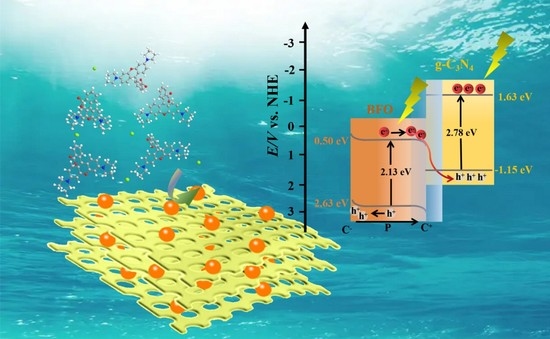
Facilitated Photocatalytic Degradation of Rhodamine B over One-Step Synthesized Honeycomb-Like BiFeO3/g-C3N4 Catalyst
Abstract
1. Introduction
2. Experimental
2.1. Synthesis of the Photocatalysts
2.2. Catalysts Characterizations
2.3. Photocatalytic Activity Test
3. Results and Discussion
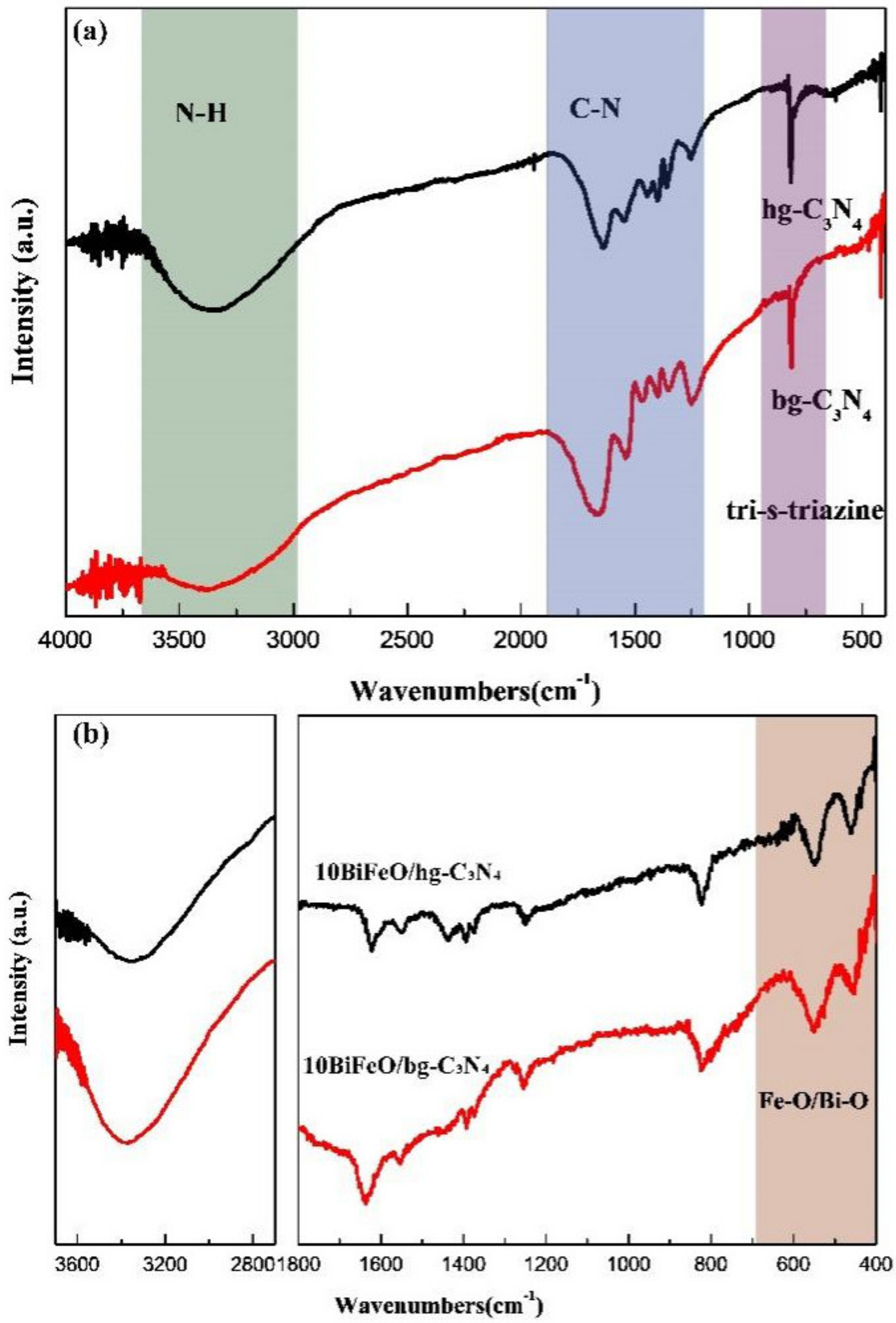
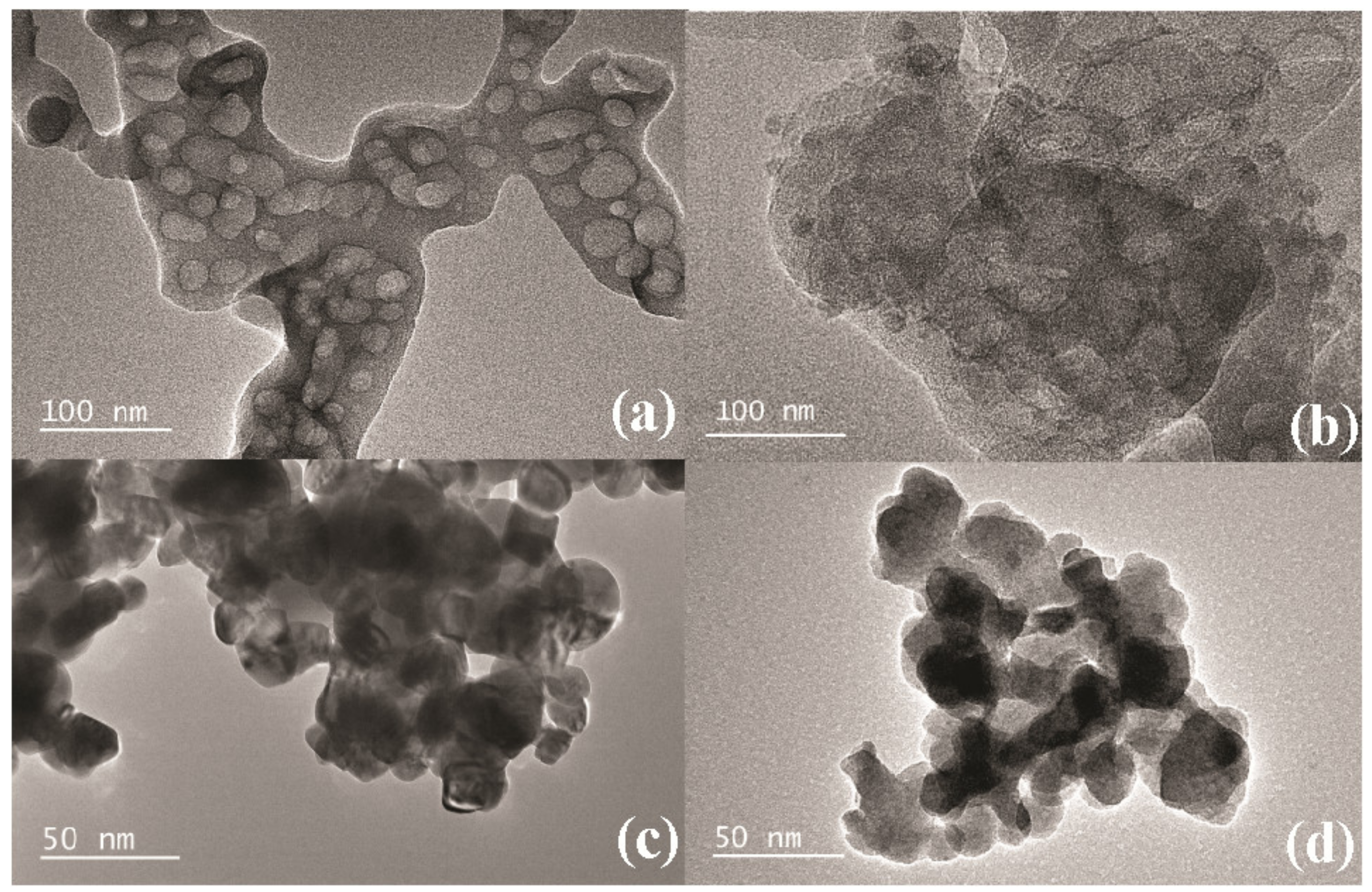
3.1. Structure and Morphology
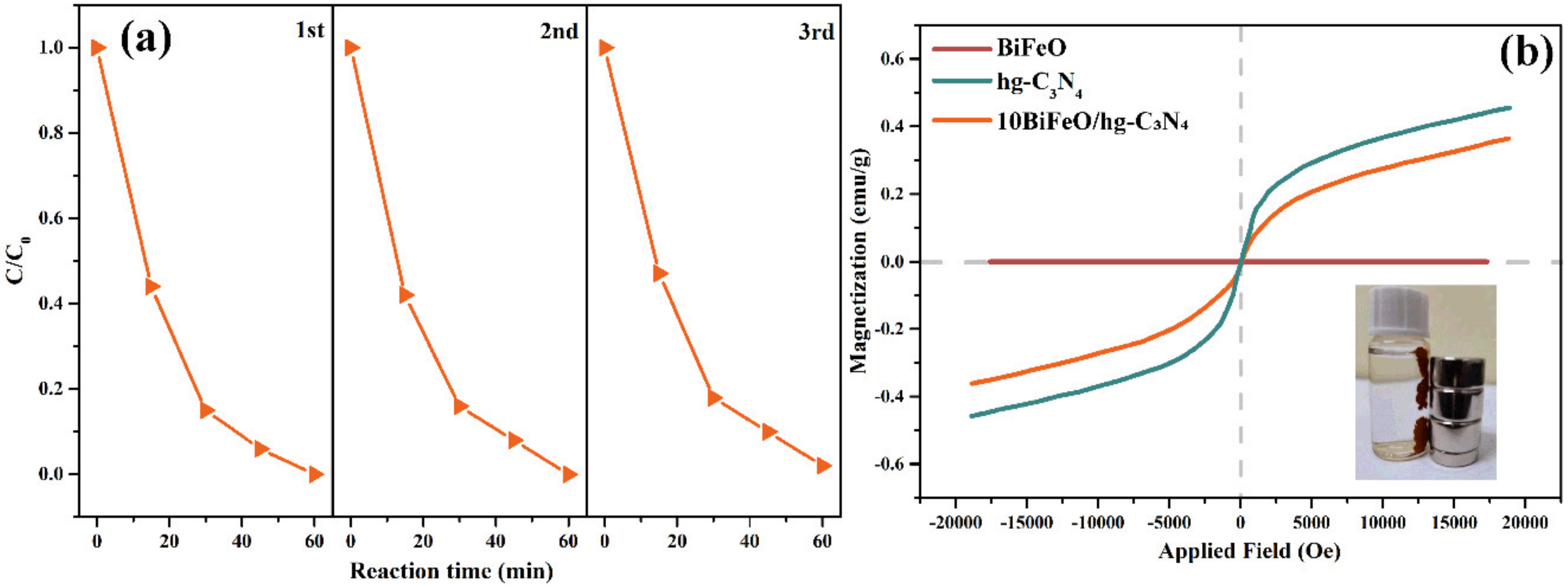
3.2. Evaluation of Photocatalytic Performance
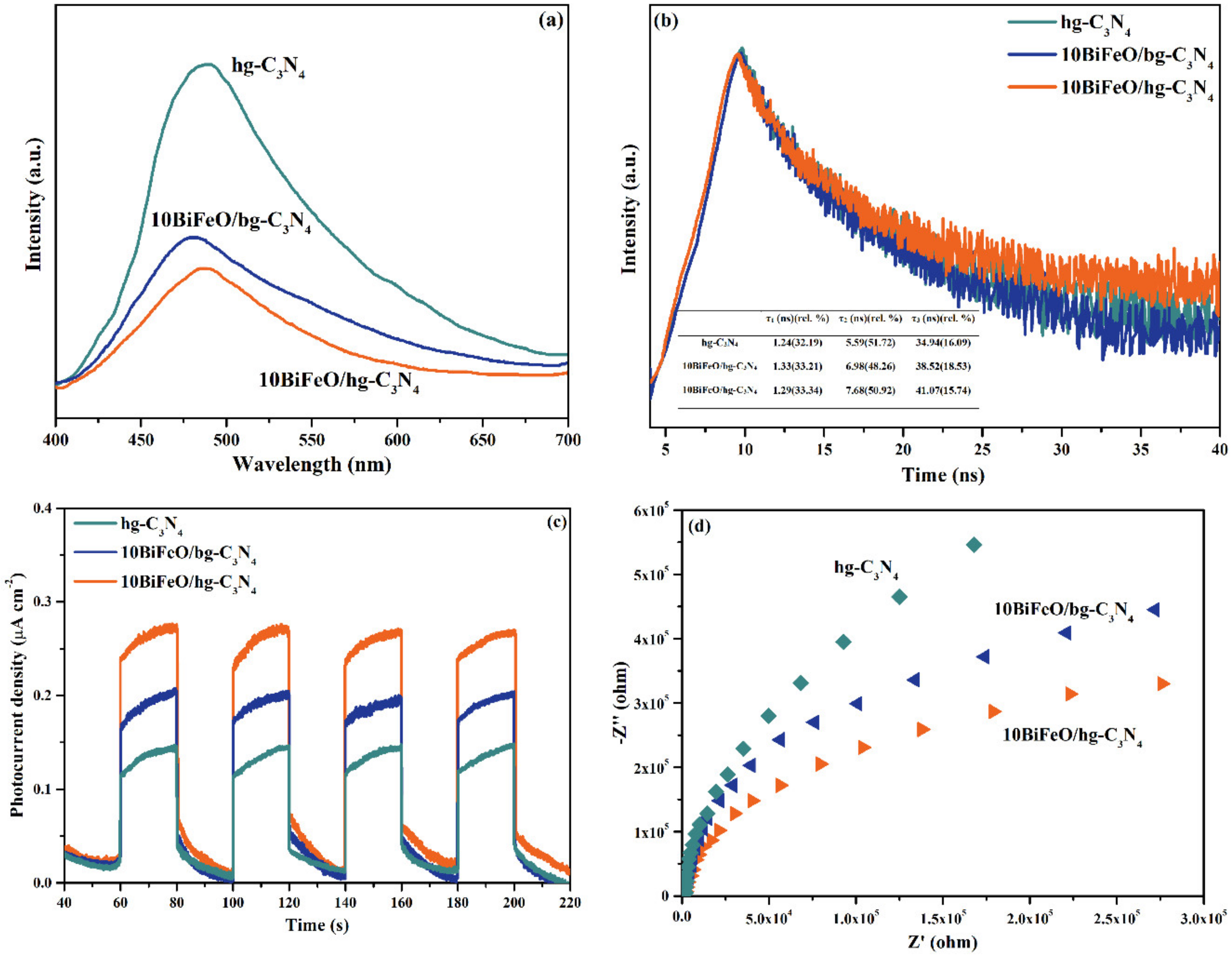
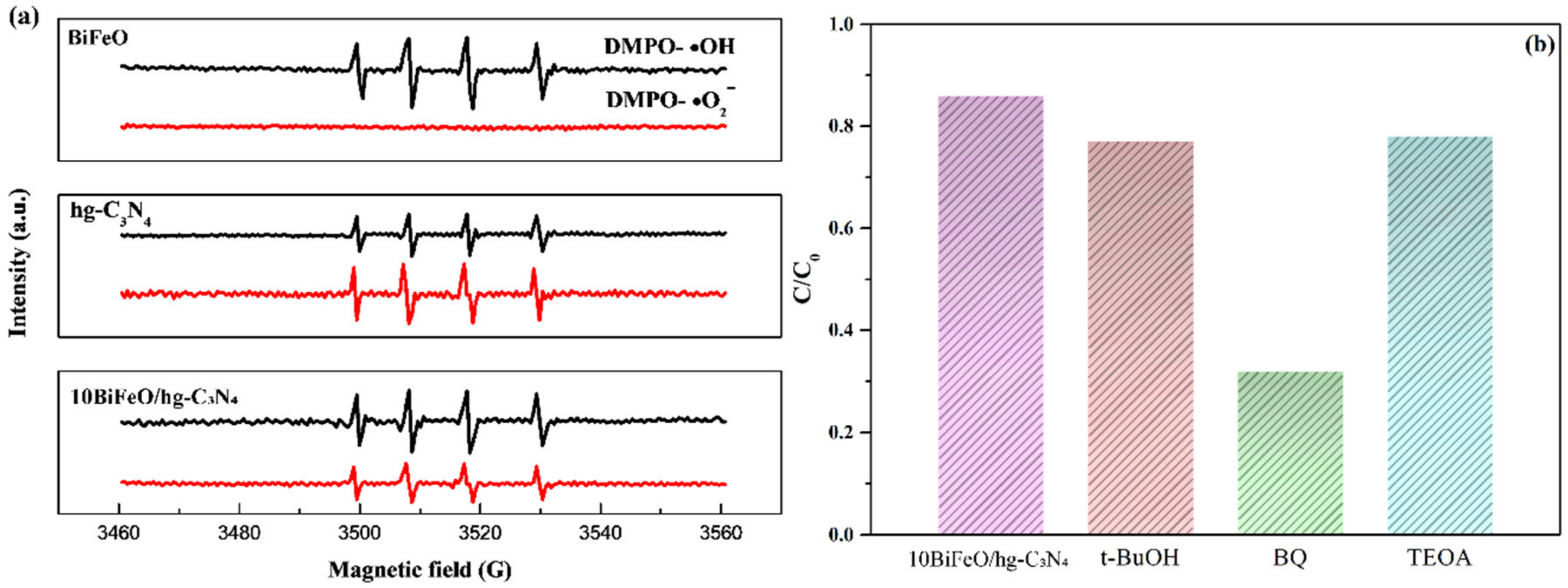
3.3. Reaction Mechanisms
3.3.1. Micro-Nano Structure
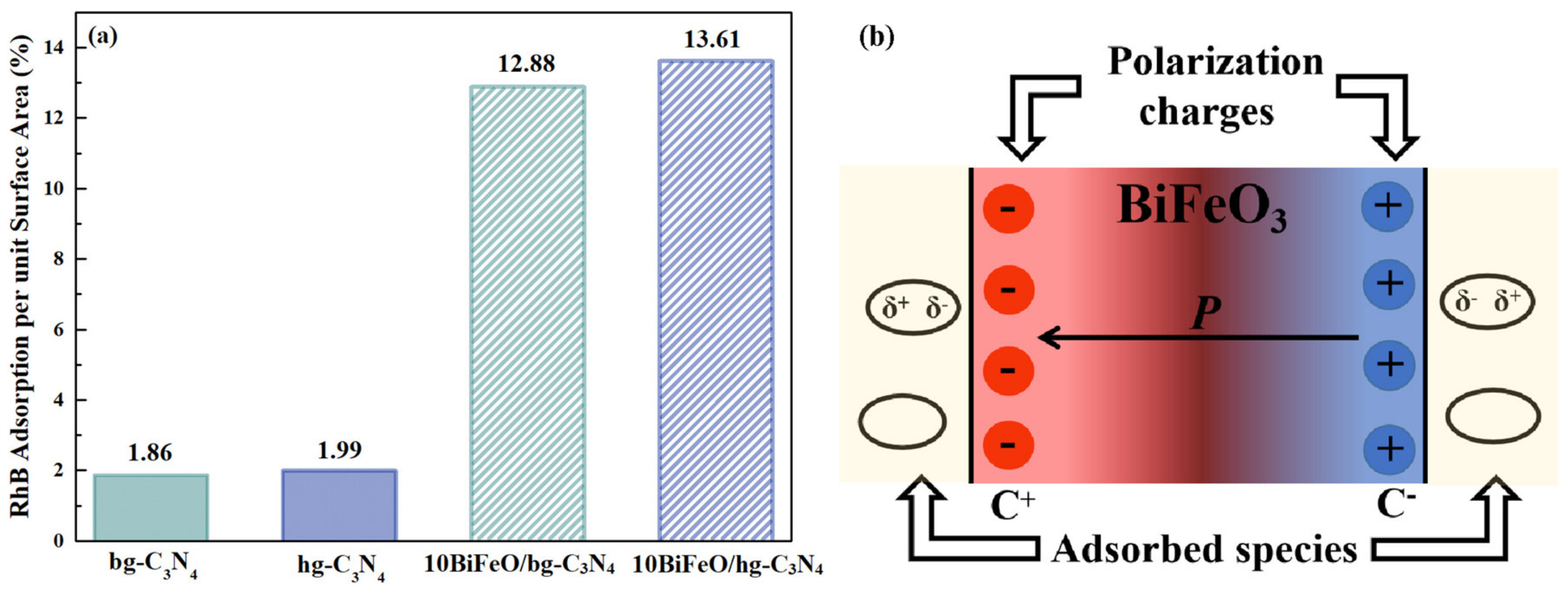
3.3.2. Adsorption of Reactant Molecules on the Catalysts
3.3.3. The Internal Field
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lops, C.; Ancona, A.; Cesare, D.K.; Dumontel, B.; Garino, N.; Canavese, G.; Hérnandez, S.; Cauda, V. Sonophotocatalytic degradation mechanisms of rhodamine B dye via radicals generation by micro- and nano-particles of ZnO. Appl. Catal. B Environ. 2019, 243, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Alakhras, F.; Alhajri, E.; Haounati, R.; Ouachtak, H.; Addi, A.A.; Saleh, T.A. A comparative study of photocatalytic degradation of rhodamine B using natural-based Zeolite composites. Surf. Interfaces 2020, 20, 100611. [Google Scholar] [CrossRef]
- Wang, T.; Liu, S.; Mao, W.; Bai, Y.; Chiang, K.; Shah, K.; Paz-Ferreiro, J. Novel Bi2WO6 loaded N-biochar composites with enhanced photocatalytic degradation of rhodamine B and Cr(VI). J. Hazard. Mater. 2020, 389, 121827. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Lei, Q.; Miao, R.; Gao, M.; Liu, H.; Yang, Q.; Liu, Y.; Song, F.; Yu, Y.; Yang, W. Bi-doped graphitic carbon nitride nnanotubes boost the photocatalytic degradation of rhodamine B. New J. Chem. 2022, 46, 3588–3594. [Google Scholar] [CrossRef]
