A Versatile Luminescent Ga-Organic Framework with Multi-Emission Centers as a Blue LED and Fluorescent Probe for Low-Temperature Detection and Selective Fe3+ Sensing
Abstract
1. Introduction
2. Experimental Section
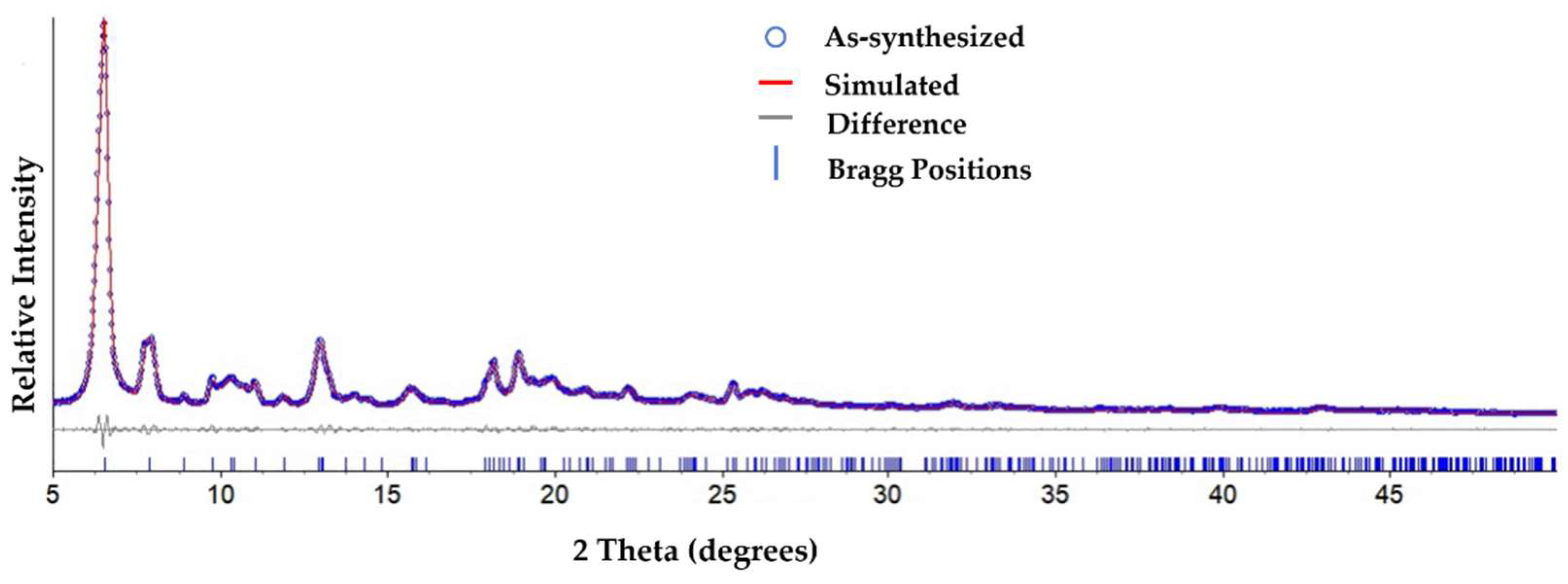
3. Results and Discussion
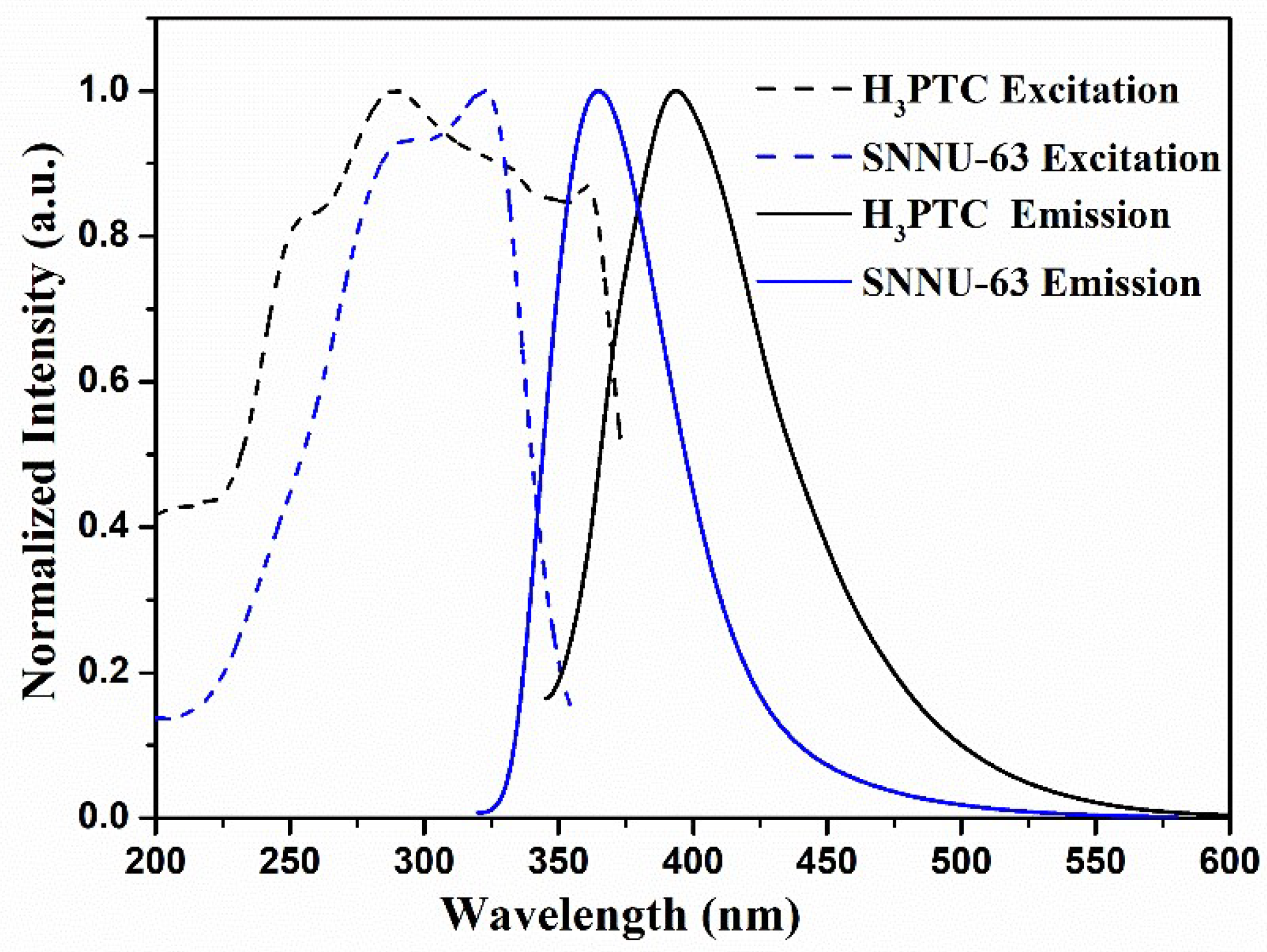
3.1. Photoluminescence Study
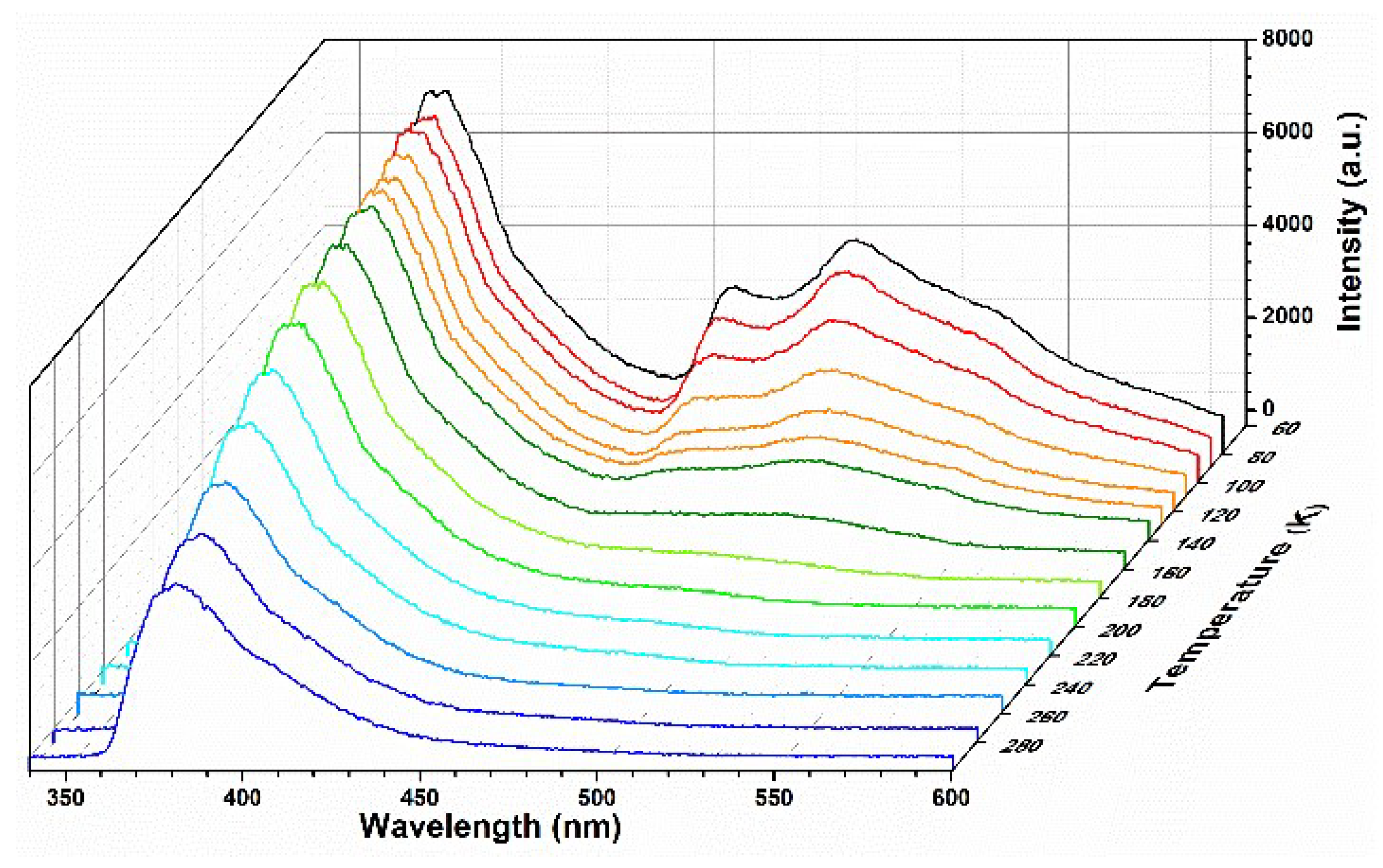
3.2. Temperature-Dependent Emission Properties
3.3. Metal Ions Sensing Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zhao, D.; Timmons, D.J.; Yuan, D.; Zhou, H.-C. Tuning the topology and functionality of metal-organic frameworks by ligand design. Acc. Chem. Res. 2011, 44, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zeng, H.; Zhu, X.-W.; Lu, W.; Li, D. Metal-organic frameworks as photoluminescent biosensing platforms: Mechanisms and applications. Chem. Soc. Rev. 2021, 50, 4484–4513. [Google Scholar] [CrossRef] [PubMed]
- Stavila, V.; Talin, A.A.; Allendorf, M.D. MOF-based electronic and opto-electronic devices. Chem. Soc. Rev. 2014, 43, 5994–6010. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, J.; He, H.; Qian, G. Photonic functional metal–organic frameworks. Chem. Soc. Rev. 2018, 47, 5740–5785. [Google Scholar] [CrossRef]
- Wu, S.; Min, H.; Shi, W.; Cheng, P. Multicenter metal-organic framework-based ratiometric fluorescent sensors. Adv. Mater. 2020, 32, 1805871. [Google Scholar] [CrossRef]
- Wu, Y.-P.; Tian, J.-W.; Liu, S.; Li, B.; Zhao, J.; Ma, L.-F.; Li, D.-S.; Lan, Y.-Q.; Bu, X. Bi-microporous metal-organic-frameworks with cubane [M4(OH)4] (M = Ni, Co) clusters and pore space partition for electrocatalytic methanol oxidation reaction. Angew. Chem. Int. Ed. 2019, 58, 12185–12189. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.-J.; Wang, N.; Zheng, P.; Fu, H.-R.; Han, M.-L.; Ma, L.-F.; Wang, L.-Y. Tetraphenylethylene-decorated metal-organic frameworks as energy-transfer platform for the detection of nitro-antibiotics and white-light emission. Inorg. Chem. 2019, 58, 12700–12706. [Google Scholar] [CrossRef]
