Gold Nanoparticles Modification with Liquid Crystalline Polybenzylic Dendrons via 1,3-Dipolar Cycloaddition
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Equipment
2.2. Synthesis of Alkyne Focal-Point Fréchet-Type Dendrons
2.3. Synthesis of AuDT, AuDT-AT and AuDT-AT-TA@L3-3,4,5 Gold Nanoparticles
2.3.1. Synthesis of AuDT Pristine Nanoparticles
2.3.2. Synthesis AuDT-AT Pristine Nanoparticles by Ligand Exchange Reaction of the AuDT Nanoparticles with the 11-Azidoundecane-1-thiol (AT)
2.3.3. Synthesis of the AuDT-AT-TA@L3-3,4,5 Gold Nanoparticles by Functionalization of AuDT-AT Nanoparticles with Alkynyl Dendritic Ligands by Huisgen 1,3-Dipolar Cycloaddition
3. Results and Discussion
3.1. Characterization of the Optical, Thermal, Thermodynamic Properties and Mesogenic Behavior of Alkyne Focal-Point Fréchet-Type Dendrons
3.2. Characterization of AuDT and AuDT-AT Gold Nanoparticles
3.3. Characterization of AuDT-AT-TA@Ln Gold Nanoparticles
3.4. Isothermal Treatment of the Gold Nanoparticles
3.4.1. NMR Studies
3.4.2. UV-Vis Study of the Thermally-Treated Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Percec, V.; Kawasumi, M. Synthesis and characterization of a thermotropic nematic liquid crystalline dendrimeric polymer. Macromolecules 1992, 25, 3843–3850. [Google Scholar] [CrossRef]
- Ponomarenko, S.A.; Rebrov, E.A.; Boiko, N.I.; Vasilenko, N.G.; Muzafarov, A.M.; Freidzon, Y.S.; Shibaev, V.P. Synthesis of cholesterol-containing polyorganosiloxane dendrimers. Polym. Sci. A 1994, 3, 896–901. [Google Scholar]
- Percec, V.; Glodde, M.; Bera, T.K.; Miura, Y.; Shiyanovskaya, I.; Singer, K.D.; Balagurusamy, V.S.K.; Heiney, P.A.; Schnell, I.; Rapp, A.; et al. Self-organization of supramolecular helical dendrimers into complex electronic materials. Nature 2002, 419, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Percec, V.; Dulcey, A.E.; Balagurusamy, V.S.K.; Miura, Y.; Smidrkal, J.; Peterca, M.; Nummelin, S.; Edlund, U.; Hudson, S.D.; Heiney, P.A.; et al. Self-assembly of amphiphilic dendritic dipeptides into helical pores. Nature 2004, 430, 764–768. [Google Scholar] [CrossRef]