- Al-Buriahi, A.K.; Al-Gheethi, A.A.; Senthil Kumar, P.; Radin Mohamed, R.M.S.; Yusof, H.; Alshalif, A.F.; Khalifa, N.A. Elimination of rhodamine B from textile wastewater using nanoparticle photocatalysts: A review for sustainable approaches. Chemosphere 2022, 287, 132162. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Sun, M.; Yuan, X.; Zhu, Y.; Lin, X.; Anandan, S. One-step hydrothermal synthesis of N/Ti3+ co-doping multiphasic TiO2/BiOBr heterojunctions towards enhanced sonocatalytic performance. Ultrason. Sonochem. 2018, 49, 69–78. [Google Scholar] [CrossRef]
- Xu, D.; Ma, H. Degradation of rhodamine B in water by ultrasound-assisted TiO2 photocatalysis. J. Clean. Prod. 2021, 313, 127758. [Google Scholar] [CrossRef]
- Wenderich, K.; Mul, G. Methods, mechanism, and applications of photodeposition in photocatalysis: A review. Chem. Rev. 2016, 116, 14587–14619. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Fattahimoghaddam, H.; Mahvelati-Shamsabadi, T.; Lee, B.-K. Efficient photodegradation of rhodamine B and tetracycline over robust and green g-C3N4 nanostructures: Supramolecular design. J. Hazard. Mater. 2021, 403, 123703. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, X.; Wang, H.; Zhang, J.; Pan, B.; Xie, Y. Enhanced photoresponsive ultrathin graphitic-phase C3N4 nanosheets for bioimaging. J. Am. Chem. Soc. 2013, 135, 18–21. [Google Scholar] [CrossRef]
- Cheng, F.; Wang, H.; Dong, X. The amphoteric properties of g-C3N4 nanosheets and fabrication of their relevant heterostructure photocatalysts by an electrostatic re-assembly route. Chem. Commun. 2015, 51, 7176–7179. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Y.; An, X.; Hou, L. A critical review of g-C3N4-based photocatalytic membrane for water purification. Chem. Eng. J. 2021, 412, 128663. [Google Scholar] [CrossRef]
