Highly Efficient Photocatalytic Hydrogen Evolution over Mo-Doped ZnIn2S4 with Sulfur Vacancies
Abstract
1. Introduction
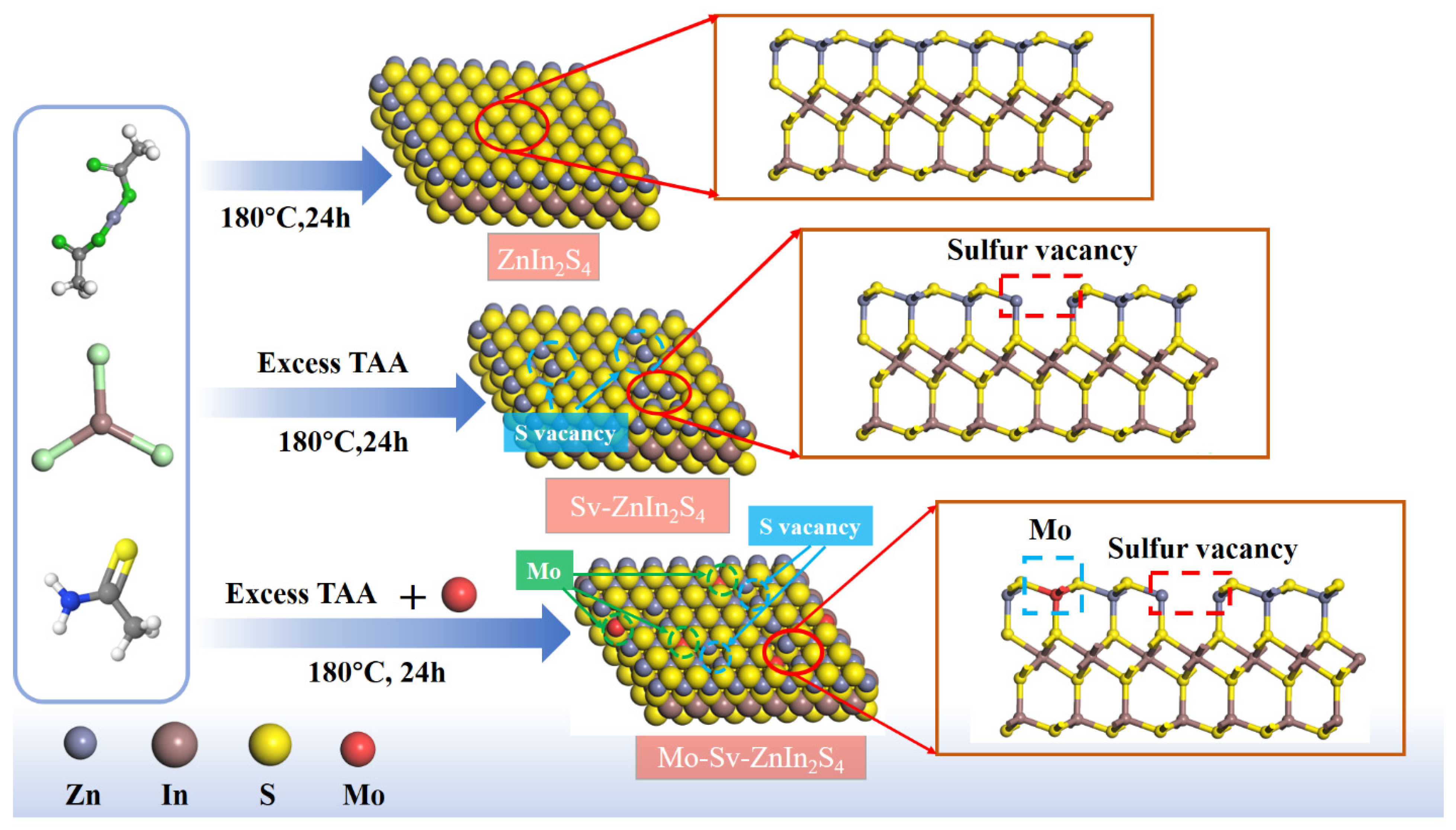
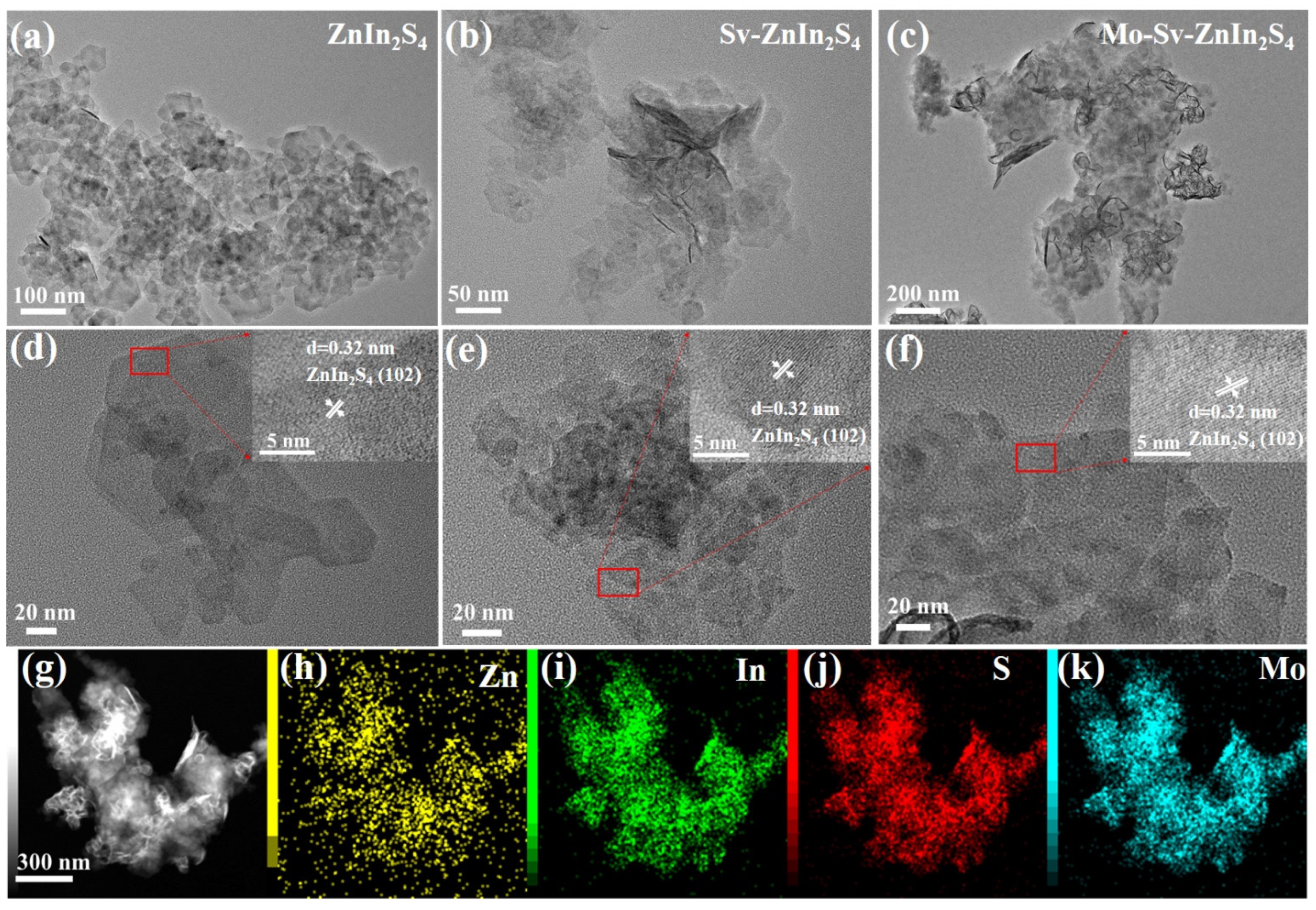
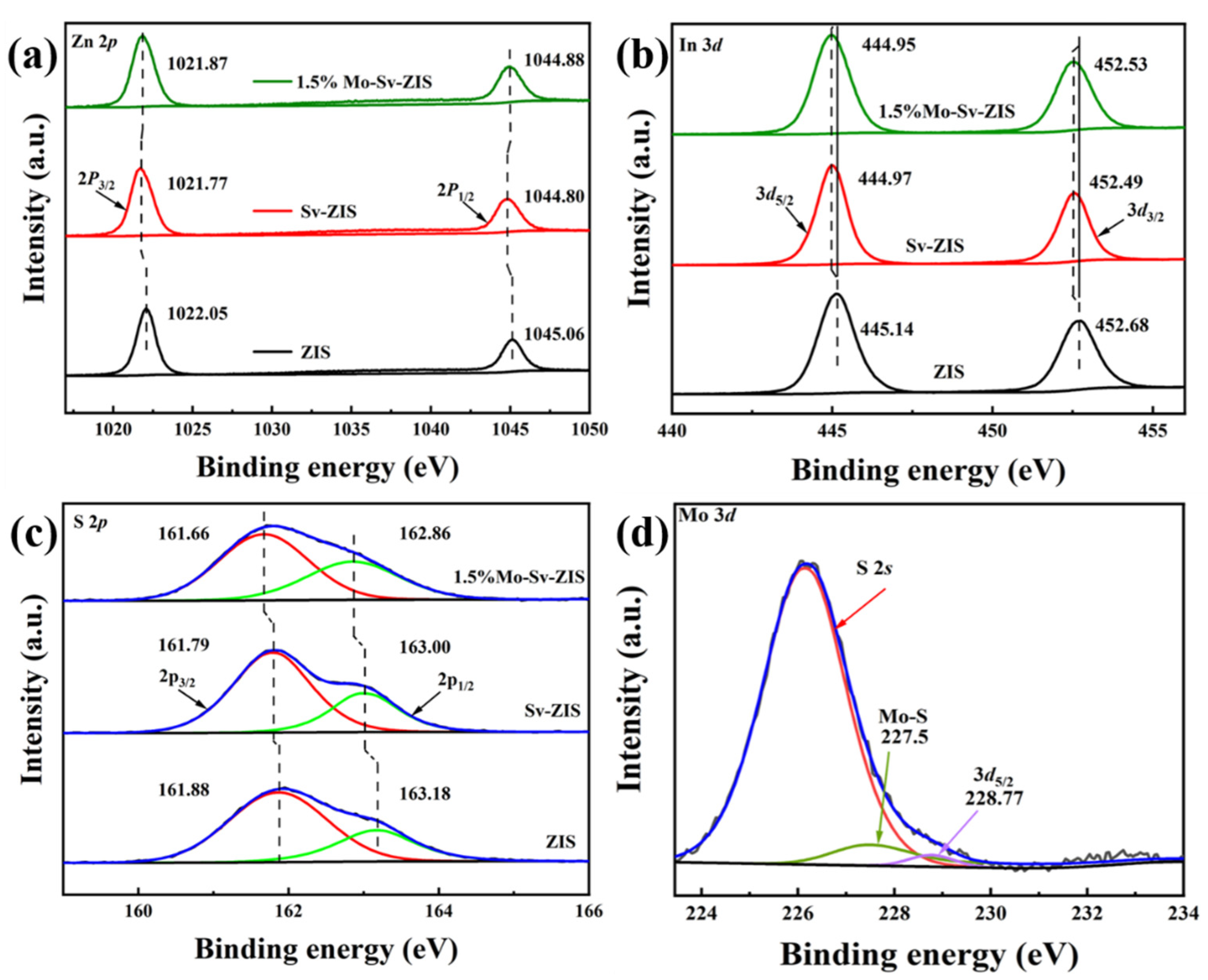
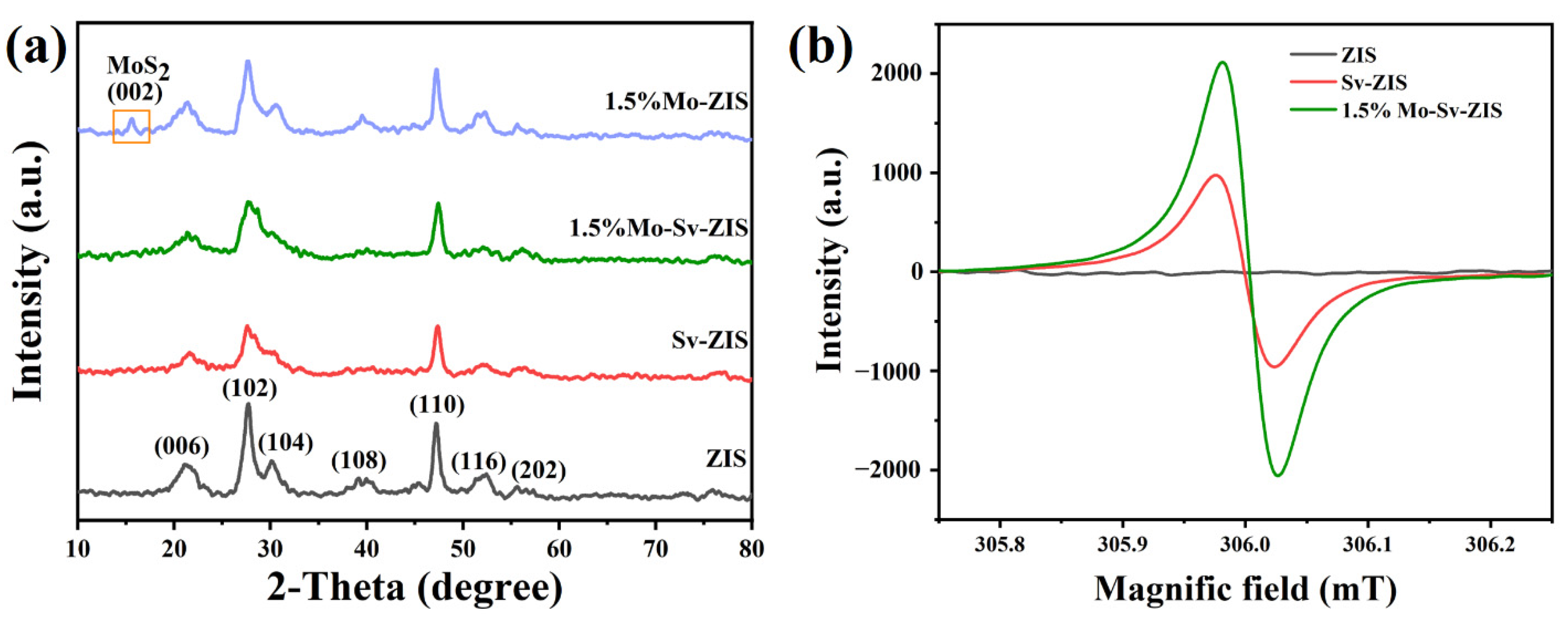
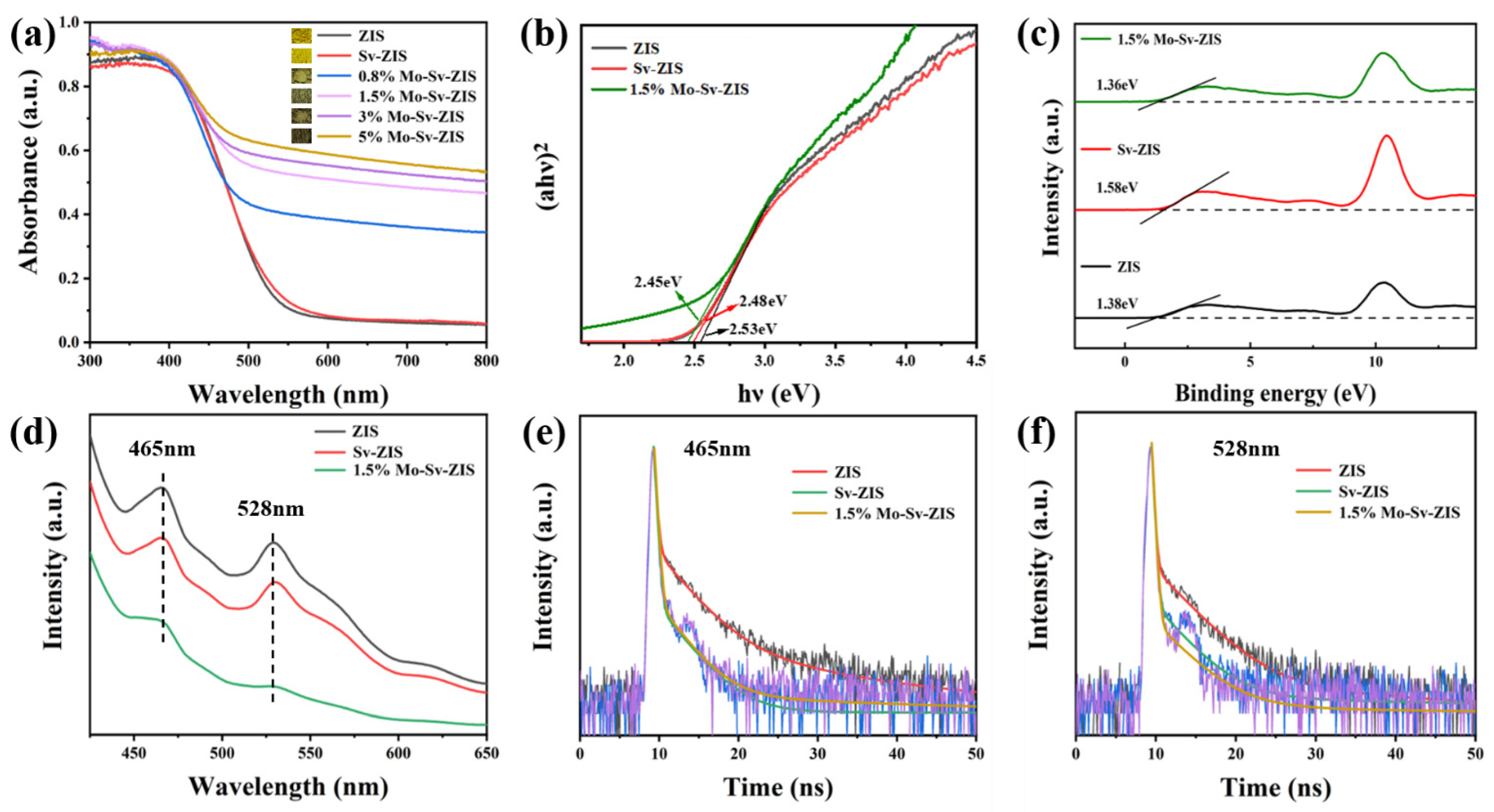
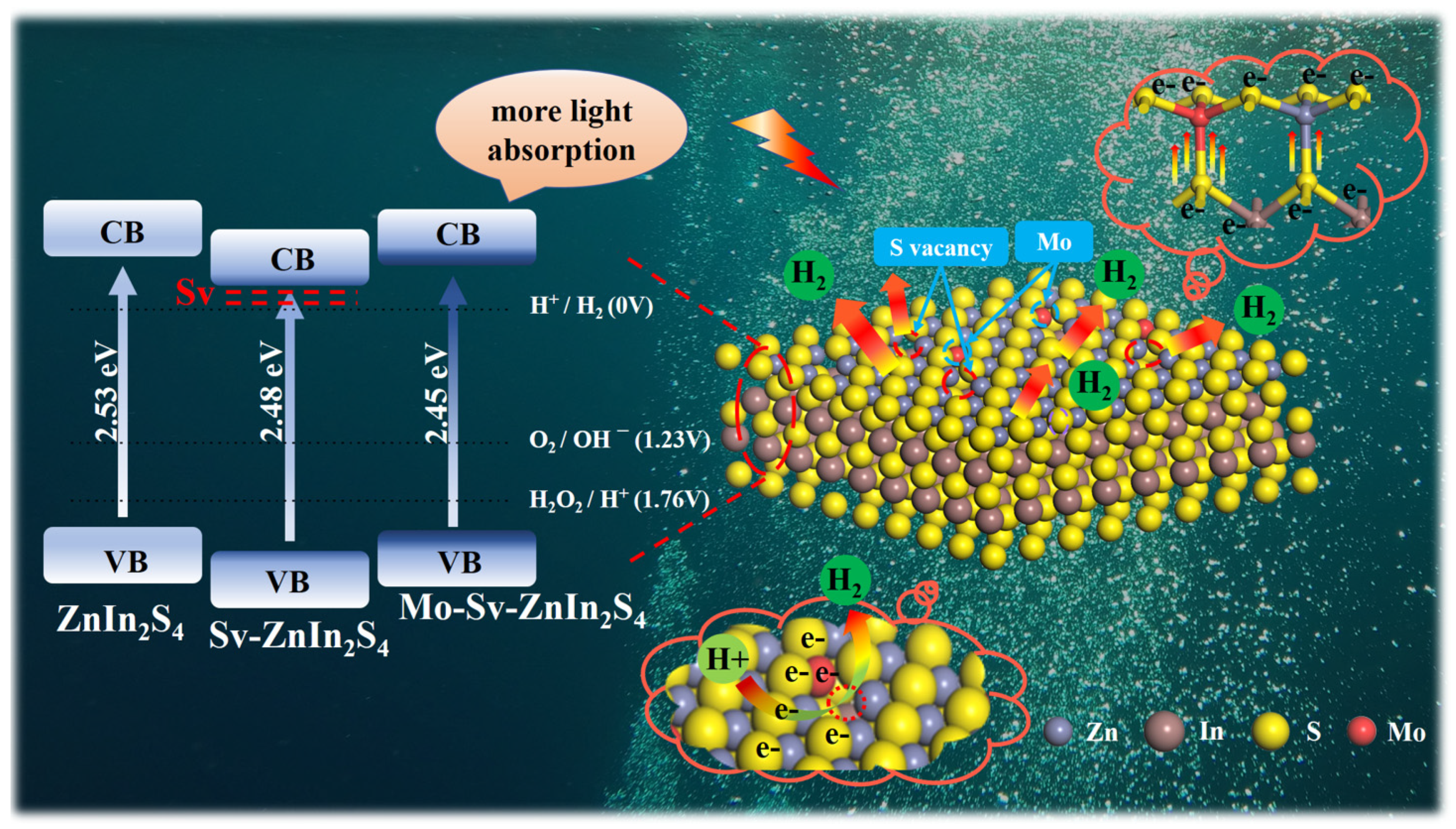
2. Results
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Liu, L.; Ma, T.; Zhang, Y.; Huang, H. 2D Graphitic Carbon Nitride for Energy Conversion and Storage. Adv. Funct. Mater. 2021, 31, 1807013. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, T.; Zheng, B.; Shi, J.; Cai, C.; Mao, L.; Cheng, C.; Zong, S.; Guo, X.; Chen, Q. EDTA-dominated hollow tube-like porous graphitic carbon nitride towards enhanced photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2022, 619, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Bai, X.; Ci, Q.; Du, L.; Ren, X.; Phillips, D.L. Near-Field Drives Long-Lived Shallow Trapping of Polymeric C3N4 for Efficient Photocatalytic Hydrogen Evolution. Adv. Funct. Mater. 2021, 31, 2103978. [Google Scholar] [CrossRef]
- Jia, R.; Gui, Q.; Sui, L.; Huang, Y.; Lu, H.; Dong, H.; Ma, S.; Gan, Z.; Dong, L.; Yu, L. Active sites provided by the surface autocatalytic effect and quantum confinement for stable and efficient photocatalytic hydrogen generation. J. Mater. Chem. A 2021, 9, 14768–14774. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Huang, J.; Li, S.; Meng, A.; Li, Z. Interfacial chemical bond and internal electric field modulated Z-scheme Sv-ZnIn2S4/MoSe2 photocatalyst for efficient hydrogen evolution. Nat. Commun. 2021, 12, 4112. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, H.; Li, S.; Wang, R.; Sun, X.; Zhou, M.; Zhou, J.; Lou, X.W.; Xie, Y. Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv. Mater. 2013, 25, 5807–5813. [Google Scholar] [CrossRef]
- Du, C.; Yan, B.; Yang, G. Self-integrated effects of 2D ZnIn2S4 and amorphous Mo2C nanoparticles composite for promoting solar hydrogen generation. Nano Energy 2020, 76, 105031. [Google Scholar] [CrossRef]
- Xiao, F.-X.; Miao, J.; Liu, B. Layer-by-layer self-assembly of CdS quantum dots/graphene nanosheets hybrid films for photoelectrochemical and photocatalytic applications. J. Am. Chem. Soc. 2014, 136, 1559–1569. [Google Scholar] [CrossRef]
