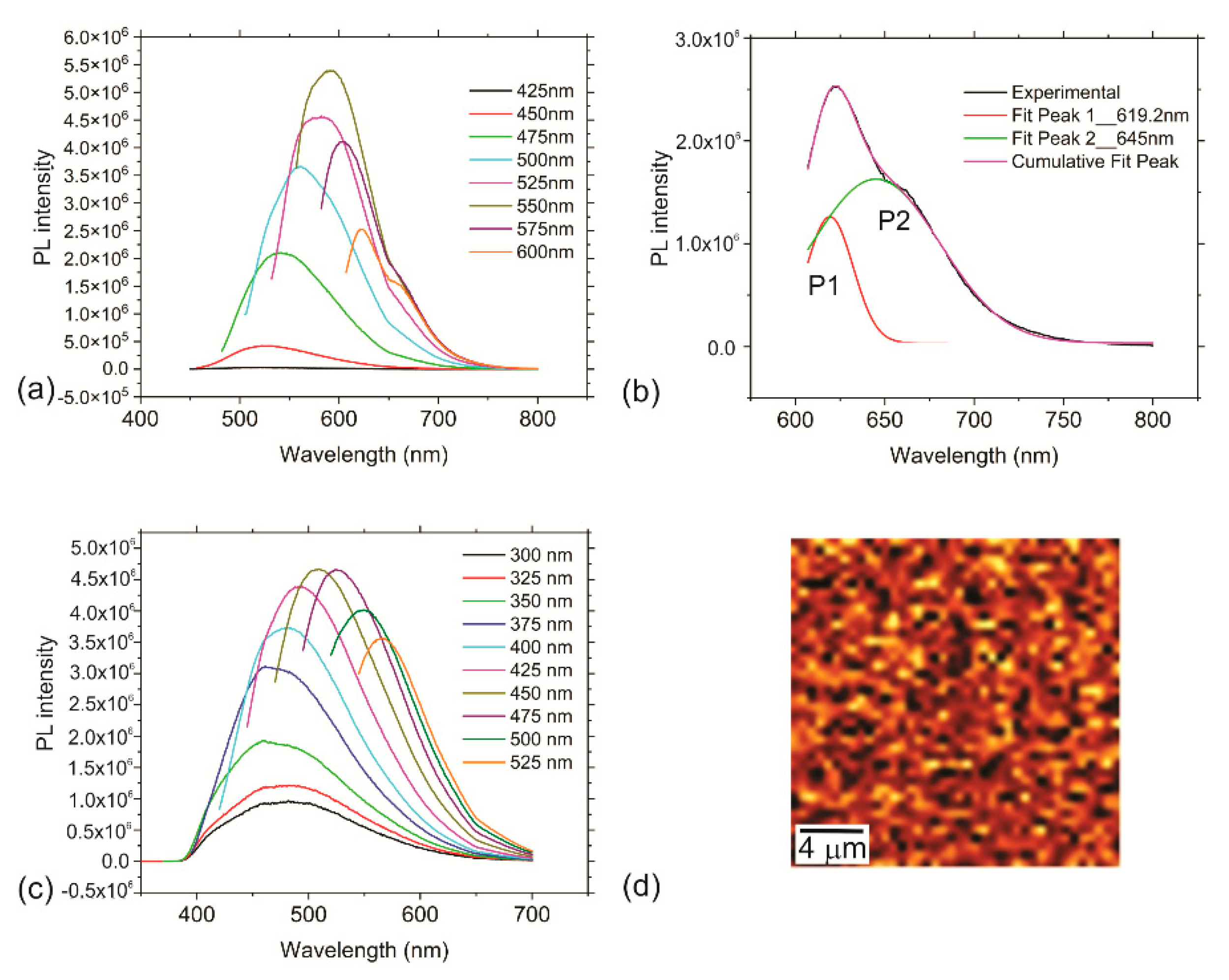
3.4. Optical Properties of the RF-CPDs and RF-CPD/PU Composites
3.5. Reactive Oxygen Species Production of RF-CPDs and RF-CPD/PU Composites
3.6. Photocatalytic Activity of RF-CPD/PU Composites
3.7. Contact Angle of the PU and RF-CPD/PU Composites
3.8. Antibacterial Testing of RF-CPDs/PU Composites
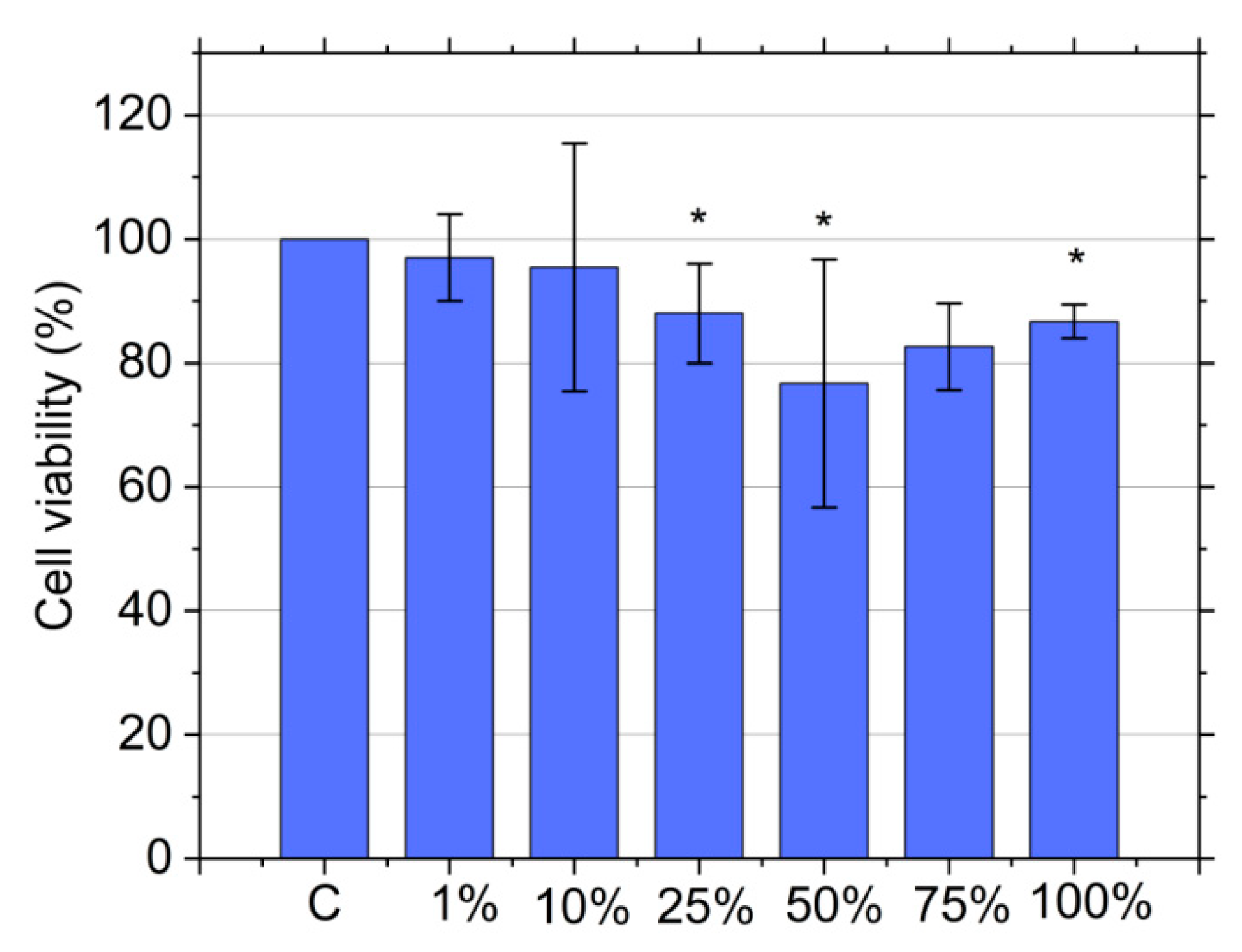
3.9. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Curtis, A.; Moore, Z.; Patton, D.; O’Connor, T.; Nugent, L. Does using a cellular mobile phone increase the risk of nosocomial infections in the neonatal intensive care unit: A systematic review. J. Neonatal Nurs. 2018, 24, 247–252. [Google Scholar] [CrossRef]
- Pal, S.; Juyal, D.; Adekhandi, S.; Sharma, M.; Prakash, R.; Sharma, N.; Rana, A.; Parihar, A. Mobile phones: Reservoirs for the transmission of nosocomial pathogens. Adv. Biomed. Res. 2015, 4, 144. [Google Scholar] [CrossRef] [PubMed]
- Al Momani, W.; Khatetbeh, M.; Altaany, Z. Antibiotic susceptibility of bacterial pathogens recovered from the hand and mobile phones of university students. Germs 2019, 9, 9–16. [Google Scholar] [CrossRef]
- Brady, R.R.; Hunt, A.C.; Visvanathan, A.; Rodrigues, M.A.; Graham, C.; Rae, C.; Kalima, P.; Peterson, H.M.; Gibb, A.P. Mobile phone technology and hospitalized patients: A cross-sectional surveillance study of bacterial colonization, and patient opinions and behaviours. Clin. Microbiol. Infect. 2011, 17, 830–835. [Google Scholar] [CrossRef]
- Shah, P.D.; Shaikh, N.M.; Dholaria, K.V. Microorganisms isolated from mobile phones and hands of health-care workers in a tertiary care hospital of Ahmedabad, Gujarat, India. Indian J. Public Health 2019, 63, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.publichealthontario.ca/-/media/documents/a/2018/at-a-glance-ipac-pss-disinfectant-tables.pdf?la=en (accessed on 29 October 2022).