- Lan, H.; Li, L.; An, X.; Liu, F.; Chen, C.; Liu, H.; Qu, J. Microstructure of carbon nitride affecting synergetic photocatalytic sctivity: Hydrogen bonds vs. structural defects. Appl. Catal. B Environ. 2017, 204, 49–57. [Google Scholar] [CrossRef]
- Zhao, T.; Xing, Z.; Xiu, Z.; Li, Z.; Yang, S.; Zhou, W. Oxygen-doped MoS2 nanospheres/CdS quantum dots/g-C3N4 nanosheets super-architectures for prolonged charge lifetime and enhanced visible-light-driven photocatalytic performance. ACS Appl. Mater. Interfaces 2019, 11, 7104–7111. [Google Scholar] [CrossRef]
- Wu, M.; He, X.; Jing, B.; Wang, T.; Wang, C.; Qin, Y.; Ao, Z.; Wang, S.; An, T. Novel carbon and defects co-modified g-C3N4 for highly efficient photocatalytic degradation of bisphenol A under visible light. J. Hazard. Mater. 2020, 384, 121323. [Google Scholar] [CrossRef]
- Katsumata, K.; Motoyoshi, R.; Matsushita, N.; Okada, K. Preparation of graphitic carbon nitride (g-C3N4)/WO3 composites and enhanced visible-light-driven photodegradation of acetaldehyde gas. J. Hazard. Mater. 2013, 260, 475–482. [Google Scholar] [CrossRef]
- Zhou, C.; Lai, C.; Huang, D.; Zeng, G.; Zhang, C.; Cheng, M.; Hu, L.; Wan, J.; Xiong, W.; Wen, M.; et al. Highly porous carbon nitride by supramolecular preassembly of monomers for photocatalytic removal of sulfamethazine under visible light driven. Appl. Catal. B Environ. 2018, 220, 202–210. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Wei, H.; McMaster, W.A.; Tan, J.Z.Y.; Cao, L.; Chen, D.; Caruso, R.A. Mesoporous TiO2/g-C3N4 microspheres with enhanced visible-light photocatalytic activity. J. Phys. Chem. C. 2017, 121, 22114–22122. [Google Scholar] [CrossRef]