- Maeda, K.; Higashi, M.; Lu, D.; Abe, R.; Domen, K. Efficient nonsacrificial water splitting through two-step photoexcitation by visible light using a modified oxynitride as a hydrogen evolution photocatalyst. J. Am. Chem. Soc. 2010, 132, 5858–5868. [Google Scholar] [CrossRef]
- Maeda, K.; Takata, T.; Hara, M.; Saito, N.; Inoue, Y.; Kobayashi, H.; Domen, K. GaN: ZnO solid solution as a photocatalyst for visible-light-driven overall water splitting. J. Am. Chem. Soc. 2005, 127, 8286–8287. [Google Scholar] [CrossRef]
- Kim, Y.; Coy, E.; Kim, H.; Mrówczyński, R.; Torruella, P.; Jeong, D.-W.; Choi, K.S.; Jang, J.H.; Song, M.Y.; Jang, D.-J. Efficient photocatalytic production of hydrogen by exploiting the polydopamine-semiconductor interface. Appl. Catal. B Environ. 2021, 280, 119423. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Zhang, S.L.; Zang, S.Q.; Lou, X.W. Supporting ultrathin ZnIn2S4 nanosheets on Co/N-Doped graphitic carbon nanocages for efficient photocatalytic H2 generation. Adv. Mater. 2019, 31, 1903404. [Google Scholar] [CrossRef] [PubMed]
- Kageshima, Y.; Shiga, S.; Ode, T.; Takagi, F.; Shiiba, H.; Htay, M.T.; Hashimoto, Y.; Teshima, K.; Domen, K.; Nishikiori, H. Photocatalytic and Photoelectrochemical Hydrogen Evolution from Water over Cu2SnxGe1–xS3 Particles. J. Am. Chem. Soc. 2021, 143, 5698–5708. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Geng, M.; Yu, J.; Zhang, Y.; Tian, F.; Guo, Y.n.; Zhang, D.; Yang, X.; Li, Z.; Li, Z.; et al. Vacancy-induced 2H@1T MoS2 phase-incorporation on ZnIn2S4 for boosting photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2021, 298, 120570. [Google Scholar] [CrossRef]
- Gou, X.; Cheng, F.; Shi, Y.; Zhang, L.; Peng, S.; Chen, J.; Shen, P. Shape-controlled synthesis of ternary chalcogenide ZnIn2S4 and CuIn(S, Se)2 nano-/microstructures via facile solution route. J. Am. Chem. Soc. 2006, 128, 7222–7229. [Google Scholar] [CrossRef]
- Shi, L.; Yin, P.; Dai, Y. Synthesis and photocatalytic performance of ZnIn2S4 nanotubes and nanowires. Langmuir 2013, 29, 12818–12822. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Zhou, W.; Tian, G.; Xiao, Y.; Fu, H.; Fu, H. Cubic quantum dot/hexagonal microsphere ZnIn2S4 heterophase junctions for exceptional visible-light-driven photocatalytic H2 evolution. J. Mater. Chem. A 2017, 5, 8451–8460. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Zhang, L.; Cao, M.; Yang, F.; Dai, W.