- Sun, H.-J.; Zhang, S.; Percec, V. From structure to function via complex supramolecular dendrimer systems. Chem. Soc. Rev. 2015, 44, 3900–3923. [Google Scholar] [CrossRef]
- Ponomarenko, S.A.; Boiko, N.I.; Shibaev, V.P.; Richardson, R.M.; Whitehouse, I.J.; Rebrov, E.A.; Muzafarov, A.M. Carbosilane Liquid Crystalline Dendrimers: From Molecular Architecture to Supramolecular Nanostructures. Macromolecules 2000, 33, 5549–5558. [Google Scholar] [CrossRef]
- Donnio, B.; Buathong, S.; Bury, I.; Guillon, D. Liquid crystalline dendrimers. Chem. Soc. Rev. 2007, 36, 1495–1513. [Google Scholar] [CrossRef]
- Hernández-Ainsa, S.; Marcos, M.; Serrano, J.L. Dendrimeric and Hyperbranched Liquid Crystal Structures. In Handbook of Liquid Crystals; Wiley-VCH: Weinheim, Germany, 2014; Volume 7, pp. 1–14. [Google Scholar]
- Guerra, S.; Nguyen, T.L.A.; Furrer, J.; Nierengarten, J.-F.; Barberá, J.; Deschenaux, R. Liquid-Crystalline Dendrimers Designed by Click Chemistry. Macromolecules 2016, 49, 3222–3231. [Google Scholar] [CrossRef]
- Gröhn, F.; Bauer, B.J.; Akpalu, Y.A.; Jackson, C.L.; Amis, E.J. Dendrimer Templates for the Formation of Gold Nanoclusters. Macromolecules 2000, 33, 6042–6050. [Google Scholar] [CrossRef]
- Garcia-Martinez, J.C.; Crooks, R.M. Extraction of Au Nanoparticles Having Narrow Size Distributions from within Dendrimer Templates. J. Am. Chem. Soc. 2004, 126, 16170–16178. [Google Scholar] [CrossRef]
- Scott, R.W.J.; Wilson, O.M.; Crooks, R.M. Synthesis, Characterization, and Applications of Dendrimer-Encapsulated Nanoparticles. J. Phys. Chem. B 2005, 109, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Elbert, K.C.; Lee, J.D.; Wu, Y.; Murray, C.B. Improved Chemical and Colloidal Stability of Gold Nanoparticles through Dendron Capping. Langmuir 2018, 34, 13333–13338. [Google Scholar] [CrossRef] [PubMed]
- Jishkariani, D.; Wu, Y.; Wang, D.; Liu, Y.; van Blaaderen, A.; Murray, C.B. Preparation and Self-Assembly of Dendronized Janus Fe3O4–Pt and Fe3O4–Au Heterodimers. ACS Nano 2017, 11, 7958–7966. [Google Scholar] [CrossRef]
