A Stable Rechargeable Aqueous Zn–Air Battery Enabled by Heterogeneous MoS2 Cathode Catalysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Material Preparation
2.3. Material Characterization
2.4. Electrode Preparation and Battery Assembly
2.5. Electrochemical Test
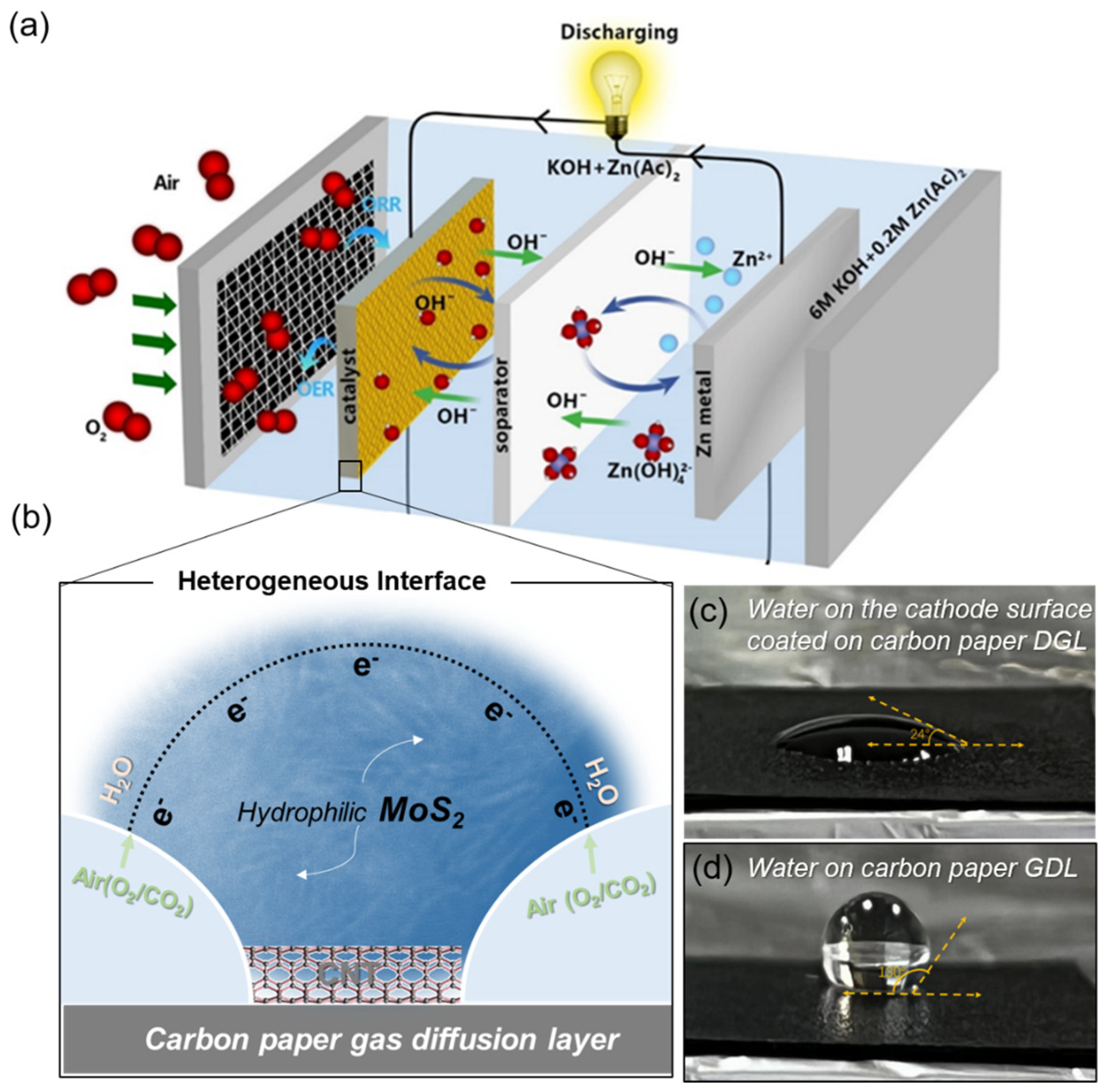
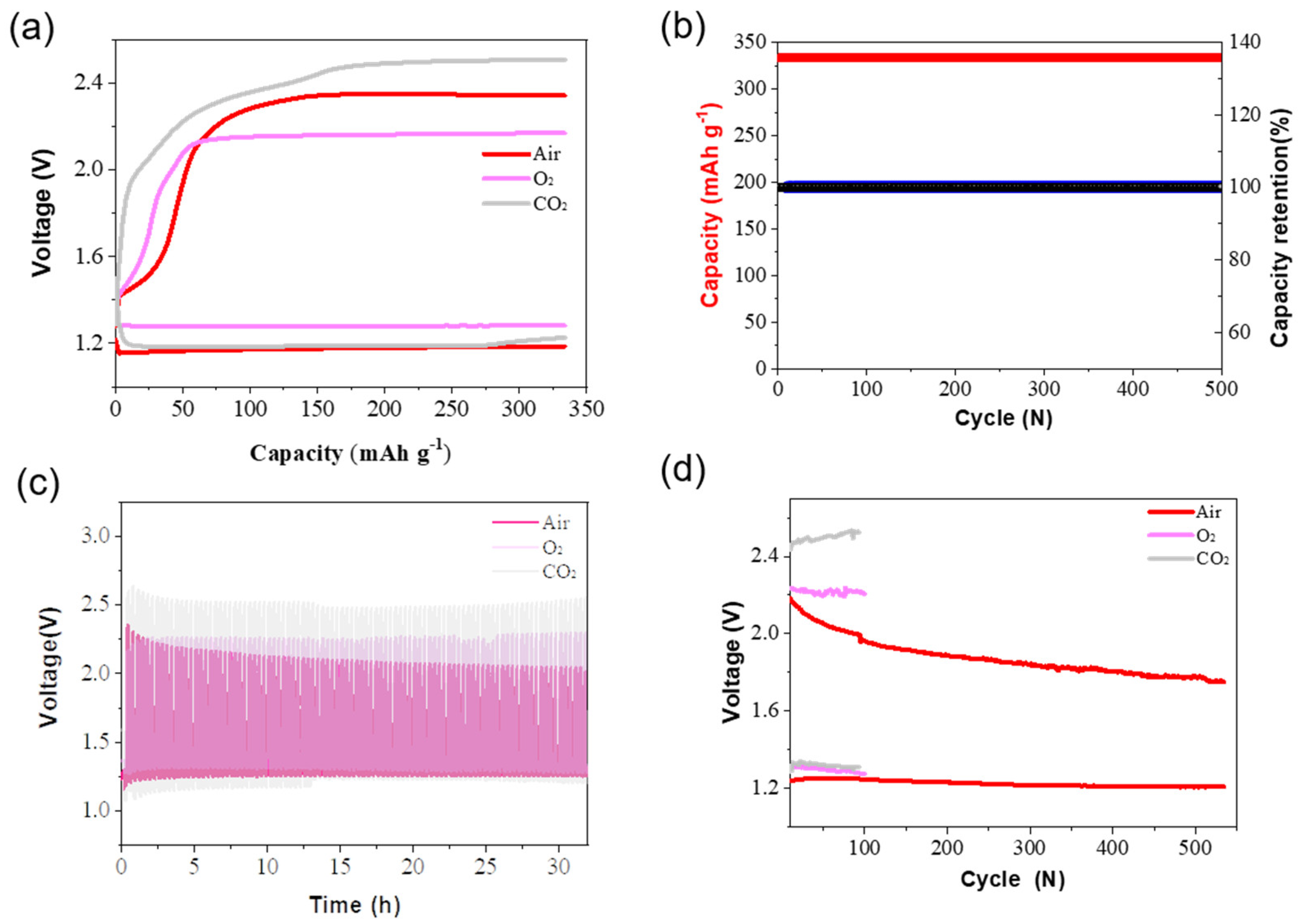
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yi, Z.; Chen, G.; Hou, F.; Wang, L.; Liang, J. Zinc-Ion Batteries: Strategies for the Stabilization of Zn Metal Anodes for Zn-Ion Batteries. Adv. Energy Mater. 2021, 11, 2170001. [Google Scholar] [CrossRef]
- Cao, R.; Lee, J.-S.; Liu, M.; Cho, J. Recent progress in non-precious catalysts for metal-air batteries. Adv. Energy Mater. 2012, 2, 816–829. [Google Scholar] [CrossRef]
- Li, Y.; Dai, H. Recent advances in zinc-air batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, Y.; Ni, Z.; Zhang, Y.; Xu, J.; Kong, T.Y.; Huang, J.H.; Li, W.; Ma, J.; Wang, Y.G. Chemically self-charging aqueous zinc-organic battery. J. Am. Chem. Soc. 2021, 143, 15369–15377. [Google Scholar] [CrossRef]
- Liu, W.; Feng, J.; Wei, T.; Liu, Q.; Zhang, S.; Luo, Y.; Luo, J.; Liu, X. Active-site and interface engineering of cathode materials for aqueous Zn—gas batteries. Nano Res. 2022, 1–22. [Google Scholar] [CrossRef]
- Liu, J.N.; Zhao, C.X.; Ren, D.; Wang, J.; Zhang, R.; Wang, S.H.; Zhao, C.; Li, B.Q.; Zhang, Q. Preconstructing asymmetric interface in air cathodes for high-performance rechargeable Zn—air batteries. Adv. Mater. 2022, 34, 2109407. [Google Scholar] [CrossRef]
- Lee, J.S.; Tai Kim, S.; Cao, R.; Choi, N.S.; Liu, M.; Lee, K.T.; Cho, J. Metal–air batteries with high energy density: Li–air versus Zn–air. Adv. Energy Mater. 2011, 1, 34–50. [Google Scholar] [CrossRef]
- Venezia, E.; Salimi, P.; Chauque, S.; Proietti Zaccaria, R. Sustainable Synthesis of Sulfur-Single Walled Carbon Nanohorns Composite for Long Cycle Life Lithium-Sulfur Battery. Nanomaterials 2022, 12, 3933. [Google Scholar] [CrossRef]
