Preparation and Adsorption Performance Study of Graphene Quantum Dots@ZIF-8 Composites for Highly Efficient Removal of Volatile Organic Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of GQDs
2.3. Preparation of ZIF-8
2.4. Preparation of GQDs@ZIF-8 Composites
2.5. Characterization of GQDs@ZIF-8 Composites
2.6. Adsorption Test of GQDs@ZIF-8 Composites for Toluene and Ethyl Acetate
3. Results and Discussion
3.1. Morphology Analysis of GQDs@ZIF-8 Composites
3.2. Crystalline Structure and Chemical Bond Analysis of GQDs@ZIF-8 Composites
3.3. Chemical Components Analysis of GQDs@ZIF-8 Composites
3.4. Specific Surface Area and Pore Structure Analysis of GQDs@ZIF-8 Composites
3.5. Thermal Stability Analysis of GQDs@ZIF-8 Composites
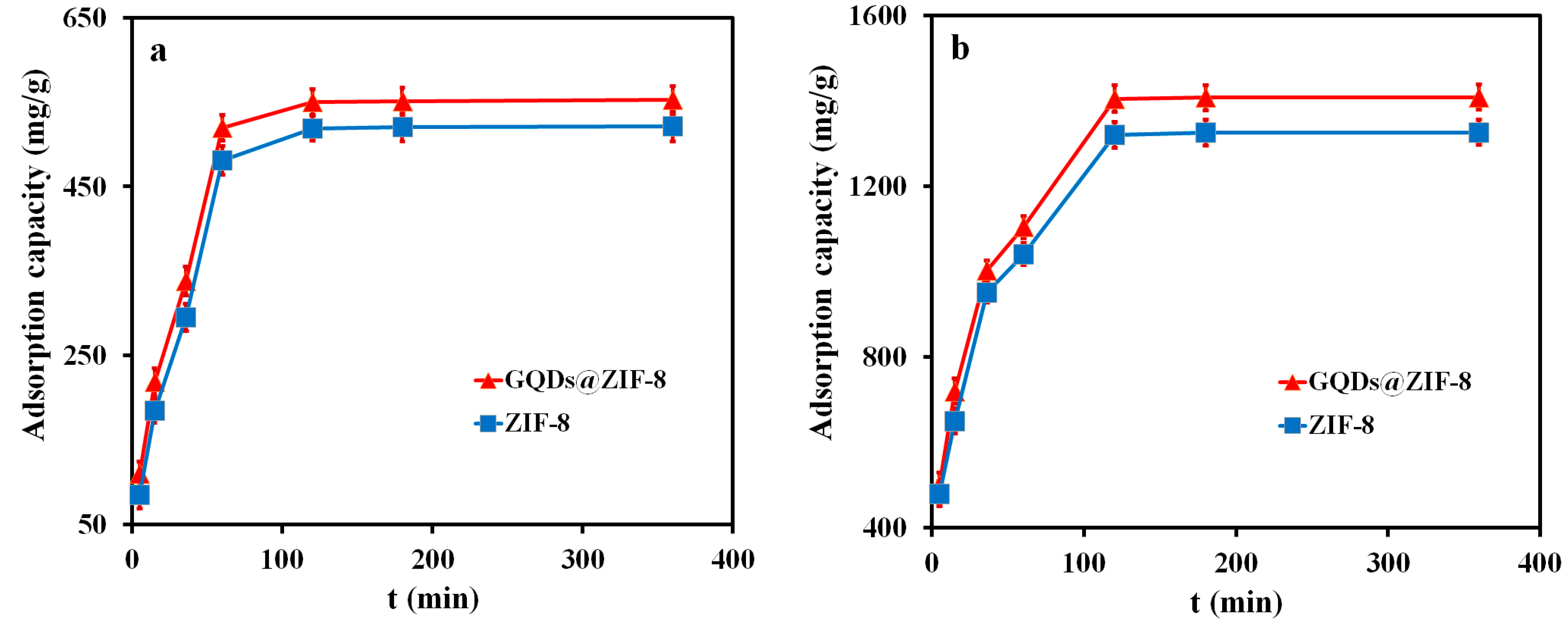
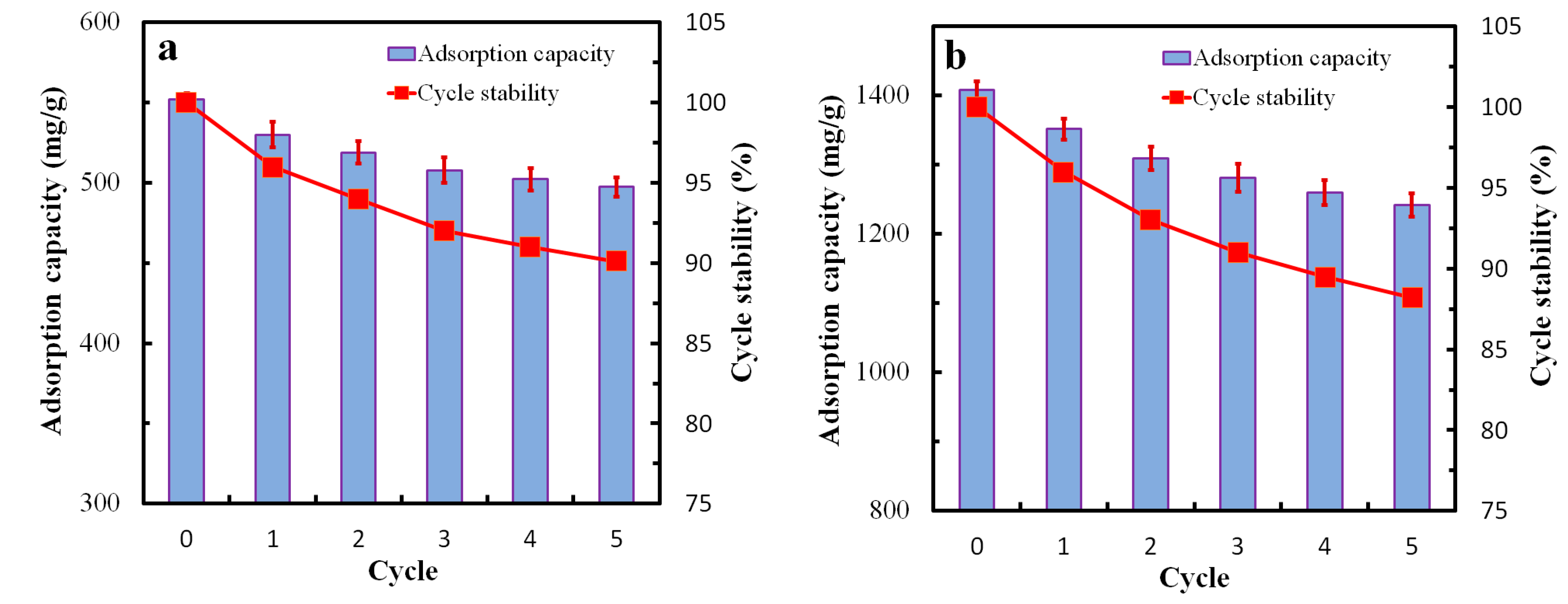
3.6. Adsorption Performance of GQDs@ZIF-8 Composites for Toluene and Ethyl Acetate
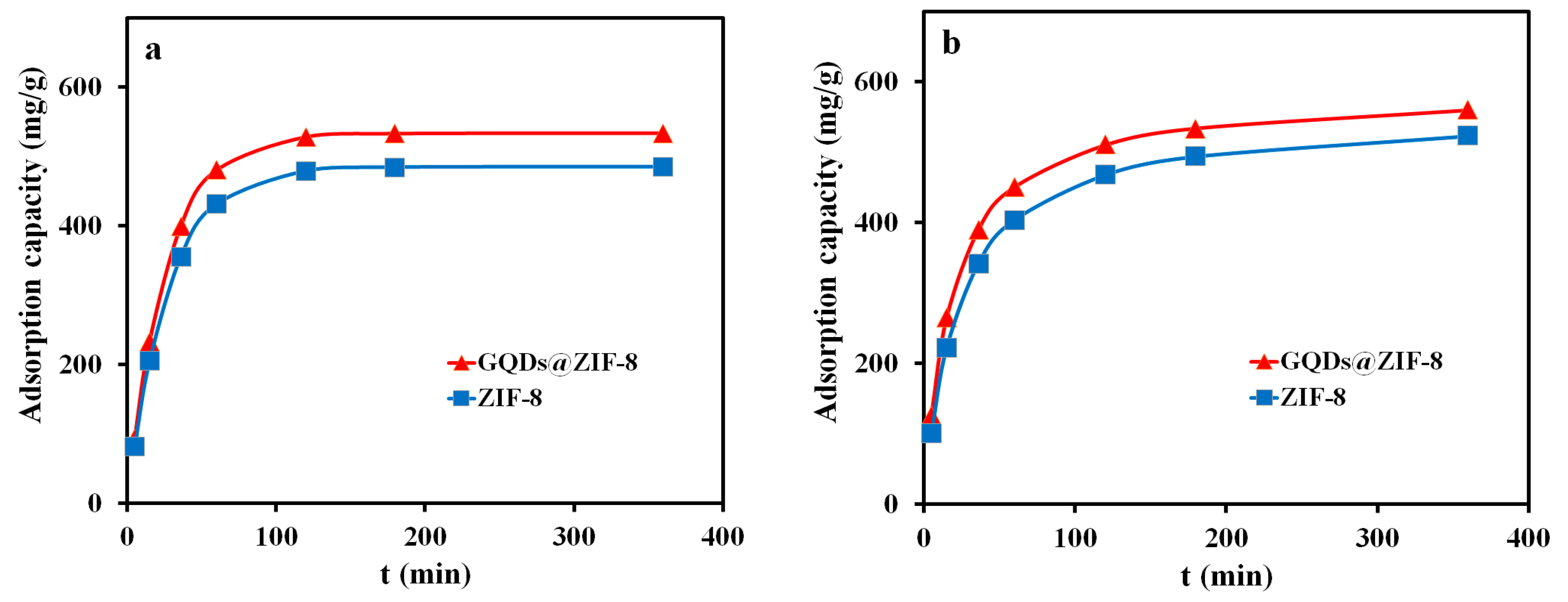
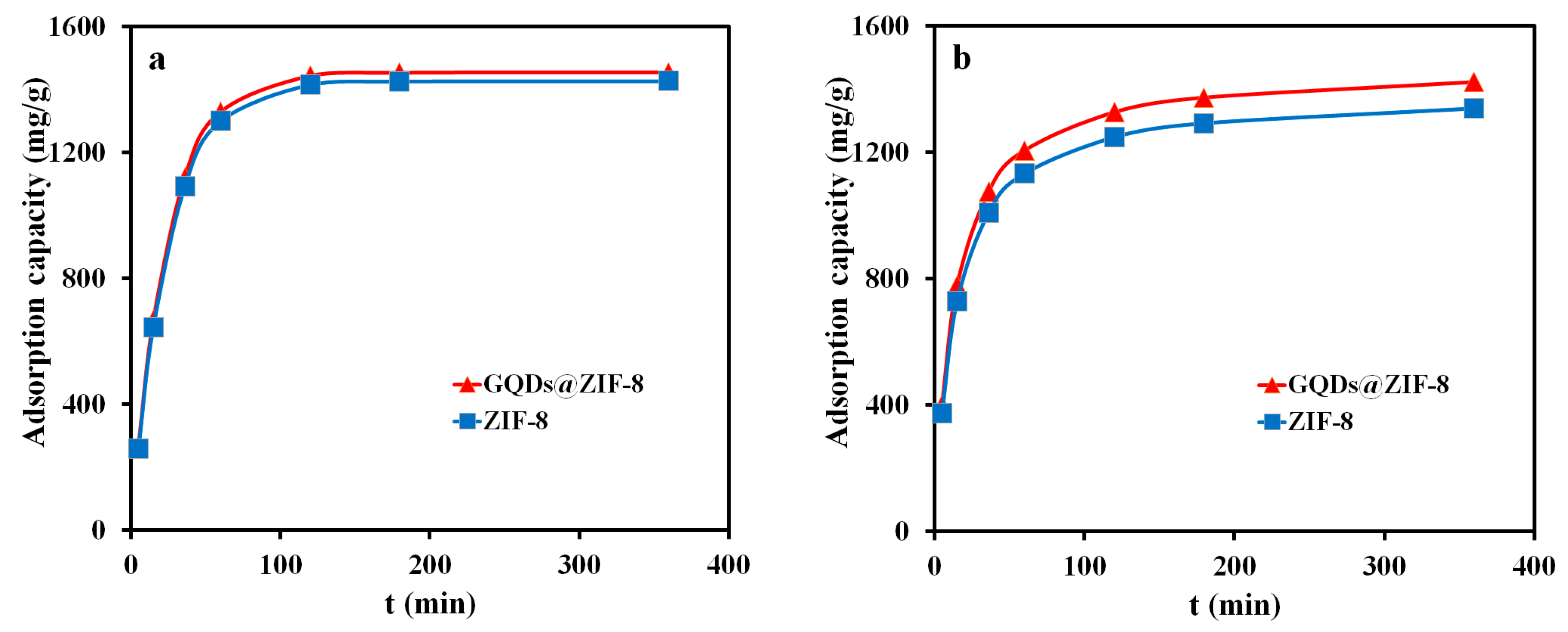
3.7. Adsorption Kinetics Study of GQDs@ZIF-8 Composites
3.8. Adsorption Isotherm Study of GQDs@ZIF-8 Composites
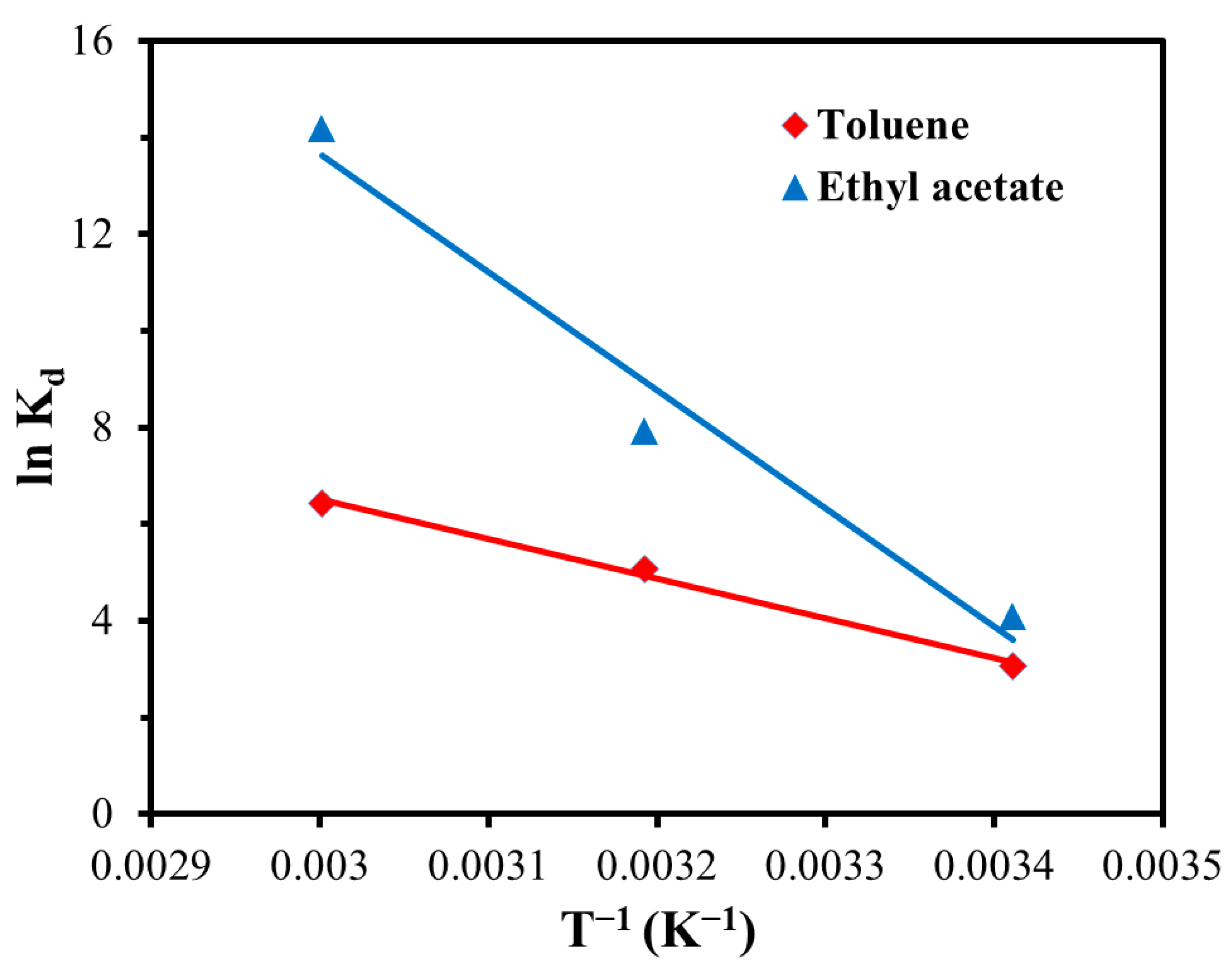
3.9. Adsorption Thermodynamic Study of GQDs@ZIF-8 Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Q.Q.; Su, G.J.; Li, C.Q.; Liu, P.F.; Zhao, X.X.; Zhang, C.L.; Sun, X.; Mu, Y.J.; Wu, M.G.; Wang, Q.L.; et al. An investigation into the role of VOCs in SOA and ozone production in Beijing, China. Sci. Total. Environ. 2020, 720, 137536. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Harrad, S.; Harrison, R.M. Concentrations and sources of VOCs in urban domestic and public microenvironments. Environ. Sci. Technol. 2016, 35, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.F.; Zhang, M.X.; Yang, C.; Xiang, R.Y.; Yang, X.D.; Cui, L.Z. Performance evaluation and prediction of activated carbon for VOCs via experiments and LFER methods. J. Ind. Eng. Chem. 2022, 116, 385–392. [Google Scholar] [CrossRef]
- Shen, X.Q.; Du, X.S.; Yang, D.F.; Ran, J.Y.; Yang, Z.Q.; Chen, Y.R. Influence of physical structures and chemical modification on VOCs adsorption characteristics of molecular sieves. J. Environ. Chem. Eng. 2021, 9, 106729. [Google Scholar] [CrossRef]
- Mashabi, R.A.; Khan, Z.A.; Elwakeel, K.Z. Chitosan- or glycidyl methacrylate-based adsorbents for the removal of dyes from aqueous solutions: A review. Mater. Adv. 2022, 3, 5645–5671. [Google Scholar] [CrossRef]
- Deng, H.; Pan, T.T.; Zhang, Y.; Wang, L.; Wu, Q.M.; Ma, J.Z.; Shan, W.P.; He, H. Adsorptive removal of toluene and dichloromethane from humid exhaust on MFI, BEA and FAU zeolites: An experimental and theoretical study. Chem. Eng. J. 2020, 394, 124986. [Google Scholar] [CrossRef]