- Guan, R.; Li, J.; Zhang, J.; Zhao, Z.; Wang, D.; Zhai, H.; Sun, D. Photocatalytic performance and mechanistic research of ZnO/g-C3N4 on degradation of methyl orange. ACS Omega 2019, 4, 20742–20747. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhu, J.; He, Y.; Li, M.; Liu, Y.; Chen, M.; Cao, D. Charge transfer in photocatalysis of direct Z-scheme g-C3N4-based ferroelectric heterojunction. J. Alloys Compd. 2022, 893, 162270. [Google Scholar] [CrossRef]
- Li, Y.; Qin, T.; Chen, W.; Huang, M.; Xu, J.; Lv, J. Construction of a switchable g-C3N4/BiVO4 heterojunction from the Z-Scheme to the type II by incorporation of pyromellitic diimide. Cryst. Growth Des. 2022, 22, 1645–1653. [Google Scholar] [CrossRef]
- Subhiksha, V.; Kokilavani, S.; Sudheer Khan, S. Recent advances in degradation of organic pollutant in aqueous solutions using bismuth based photocatalysts: A review. Chemosphere 2022, 290, 133228. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Ai, Y.; Li, D.; Tang, Z.; Shao, Z.; Liang, Q. Bismuth iron oxide nanocomposite supported on graphene oxides as the high efficient, stable and reusable catalysts for the reduction of nitroarenes under continuous flow conditions. Chem. Eng. J. 2017, 314, 328–335. [Google Scholar] [CrossRef]
- Irfan, S.; Zheng, Z.; Li, F.; Chen, Y.X.; Liang, G.X.; Luo, J.T.; Ping, F. Critical review: Bismuth ferrite as an emerging visible light active nanostructured photocatalyst. J. Mater. Res. Technol. 2019, 8, 6375–6389. [Google Scholar] [CrossRef]
- Zou, X.; You, L.; Chen, W.; Ding, H.; Wu, D.; Wu, T.; Chen, L.; Wang, J. Mechanism of polarization fatigue in BiFeO3. ACS Nano 2012, 6, 8997–9004. [Google Scholar] [CrossRef]