- Yang, X.-G.; Lu, X.M.; Zhai, Z.-M.; Zhao, Y.; Liu, X.-Y.; Ma, L.-F.; Zang, S.-Q. Facile synthesis of micro-scale MOF host-guest with long-last phosphorescence and enhanced optoelectronic performance. Chem. Commun. 2018, 54, 13563–13566. [Google Scholar]
- Sun, Z.; Khurshid, A.; Sohail, M.; Qiu, W.; Cao, D.; Su, S.-J. Encapsulation of dyes in luminescent metal-organic frameworks for white light emitting diodes. Nanomaterials 2021, 11, 2761. [Google Scholar] [CrossRef]
- Cai, K.; Zhao, N.; Zhang, N.; Sun, F.-X.; Zhao, Q.; Zhu, G.-S. A homochiral multifunctional metal-organic framework with rod-shaped secondary building units. Nanomaterials 2017, 7, 88. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Li, H.; Li, J.-Q.; Chen, Y.; Qu, P.; Zhai, Q.-G. Enhancement of the fluorescence properties via introducing the tetraphenylethylene chromophores into a novel Mn-organic framework with a rare [Mn4(μ3-OH)2] cluster. Dalton Trans. 2021, 50, 17482–17486. [Google Scholar] [CrossRef] [PubMed]
- Dawood, S.; Dorris, A.; Davis, K.; Hammer, N.I.; Rathnayake, H. Synthesis, characterization, and photophysics of self-assembled Mn(II)-MOF with naphthalene chromophore. J. Phys. Chem. C 2021, 125, 792–802. [Google Scholar] [CrossRef]
- Wei, Z.; Gu, Z.-Y.; Arvapally, R.K.; Chen, Y.-P.; McDougald, R.N.; Ivy, J.F.; Yakovenko, A.A.; Feng, D.; Omary, M.A.; Zhou, H.-C. Rigidifying fluorescent linkers by metal-organic framework formation for fluorescence blue shift and quantum yield enhancement. J. Am. Chem. Soc. 2014, 136, 8269–8276. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wang, H.; Liu, W.; Teat, S.J.; Li, J. Blue-light-excitable, quantum yield enhanced, yellow-emitting, zirconium-based metal–organic framework phosphors formed by immobilizing organic chromophores. Cryst. Growth Des. 2019, 19, 6850–6854. [Google Scholar] [CrossRef]