- Chen, D.S.; LI, Y.T.; Zhong, X.M.; Zeng, H.M.; Chen, Z.H.; Tang, Z.J.; Cen, C.P. Preparation of novel MOF with multipolar pore and adsorption properties of VOCs. IOP Conf. Ser. Earth Environ. Sci. 2019, 300, 032008. [Google Scholar] [CrossRef]
- Wang, H.; Bai, J.Q.; Yin, Y.; Wang, S.F. Experimental and numerical study of SO2 removal from a CO2/SO2 gas mixture in a Cu-BTC metal organic framework. J. Mol. Graph. Model. 2020, 96, 107533. [Google Scholar] [CrossRef]
- Jun, B.M.; Al-Hamadani, Y.A.J.; Son, A.; Park, C.M.; Jang, M.; Jang, A.; Kim, N.C.; Yoon, Y. Applications of metal-organic framework based membranes in water purification: A review. Separ. Purif. Technol. 2020, 247, 116947. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, P.; Zhang, Y.L.; Liu, B.Y.; Gao, P.; Guo, S.J. 3D star-like atypical hybrid MOF derived single-atom catalyst boosts oxygen reduction catalysis. J. Energy Chem. 2021, 55, 355–360. [Google Scholar] [CrossRef]
- Ahmad, N.; Samavati, A.; Nordin, N.A.H.M.; Jaafar, J.; Ismail, A.F.; Malek, N.A.N.N. Enhanced performance and antibacterial properties of amine-functionalized ZIF-8-decorated GO for ultrafiltration membrane. Separ. Purif. Technol. 2020, 239, 116554. [Google Scholar] [CrossRef]
- Meng, X.; Duan, C.; Zhang, Y.L.; Lu, W.L.; Wang, W.L.; Ni, Y.H. Corncob-supported AgNPs@ ZIF-8 nanohybrids as multifunction biosorbents for wastewater remediation: Robust adsorption, catalysis and antibacterial activity. Compos. Sci. Technol. 2020, 200, 108384. [Google Scholar] [CrossRef]
- Beni, F.A.; Gholami, A.; Ayati, A.; Shahrak, M.N.; Sillanpaa, M. UV-switchable phosphotungstic acid sandwiched between ZIF-8 and Au nanoparticles to improve simultaneous adsorption and UV light photocatalysis toward tetracycline degradation. Microporous Mesoporous Mater. 2020, 303, 110275. [Google Scholar] [CrossRef]
- Li, S.Q.; Zhang, X.D.; Huang, Y.M. Zeolitic imidazolate framework-8 derived nanoporous carbon as an effective and recyclable adsorbent for removal of ciprofloxacin antibiotics from water. J. Hazard. Mater. 2017, 321, 711–719. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, L.; Zhang, X.H.; Chen, Y.L. Preparation of zeolitic imidazolate framework-8/graphene oxide composites with enhanced VOCs adsorption capacity. Microporous Mesoporous Mater. 2016, 225, 488–493. [Google Scholar] [CrossRef]
- Qiu, J.T.; Xu, X.; Liu, B.G.; Guo, Y.; Wang, H.J.; Yu, L.Y.; Jiang, Y.W.; Huang, C.S.; Fan, B.F.; Zeng, Z.; et al. Size-controllable synthesis of zif-8 and derived nitrogen-rich porous carbon for CO2 and vocs adsorption. Chemistryselect. 2022, 7, e202203273. [Google Scholar] [CrossRef]