-L. Facile construction of flower-like black phosphorus nanosheet@ ZnIn2S4 composite with highly efficient catalytic performance in hydrogen production. Appl. Surf. Sci. 2020, 504, 144366. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, K.; Wang, L.; Bai, B.; Liu, H.; Wang, Q. Aminated flower-like ZnIn2S4 coupled with benzoic acid modified g-C3N4 nanosheets via covalent bonds for ameliorated photocatalytic hydrogen generation. Appl. Catal. B Environ. 2020, 268, 118462. [Google Scholar] [CrossRef]
- Xiao, Y.; Peng, Z.; Zhang, W.; Jiang, Y.; Ni, L. Self-assembly of Ag2O quantum dots on the surface of ZnIn2S4 nanosheets to fabricate pn heterojunctions with wonderful bifunctional photocatalytic performance. Appl. Surf. Sci. 2019, 494, 519–531. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, L.; Xie, J.; Zhang, X.; Liu, Q.; Yao, T.; Wei, S.; Zhang, Q.; Xie, Y. Enhanced photoexcited carrier separation in oxygen-doped ZnIn2S4 nanosheets for hydrogen evolution. Angew. Chem. Int. Ed. 2016, 55, 6716–6720. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Mao, L.; Yang, P.; Zheng, H.; Fujitsuka, M.; Zhang, J.; Majima, T. Ultrathin ZnIn2S4 nanosheets with active (110) facet exposure and efficient charge separation for cocatalyst free photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2020, 265, 118616. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, S.; Lei, F.; Xie, Y. Atomically-thin two-dimensional sheets for understanding active sites in catalysis. Chem. Soc. Rev. 2015, 44, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Wu, X.; Wang, P.; Yu, H.; Zhu, B.; Fan, J.; Yu, J. Selenium-enriched amorphous NiSe1+ x nanoclusters as a highly efficient cocatalyst for photocatalytic H2 evolution. Chem. Eng. J. 2021, 408, 127230. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Z.; Si, Y.; Li, B.; Deng, F.; Yang, L.; Liu, X.; Dai, W.; Luo, S. Gradient Hydrogen Migration Modulated with Self-Adapting S Vacancy in Copper-Doped ZnIn2S4 Nanosheet for Photocatalytic Hydrogen Evolution. ACS Nano 2021, 15, 15238–15248. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, G.; Guan, Z.; Zhao, Q.; Li, G.; Yang, J.; Li, Q.; Zou, Z. Anchoring Ni single atoms on sulfur-vacancy-enriched ZnIn2S4 nanosheets for boosting photocatalytic hydrogen evolution. J. Energy Chem. 2021, 58, 408–414. [Google Scholar] [CrossRef]