- Yuan, S.; Qin, J.-S.; Lollar, C.T.; Zhou, H.-C. Stable metal-organic frameworks with group 4 metals: Current status and trends. ACS Cent. Sci. 2018, 4, 440–450. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Ji, W.-J.; Hu, M.-C.; Li, S.-N.; Jiang, Y.-C.; Zhang, X.-M.; Qu, P.; Zhai, Q.-G. A superstable 3p-block metal-organic framework platform towards prominent CO2 and C1/C2-hydrocarbon uptake and separation performance and strong lewis acid catalysis for CO2 fixation. Inorg. Chem. Front. 2019, 6, 813–819. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.-N.; Dong, W.-W.; Wu, Y.-P.; Li, D.-S.; Zhang, Q.-C. A robust luminescent Tb(III)-MOF with lewis basic pyridyl sites for the highly sensitive detection of metal Ions and small molecules. Inorg. Chem. 2016, 55, 3265–3271. [Google Scholar] [CrossRef]
- Wang, B.; Yang, Q.; Guo, C.; Sun, Y.; Xie, L.-H.; Li, J.-R. Stable Zr(IV)-based metal-organic frameworks with predesigned functionalized ligands for highly selective detection of Fe(III) ions in water. ACS Appl. Mater. Interfaces 2017, 9, 10286–10295. [Google Scholar] [CrossRef]
- Xu, N.; Tan, Y.-X.; El-Sayed, E.-S.M.; Yuan, D. Two zirconium metal organic cages with S4 and D2d symmetry: Construction and detection of antibiotics. Cryst. Growth Des. 2022, 22, 2768–2773. [Google Scholar] [CrossRef]
- Cairns, A.J.; Eckert, J.; Wojtas, L.; Thommes, M.; Wallacher, D.; Georgiev, P.A.; Forster, P.M.; Belmabkhout, Y.; Ollivier, J.; Eddaoudi, M. Gaining insights on the H2-sorbent interactions: Robust soc-MOF platform as a case study. Chem. Mater. 2016, 28, 7353–7361. [Google Scholar] [CrossRef]
- Yan, B. Lanthanide-functionalized metal–organic framework hybrid systems to create multiple luminescent centers for chemical sensing. Acc. Chem. Res. 2017, 50, 2789–2798. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Song, R.; Yu, J.; Liu, M.; Wang, Z.; Wu, C.; Yang, Y.; Wang, Z.; Chen, B.; Qian, G. Dual-emitting MOF⊃dye composite for ratiometric temperature sensing. Adv. Mater. 2015, 27, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Fan, H.-T.; Zang, S.-Q.; Li, H.-Y.; Wang, L.-Y. Metal–containing crystalline luminescent thermochromic materials. Coord. Chem. Rev. 2018, 377, 307–329. [Google Scholar]