- Deng, X.-Z.; Song, C.; Tong, Y.-L.; Yuan, G.; Gao, F.; Liu, D.-Q.; Zhang, S.-T. Enhanced photocatalytic efficiency of C3N4/BiFeO3 heterojunctions: The synergistic effects of band alignment and ferroelectricity. Phys. Chem. Chem. Phys. 2018, 20, 3648–3657. [Google Scholar] [CrossRef]
- Lan, H.; Tang, Y.; Zhang, X.; You, S.; Tang, Q.; An, X.; Liu, H.; Qu, J. Decomplexation of Cu(II)-EDTA over oxygen-doped g-C3N4: An available resource towards environmental sustainability. Chem. Eng. J. 2018, 345, 138–146. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Y.; An, X.; Zhao, J.; Bao, Y.; Hou, L. Exfoliation method matters: The microstructure-dependent photoactivity of g-C3N4 nanosheets for water purification. J. Hazard. Mater. 2022, 424, 127424. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.-Q.; Bao, S.-J.; Lu, S.; Xu, M.; Long, D.; Pu, S. Tuning and thermal exfoliation graphene-like carbon nitride nanosheets for superior photocatalytic activity. Ceram. Int. 2016, 42, 18521–18528. [Google Scholar] [CrossRef]
- Zou, L.-R.; Huang, G.-F.; Li, D.-F.; Liu, J.-H.; Pan, A.-L.; Huang, W.-Q. A facile and rapid route for synthesis of g-C3N4 nanosheets with high adsorption capacity and photocatalytic activity. RSC Adv. 2016, 6, 86688–86694. [Google Scholar] [CrossRef]
- Xian, T.; Yang, H.; Shen, X.; Jiang, J.L.; Wei, Z.Q.; Feng, W.J. Preparation of high-quality BiFeO3 nanopowders via a polyacrylamide gel route. J. Alloys Compd. 2009, 480, 889–892. [Google Scholar] [CrossRef]
- Lan, F.; Aarons, J.; Shu, Y.; Zhou, X.; Jiao, H.; Wang, H.; Guan, Q.; Li, W. Anchoring strategy for highly active copper nanoclusters in hydrogenation of renewable biomass-derived compounds. Appl. Catal. B Environ. 2021, 299, 120651. [Google Scholar] [CrossRef]