- Abreu, A.C.; Tavares, R.R.; Borges, A.; Mergulhao, F.; Simoes, M. Current and emergent strategies for disinfection of hospital enviroments. J. Antimicrob. Chemother. 2013, 68, 2718–2732. [Google Scholar] [CrossRef]
- Marković, Z.M.; Kováčová, M.; Humpolíček, P.; Budimir, M.D.; Vajďákc, J.; Kubát, P.; Mičušík, M.; Švajdlenková, H.; Danko, M.; Capáková, Z.; et al. Antibacterial photodynamic activity of carbon quantum dots/polydimethylsiloxane nanocomposites against Staphylococcus aureus, Escherichia coli and Klebsiella pneumonia. Photodiagn. Photodyn. Ther. 2019, 26, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Marković, Z.; Kováčová, M.; Mičušík, M.; Danko, M.; Švajdlenková, H.; Kleinová, A.; Humpolíček, P.; Lehocký, M.; Todorović Marković, B.; Špitalský, Z. Structural, mechanical, and antibacterial features of curcumin/polyurethane nanocomposites. J. Appl. Polym. Sci. 2019, 136, 47283. [Google Scholar] [CrossRef]
- Kováčová, M.; Marković, Z.M.; Humpolíček, P.; Mičušík, M.; Švajdlenková, H.; Kleinová, A.; Danko, M.; Kubát, P.; Vajďák, J.; Capáková, Z.; et al. Carbon quantum dots modified polyurethane nanocomposites as effective photocatalytic and antibacterial agents. ACS Biomater. Sci. Eng. 2018, 4, 3983–3993. [Google Scholar] [CrossRef] [PubMed]
- Kováčová, M.; Špitalská, E.; Markovic, Z.; Špitálský, Z. Carbon quantum dots as antibacterial photosensitizers and their polymer nanocomposite applications. Part. Part. Syst. Char. 2020, 37, 1900348. [Google Scholar] [CrossRef]
- Budimir, M.; Marković, Z.; Vajdak, J.; Jovanović, S.; Kubat, P.; Humpoliček, P.; Mičušik, M.; Danko, M.; Barras, A.; Milivojević, D.; et al. Enhanced visible light-triggered antibacterial activity of carbon quantum dots/polyurethane nanocomposites by gamma rays induced pre-treatment. Radiat. Phys. Chem. 2021, 185, 109499. [Google Scholar] [CrossRef]
- Zmejkoski, D.Z.; Marković, Z.M.; Budimir, M.D.; Zdravković, N.M.; Trišić, D.D.; Bugárová, N.; Danko, M.; Kozyrovska, N.O.; Špitalský, Z.; Kleinová, A.; et al. Photoactive and antioxidant nanochitosan dots/biocellulose hydrogels for wound healing treatment. Mater. Sci. Eng. C 2021, 122, 111925. [Google Scholar] [CrossRef] [PubMed]
- Stanković, N.K.; Bodik, M.; Šiffalovic, P.; Kotlar, M.; Micusik, M.; Spitalsky, Z.; Danko, M.; Milivojević, D.D.; Kleinova, A.; Kubat, P.; et al. Antibacterial and antibiofouling properties of light triggered fluorescent hydrophobic carbon quantum dots Langmuir−Blodgett thin films. ACS Sustain. Chem. Eng. 2018, 6, 4154–4163. [Google Scholar] [CrossRef]
- Available online: https://www.fao.org/fileadmin/user_upload/jecfa_additives/docs/Monograph1/Additive-377.pdf (accessed on 29 October 2022).