- Glaydson, S.D.R.; Chandrasekar, M.S.; Angélica, D.C.; Sylvia, H.L.; Mikael, T.; Ulla, L.; Flaviano, G.A. Facile Synthesis of Sustainable Activated Biochars with Different Pore Structures as Efficient Additive-Carbon-Free Anodes for Lithium- and Sodium-Ion Batteries. ACS Omega 2022. [Google Scholar] [CrossRef]
- Amiinu, I.S.; Pu, Z.; Liu, X.; Owusu, K.A.; Monestel, H.G.R.; Boakye, F.O.; Zhang, H.; Mu, S. Multifunctional Mo–N/C@MoS2 Electrocatalysts for HER, OER, ORR, and Zn–Air Batteries. Adv. Funct. Mater. 2017, 27, 1702300. [Google Scholar] [CrossRef]
- Liu, Q.; Jin, J.; Zhang, J. NiCo2S4@graphene as a Bifunctional Electrocatalyst for Oxygen Reduction and Evolution Reactions. ACS Appl. Mater. Interfaces 2013, 5, 5002–5008. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.Y.; Yan, Y.; Li, N.; Wu, H.B.; Lou, X.W.; Wang, X. A metal–organic framework-derived bifunctional oxygen electrocatalyst. Nat. Energy 2016, 1, 15006. [Google Scholar] [CrossRef]
- Li, L.; Tsang, Y.C.A.; Xiao, D.; Zhu, G.; Zhi, C.; Chen, Q. Phase-transition tailored nanoporous zinc metal electrodes for rechargeable alkaline zinc-nickel oxide hydroxide and zinc-air batteries. Nat. Commun. 2022, 13, 2870. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Kunimoto, M.; Takanori, M.; Masahiro, Y.; Niikura, J.; Takahashi, I.; Morita, M.; Abe, T.; Homma, M. Carbonate formation on carbon electrode in rechargeable zinc-air battery revealed by in-situ Raman measurements. J. Power Sources 2022, 533, 231237. [Google Scholar] [CrossRef]
- Gauthier, M.; Carney, T.J.; Grimaud, A.; Giordano, L.; Pour, N.; Chang, H.H.; Fenning, D.P.; Lux, S.F.; Paschos, O.; Bauer, C.; et al. Electrode-electrolyte interface in Li-ion batteries: Current understanding and new insights. J. Phys. Chem. Lett. 2015, 6, 4653–4672. [Google Scholar] [CrossRef]