- Wang, H.; Thangamuthu, M.; Wu, Z.; Yang, J.; Yuan, H.; Tang, J. Self-assembled sulphur doped carbon nitride for photocatalytic water reforming of methanol. Chem. Eng. J. 2022, 445, 136790. [Google Scholar] [CrossRef]
- Zhou, T.; Li, T.; Hou, J.; Wang, Y.; Hu, B.; Sun, D.; Wu, Y.; Jiang, W.; Che, G.; Liu, C. Tailoring boron doped intramolecular donor–acceptor integrated carbon nitride skeleton with propelling photocatalytic activity and mechanism insight. Chem. Eng. J. 2022, 445, 136643. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.; Zhou, X.; Jin, X.; Li, B.; Liu, J.; Chen, G. Cu doped SnS2 nanostructure induced sulfur vacancy towards boosted photocatalytic hydrogen evolution. Chem. Eng. J. 2021, 407, 127180. [Google Scholar] [CrossRef]
- Li, W.; Wang, F.; Liu, X.-y.; Dang, Y.-y.; Li, J.-y.; Ma, T.-h.; Wang, C.-y. Promoting body carriers migration of CdS nanocatalyst by N-doping for improved hydrogen production under simulated sunlight irradiation. Appl. Catal. B Environ. 2022, 313, 121470. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Zhao, S.; Huang, Z.; Chen, W.; Zhou, Y.; Lv, X.; Yuan, S. Bio-template synthesis of Mo-doped polymer carbon nitride for photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2019, 248, 44–53. [Google Scholar] [CrossRef]
- Zhang, N.; Jalil, A.; Wu, D.; Chen, S.; Liu, Y.; Gao, C.; Ye, W.; Qi, Z.; Ju, H.; Wang, C. Refining defect states in W18O49 by Mo doping: A strategy for tuning N2 activation towards solar-driven nitrogen fixation. J. Am. Chem. Soc. 2018, 140, 9434–9443. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, L.; Wang, K.; Sun, X.; Wang, W. Enhanced photocatalytic CO2 reduction to methane over WO3·0.33 H2O via Mo doping. Appl. Catal. B Environ. 2019, 243, 771–779. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Wang, Y.; Qin, H.; Li, X.; Zuo, Y.; Kang, S.; Cui, L. Synthesis of Mo-doped graphitic carbon nitride catalysts and their photocatalytic activity in the reduction of CO2 with H2O. Catal. Commun. 2016, 74, 75–79. [Google Scholar] [CrossRef]
- Etogo, A.; Liu, R.; Ren, J.; Qi, L.; Zheng, C.; Ning, J.; Zhong, Y.; Hu, Y. Facile one-pot solvothermal preparation of Mo-doped Bi2WO6 biscuit-like microstructures for visible-light-driven photocatalytic water oxidation. J. Mater. Chem. A 2016, 4, 13242–13250. [Google Scholar] [CrossRef]