- Liu, Y.; Lu, Y.-K.; Zhang, B.; Hou, L.; Wang, Y.-Y. Post-synthetic functionalization of Ni-MOF by Eu3+ ions: Luminescent probe for aspartic acid and magnetic property. Inorg. Chem. 2020, 59, 7531–7538. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Gao, Y.; Lin, H.; Huang, F.; Wang, Y. Strategy design for ratiometric luminescence thermometry: Circumventing the limitation of thermally coupled levels. J. Mater. Chem. C 2018, 6, 7462–7478. [Google Scholar] [CrossRef]
- Wu, J.-X.; Yan, B. A dual-emission probe to detect moisture and water in organic solvents based on green-Tb3+ post-coordinated metal–organic frameworks with red carbon dots. Dalton Trans. 2017, 46, 7098–7105. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-T.; Zhang, J.-W.; Lv, H.-J.; Hu, M.-C.; Li, S.-N.; Jiang, Y.-C.; Zhai, Q.-G. Tailoring the pore environment of a robust Ga-MOF by deformed [Ga3O(COO)6] cluster for boosting C2H2 uptake and separation. Inorg. Chem. 2020, 59, 10368–10373. [Google Scholar] [CrossRef]
- Coelho, A. Topas-A: General Profile and Structure Analysis Software for Powder Diffraction Data; Bruker-AXS: Madison, WI, USA, 2005. [Google Scholar]
- Wu, Z.; Li, C.; Zhang, F.; Huang, S.; Wang, F.; Wang, X.; Jiao, H. High-performance ultra-narrow-band green-emitting phosphor LaMgAl11O19:Mn2+ for wide color-gamut WLED backlight displays. J. Mater. Chem. C 2022, 10, 7443–7448. [Google Scholar] [CrossRef]
- Zhao, D.; Rao, X.; Yu, J.; Cui, Y.; Yang, Y.; Qian, G. Design and synthesis of an MOF thermometer with high sensitivity in the physiological temperature range. Inorg. Chem. 2015, 54, 11193–11199. [Google Scholar] [CrossRef]