- Meng, X.M.; Yang, L.; Jiang, W.J.; Yao, L. Adsorption of acetone and toluene by N-functionalized porous carbon derived from ZIF-8. J. Ind. Eng. Chem. 2022, 111, 137–146. [Google Scholar] [CrossRef]
- Song, Y.C.; Wang, N.; Yang, L.Y.; Wang, Y.G.; Yu, D.; Ouyang, X.K. Facile fabrication of ZIF-8/calcium alginate microparticles for highly efficient adsorption of Pb(Ⅱ) from Aqueous solutions. Ind. Eng. Chem. Res. 2019, 58, 6394–6401. [Google Scholar] [CrossRef]
- Wang, C.L.; Yang, R.Q.; Wang, H.F. Synthesis of ZIF-8/fly ash composite for adsorption of Cu2+, Zn2+ and Ni2+ from aqueous solutions. Materials 2020, 13, 214. [Google Scholar] [CrossRef]
- Huang, H.D.; Liao, L.; Xu, X.; Zou, M.J.; Liu, F.; Li, N. The electron-transfer based interaction between transition metal ions and photoluminescent graphene quantum dots (GQDs): A platform for metal ion sensing. Talanta 2013, 117, 152–157. [Google Scholar] [CrossRef]
- Liu, R.L.; Wu, D.Q.; Feng, X.L.; Mullen, K. Bottom-up fabrication of photoluminescent graphene quantum dots with uniform morphology. J. Am. Chem. Soc. 2011, 133, 15221–15223. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.Y.; Lyu, J.; Tian, F.; Yang, M. A fluorescence turn-on biosensor based on graphene quantum dots (GQDs) and molybdenum disulfide (MoS2) nanosheets for epithelial cell adhesion molecule (EpCAM) detection. Biosens. Bioelectron. 2016, 93, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Sangam, S.; Gupta, A.; Shakeel, A.; Bhattacharya, R.; Sharma, A.K.; Suhag, D.; Chakrabarti, S.; Garg, S.K.; Chattopadhyay, S.; Basu, B.; et al. Sustainable synthesis of single crystalline sulphur-doped graphene quantum dots for bioimaging and beyond. Green. Chem. 2018, 20, 4245–4259. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Zhang, J.; Chen, N.; Qu, L.T. Graphene quantum dots: An emerging material for energy-related applications and beyond. Energy Environ. Sci. 2012, 5, 8869–8890. [Google Scholar] [CrossRef]
- Zhou, X.J.; Zhang, Y.; Wang, C.; Wu, X.C.; Yang, Y.Q.; Zheng, B.; Wu, H.X.; Guo, S.W.; Zhang, J.Y. Photo-Fenton reaction of graphene oxide: A new strategy to prepare graphene quantum dots for DNA cleavage. ACS Nano 2012, 6, 6592–6599. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, S.J.; Wang, H.Y.; Qu, S.N.; Zhang, Y.L.; Zhang, J.H.; Chen, Q.D.; Xu, H.L.; Han, W.; Yang, B.; et al. Common origin of green luminescence in carbon nanodots and graphene quantum dots. ACS Nano 2014, 8, 2541–2547. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Fekry, N.A.; El-Latif, M.M.A. Nanocomposites of nanosilica-immobilized-nanopolyaniline and crosslinked nanopolyaniline for removal of heavy metals. Chem. Eng. J. 2016, 304, 679–691. [Google Scholar] [CrossRef]