- Xu, Y.; Sumboja, A.; Zong, Y.; Darr, J. Bifunctionally active nanosized spinel cobalt nickel sulfides for sustainable secondary zinc–air batteries: Examining the effects of compositional tuning on OER and ORR activity. Catal. Sci. Technol. 2020, 10, 2173–2182. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, G.; Qiao, J.; Chen, N.; Chen, W.; Sun, S. Ultra-Long Life Rechargeable Zinc–Air Battery Based on High-Performance Trimetallic Nitride and NCNT Hybrid Bifunctional Electrocatalysts. Nano Energy 2019, 61, 86–95. [Google Scholar] [CrossRef]
- Turney, D.; Gallaway, J.; Yadav, G.; Ramirez, R.; Nyce, M.; Banerjee, S.; Chen-Wiegart, Y.; Wang, J.; Ambrose, M.; Kolhekar, S.; et al. Rechargeable Zinc Alkaline Anodes for Long-Cycle Energy Storage. Chem. Mater. 2017, 29, 4819–4832. [Google Scholar] [CrossRef]
- Li, X.; Lin, Z.; Cheng, L.; Chen, X. Layered MoSi2N4 as Electrode Material of Zn–Air Battery. Phys. Status Solidi RRL 2022, 16, 2200007. [Google Scholar] [CrossRef]
- Sivanantham, A.; Ganesan, P.; Shanmugam, S. Hierarchical NiCo2S4 Nanowire Arrays Supported on Ni Foam: An Efficient and Durable Bifunctional Electrocatalyst for Oxygen and Hydrogen Evolution Reactions. Adv. Funct. Mater. 2016, 26, 4661–4672. [Google Scholar] [CrossRef]
- Wagh, N.; Lee, C.; Kim, D.; Kim, S.; Shinde, S.; Lee, J. Heuristic Iron–Cobalt-Mediated Robust pH-Universal Oxygen Bifunctional Lusters for Reversible Aqueous and Flexible Solid-State Zn–Air Cells. ACS Nano 2021, 15, 14683–14696. [Google Scholar] [CrossRef] [PubMed]
- Puglia, M.K.; Malhotra, M.; Kumar, C.V. Engineering functional inorganic nanobiomaterials: Controlling interactions between 2D-nanosheets and enzymes. Dalton Trans. 2020, 49, 3917–3933. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.J.; Wang, Y.; Wang, X.L.; Li, S.L.; Huang, W.; Dong, L.Z.; Liu, C.H.; Li, Y.F.; Lan, Y.Q. Molybdenum Disulfide/Nitrogen-Doped Reduced Graphene Oxide Nanocomposite with Enlarged Interlayer Spacing for Electrocatalytic Hydrogen Evolution. Adv. Energy Mater. 2016, 6, 1600116. [Google Scholar] [CrossRef]