- Lian, X.; Zhao, D.; Cui, Y.; Yang, Y.; Qian, G. A near infrared luminescent metal-organic framework for temperature sensing in the physiological range. Chem. Commun. 2015, 51, 17676–17679. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, D.; Cui, Y.; Yang, Y.; Qian, G. A Eu/Tb-mixed MOF for luminescent high-temperature sensing. J. Solid State Chem. 2017, 246, 341–345. [Google Scholar] [CrossRef]
- Huangfu, M.; Tian, X.; Zhao, S.; Wu, P.; Chu, H.; Zheng, X.; Tang, J.; Wang, J. Post-synthetic modification of a Tb-based metal-organic framework for highly selective and sensitive detection of metal ions in aqueous solution. New J. Chem. 2019, 43, 10232–10236. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, W.; Liang, L.; Zhang, J.; Ye, H.; Hu, X.; Zhang, J.; Wei, W. A Versatile Luminescent Ga-Organic Framework with Multi-Emission Centers as a Blue LED and Fluorescent Probe for Low-Temperature Detection and Selective Fe3+ Sensing. Nanomaterials 2022, 12, 4009. https://doi.org/10.3390/nano12224009
Shi W, Liang L, Zhang J, Ye H, Hu X, Zhang J, Wei W. A Versatile Luminescent Ga-Organic Framework with Multi-Emission Centers as a Blue LED and Fluorescent Probe for Low-Temperature Detection and Selective Fe3+ Sensing. Nanomaterials. 2022; 12(22):4009. https://doi.org/10.3390/nano12224009
Chicago/Turabian StyleShi, Weiwei, Lei Liang, Jinping Zhang, Haihan Ye, Xincheng Hu, Jianwei Zhang, and Wei Wei. 2022. "A Versatile Luminescent Ga-Organic Framework with Multi-Emission Centers as a Blue LED and Fluorescent Probe for Low-Temperature Detection and Selective Fe3+ Sensing" Nanomaterials 12, no. 22: 4009. https://doi.org/10.3390/nano12224009
APA StyleShi, W., Liang, L., Zhang, J., Ye, H., Hu, X., Zhang, J., & Wei, W. (2022). A Versatile Luminescent Ga-Organic Framework with Multi-Emission Centers as a Blue LED and Fluorescent Probe for Low-Temperature Detection and Selective Fe3+ Sensing. Nanomaterials, 12(22), 4009. https://doi.org/10.3390/nano12224009