- Manna, S.; Bhattacharya, S.; Sengupta, S.; Das, P. Synthesis of graphene oxide dots coated biomatrices and its application for the removal of multiple pollutants present in wastewater. J. Clean. Prod. 2018, 203, 83–88. [Google Scholar] [CrossRef]
- Nagaraj, A.; Munusamy, M.A.; Al-Arfaj, A.A.; Rajan, M. Functional ionic liquid- capped graphene quantum dots for chromium removal from chromium contaminated water. J. Chem. Eng. Data 2018, 64, 651–667. [Google Scholar] [CrossRef]
- Shao, S.F.; Wang, W.; Zhou, K.; Jiang, F.; Wu, H.Y.; Koehn, R. GQDs-TiO2 heterojunction based thin films for volatile organic compounds sensor with excellent performance at room temperature. Mater. Lett. 2017, 186, 193–197. [Google Scholar] [CrossRef]
- Alivand, M.S.; Tehrani, N.H.M.H.; Askarieh, M.; Ghasemy, E.; Esrafili, M.D.; Ahmadi, R.; Anisi, H.; Tavakoli, O.; Rashidi, A. Defect engineering-induced porosity in graphene quantum dots embedded metal-organic frameworks for enhanced benzene and toluene adsorption. J. Hazard. Mater. 2021, 416, 125973. [Google Scholar] [CrossRef]
- Chowdhury, A.D.; Doong, R. Highly sensitive and selective detection of nanomolar ferric ions using dopamine functionalized graphene quantum dots. ACS Appl. Mater. Inter. 2016, 8, 21002–21010. [Google Scholar] [CrossRef]
- Zhang, D.; Xiang, Q. Simple and efficient size-controllable engineering of zeolitic imidazolate framework (ZIF-8) and M-ZIF-8 by changing addition order. Mater. Lett. 2021, 289, 129418. [Google Scholar] [CrossRef]
- Saliba, D.; Ammar, M.; Rammal, M.; Al-Ghoul, M.; Hmadeh, M. Crystal growth of ZIF-8, ZIF-67, and their mixed-metal derivatives. J. Am. Chem. Soc. 2018, 140, 1812–1823. [Google Scholar] [CrossRef]
- Ahmad, N.; Nordin, N.A.; Jaafar, J.; Ismail, A.F.; Ramli, M.K. Significant improvement in antibacterial property of ZIF-8 decorated graphene oxide by post-synthetic modification process. J. Environ. Chem. Eng. 2021, 9, 105887. [Google Scholar] [CrossRef]
- Chen, K.W.; Wang, P.; Gu, A.T.; Miensah, E.D.; Gong, C.H.; Mao, P.; Jiao, Y.; Chen, K.; Liu, Y.; Yang, Y. Core-shell Bi2S3 nanorods loaded ZIF-8 nanocomposites for efficient and reversible capture of radioactive iodine. Microporous Mesoporous Mater. 2022, 339, 111983. [Google Scholar] [CrossRef]
- Hassanpouraghdam, Y.; Pooresmaeil, M.; Namazi, H. In-vitro evaluation of the 5-fluorouracil loaded GQDs@Bio-MOF capped with starch biopolymer for improved colon-specific delivery. Int. J. Biol. Macromol. 2022, 221, 256–267. [Google Scholar] [CrossRef]