- Shimizu, R.; Yagi, M.; Kikuch, A. Suppression of riboflavin-sensitized singlet oxygen generation by l-ascorbic acid, 3-o-ethyl-l-ascorbic acid and trolox. J. Photochem. PhotoBiol. B 2019, 191, 116–122. [Google Scholar] [CrossRef]
- Knak, A.; Regensburger, J.; Maisch, T.; Bäumler, W. Exposure of vitamins to UVB and UVA radiation generates singlet oxygen. Photochem. Photobiol. Sci. 2014, 13, 820–829. [Google Scholar] [CrossRef]
- Farah, N.; Chin, V.K.; Chong, P.P.; Lim, W.F.; Lim, C.W.; Basir, R.; Chang, S.K.; Lee, T.Y. Riboflavin as a promising antimicrobial agent? A multi-perspective review. Curr. Res. Microb. Sci. 2022, 3, 100111. [Google Scholar] [CrossRef]
- Makdoumi, K.; Bäckman, A.; Mortensen, J.; Crafoord, S. Evaluation of antibacterial efficacy of photo-activated riboflavin using ultraviolet light (UVA). Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 207–212. [Google Scholar] [CrossRef]
- Li, H.; Tan, L.; Chen, B.; Huang, J.; Zeng, Q.; Liu, H.; Zhao, Y.; Wang, J.J. Antibacterial potency of riboflavin-mediated photodynamic inactivation against Salmonella and its influences on tuna quality. LWT 2021, 146, 111462. [Google Scholar] [CrossRef]
- Khan, S.; Rayis, M.P.; Rizvi, A.; Alam, M.M.; Rizvi, M.; Naseem, I. ROS mediated antibacterial activity of photoilluminated riboflavin: A photodynamic mechanism against nosocomial infections. Toxicol. Rep. 2019, 6, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.A.R.; Combs, J.C.; Noguera, G.; Camacho, W.; Wittmann, P.; Walther, R.; Cano, M.; Dick, J.; Behrens, A. Antimicrobial efficacy of riboflavin/UVA combination (365 nm) in vitro for bacterial and fungal isolates: A potential new treatment for infectious keratitis. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3402–3408. [Google Scholar] [CrossRef]
- Tunçcan, Ö.G.; Kalkancı, A.; Unal, E.A.; Abdulmajed, O.; Erdoğan, M.; Dizbay, M.; Çağlar, K. The in vitro effect of antimicrobial photodynamic therapy on Candida and Staphylococcus Biofilms. Turk. J. Med. Sci. 2018, 48, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Ghosh, D.; Vishakha, K.; Das, S.; Mondal, S.; Ganguli, A. Photodynamic antimicrobial chemotherapy (PACT) using riboflavin inhibits the mono and dual species biofilm produced by antibiotic resistant Staphylococcus aureus and Escherichia coli. Photodiag. Photodyn. Therap. 2020, 32, 102002. [Google Scholar] [CrossRef]
- Sun, J.; Peng, W.; Fan, B.; Gan, D.; Li, L.; Liu, P.; Shen, J. Tertiary amines convert 1O2 to H2O2 with enhanced photodynamic antibacterial efficiency. J. Hazard. Mater. 2022, 435, 128948. [Google Scholar] [CrossRef] [PubMed]
- Romanova, Y.; Khaydukov, E.V.; Tolordava, E.R.; Nikolaeva, M.; Solov’ev, A.I.; Panchenko, V.Y.; Borodina, T. Antimicrobial photodynamic activity of hydrophilic riboflavin derivatives. Mol. Genet. Microbiol. Virol. 2021, 36, 176–180. [Google Scholar] [CrossRef]
- Mirshafiee, H.; Sharifi, Z.; Hosseini, S.M.; Yari, F.; Nikbakht, H.; Latifi, H. The effects of ultraviolet light and riboflavin on inactivation of viruses and the quality of platelet concentrates at laboratory scale. Avicenna J. Med. Biotech. 2015, 7, 57–63. [Google Scholar]
- Keil, S.D.; Bowen, R.; Marschner, S. Inactivation of middle east respiratory syndrome coronavirus (Mers-Cov) in plasma products using a riboflavin-based and ultraviolet light-based photochemical treatment. Transfusion 2016, 56, 2948–2952. [Google Scholar] [CrossRef] [PubMed]