- Xie, P.; Yang, F.; Li, R.; Ai, C.; Lin, C.; Lin, S. Improving hydrogen evolution activity of perovskite BaTiO3 with Mo doping: Experiments and first-principles analysis. Int. J. Hydrog. Energy 2019, 44, 11695–11704. [Google Scholar] [CrossRef]
- Zhang, R.; Li, P.; Wang, F.; Ye, L.; Gaur, A.; Huang, Z.; Zhao, Z.; Bai, Y.; Zhou, Y. Atomically dispersed Mo atoms on amorphous g-C3N4 promotes visible-light absorption and charge carriers transfer. Appl. Catal. B Environ. 2019, 250, 273–279. [Google Scholar] [CrossRef]
- Xing, F.; Liu, Q.; Huang, C. Mo-Doped ZnIn2S4 Flower-Like Hollow Microspheres for Improved Visible Light-Driven Hydrogen Evolution. Sol. RRL 2019, 4, 1900483. [Google Scholar] [CrossRef]
- Qiu, B.; Huang, P.; Lian, C.; Ma, Y.; Xing, M.; Liu, H.; Zhang, J. Realization of all-in-one hydrogen-evolving photocatalysts via selective atomic substitution. Appl. Catal. B Environ. 2021, 298, 120518. [Google Scholar] [CrossRef]
- Du, C.; Zhang, Q.; Lin, Z.; Yan, B.; Xia, C.; Yang, G. Half-unit-cell ZnIn2S4 monolayer with sulfur vacancies for photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2019, 248, 193–201. [Google Scholar] [CrossRef]
- Luan, Q.; Xue, X.; Li, R.; Gu, L.; Dong, W.; Zhou, D.; Wang, X.; Li, B.; Wang, G.; Hou, C. Boosting photocatalytic hydrogen evolution: Orbital redistribution of ultrathin ZnIn2S4 nanosheets via atomic defects. Appl. Catal. B Environ. 2022, 305, 121007. [Google Scholar] [CrossRef]
- Jiao, X.; Chen, Z.; Li, X.; Sun, Y.; Gao, S.; Yan, W.; Wang, C.; Zhang, Q.; Lin, Y.; Luo, Y.; et al. Defect-Mediated Electron-Hole Separation in One-Unit-Cell ZnIn2S4 Layers for Boosted Solar-Driven CO2 Reduction. J. Am. Chem. Soc. 2017, 139, 7586–7594. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Wu, Z.; Jin, C.; Li, W.; Wei, Q.; Chen, Y.; Wang, T. Flower-Like Dual-Defective Z-Scheme Heterojunction g-C3N4/ZnIn2S4 High-Efficiency Photocatalytic Hydrogen Evolution and Degradation of Mixed Pollutants. Nanomaterials 2021, 11, 2483. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Liu, M.; Zheng, C.; Long, F.; Min, Z.; Fu, F.; Yu, D.; Li, J.; Lee, J.H.; Kim, N.H. One-Pot Hydrothermal Synthesis of La-Doped ZnIn2S4 Microspheres with Improved Visible-Light Photocatalytic Performance. Nanomaterials 2020, 10, 2026. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Shang, C.; Chen, Z.; Jin, M.; Zhang, Y.; Zhang, Z.; Wang, X.; Zhou, G. Enhanced Photocatalytic H2 Evolution over ZnIn2S4 Flower-Like Microspheres Doped with Black Phosphorus Quantum Dots. Nanomaterials 2019, 9, 1266. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Liu, C.; Luo, S.; Wang, L.; Cai, T.; Zeng, Y.; Yuan, J.; Dong, W.; Pei, Y.; et al. MoS2 Quantum Dot Growth Induced by S Vacancies in a ZnIn2S4 Monolayer: Atomic-Level Heterostructure for Photocatalytic Hydrogen Production. ACS Nano 2018, 12, 751–758. [Google Scholar] [CrossRef]
- Turner, N.; Single, A. Determination of peak positions and areas from wide-scan XPS spectra. Surf. Interface Anal. 1990, 15, 215–222. [Google Scholar] [CrossRef]
- Duan, Y.; Xue, J.; Dai, J.; Wei, Y.; Wu, C.; Chang, S.-H.; Ma, J. Interface engineering of ZnO/In2O3 Z-scheme heterojunction with yolk-shell structure for efficient photocatalytic hydrogen evolution. Appl. Surf. Sci. 2022, 592, 153306. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, J.; Zhu, B.; Fedin, M.V.; Cheng, B.; Yu, J.; Zhang, L. ZnO/COF S-scheme heterojunction for improved photocatalytic H2O2 production performance. Chem. Eng. J. 2022, 444, 136584. [Google Scholar] [CrossRef]
- Wang, P.; Shen, Z.; Xia, Y.; Wang, H.; Zheng, L.; Xi, W.; Zhan, S. Atomic Insights for Optimum and Excess Doping in Photocatalysis: A Case Study of Few-Layer Cu- ZnIn2S4. Adv. Funct. Mater. 2019, 29, 1807013. [Google Scholar] [CrossRef]
- Zhong, X.; Sun, Y.; Chen, X.; Zhuang, G.; Li, X.; Wang, J.-G. Mo Doping Induced More Active Sites in Urchin-Like W18O49Nanostructure with Remarkably Enhanced Performance for Hydrogen Evolution Reaction. Adv. Funct. Mater. 2016, 26, 5778–5786. [Google Scholar] [CrossRef]
- Huang, X.; Gu, W.; Ma, Y.; Liu, D.; Ding, N.; Zhou, L.; Lei, J.; Wang, L.; Zhang, J. Recent advances of doped graphite carbon nitride for photocatalytic reduction of CO2: A review. Res. Chem. Intermed. 2020, 46, 5133–5164. [Google Scholar] [CrossRef]
- Ye, L.; Fu, J.; Xu, Z.; Yuan, R.; Li, Z. Facile one-pot solvothermal method to synthesize sheet-on-sheet reduced graphene oxide (RGO)/ZnIn2S4 nanocomposites with superior photocatalytic performance. ACS Appl. Mater. Interfaces 2014, 6, 3483–3490. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kang, B.; Dong, F.; Zhang, Z.; Luo, X.; Han, L.; Huang, J.; Feng, Z.; Chen, Z.; Xu, J.; et al. Enhanced photocatalytic degradation and H2/H2O2 production performance of S-pCN/WO2.72 S-scheme heterojunction with appropriate surface oxygen vacancies. Nano Energy 2021, 81, 105671. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.; Lu, L.; Zhou, C.; Xin, Z.; Wang, J.; Ke, X.-k.; Sheng, G.; Yan, S.; Zou, Z. Oxygen-Vacancy-Activated CO2 Splitting over Amorphous Oxide Semiconductor Photocatalyst. ACS Catal. 2017, 8, 516–525. [Google Scholar] [CrossRef]