- Wei, X.Y.; Wang, Y.W.; Huang, Y.; Fan, C.M. Composite ZIF-8 with CQDs for boosting visible-light-driven photocatalytic removal of NO. J. Alloys Compd. 2019, 802, 467–476. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Datta, K.K.R.; Rosler, C.; Petr, M.; Otyepka, M.; Zboril, R.; Fischer, R.A. Biomimetic superhydrophobic/superoleophilic highly fluorinated graphene oxide and ZIF-8 composites for oil-water separation. Angew. Chem. Int. Edit. 2016, 55, 1178–1182. [Google Scholar] [CrossRef]
- Ono, H.; Ikarashi, T.; Miura, Y.; Hasegawa, E.; Ando, K.; Kitano, T. Bonding configurations of nitrogen absorption peak at 960 cm(-1) in silicon oxynitride films. Appl. Phys. Lett. 1999, 74, 203–205. [Google Scholar] [CrossRef]
- Li, P.Z.; Aranishi, K.; Xu, Q. ZIF-8 immobilized nickel nanoparticles: Highly effective catalysts for hydrogen generation from hydrolysis of ammonia borane. Chem. Commun. 2012, 48, 3173–3175. [Google Scholar] [CrossRef] [PubMed]
- Jian, M.P.; Liu, B.; Zhang, G.S.; Liu, R.P.; Zhang, X.W. Adsorptive removal of arsenic from aqueous solution by zeolitic imidazolate framework-8 (ZIF-8) nanoparticles. Colloid Surfaces A Physicochem. Eng. Asp. 2015, 465, 67–76. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Song, J.Y.; Lee, S.K.; Hwang, Y.K.; Jhung, S.H. Adsorptive removal of aromatic hydrocarbons from water over metal azolate framework-6-derived carbons. J. Hazard. Mater. 2018, 344, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Abussaud, B.A.; Ihsanullah; Al-Baghli, N.A.H.; Redhwi, H.H. Adsorption of toluene and paraxylene from aqueous solution using pure and iron oxide impregnated carbon nanotubes: Kinetics and isotherms study. Bioinorg. Chem. Appl. 2017, 2017, 2853925. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; McKay, G.; Al-Musawi, T.J.; Salehi, S.; Balarak, D. Removal of benzene and toluene from synthetic wastewater by adsorption onto magnetic zeolitic imidazole framework nanocomposites. Nanomaterials 2022, 12, 3049. [Google Scholar] [CrossRef]
- Abussaud, B.A. Synthesis, Characterization and application of carbon nanotubes decorated with zinc oxide nanoparticles for removal of benzene, toluene and p-xylene from aqueous solution. Sustainability 2021, 13, 11716. [Google Scholar] [CrossRef]
- Wang, Y.L.; Li, Z.H.; Tang, C.; Ren, H.X.; Zhang, Q.; Xue, M.; Xiong, J.; Wang, D.B.; Yu, Q.; He, Z.Y.; et al. Few-layered mesoporous graphene for high-performance toluene adsorption and regeneration. Environ. Sci.-Nano 2019, 6, 3113–3122. [Google Scholar] [CrossRef]
- Chen, Y.X.; Fan, C.M.; Li, X.L.; Ren, J.M.; Zhang, G.Z.; Xie, H.M.; Liu, B.Y.; Zhou, G.L. Preparation of carbon black-based porous carbon adsorbents and study of toluene adsorption properties. J. Chem. Technol. Biot. 2022. [Google Scholar] [CrossRef]