- Avila-Salas, F.; González, R.I.; Ríos, P.L.; Araya-Durán, I.; Camarada, M.B. Effect of the Generation of PAMAM Dendrimers on the Stabilization of Gold Nanoparticles. J. Chem. Inf. Model. 2020, 60, 2966–2976. [Google Scholar] [CrossRef] [PubMed]
- Najafi, F.; Ghasemian, N.; Safari, M.; Salami-Kalajahi, M. Poly(propylene imine) dendrimer as reducing agent for chloroauric acid to fabricate and stabilize gold nanoparticles. J. Phys. Chem. Solids 2021, 148, 109682. [Google Scholar] [CrossRef]
- Ulloa, J.A.; Lorusso, G.; Evangelisti, M.; Camón, A.; Barberá, J.; Serrano, J.L. Magnetism of Dendrimer-Coated Gold Na-noparticles: A Size and Functionalization Study. J. Phys. Chem. C 2021, 125, 20482–20487. [Google Scholar] [CrossRef]
- Won, J.; Ihn, K.J.; Kang, Y.S. Gold Nanoparticle Patterns on Polymer Films in the Presence of Poly(amidoamine) Den-drimers. Langmuir 2002, 18, 8246–8249. [Google Scholar] [CrossRef]
- Gopidas, K.R.; Whitesell, J.K.; Fox, M.A. Metal-Core−Organic Shell Dendrimers as Unimolecular Micelles. J. Am. Chem. Soc. 2003, 125, 14168–14180. [Google Scholar] [CrossRef]
- Gopidas, K.R.; Whitesell, J.K.; Fox, M.A. Synthesis, Characterization, and Catalytic Applications of a Palladi-um-Nanoparticle-Cored Dendrimer. Nano Lett. 2003, 3, 1757–1760. [Google Scholar] [CrossRef]
- Li, D.; Li, J. Fréchet-type dendrons-capped gold clusters. Colloids Surf. A Physicochem. Eng. Asp. 2005, 257, 255–259. [Google Scholar] [CrossRef]
- Love, C.S.; Ashworth, I.; Brennan, C.; Chechik, V.; Smith, D.K. Dendron-protected Au nanoparticles—Effect of dendritic structure on chemical stability. J. Colloid Interface Sci. 2006, 302, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Wang, L.; Chen, W. Studies on the preparation and characterization of gold nanoparticles protected by dendrons. Mater. Lett. 2007, 61, 278–283. [Google Scholar] [CrossRef]
- Kumar, W.K.R.; Gopidas, K.R. Synthesis and Characterization of Gold-Nanoparticle-Cored Dendrimers Stabilized by Metal–Carbon Bonds. Chem. Asian J. 2010, 5, 887–896. [Google Scholar] [CrossRef]