- Wang, B.; Yue, Y.; Jin, C.; Lu, J.; Wang, S.; Yu, L.; Guo, L.; Li, R.; Hu, Z.-T.Z.; Pan, Z.; et al. Hydrochlorination of acetylene on single-atom Pd/N-doped carbon catalysts: Importance of pyridinic-N synergism. Appl. Catal. B Environ. 2020, 272, 118944. [Google Scholar] [CrossRef]
- Wang, Z.; Guan, W.; Sun, Y.; Dong, F.; Zhou, Y.; Ho, W.-K. Water-assisted production of honeycomb-like g-C3N4 with ultralong carrier: Lifetime and outstanding photocatalytic activity. Nanoscale 2015, 7, 2471–2479. [Google Scholar] [CrossRef]
- Wang, Z.; Ban, L.; Meng, P.; Li, H.; Zhao, Y. Ethynylation of formaldehyde over CuO/SiO2 catalysts modified by Mg species: Effects of the existential states of Mg species. Nanomaterials 2019, 9, 1137. [Google Scholar] [CrossRef]
- Wang, S.; Chen, D.; Niu, F.; Zhang, N.; Qin, L.; Huang, Y. Hydrogenation-induced surface oxygen vacancies in BiFeO3 nanoparticles for enhanced visible light photocatalytic performance. J. Alloys Compd. 2016, 688, 399–406. [Google Scholar] [CrossRef]
- Ge, L.; Xu, Y.; Ding, L.; You, F.; Liu, Q.; Wang, K. Perovskite-type BiFeO3/ultrathin graphite-like carbon nitride nanosheets p-n heterojunction: Boosted visible-lightdriven photoelectrochemical activity for fabricating ampicillin aptasensor. Biosens. Bioelectron. 2019, 124, 33–39. [Google Scholar] [CrossRef]
- Vignesh, S.; Muppudathi, A.L.; Sundar, J.K. Multifunctional performance of gC3N4-BiFeO3-Cu2O hybrid nanocomposites for magnetic separable photocatalytic and antibacterial activity. J. Mater. Sci. Mater. Electron. 2018, 29, 10784–10801. [Google Scholar] [CrossRef]