- Prins, R.; Debeer, V.H.J.G.; Somorjai, A. Structure and Function of the Catalyst and the Promoter in Co—Mo Hydrodesulfurization Catalysts. Catal. Rev. Sci. Eng. 1989, 31, 1. [Google Scholar] [CrossRef]
- Bai, J.; Meng, T.; Guo, D.L.; Wang, S.G.; Mao, B.G.; Cao, M.H. Co9S8@MoS2 Core–Shell Heterostructures as Trifunctional Electrocatalysts for Overall Water Splitting and Zn–Air Batteries. ACS Appl. Mater. Interfaces 2018, 10, 1678–1689. [Google Scholar] [CrossRef]
- Puglia, M.K.; Malhotra, M.; Chivukula, A.; Kumar, C.V. “Simple-Stir” Heterolayered MoS2/Graphene Nanosheets for Zn–Air Batteries. ACS Appl. Nano Mater. 2021, 4, 10389–10398. [Google Scholar] [CrossRef]
- Drew, A.A.; Yi, S.; Douglas, G. Zn-Based Oxides Anchored to Nitrogen-Doped Carbon Nanotubes as Efficient Bifunctional Catalysts for Zn–Air Batteries. ChemElectroChem 2020, 7, 2283. [Google Scholar]
- Hu, H.; Xu, J.C.; Zheng, Y.H.; Yao Yao, Z.; Rong, J.; Zhang, T.; Yang, D.Y.; Qiu, F.X. NiS2-Coated Carbon Fiber Paper Decorated with MoS2 Nanosheets for Hydrogen Evolution. ACS Appl. Nano Mater. 2022, 5, 10933–10940. [Google Scholar] [CrossRef]
- Hou, X.B.; Zhou, H.M.; Zhao, M.; Cai, Y.B.; Wei, Q.F. MoS2 Nanoplates Embedded in Co–N-Doped Carbon Nanocages as Efficient Catalyst for HER and OER. ACS Sustain. Chem. Eng. 2020, 8, 5724–5733. [Google Scholar] [CrossRef]
- Li, J.C.; Zhang, C.; Ma, H.J.; Wang, T.H.; Guo, Z.Q.; Yang, Y.; Wang, Y.Y.; Ma, H.X. Modulating interfacial charge distribution of single atoms confined in molybdenum phosphosulfide heterostructures for high efficiency hydrogen evolution. Chem. Eng. J. 2021, 414, 128834. [Google Scholar] [CrossRef]
- Wen, F.S.; Li, Y.J.; Liu, W.L.; Liu, G.D.; Zhang, T.; Xu, Y.; Zhang, X.Y. Comprehensive experiment on simple morphology regulation and photocatalytic degradation of Rhodamine B by molybdenum disulfide. Exp. Technol. Manag. 2022, 39, 157–161. (In Chinese) [Google Scholar]
- Tan, J.W.; Zhang, W.B.; Shu, Y.J.; Lu, H.Y.; Tang, Y.; Gao, Q.S. Interlayer engineering of molybdenum disulfide toward efficient electrocatalytic hydrogenation. Sci. Bull. 2021, 66, 1003–1012. [Google Scholar] [CrossRef]