- Enciso, A.E.; Doni, G.; Nifosi, R.; Palazzesi, F.; Gonzalez, R.; Ellsworth, A.A.; Coffer, J.L.; Walker, A.V.; Pavan, G.M.; Mohamed, A.A.; et al. Facile synthesis of stable, water soluble, dendron-coated gold nanoparticles. Nanoscale 2017, 9, 3128–3132. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.-C.; Ruiz, J.; Nlate, S.; Blais, J.-C.; Astruc, D. Nanoscopic Assemblies between Supramolecular Redox Active Metallodendrons and Gold Nanoparticles: Synthesis, Characterization, and Selective Recognition of H2PO4−, HSO4−, and Adenosine-5‘-Triphosphate (ATP2−) Anions. J. Am. Chem. Soc. 2003, 125, 2617–2628. [Google Scholar] [CrossRef] [PubMed]
- Shon, Y.-S.; Choi, D.; Dare, J.; Dinh, T. Synthesis of Nanoparticle-Cored Dendrimers by Convergent Dendritic Functionali-zation of Monolayer-Protected Nanoparticles. Langmuir 2008, 24, 6924–6931. [Google Scholar] [CrossRef] [PubMed]
- Cho, T.J.; Zangmeister, R.A.; MacCuspie, R.I.; Patri, A.K.; Hackley, V.A. Newkome-Type Dendron-Stabilized Gold Na-noparticles: Synthesis, Reactivity, and Stability. Chem. Mater. 2011, 23, 2665–2676. [Google Scholar] [CrossRef]
- Ulloa, J.A.; Barberá, J.; Serrano, J.L. Controlled Growth of Dendrimer-Coated Gold Nanoparticles: A Solvent-Free Process in Mild Conditions. ACS Omega 2021, 6, 348–357. [Google Scholar] [CrossRef]
- Boisselier, E.; Salmon, L.; Ruiz, J.; Astruc, D. How to very efficiently functionalize gold nanoparticles by “click” chemistry. Chem. Commun. 2008, 2008, 5788–5790. [Google Scholar] [CrossRef]
- Mischler, S.; Guerra, S.; Deschenaux, R. Design of liquid-crystalline gold nanoparticles by click chemistry. Chem. Commun. 2012, 48, 2183–2185. [Google Scholar] [CrossRef]
- Kanie, K.; Matsubara, M.; Zeng, X.; Liu, F.; Ungar, G.; Nakamura, H.; Muramatsu, A. Simple Cubic Packing of Gold Na-noparticles through Rational Design of Their Dendrimeric Corona. J. Am. Chem. Soc. 2012, 134, 808–8112. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Albert, S.; Nguyen, T.L.A.; Deschenaux, R. Liquid-crystalline fullerene-gold nanoparticles. RSC Adv. 2015, 5, 27224–27228. [Google Scholar] [CrossRef][Green Version]