- Ragan, I.; Hartson, L.; Pidcoke, H.; Bowen, R.; Goodrich, R. Pathogen reduction of SARS-Cov-2 virus in plasma and whole blood using riboflavin and UV light. PLoS ONE 2020, 15, e0233947. [Google Scholar] [CrossRef]
- Sheraz, M.A.; Kazi, S.H.; Ahmed, S.; Anwar, Z.; Ahmad, I. Photo, thermal and chemical degradation of riboflavin. Beilstein J. Org. Chem. 2014, 10, 1999–2012. [Google Scholar] [CrossRef]
- Moore, W.W.; Ireton, R.C. The photochemistry of riboflavin—V. The photodegradation of isoalloxazines in alcoholic solvents. Photochem. Photobiol. 1977, 25, 347–356. [Google Scholar] [CrossRef]
- Yue, J.; Li, L.; Jiang, C.; Mei, Q.; Dong, W.; Yan, R. Riboflavin-based carbon dots with high singlet oxygen generation for photodynamic therapy. J. Mater. Chem. B 2021, 9, 7972–7978. [Google Scholar] [CrossRef]
- Pei, J.; Zhu, S.; Liu, Y.; Song, Y.; Xue, F.; Xiong, X.; Li, C. Photodynamic effect of riboflavin on chitosan coatings and the application in pork preservation. Molecules 2022, 27, 1355. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Huang, J.; Li, H.; Pan, Y.; Zhu, B.; Zhao, Y.; Liu, H. Chitosan-riboflavin composite film based on photodynamic inactivation technology for antibacterial food packaging. Int. J. Biol. Macromol. 2021, 172, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Orsuwan, A.; Kwon, S.; Bumbudsanpharoke, N.; Ko, S. Novel LDPE-riboflavin composite film with dual function of broad-spectrum light barrier and antimicrobial activity. Food Control 2019, 100, 176–182. [Google Scholar] [CrossRef]
- Zhang, Z.; Pan, B.; Wang, L.; Sun, G. Photoactivities of two vitamin B derivatives and their applications in the perpetration of photoinduced antibacterial nanofibrous membranes. ACS Appl. Bio Mater. 2021, 4, 8584–8596. [Google Scholar] [CrossRef]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and synthesis of carbon dots: From carbon dots to carbonized polymer dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef]
- Tao, S.; Zhu, S.; Feng, T.; Xia, C.; Song, Y.; Yang, B. The polymeric characteristics and photoluminescence mechanism in polymer carbon dots: A review. Mater. Today Chem. 2017, 6, 13–25. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, S.; Zhang, S.; Fu, Y.; Wang, L.; Zhao, X.; Yang, B. Investigation from chemical structure to photoluminescent mechanism: A type of carbon dots from the pyrolysis of citric acid and an amine. J. Mater. Chem. C 2015, 3, 5976–5984. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, S.; Shao, J.; Yang, B. Polymer carbon dots-a highlight reviewing their unique structure, bright emission and probable photoluminescence mechanism. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 610–615. [Google Scholar] [CrossRef]
- Ai, L.; Yang, Y.; Wang, B.; Chang, J.; Tang, Z.; Yang, B.; Lu, S. Insights into photoluminescence mechanisms of carbon dots: Advances and perspectives. Sci. Bull. 2021, 66, 839–856. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: Open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Available online: http://www.casaxps.com/berlin/software (accessed on 29 October 2022).
- Martí, C.; Jürgens, O.; Cuenca, O.; Casals, M.; Nonell, S. Aromatic ketones as standards for singlet molecular oxygen O2 (1Δg) photosensitization. Time-resolved photoacoustic and near-IR emission studies. J. Photochem. Photobiol. A 1996, 97, 11–18. [Google Scholar] [CrossRef]
- ISO 22196:2007; Plastics—Measurement of Antibacterial Activity on Plastics Surfaces. ISO Central Secretariat: Vernier, Geneva, 2007. Available online: https://www.iso.org/standard/40759.html (accessed on 29 October 2022).