- Pan, T.T.; Wang, Y.Q.; Liu, F.; Liu, C.S.; Li, W.X. Stable Metal-Organic Frameworks based mixed tetramethylammonium hydroxide for toluene adsorption. J. Solid State Chem. 2022, 306, 122732. [Google Scholar] [CrossRef]
- Zhang, J.J.; Shao, J.A.; Zhang, X.; Rao, G.; Li, G.Y.; Yang, H.P.; Zhang, S.H.; Chen, H.P. Facile synthesis of Cu-BTC@biochar with controlled morphology for effective toluene adsorption at medium–high temperature. Chem. Eng. J. 2022, 452, 139003. [Google Scholar] [CrossRef]
- Li, W.X.; Wang, W.L.; Sun, J.; Ma, X.L.; Dong, Y.L. Hydrophobic modification of UiO-66 by naphthyl ligand substitution for efficient toluene adsorption in a humid environment. Microporous Mesoporous Mater. 2021, 326, 111357. [Google Scholar] [CrossRef]
- Stoycheva, I.G.; Tsyntsarski, B.G.; Petrova, B.N.; Kumanek, B.; Budinova, T.K.; Petrov, N.V. Adsorption of Ethyl Acetate from Water by Nanoporous Carbon Prepared from Waste Materials. Water Air Soil Poll. 2017, 227, 452. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Sui, H.; Yang, H.W.; Li, X.G.; He, L.; Liu, J.J. Adsorption-desorption behaviors of methanol and ethyl acetate on silica gel: Modeling and experimental tests. Ind. Eng. Chem. Res. 2021, 60, 1829–1838. [Google Scholar] [CrossRef]
- Yu, S.Y.; Wang, X.Y.; Liu, F.; Xiao, K.K.; Kang, C.L. Adsorption of acetone, ethyl acetate and toluene by beta zeolite/diatomite composites: Preparation, characterization and adsorbability. Environ. Sci. Pollut. Res. 2022, 29, 80646–80656. [Google Scholar] [CrossRef]
- Sun, L.; Yuan, D.; Liu, R.R.; Wan, S.G.; Lu, X.J. Coadsorption of gaseous xylene, ethyl acetate and water onto porous biomass carbon foam pellets derived from liquefied Vallisneria natans waste. J. Chem. Technol. Biot. 2020, 95, 1348–1360. [Google Scholar] [CrossRef]
- Liu, Y.T.; Tian, T. Fabrication of diatomite/silicalite-1 composites and their property for vocs adsorption. Materials 2019, 12, 551. [Google Scholar] [CrossRef]
- Chen, H.; Mu, X.Y.; Ma, J.; Yao, M.Q.; Liu, F. Performance evaluation of functionalized ordered mesoporous silica for VOCs adsorption by molecular dynamics simulation. Arab. J. Chem. 2022, 15, 104192. [Google Scholar] [CrossRef]
- Manjare, S.; Ghoshal, A. Studies on dynamic adsorption behaviour of ethyl acetate from air on 5a and 13x molecular sieves. Can. J. Chem. Eng. 2005, 83, 232–241. [Google Scholar] [CrossRef]
- Anasthas, H.; Gaikar, V. Removal of acetic acid impurities from ethyl acetate by adsorption on ion exchange resins. Sep. Sci. Technol. 2007, 36, 2623–2646. [Google Scholar] [CrossRef]