- Boles, M.A.; Ling, D.; Hyeon, T.; Talapin, D.V. The surface science of nanocrystals. Nat. Mater. 2016, 15, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Jishkariani, D.; Diroll, B.T.; Cargnello, M.; Klein, D.R.; Hough, L.A.; Murray, C.B.; Donnio, B. Dendron-Mediated Engineering of Interparticle Separation and Self-Assembly in Dendronized Gold Nanoparticles Superlattices. J. Am. Chem. Soc. 2015, 137, 10728–10734. [Google Scholar] [CrossRef] [PubMed]
- Cseh, L.; Mehl, G.H. Structure–property relationships in nematic gold nanoparticles. J. Mater. Chem. 2007, 17, 311–315. [Google Scholar] [CrossRef]
- Donnio, B.; García-Vázquez, P.; Gallani, J.-L.; Guillon, D.; Terazzi, E. Dendronized Ferromagnetic Gold Nanoparticles Self-Organized in a Thermotropic Cubic Phase. Adv. Mater. 2007, 19, 3534–3539. [Google Scholar] [CrossRef]
- Frein, S.; Boudon, J.; Vonlanthen, M.; Scharf, T.; Barberá, J.; Süss-Fink, G.; Bürgi, T.; Deschenaux, R. Liquid-Crystalline Thiol- and Disulfide-Based Dendrimers for the Functionalization of Gold Nanoparticles. Helv. Chim. Acta 2008, 91, 2321–2337. [Google Scholar] [CrossRef]
- Stamatoiu, O.; Mirzaei, J.; Feng, X.; Hegmann, T. Nanoparticles in Liquid Crystals and Liquid Crystalline Nanoparticles. In Liquid Crystals. Topics in Current Chemistry; Tschierske, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 318. [Google Scholar] [CrossRef]
- Nealon, G.L.; Greget, R.; Dominguez, C.; Nagy, Z.T.; Guillon, D.; Gallani, J.-L.; Donnio, B. Liquid-crystalline nanoparticles: Hybrid design and mesophase structures. Beilstein J. Org. Chem. 2012, 8, 349–370. [Google Scholar] [CrossRef]
- Lewandowski, W.; Wójcik, M.; Górecka, E. Metal Nanoparticles with Liquid-Crystalline Ligands: Controlling Nanoparticle Superlattice Structure and Properties. ChemPhysChem 2014, 15, 1283–1295. [Google Scholar] [CrossRef]
- Xue, C.; Li, Q. Liquid Crystal-Gold Nanoparticle Hybrid Materials. In Nanoscience with Liquid Crystals. NanoScience and Technology; Li, Q., Ed.; Springer: Cham, Switzerland, 2014. [Google Scholar] [CrossRef]