- Huo, H.; Hu, X.; Wang, H.; Li, J.; Xie, G.; Tan, X.; Jin, Q.; Zhou, D.; Li, C.; Qiu, G.; et al. Synergy of photocatalysis and adsorption for simultaneous removal of hexavalent chromium and methylene blue by g-C3N4/BiFeO3/carbon nanotubes ternary composites. Int. J. Environ. Res. Public Health 2019, 16, 3219. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zhou, Q. Nitrogen doped g-C3N4 with the extremely narrow band gap for excellent photocatalytic sctivities under visible light. Appl. Catal. B Environ. 2021, 281, 119474. [Google Scholar] [CrossRef]
- Khan, M.A.; Nadeem, M.A.; Idriss, H. Ferroelectric polarization effect on surface chemistry and photo-catalytic activity: A review. Surf. Sci. Rep. 2016, 71, 1–31. [Google Scholar] [CrossRef]
- Cui, Y.; Briscoe, J.; Dunn, S. Effect of Ferroelectricity on Solar-Light-Driven Photocatalytic activity of BaTiO3—Influence on the carrier separation and stern layer formation. Chem. Mater. 2013, 25, 4215–4223. [Google Scholar] [CrossRef]
- Dunn, S.; Jones, P.M.; Gallardo, D.E. Photochemical growth of silver nanoparticles on c- and c+ domains on lead Zirconate Titanate thin films. J. Am. Chem. Soc. 2007, 129, 8724–8728. [Google Scholar] [CrossRef] [PubMed]
- Dunn, S.; Shaw, C.P.; Huang, Z.; Whatmore, R.W. Ultrahigh resolution of lead Zirconate Titanate 30/70 domains as imaged by piezoforce microscopy. Nanotechnology 2002, 13, 456–459. [Google Scholar] [CrossRef]
- Dunn, S.; Tiwari, D.; Jones, P.M.; Gallardo, D.E. Insights into the relationship between inherent materials properties of PZT and photochemistry for the development of nanostructured silver. J. Mater. Chem. 2007, 17, 4460–4463. [Google Scholar] [CrossRef]
- Cai, X.; Wang, F.; Wang, R.; Xi, Y.; Wang, A.; Wang, J.; Teng, B.; Bai, S. Synergism of surface strain and interfacial polarization on Pd@Au core–shell cocatalysts for highly efficient photocatalytic CO2 reduction over TiO2. J. Mater. Chem. A 2020, 8, 7350–7359. [Google Scholar] [CrossRef]