- Chen, B.; Lu, H.; Zhou, J.; Ye, C.; Shi, C.; Zhao, N.; Qiao, S.-Z. Porous MoS2/Carbon Spheres Anchored on 3D Interconnected Multiwall Carbon Nanotube Networks for Ultrafast Na Storage. Adv. Energy Mater. 2018, 8, 1702909. [Google Scholar] [CrossRef]
- Bau, J.A.; Emwas, A.H.; Nikolaienko, P.; Aljarb, A.A.; Tung, V.; Rueping, M. Mo3+ hydride as the commonorigin of H2 evolution and selective NADH regeneration in molybdenum sulfide electrocatalysts. Nat. Catal. 2022, 5, 397–404. [Google Scholar] [CrossRef]
- Li, L.; Qin, Z.; Ries, L.; Hong, S.; Michel, T.; Yang, J.; Salameh, C.; Bechelany, M.; Miele, P.; Kaplan, D.; et al. Role of Sulfur Vacancies and Undercoordinated Mo Regions in MoS2 Nanosheets toward the Evolution of Hydrogen. ACS Nano 2019, 13, 6824–6834. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; He, X.; Yin, F.; Wang, H.; Liu, D.-J.; Shi, R.; Chen, J.; Yin, H. MO-Co@N-Doped Carbon (M = Zn or Co): Vital Roles of Inactive Zn and Highly Efficient Activity toward Oxygen Reduction/Evolution Reactions for Rechargeable Zn–Air Battery. Adv. Funct. Mater. 2017, 27, 1700795. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X. Recent Advances of Transition Metal Chalcogenides as Cathode Materials for Aqueous Zinc-Ion Batteries. Nanomaterials 2022, 12, 3298. [Google Scholar] [CrossRef]
- Mohammad, A.; Bijandra, K.; Liu, C.; Patrick, P.; Poya, Y.; Amirhossein, B.; Peter, Z.; Robert, F.K.; Larry, A.C.; Amin, S.K. Cathode Based on Molybdenum Disulfide Nanoflakes for Lithium–Oxygen Batteries. ACS Nano 2016, 10, 2167–2175. [Google Scholar]
- Zoya, S.; Liu, J.P.; Zhao, L.; Francesco, C.; Jang-Kyo, K. Metallic MoS2 nanosheets: Multifunctional electrocatalyst for the ORR, OER and Li–O2 batteries. Nanoscale 2018, 10, 22549–22559. [Google Scholar]