- Omer, A.S.; Naeem, G.; Abd-Elhamid, A.; Farahat, O.; ElBardan, A.; Soliman, H.; Nayl, A. Adsorption of crystal violet and methylene blue dyes using a cellulose-based adsorbent from sugercane bagasse: Characterization, kinetic and isotherm studies. J. Mater. Res. Technol. 2022, 19, 3241–3254. [Google Scholar] [CrossRef]
- Neolaka, Y.; Lawa, Y.; Naat, J.; Riwu, A.; Iqbal, M.; Darmokoesoemo, H.; Kusuma, H.S. The adsorption of Cr(VI) from water samples using graphene oxide-magnetic (GO-Fe3O4) synthesized from natural cellulose-based graphite (kusambi wood or Schleichera oleosa): Study of kinetics, isotherms and thermodynamics. J. Mater. Res. Technol. 2020, 9, 6544–6556. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Al-Mur, B.A.; Akhdhar, A.; El-Sadik, H.A.; El-Liethy, M.A.; Elwakeel, K.Z.; Salama, A.M. Biosorption kinetics of cerium(III) and cobalt(II) from liquid wastes using individual bacterial species isolated from low-level liquid radioactive wastes. Environ. Sci. Pollut. Res. 2022, 23241. [Google Scholar] [CrossRef] [PubMed]
- Shafiei, M.; Alivand, M.S.; Rashidi, A.; Samimi, A.; Mohebbi-Kalhori, D. Synthesis and adsorption performance of a modified micro-mesoporous MIL-101(Cr) for VOCs removal at ambient conditions. Chem. Eng. J. 2018, 341, 164–174. [Google Scholar] [CrossRef]
- Saini, V.K.; Pires, J. Development of metal organic fromwork-199 immobilized zeolite foam for adsorption of common indoor VOCs. J. Environ. Sci. 2017, 55, 321–330. [Google Scholar] [CrossRef]
- Vellingiri, K.; Kumar, P.; Deep, A.; Kim, K.H. Metal-organic frameworks for the adsorption of gaseous toluene under ambient temperature and pressure. Chem. Eng. J. 2017, 307, 1116–1126. [Google Scholar] [CrossRef]
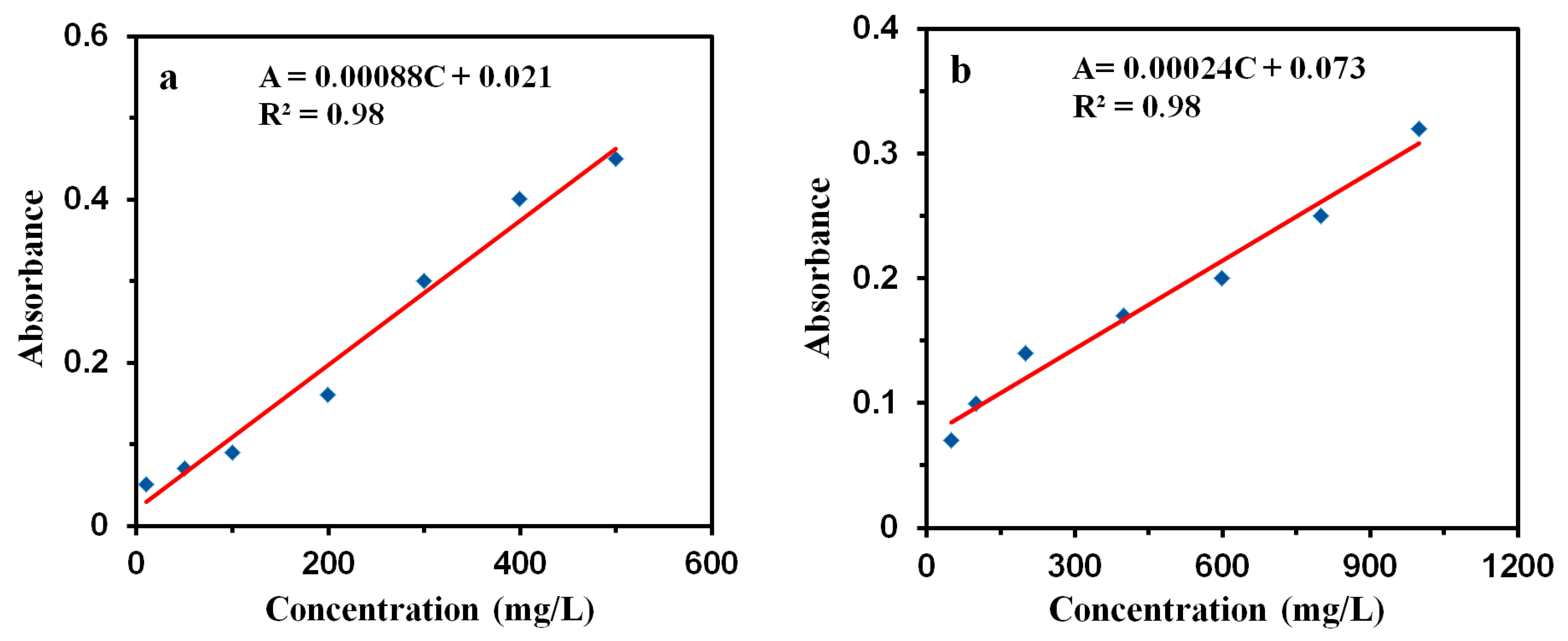
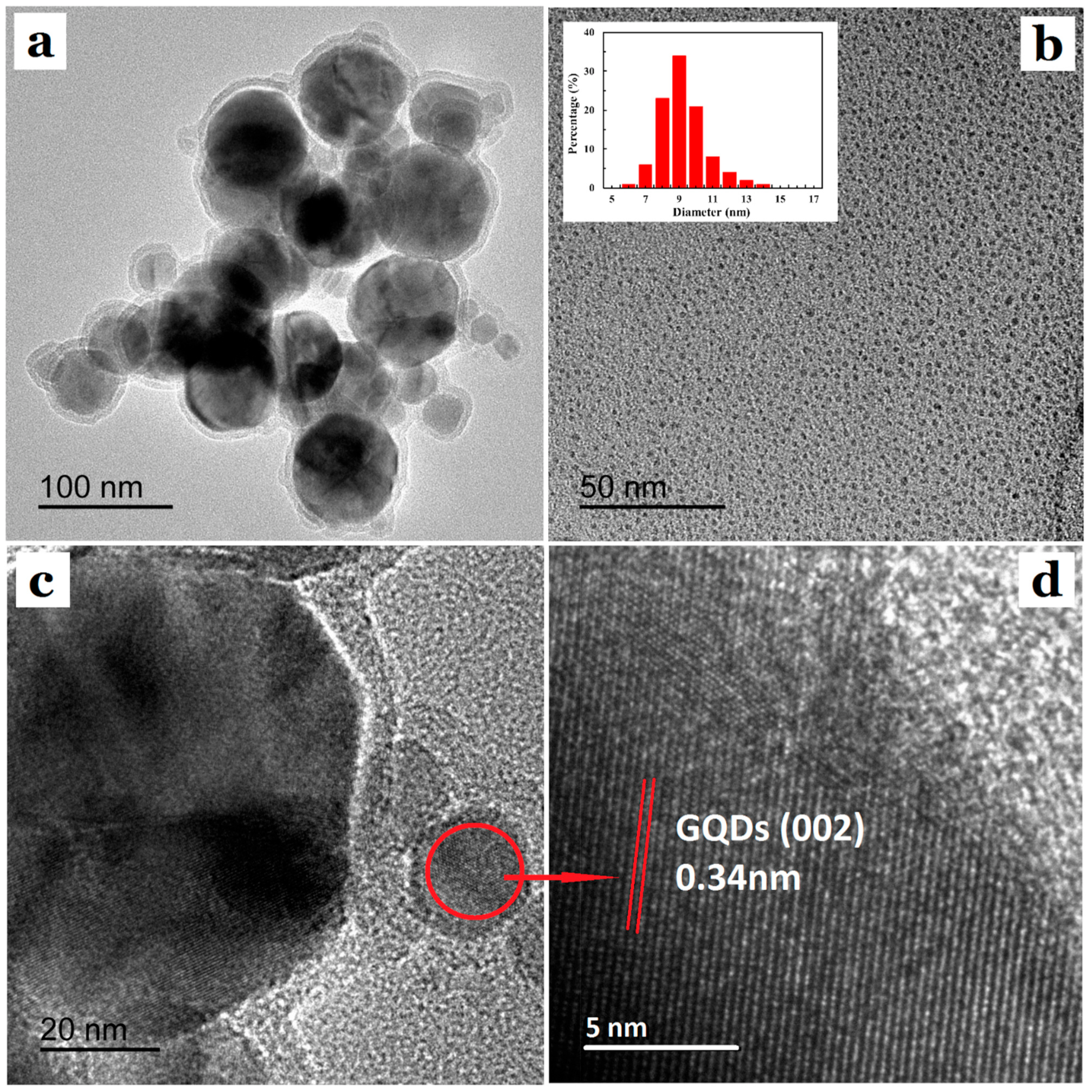
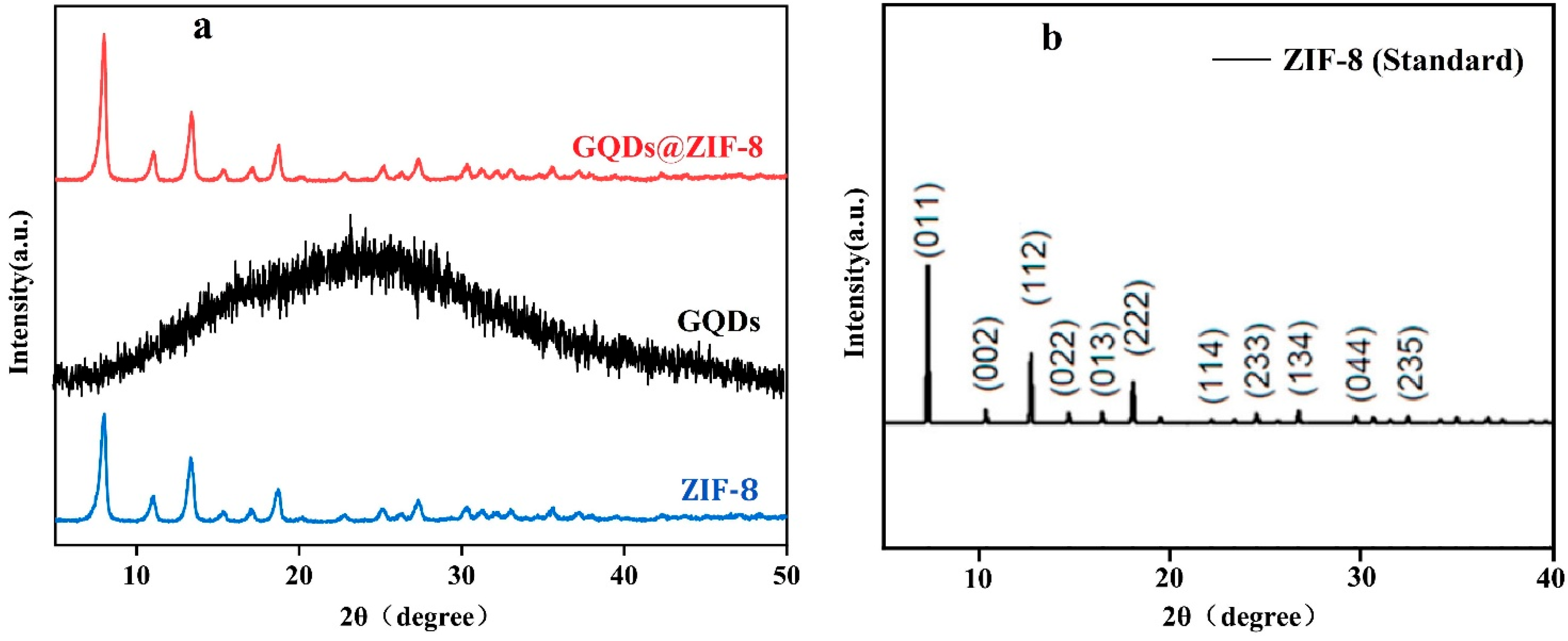
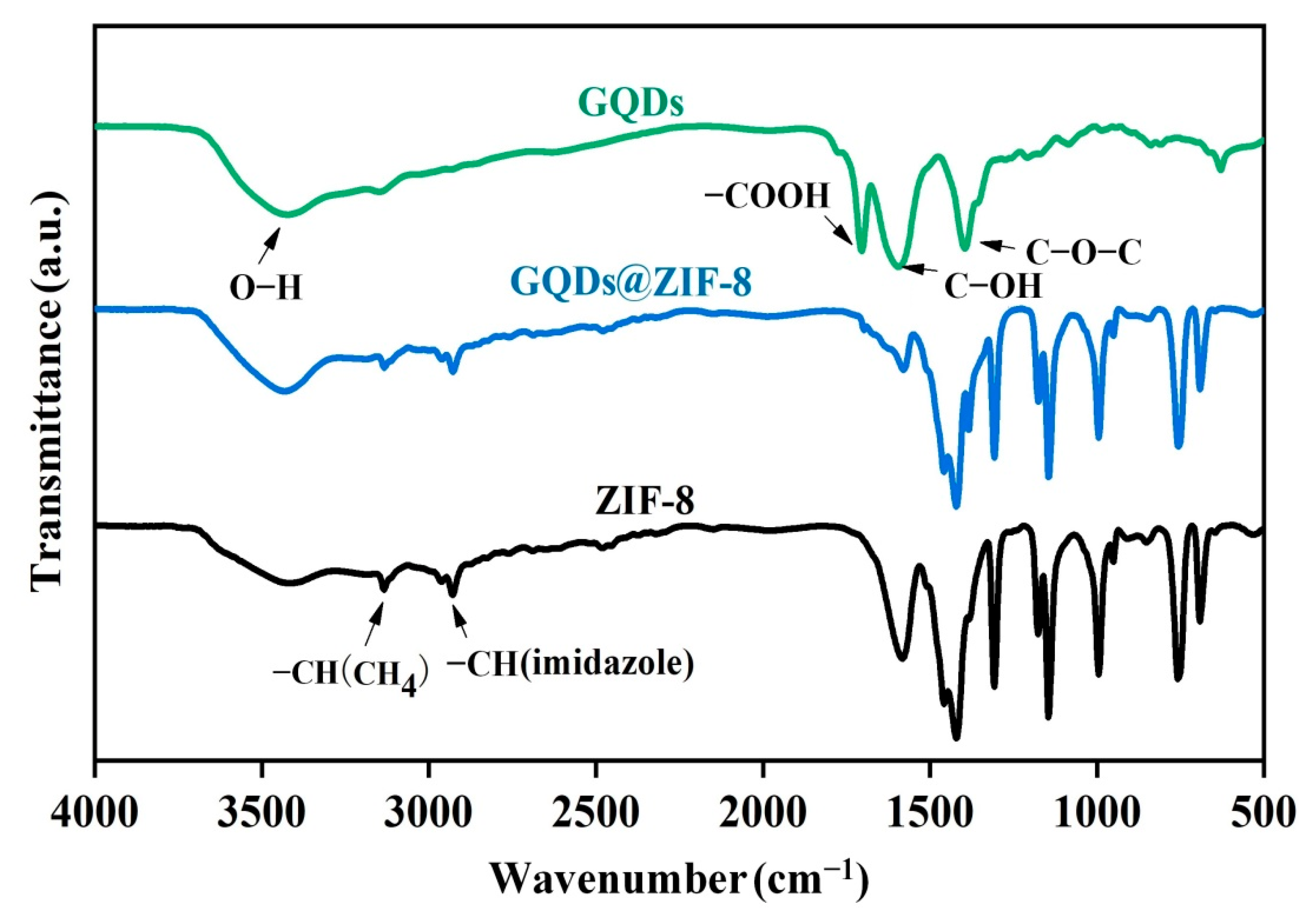
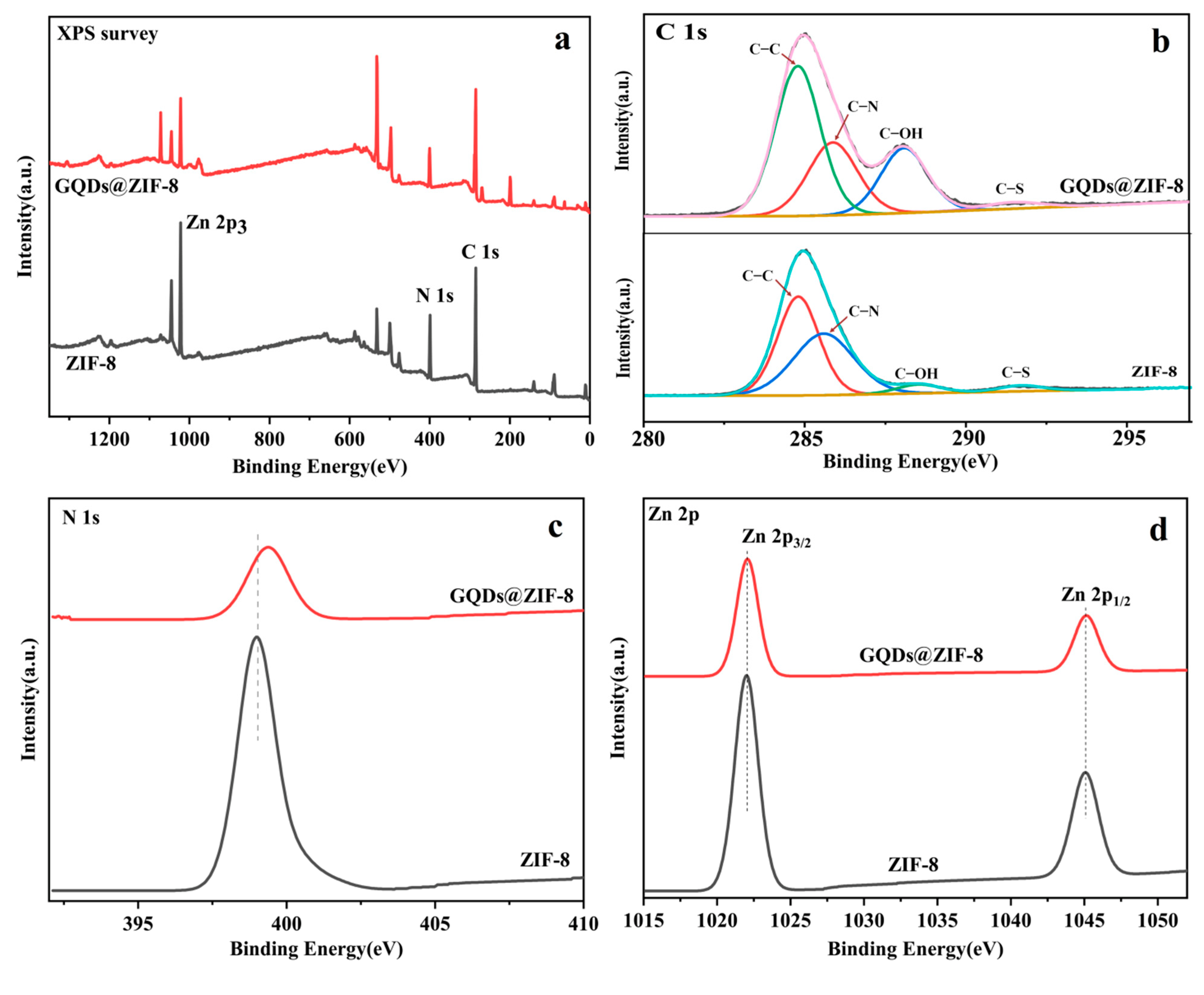
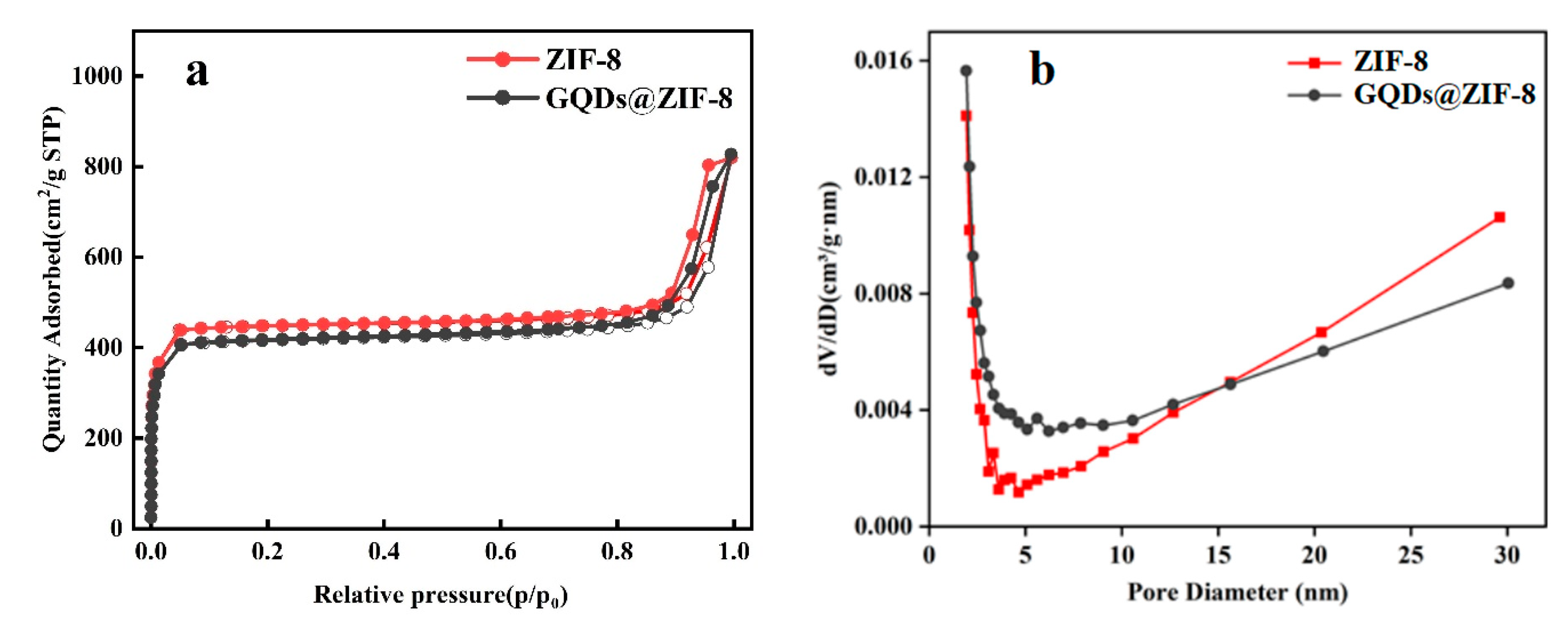

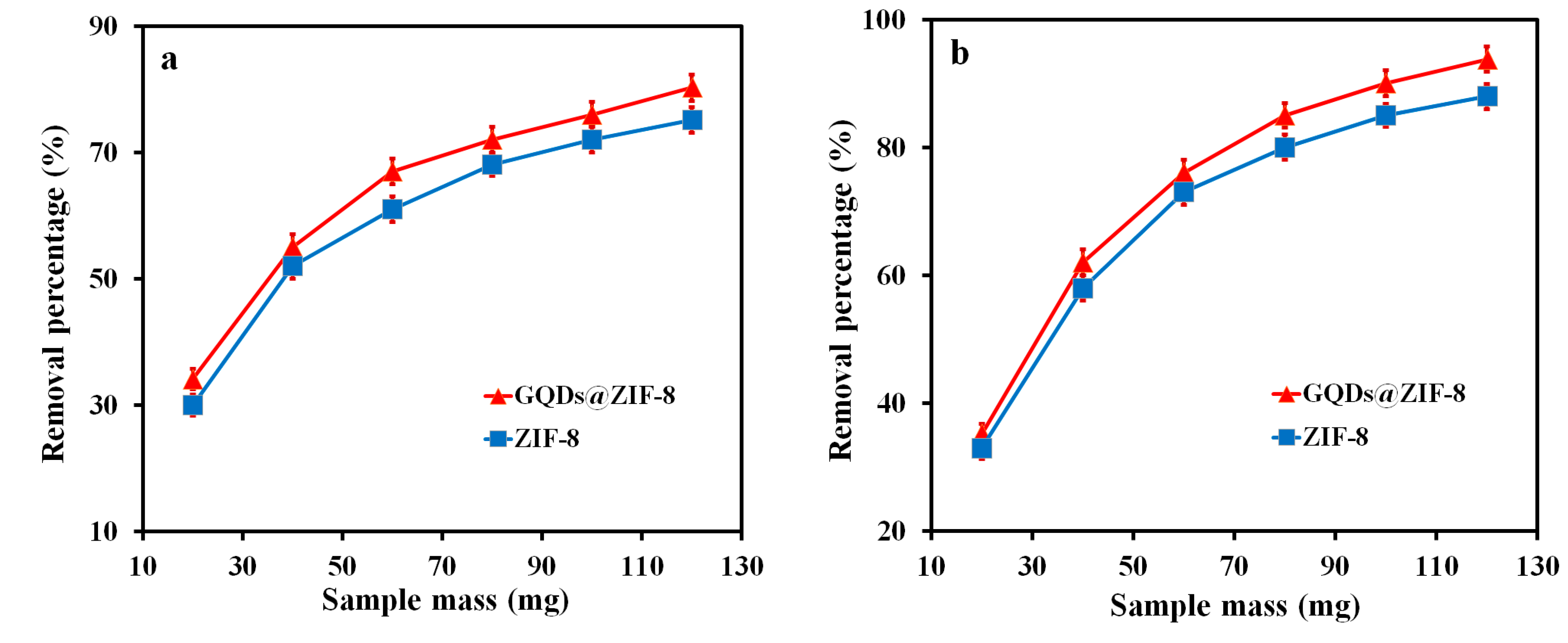
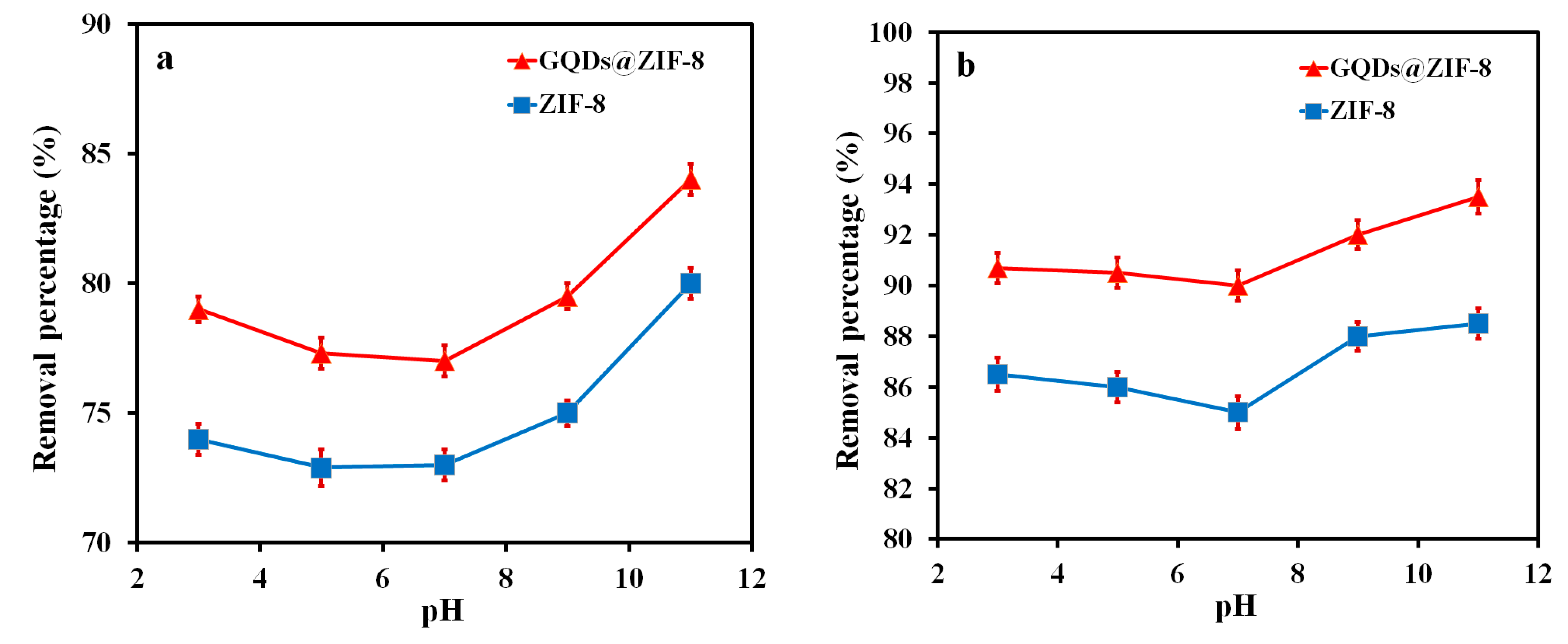

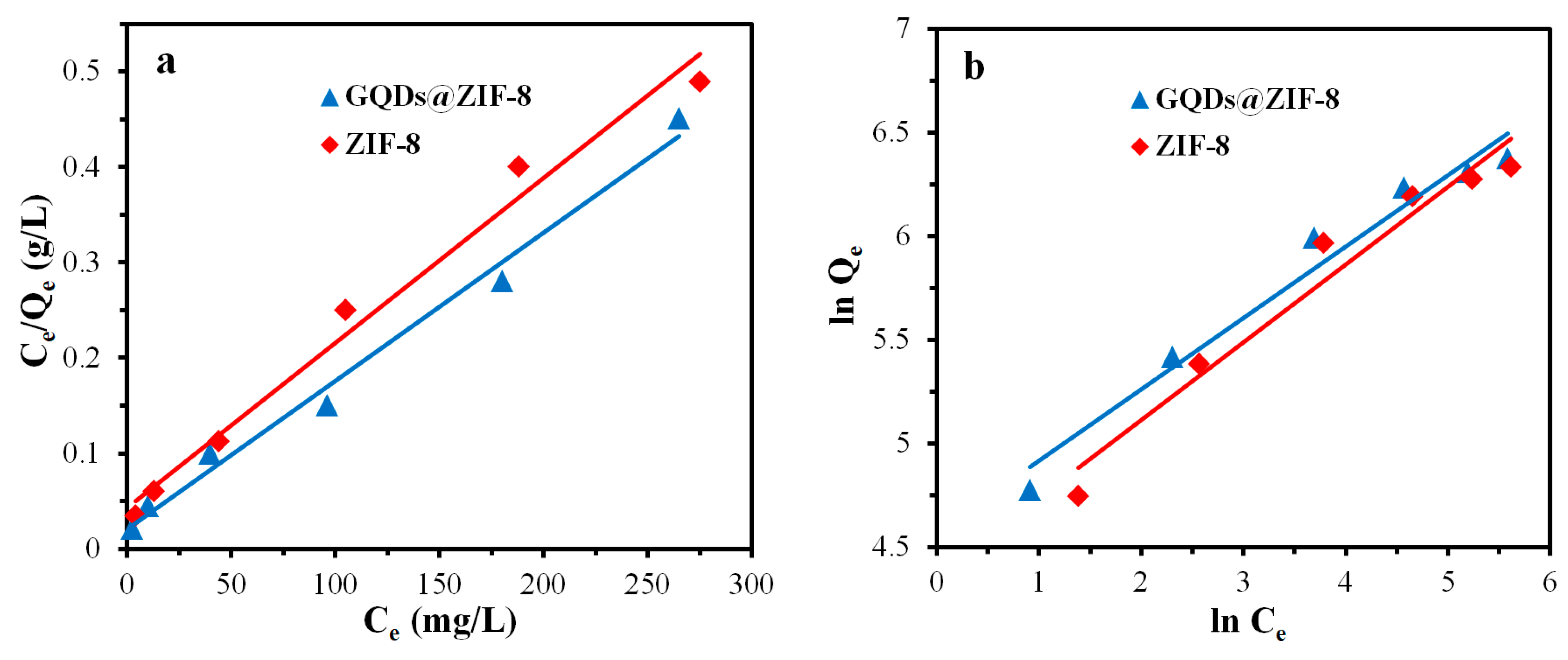


| Sample | SBET (m2/g) | Smic (m2/g) | Vtot (cm3/g) | Vmic (cm3/g) | D (nm) |
|---|---|---|---|---|---|
| ZIF-8 | 1368.74 | 1298.83 | 1.267 | 0.296 | 3.7 |
| GQDs@ZIF-8 | 1312.05 | 1228.81 | 1.280 | 0.607 | 3.9 |
| Adsorbate | Experimental Adsorption Capacity Qe,exp (mg/g) | Pseudo-First-Order Kinetic Equation | Pseudo-Second-Order Kinetic Equation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| k1 (min−1) | Qe,cal (mg/g) | R2 | χ2 | k2 (g/(mg·min)) | Qe,cal (mg/g) | R2 | χ2 | ||
| ZIF-8 | 521.02 | 0.036 | 485.46 | 0.99 | 2.60 | 0.000079 | 564.97 | 0.94 | 3.42 |