- Israelachvili, J.N. Intermolecular and Surface Forces; Academic Press: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Tepliakov, N.V.; Kundelev, E.V.; Khavlyuk, P.D.; Xiong, Y.; Leonov, M.Y.; Zhu, W.; Baranov, A.V.; Fedorov, A.V.; Rogach, A.L.; Rukhlenko, I.D. sp2−sp3-hybridized atomic domains determine optical features of carbon dots. ACS Nano 2019, 13, 10737–10744. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Rong, M.; Li, S.; Song, X.; Zhong, Y.; Yan, J.; Wang, Y.; Chen, X. A facile synthesis of highly luminescent nitrogen-doped graphene quantum dots for the detection of 2,4,6-trinitrophenol in aqueous solution. Nanoscale 2015, 7, 1872–1878. [Google Scholar] [CrossRef] [PubMed]
- Marković, Z.M.; Jovanović, S.P.; Mašković, P.Z.; Danko, M.; Mičušik, M.; Pavlović, V.B.; Milivojević, D.D.; Kleinova, A.; Špitalsky, Z.; Todorović Marković, B.M. Photo-induced antibacterial activity of four graphene based nanomaterials on a wide range of bacteria. RSC Adv. 2018, 8, 31337. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, J.; Yuan, Y.; Li, L.; Zhang, M.; Zhou, C.; Lin, Z. A rapid microwave-assisted thermolysis route to highly crystalline carbon nitrides for efficient hydrogen generation. Angew. Chem. 2016, 128, 14913–14917. [Google Scholar] [CrossRef]
- IR Spectrum Table & Chart. Available online: https://www.sigmaaldrich.com/RS/en/technical-documents/technical-article/analytical-chemistry/photometry-and-reflectometry/ir-spectrum-table (accessed on 29 October 2022).
- Table of Characteristic IR Absorptions. Available online: https://ih.pmf.ukim.edu.mk (accessed on 29 October 2022).
- Wang, X.; Qu, K.; Xu, B.; Ren, J.; Qu, X. Microwave assisted one-step green synthesis of cell-permeable multicolor photoluminescent carbon dots with-out surface passivation reagents. J. Mater. Chem. 2011, 21, 2445–2450. [Google Scholar] [CrossRef]
- Ionita, G.; Matei, I. Application of Riboflavin Photochemical Properties in Hydrogel Synthesis. In Hydrogels, Microgels and Nanogels; IntechOpen: Rijeka, Croatia, 2019; pp. 1–14. [Google Scholar] [CrossRef]
- Gao, Y.; Jiao, Y.; Lu, W. Carbon dots with red emission as a fluorescent and colorimetric dual-readout probe for the detection of chromium (VI) and cysteine and its logic gate operation. J. Mater. Chem. B 2018, 6, 6099–6107. [Google Scholar] [CrossRef]