- Jia, R.; Lu, H.; Wang, C.; Guan, W.; Dong, H.; Pang, B.; Sui, L.; Gan, Z.; Dong, L.; Yu, L. Construction of 2D-layered quantum dots/2D-nanosheets heterostructures with compact interfaces for highly efficient photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2022, 608, 284–293. [Google Scholar] [CrossRef]
- Xue, J.; Liu, H.; Zeng, S.; Feng, Y.; Zhang, Y.; Zhu, Y.; Cheng, M.; Zhang, H.; Shi, L.; Zhang, G. Bifunctional Cobalt-Doped ZnIn2S4 Hierarchical Nanotubes Endow Noble-Metal Cocatalyst-Free Photocatalytic H2 Production Coupled with Benzyl Alcohol Oxidation. Solar RRL 2022, 6, 2101042. [Google Scholar] [CrossRef]
- Zhan, X.; Zheng, Y.; Li, B.; Fang, Z.; Yang, H.; Zhang, H.; Xu, L.; Shao, G.; Hou, H.; Yang, W. Rationally designed Ta3N5/ZnIn2S4 1D/2D heterojunctions for boosting Visible-Light-driven hydrogen evolution. Chem. Eng. J. 2022, 431, 134053. [Google Scholar] [CrossRef]
- Su, H.; Lou, H.; Zhao, Z.; Zhou, L.; Pang, Y.; Xie, H.; Rao, C.; Yang, D.; Qiu, X. In-situ Mo doped ZnIn2S4 wrapped MoO3 S-scheme heterojunction via Mo-S bonds to enhance photocatalytic HER. Chem. Eng. J. 2022, 430, 132770. [Google Scholar] [CrossRef]
- Bhavani, P.; Praveen Kumar, D.; Hussain, M.; Aminabhavi, T.M.; Park, Y.-K. Eco-friendly rice husk derived biochar as a highly efficient noble Metal-Free cocatalyst for high production of H2 using solar light irradiation. Chem. Eng. J. 2022, 434, 134743. [Google Scholar] [CrossRef]
- Kong, D.; Hu, X.; Geng, J.; Zhao, Y.; Fan, D.; Lu, Y.; Geng, W.; Zhang, D.; Liu, J.; Li, H.; et al. Growing ZnIn2S4 nanosheets on FeWO4 flowers with p-n heterojunction structure for efficient photocatalytic H2 production. Appl. Surf. Sci. 2022, 591, 153256. [Google Scholar] [CrossRef]
- She, P.; Qin, J.S.; Sheng, J.; Qi, Y.; Rui, H.; Zhang, W.; Ge, X.; Lu, G.; Song, X.; Rao, H. Dual-Functional Photocatalysis for Cooperative Hydrogen Evolution and Benzylamine Oxidation Coupling over Sandwiched-Like Pd@TiO2@ ZnIn2S4 Nanobox. Small 2022, 18, e2105114. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Rao, C.; Zhou, L.; Pang, Y.; Lou, H.; Yang, D.; Qiu, X. Mo-Doped/Ni-supported ZnIn2S4-wrapped NiMoO4 S-scheme heterojunction photocatalytic reforming of lignin into hydrogen. Green Chem. 2022, 24, 2027–2035. [Google Scholar] [CrossRef]