| GQDs@ZIF-8 | 552.31 | 0.038 | 533.57 | 0.98 | 0.66 | 0.000093 | 588.23 | 0.95 | 2.19 |
| Adsorbate | Experimental Adsorption Capacity Qe,exp (mg/g) | Pseudo-First-Order Kinetic Equation | Pseudo-Second-Order Kinetic Equation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| k1 (min−1) | Qe,cal (mg/g) | R2 | χ2 | k2 (g/(mg·min)) | Qe,cal (mg/g) | R2 | χ2 | ||
| ZIF-8 | 1326.17 | 0.040 | 1388.88 | 0.99 | 2.83 | 0.000053 | 1425.53 | 0.94 | 6.93 |
| GQDs@ZIF-8 | 1408.59 | 0.041 | 1453.93 | 0.99 | 1.41 | 0.000051 | 1474.31 | 0.97 | 2.93 |
| Adsorbent | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Qm (mg/g) | KL (L/mg) | R2 | KF (L/mg) | 1/n | R2 | |
| ZIF-8 | 588.23 | 0.047 | 0.95 | 78.34 | 0.38 | 0.98 |
| GQDs@ZIF-8 | 628.93 | 0.057 | 0.94 | 96.57 | 0.35 | 0.98 |
| Adsorbent | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Qm (mg/g) | KL (L/mg) | R2 | KF (L/mg) | 1/n | R2 | |
| ZIF-8 | 1428.57 | 0.012 | 0.95 | 93.16 | 0.44 | 0.99 |
| GQDs@ZIF-8 | 1567.39 | 0.016 | 0.96 | 123.66 | 0.41 | 0.99 |
| T (K) | Thermodynamic Parameters | ||
|---|---|---|---|
| ΔG (KJ/mol) | ΔH (KJ/mol) | ΔS (J/(mol·K)) | |
| 293.15 | −6.09 | 32.65 | 132.23 |
| 313.15 | −8.74 | ||
| 333.15 | −11.38 | ||
| T (K) | Thermodynamic Parameters | ||
|---|---|---|---|
| ΔG (KJ/mol) | ΔH (KJ/mol) | ΔS (J/(mol·K)) | |
| 293.15 | −7.18 | 58.45 | 223.98 |
| 313.15 | −11.66 | ||
| 333.15 | −16.14 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Cheng, Y.; Li, J.; Li, T.; Zhu, J.; Deng, W.; Zhu, J.; He, D. Preparation and Adsorption Performance Study of Graphene Quantum Dots@ZIF-8 Composites for Highly Efficient Removal of Volatile Organic Compounds. Nanomaterials 2022, 12, 4008. https://doi.org/10.3390/nano12224008
Li H, Cheng Y, Li J, Li T, Zhu J, Deng W, Zhu J, He D. Preparation and Adsorption Performance Study of Graphene Quantum Dots@ZIF-8 Composites for Highly Efficient Removal of Volatile Organic Compounds. Nanomaterials. 2022; 12(22):4008. https://doi.org/10.3390/nano12224008
Chicago/Turabian StyleLi, Hao, Youliang Cheng, Jiaxian Li, Tiehu Li, Jia Zhu, Weibin Deng, Jiajia Zhu, and Delong He. 2022. "Preparation and Adsorption Performance Study of Graphene Quantum Dots@ZIF-8 Composites for Highly Efficient Removal of Volatile Organic Compounds" Nanomaterials 12, no. 22: 4008. https://doi.org/10.3390/nano12224008
APA StyleLi, H., Cheng, Y., Li, J., Li, T., Zhu, J., Deng, W., Zhu, J., & He, D. (2022). Preparation and Adsorption Performance Study of Graphene Quantum Dots@ZIF-8 Composites for Highly Efficient Removal of Volatile Organic Compounds. Nanomaterials, 12(22), 4008. https://doi.org/10.3390/nano12224008