- Langer, M.; Paloncýová, M.; Medved, M.; Pykal, M.; Nachtigallová, D.; Shid, B.; Aquino, A.J.A.; Lischka, H.; Otyepka, M. Progress and challenges in understanding of photoluminescence properties of carbon dots based on theoretical computations. Appl. Mater. Today 2021, 22, 100924. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, L.; Zhou, N.; Zhao, X.; Song, Y.; Maharjan, S.; Zhang, J.; Lu, L.; Wang, H.; Yang, B. The crosslink enhanced emission (CEE) in non-conjugated polymer dots: From the photoluminescence mechanism to the cellular uptake mechanism and internalization. Chem. Commun. 2014, 50, 13845–13848. [Google Scholar] [CrossRef]
- Grodowski, M.S.; Veyret, B.; Weiss, K. Photochemistry of flavins-II. Photophysical properties of alloxazines and isoalloxazines. Photochem. Photobiol. 1977, 26, 341–352. [Google Scholar] [CrossRef]
- Weber, G. Fluorescence of riboflavin and flavin adenine dinucleotide. Biochem. J. 1950, 47, 114–121. [Google Scholar] [CrossRef]
- Naman, S.A.; Tegnér, L. Decay kinetics of the triplet excited state of lumiflavin. Photochem. Photobiol. 1986, 43, 331–333. [Google Scholar] [CrossRef]
- Wen, X.; Yu, P.; Toh, Y.R.; Hao, X.; Tang, J. Intrinsic and extrinsic fluorescence in carbon nanodots: Ultrafast time-resolved fluorescence and carrier dynamics. Adv. Opt. Mater. 2013, 1, 173–178. [Google Scholar] [CrossRef]
- Ge, J.; Lan, M.; Zhou, B.; Liu, W.; Guo, L.; Wang, H.; Jia, Q.; Niu, G.; Huang, X.; Zhou, H.; et al. A Graphene quantum dot photodynamic therapy agent with high singlet oxygen generation. Nat. Commun. 2014, 5, 4596. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, F.; Helman, W.P.; Ross, A.B. Quantum yields for the photosensitized formation of the lowest electronically excited singlet state of molecular oxygen in solution. J. Phys. Chem. Ref. Data 1993, 22, 113–262. [Google Scholar] [CrossRef]
- Chong, Y.; Ge, C.; Fang, G.; Tian, X.; Ma, X.; Wen, T.; Wamer, W.G.; Chen, C.; Chai, Z.; Yin, J.J. Crossover between anti- and pro-oxidant activities of graphene quantum dots in the absence or presence of light. ACS Nano 2016, 10, 8690–8699. [Google Scholar] [CrossRef]
- Pirzada, T.; Kanhar, F.H.; Munsif, M.; Talpur, A.; Chandio, W.A.; Jumani, Z.; Chandio, I.A. An effective photocatalytic degradation of Rose Bengal and Eosin Y by using ZnO nanoparticles as a photocatalysts. Ann. Rom. Soc. Cell Biol. 2021, 25, 1473–1488. [Google Scholar]
- Varghese, M.; Balachandran, M. Antibacterial efficiency of carbon dots against gram-positive and gram-negative bacteria: A review. J. Environ. Chem. Eng. 2021, 9, 106821. [Google Scholar] [CrossRef]
- Bianco, G.V.; Sacchetti, A.; Grande, M.; D’Orazio, A.; Milella, A.; Bruno, G. Effective hole conductivity in nitrogen-doped CVD-graphene by singlet oxygen treatment under photoactivation conditions. Sci. Rep. 2022, 12, 8703. [Google Scholar] [CrossRef]