- Matsubara, M.; Miyazaki, A.; Zeng, X.; Muramatsu, A.; Ungar, G.; Kanie, K. Rheology of Thermotropic Liquid-Crystalline Dendron-Modified Gold Nanoparticles. Mol. Cryst. Liq. Cryst. 2015, 617, 50–57. [Google Scholar] [CrossRef]
- Erk, C.; Eric Yau, M.Y.; Lange, H.; Thomsen, C.; Miclea, P.; Wehrspohn, R.B.; Schlecht, S.; Steinhart, M. Formation of gold nanoparticles in polymeric nanowires by low-temperature thermolysis of gold mesitylene. J. Mater. Chem. 2012, 22, 684–690. [Google Scholar] [CrossRef][Green Version]
- Ranjbar, M.; Yousefi, M. Synthesis and Characterization of Lanthanum Oxide Nanoparticles from Thermolysis of Nano-sized Lanthanum(III) Supramolecule as a Novel Precursor. J. Inorg. Organomet. Polym. Mater. 2014, 24, 652–655. [Google Scholar] [CrossRef]
- Tuchscherer, A.; Schaarschmidt, D.; Schulze, S.; Hietschold, M.; Lang, H. Gold nanoparticles generated by thermolysis of “all-in-one” gold(i) carboxylate complexes. Dalton Trans. 2012, 41, 2738–2746. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fréchet, J.M.J. Dendrimers and supramolecular chemistry. Proc. Natl. Acad. Sci. USA 2002, 99, 4782–4787. [Google Scholar] [CrossRef]
- Stadler, A.-M. Structural Features of Fréchet-Type Dendrons and Dendrimers in Single Crystals. Cryst. Growth Des. 2010, 10, 5050–5065. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J. Synthesis of thiol-derivatised gold nanoparticles in a two-phase Liquid–Liquid system. Journal of the Chemical Society. Chem. Commun. 1994, 7, 801–802. [Google Scholar] [CrossRef]
- Collman, J.P.; Devaraj, N.K.; Chidsey, C.E.D. “Clicking” Functionality onto Electrode Surfaces. Langmuir 2004, 20, 1051–1053. [Google Scholar] [CrossRef]
- Li, N.; Zhao, P.; Salmon, L.; Ruiz, J.; Zabawa, M.; Hosmane, N.S.; Astruc, D. “Click” Star-Shaped and Dendritic PEGylated Gold Nanoparticle-Carborane Assemblies. Inorg. Chem. 2013, 52, 11146–11155. [Google Scholar] [CrossRef]
- Gentry, S.T.; Kendra, S.F.; Bezpalko, M.W. Ostwald. Ripening in Metallic Nanoparticles: Stochastic Kinetics. J. Phys. Chem. C 2011, 115, 12736–12741. [Google Scholar]