- Liu, Y.; Zang, Y.P.; Liu, X.M.; Cai, J.Y.; Lu, Z.; Niu, S.W.; Pei, Z.B.; Zhai, T.; Wang, G.M. Three-Dimensional Carbon-Supported MoS2 with Sulfur Defects as Oxygen Electrodes for Li-O2 Batteries. Nano Energy 2019, 65, 103996. [Google Scholar] [CrossRef]
- Wu, L.F.; Alessandro, L.; Nelson, Y.D.; Akhil, S.; Marco, M.R.M.M.; Ageeth, A.B.; Nora, H.; Emiel, J.M.; Jan, P.H. The Origin of High Activity of Amorphous MoS2 in the Hydrogen Evolution Reaction. ChemSusChem 2019, 12, 4383. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Zhu, B.; Zhang, Z.; Xiang, Y.; Tang, C.; Ding, Q.; Wu, X. The Synthesis of Manganese Hydroxide Nanowire Arrays for a High-Performance Zinc-Ion Battery. Nanomaterials 2022, 12, 2514. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yin, Z.H.; Cui, M.; Gao, L.G.; Liu, A.M.; Su, W.N.; Chen, S.R.; Ma, T.L.; Li, Y.Q. Double shelled hollow CoS2@MoS2@NiS2 polyhedron as advanced trifunctional electrocatalyst for zinc-air battery and self-powered overall water splitting. J. Colloid Interface Sci. 2022, 610, 653–662. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Huang, X.; Yu, Z.; Zhang, P.; Zhai, C.; Song, H.; Xu, J.; Chen, K. A Stable Rechargeable Aqueous Zn–Air Battery Enabled by Heterogeneous MoS2 Cathode Catalysts. Nanomaterials 2022, 12, 4069. https://doi.org/10.3390/nano12224069
Wang M, Huang X, Yu Z, Zhang P, Zhai C, Song H, Xu J, Chen K. A Stable Rechargeable Aqueous Zn–Air Battery Enabled by Heterogeneous MoS2 Cathode Catalysts. Nanomaterials. 2022; 12(22):4069. https://doi.org/10.3390/nano12224069
Chicago/Turabian StyleWang, Min, Xiaoxiao Huang, Zhiqian Yu, Pei Zhang, Chunyang Zhai, Hucheng Song, Jun Xu, and Kunji Chen. 2022. "A Stable Rechargeable Aqueous Zn–Air Battery Enabled by Heterogeneous MoS2 Cathode Catalysts" Nanomaterials 12, no. 22: 4069. https://doi.org/10.3390/nano12224069
APA StyleWang, M., Huang, X., Yu, Z., Zhang, P., Zhai, C., Song, H., Xu, J., & Chen, K. (2022). A Stable Rechargeable Aqueous Zn–Air Battery Enabled by Heterogeneous MoS2 Cathode Catalysts. Nanomaterials, 12(22), 4069. https://doi.org/10.3390/nano12224069