- Jost, V. Packaging related properties of commercially available biopolymers—An overview of the status quo. eXPRESS Polym. Lett. 2018, 12, 429–435. [Google Scholar] [CrossRef]
- Park, H.Y.; Sin, D.D. Stress-induced premature senescence: Another mechanism involved in the process of accelerated aging in chronic obstructive pulmonary disease in inflammation. In Advancing Age and Nutrition, 1st ed.; Rahman, I., Bagchi, D., Eds.; Elsevier: London, UK, 2013; Chapter 16; pp. 193–194. [Google Scholar] [CrossRef]
- Rieske, P.; Krynska, B.; Azizi, A.S. Human fibroblast-derived cell lines have characteristics of embryonic stem cells and cells of neuro-ectodermal origin. Differentiation 2005, 73, 474–483. [Google Scholar] [CrossRef] [PubMed]

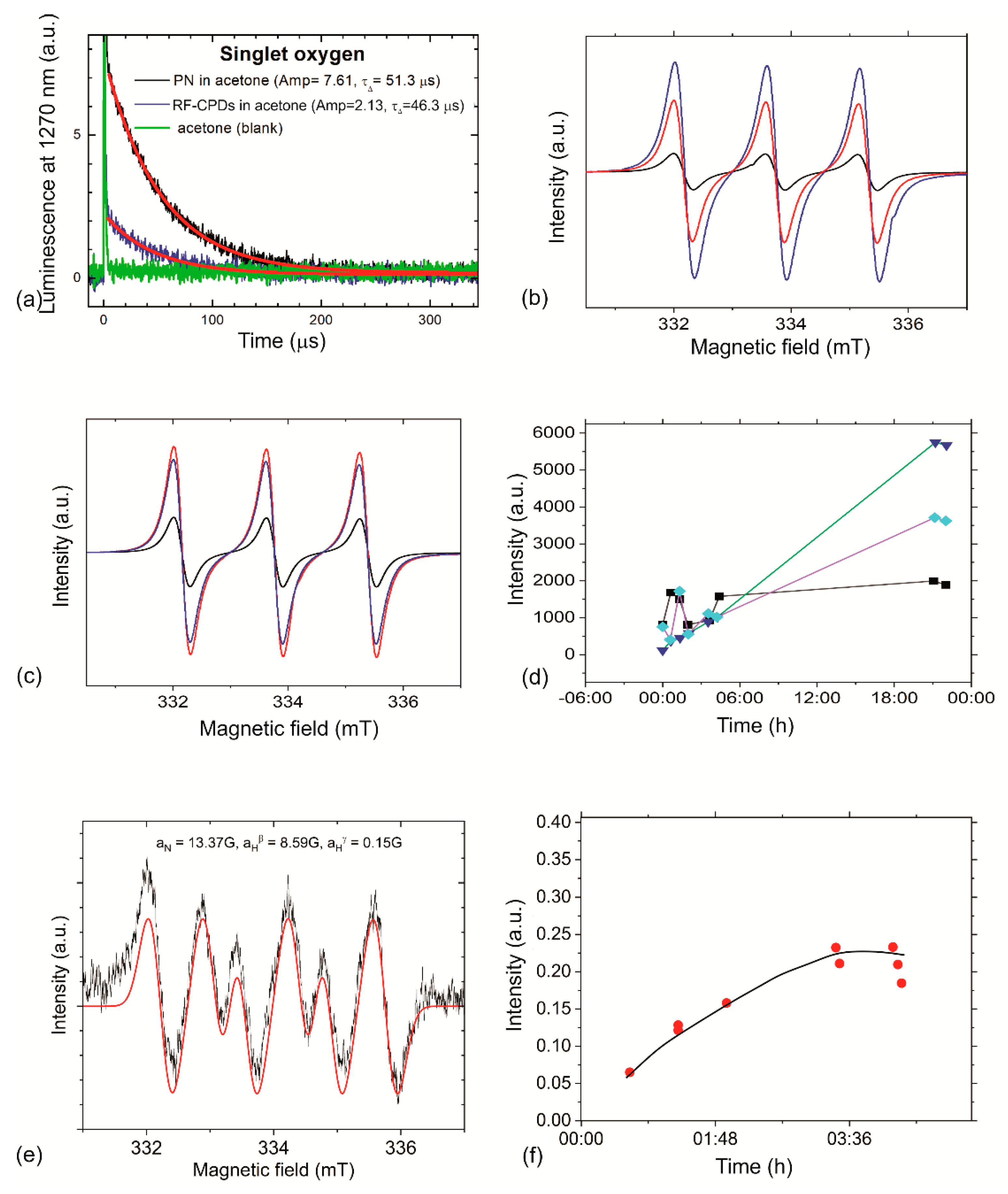
| Compound | Solvent | Amp | τΔ (μs) | τΔ (μs) [63] | ΦΔ |
|---|---|---|---|---|---|
| Phenalenone (PN) | Acetone | 7.61 | 51.3 | 30–65 | 1.00 [44] |
| RF-CPDs | Acetone | 2.13 | 46.3 | 0.28 |
| BL (min) | E. coli | S. aureus | ||
|---|---|---|---|---|
| N * (CFU/cm2) | R | N (CFU/cm2) | R | |
| 30 | 1.7 × 103 | 1.4 | 1.2 × 104 | 0.3 |
| 60 | 0.9 × 103 | 1.6 | 3.8 × 103 | 0.8 |
| 90 | 0.3 × 103 | 2.1 | 1.8 × 103 | 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marković, Z.M.; Kováčová, M.; Jeremić, S.R.; Nagy, Š.; Milivojević, D.D.; Kubat, P.; Kleinová, A.; Budimir, M.D.; Mojsin, M.M.; Stevanović, M.J.; et al. Highly Efficient Antibacterial Polymer Composites Based on Hydrophobic Riboflavin Carbon Polymerized Dots. Nanomaterials 2022, 12, 4070. https://doi.org/10.3390/nano12224070
Marković ZM, Kováčová M, Jeremić SR, Nagy Š, Milivojević DD, Kubat P, Kleinová A, Budimir MD, Mojsin MM, Stevanović MJ, et al. Highly Efficient Antibacterial Polymer Composites Based on Hydrophobic Riboflavin Carbon Polymerized Dots. Nanomaterials. 2022; 12(22):4070. https://doi.org/10.3390/nano12224070
Chicago/Turabian StyleMarković, Zoran M., Mária Kováčová, Sanja R. Jeremić, Štefan Nagy, Dušan D. Milivojević, Pavel Kubat, Angela Kleinová, Milica D. Budimir, Marija M. Mojsin, Milena J. Stevanović, and et al. 2022. "Highly Efficient Antibacterial Polymer Composites Based on Hydrophobic Riboflavin Carbon Polymerized Dots" Nanomaterials 12, no. 22: 4070. https://doi.org/10.3390/nano12224070
APA StyleMarković, Z. M., Kováčová, M., Jeremić, S. R., Nagy, Š., Milivojević, D. D., Kubat, P., Kleinová, A., Budimir, M. D., Mojsin, M. M., Stevanović, M. J., Annušová, A., Špitalský, Z., & Todorović Marković, B. M. (2022). Highly Efficient Antibacterial Polymer Composites Based on Hydrophobic Riboflavin Carbon Polymerized Dots. Nanomaterials, 12(22), 4070. https://doi.org/10.3390/nano12224070