- Gommes, C.J. Ostwald ripening of confined nanoparticles: Chemomechanical coupling in nanopores. Nanoscale 2019, 11, 7386–7393. [Google Scholar] [CrossRef]
- Vengrenovich, R.D.; Ivanskii, B.V.; Panko, I.I.; Yarema, S.V.; Kryvetskyi, V.I.; Stasyk, M.O. Ostwald Ripening of the Platinum Nanoparticles in the Framework of the Modified LSW Theory. J. Nanomater. 2014, 2014, 821584. [Google Scholar] [CrossRef]
- Pandey, S.; Goswami, G.K.; Nanda, K.K. Green synthesis of polysaccharide/gold nanoparticle nanocomposite: An efficient ammonia sensor. Carbohydr. Polym. 2013, 94, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.B.; Sivapalan, S.T.; DeVetter, B.M.; Yang, T.K.; Wen, J.; Bhargava, R.; Murphy, C.J.; Zuo, J.-M. High-Index Facets in Gold Nanocrystals Elucidated by Coherent Electron Diffraction. Nano Lett. 2013, 13, 1840–1846. [Google Scholar] [CrossRef] [PubMed]
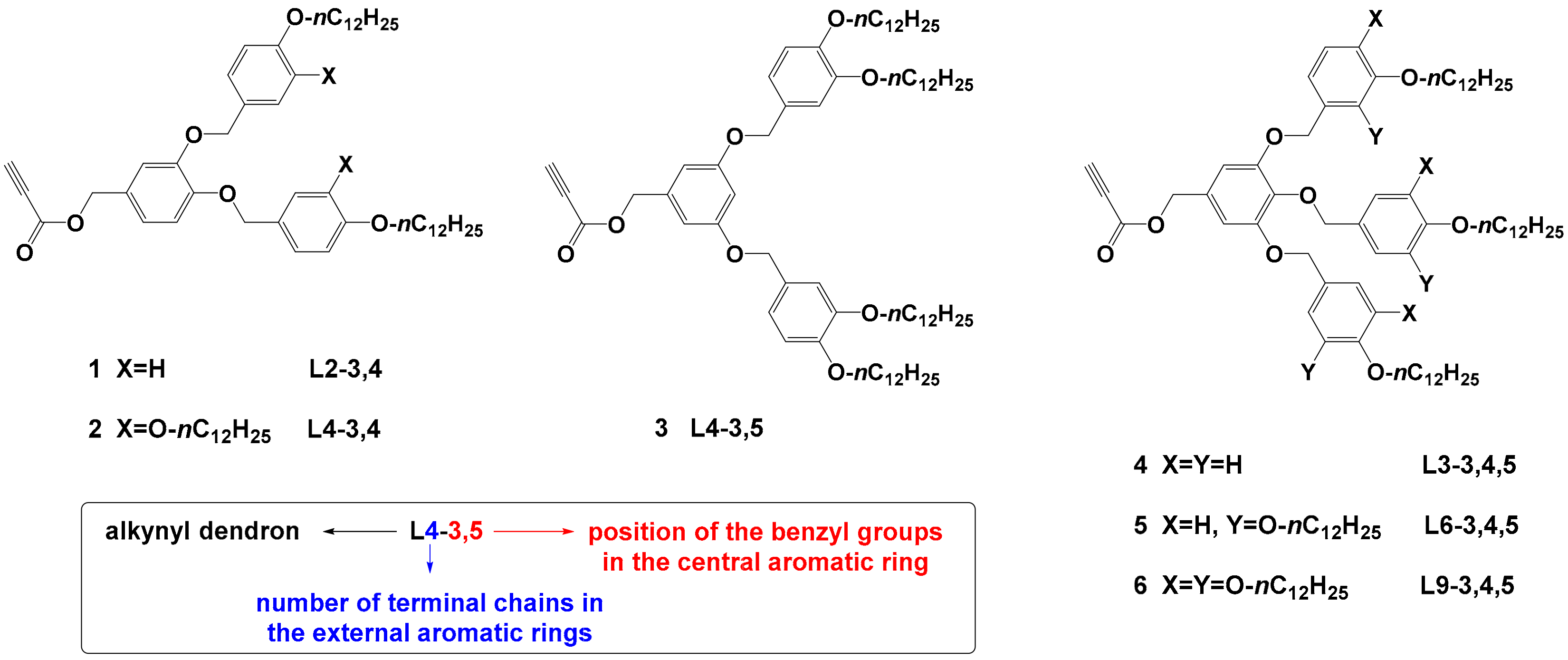
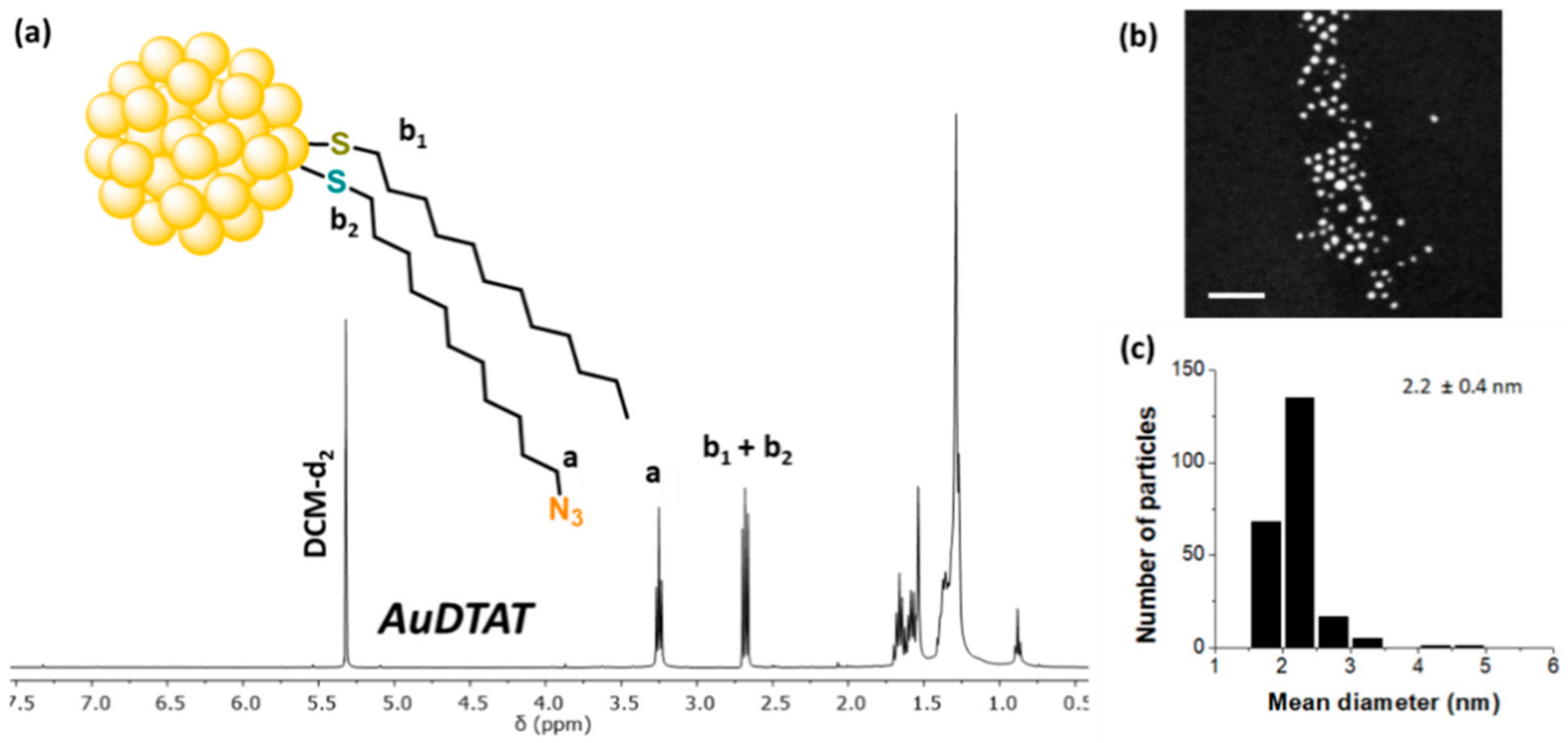

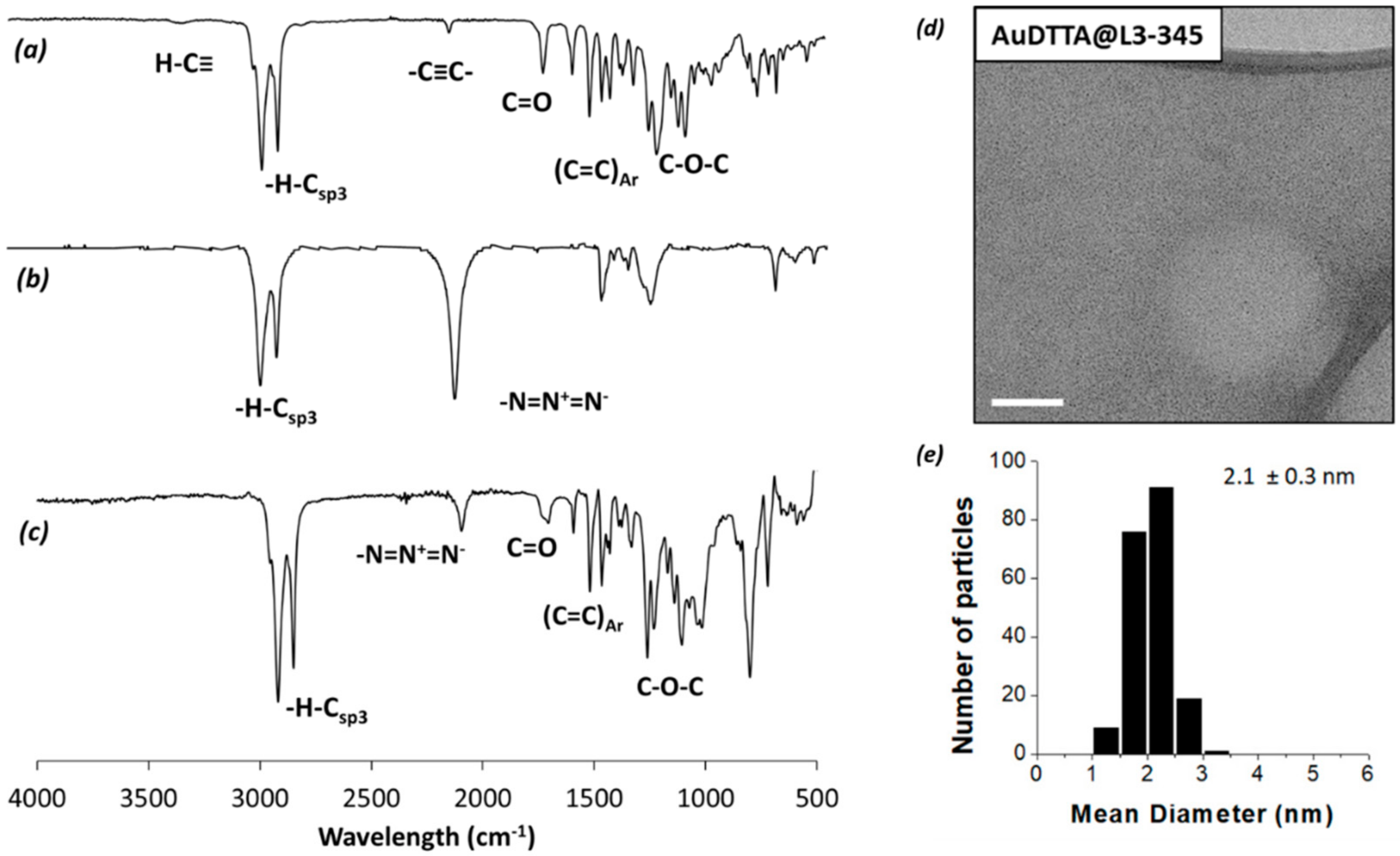
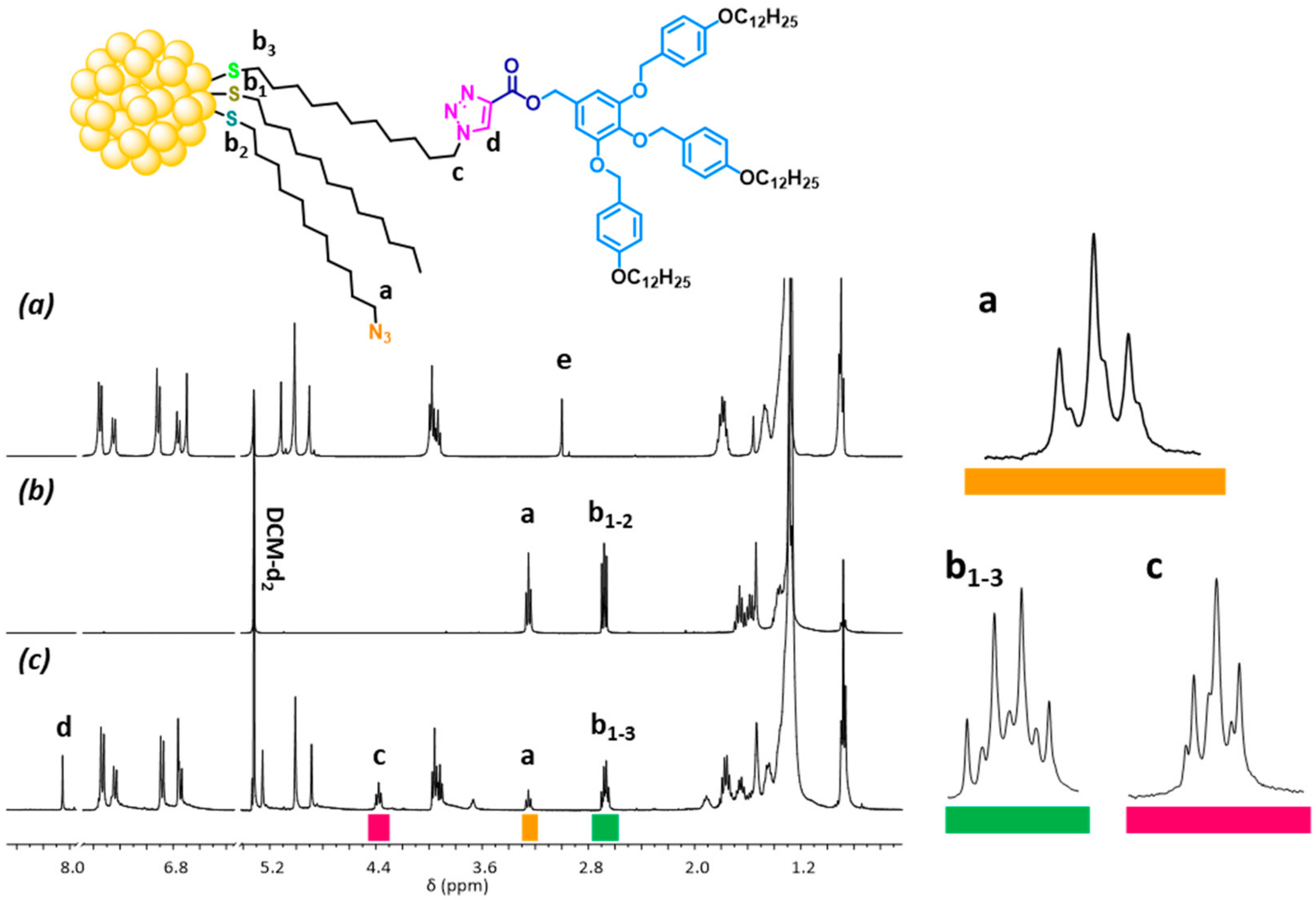


| Compound | T5% a | Phase Transitions b | T (°C) | Phase | Structural Parameters c |
|---|---|---|---|---|---|
| 1(L2-3,4) | 274 | C 50 (26.3) Colr 59 (8.8) I I 57 (9.3) Colr 20 (29.2) C | 55 | Colr | a = 128; b = 52; h = 4.8; Z = 24 |
| 2(L4-3,4) | 299 | C 8 (8.5) Colh 84 (11.2) I I 80 (10.4) Colh -2 (5.7) C | RT | Colh | a = 58; h = 4.9; Z = 8 |
| 3(L4-3,5) | 301 | C 38 (72.8) I I 28 (54.4) C | RT | - | - |
| 4(L3-3,4,5) | 279 | C1 13 (21.2) C2 30 (9.2) C3 42 (-41.0) C4 61 (60.5) I I 45 (4.6) Colh 4 (20.5) C | RT | Colh | a = 48; h = 5.0; Z = 6 |
| 5(L6-3,4,5) | 299 | C 3 (8.2) Colh 92 (7.9) I I 87 (9.6) Colh -6 (6.6) C | RT | Colh | a = 50; h = 5.0; Z = 4 |
| 6(L9-3,4,5) | 313 | C1 28 (−80.8) C2 56 (93.7) I I 18 (2.5) Colx -8 (4.8) C | - | Colx | - |
| Sample | ɸ a nm | SD a nm | % LDT b % molar | % LAT b % molar | % Ln b % molar | % Funct. c % |
|---|---|---|---|---|---|---|
| AuDT | 2.0 | 0.4 | 100 | - | - | - |
| AuDT-AT | 2.2 | 0.4 | 17 | 83 | - | - |
| AuDT-AT-TA@L2-3,4 | 2.2 | 0.3 | 17 | 35 | 48 | 58 |
| AuDT-AT-TA@L4-3,4 | 2.2 | 0.3 | 19 | 35 | 46 | 57 |
| AuDT-AT-TA@L4-3,5 | 2.2 | 0.6 | 24 (17) d | 41 (29) d | 35 (25) d | 47 |