- Dai, M.; He, Z.; Zhang, P.; Li, X.; Wang, S. ZnWO4- ZnIn2S4 S-scheme heterojunction for enhanced photocatalytic H2 evolution. J. Mater. Sci. Technol. 2022, 122, 231–242. [Google Scholar] [CrossRef]
- Cao, M.; Yang, F.; Zhang, Q.; Zhang, J.; Zhang, L.; Li, L.; Wang, X.; Dai, W.-L. Facile construction of highly efficient MOF-based Pd@UiO-66-NH2@ ZnIn2S4 flower-like nanocomposites for visible-light-driven photocatalytic hydrogen production. J. Mater. Sci. Technol. 2021, 76, 189–199. [Google Scholar] [CrossRef]
- Dhingra, S.; Sharma, M.; Krishnan, V.; Nagaraja, C.M. Design of noble metal-free NiTiO3/ZnIn2S4 heterojunction photocatalyst for efficient visible-light-assisted production of H2 and selective synthesis of 2,5-Bis(hydroxymethyl)furan. J. Colloid Interface Sci. 2022, 615, 346–356. [Google Scholar] [CrossRef]
- Liu, L.; Liu, J.; Yang, W.; Wan, J.; Fu, F.; Wang, D. Constructing a Z-scheme ZnIn2S4-S/CNTs/RP nanocomposite with modulated energy band alignment for enhanced photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2022, 608, 482–492. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, W.; Zhang, L.; Wang, P.; Wang, Y.; Wang, H.; Dong, X.; Meng, M.; Sui, L.; Gan, Z.; Dong, L.; et al. Highly Efficient Photocatalytic Hydrogen Evolution over Mo-Doped ZnIn2S4 with Sulfur Vacancies. Nanomaterials 2022, 12, 3980. https://doi.org/10.3390/nano12223980
Guan W, Zhang L, Wang P, Wang Y, Wang H, Dong X, Meng M, Sui L, Gan Z, Dong L, et al. Highly Efficient Photocatalytic Hydrogen Evolution over Mo-Doped ZnIn2S4 with Sulfur Vacancies. Nanomaterials. 2022; 12(22):3980. https://doi.org/10.3390/nano12223980
Chicago/Turabian StyleGuan, Wei, Lin Zhang, Peng Wang, Ying Wang, Haoyu Wang, Xingchen Dong, Ming Meng, Lina Sui, Zhixing Gan, Lifeng Dong, and et al. 2022. "Highly Efficient Photocatalytic Hydrogen Evolution over Mo-Doped ZnIn2S4 with Sulfur Vacancies" Nanomaterials 12, no. 22: 3980. https://doi.org/10.3390/nano12223980
APA StyleGuan, W., Zhang, L., Wang, P., Wang, Y., Wang, H., Dong, X., Meng, M., Sui, L., Gan, Z., Dong, L., & Yu, L. (2022). Highly Efficient Photocatalytic Hydrogen Evolution over Mo-Doped ZnIn2S4 with Sulfur Vacancies. Nanomaterials, 12(22), 3980. https://doi.org/10.3390/nano12223980