| AuDT-AT-TA@L3-3,4,5 | 2.1 | 0.3 | 18 | 32 | 50 | 60 |
| AuDT-AT-TA@L6-3,4,5 | 2.0 | 0.3 | 17 | 43 | 40 | 48 |
| AuDT-AT-TA@L9-3,4,5 | 2.0 | 0.3 | 28 (17) d | 22 (13) d | 50 (30) d | 69 |
| Sample | Φ (SD) a | Φ (SD) a | %LDT b | %LDT b | %LAT b | %LAT b | % Ln b | % Ln b | λsoluc c | λsoluc c | λsolid c |
|---|---|---|---|---|---|---|---|---|---|---|---|
| nm 0 min | nm 60 min | %mol 0 min | %mol 60 min | %mol 0 min | %mol 60 min | %mol 0 min | %mol 60 min | nm 60 min | nm 60 min | nm 60 min | |
| AuDT-AT-TA@L2-3,4 | 2.2 (0.3) | 2.5 (0.4) | 17 | 26 | 35 | 28 | 48 | 46 | 277 | 516 | 562 |
| AuDT-AT-TA@L4-3,4 | 2.2 (0.3) | 5.3 (1.0) | 19 | 24 | 35 | 33 | 46 | 43 | 275 | 528 | 573 |
| AuDT-AT-TA@L4-3,5 | 2.2 (0.6) | 5.8 (1.0) | 24 | 21 | 41 | 43 | 35 | 36 | 283 | 528 | 533 |
| AuDT-AT-TA@L3-3,4,5 | 2.1 (0.3) | 5.7 (1.1) | 18 | 26 | 32 | 25 | 50 | 49 | 283 | 530 | 530 |
| AuDT-AT-TA@L6-3,4,5 | 2.0 (0.3) | 8.7 (2.4) | 17 | 51 | 43 | 0 | 40 | 49 | 282 | 530 | 555 |
| AuDT-AT-TA@L9-3,4,5 | 2.0 (0.3) | 5.3 (1.3) | 28 | 29 | 22 | 25 | 50 | 46 | 271 | 525 | 533 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulloa, J.A.; Barberá, J.; Serrano, J.L. Gold Nanoparticles Modification with Liquid Crystalline Polybenzylic Dendrons via 1,3-Dipolar Cycloaddition. Nanomaterials 2022, 12, 4026. https://doi.org/10.3390/nano12224026
Ulloa JA, Barberá J, Serrano JL. Gold Nanoparticles Modification with Liquid Crystalline Polybenzylic Dendrons via 1,3-Dipolar Cycloaddition. Nanomaterials. 2022; 12(22):4026. https://doi.org/10.3390/nano12224026
Chicago/Turabian StyleUlloa, José Antonio, Joaquín Barberá, and José Luis Serrano. 2022. "Gold Nanoparticles Modification with Liquid Crystalline Polybenzylic Dendrons via 1,3-Dipolar Cycloaddition" Nanomaterials 12, no. 22: 4026. https://doi.org/10.3390/nano12224026
APA StyleUlloa, J. A., Barberá, J., & Serrano, J. L. (2022). Gold Nanoparticles Modification with Liquid Crystalline Polybenzylic Dendrons via 1,3-Dipolar Cycloaddition. Nanomaterials, 12(22), 4026. https://doi.org/10.3390/nano12224026