- He, Y.; Wang, P.; Zhu, J.; Yang, Y.; Liu, Y.; Chen, M.; Cao, D.; Yan, X. Synergistical dual strategies based on in situ-converted heterojunction and reduction-induced surface oxygen vacancy for enhanced photoelectrochemical performance of TiO2. ACS Appl. Mater. Interfaces 2019, 11, 37322–37329. [Google Scholar] [CrossRef]
- Sepahvand, H.; Sharifnia, S. Photocatalytic overall water splitting by Z-scheme g-C3N4/BiFeO3 heterojunction. Int. J. Hydrog. Energy 2019, 44, 23658–23668. [Google Scholar] [CrossRef]
- Zhu, B.; Xia, P.; Li, Y.; Ho, W.; Yu, J. Fabrication and photocatalytic activity enhanced mechanism of direct Z-scheme g-C3N4/Ag2WO4 photocatalyst. Appl. Surf. Sci. 2017, 391, 175–183. [Google Scholar] [CrossRef]
- Finkelstein, E.; Rosen, G.M.; Rauckman, E.J. Production of hydroxyl radical by decomposition of superoxide spin-trapped adducts. Mol. Pharmacol. 1982, 21, 262–265. [Google Scholar]
- Buettner, G.R. The spin trapping of superoxide and hydroxyl free radicals with DMPO (5,5-dimethylpyrroline-N-oxide): More about iron. Free Radic. Res. Commun. 1993, 19, S79–S87. [Google Scholar] [CrossRef]
- Ma, Y.; Lv, P.; Duan, F.; Sheng, J.; Lu, S.; Zhu, H.; Du, M.; Chen, M. Direct Z-scheme Bi2S3/BiFeO3 heterojunction nanofibers with enhanced photocatalytic sctivity. J. Alloys Compd. 2020, 834, 155158. [Google Scholar] [CrossRef]
- Jiang, W.; Zong, X.; An, L.; Hua, S.; Miao, X.; Luan, S.; Wen, Y.; Tao, F.F.; Sun, Z. Consciously constructing heterojunction or direct Z-Scheme photocatalysts by regulating electron flow direction. ACS Catal. 2018, 8, 2209–2217. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, Y.; Zhao, B.; Yu, Y.; Yu, Y.; Zhang, B. CuOx clusters decorated TiO2 for photocatalytic oxidation of nitrogen in air into nitric oxide under ambient conditions. J. Catal. 2022, 409, 70–77. [Google Scholar] [CrossRef]
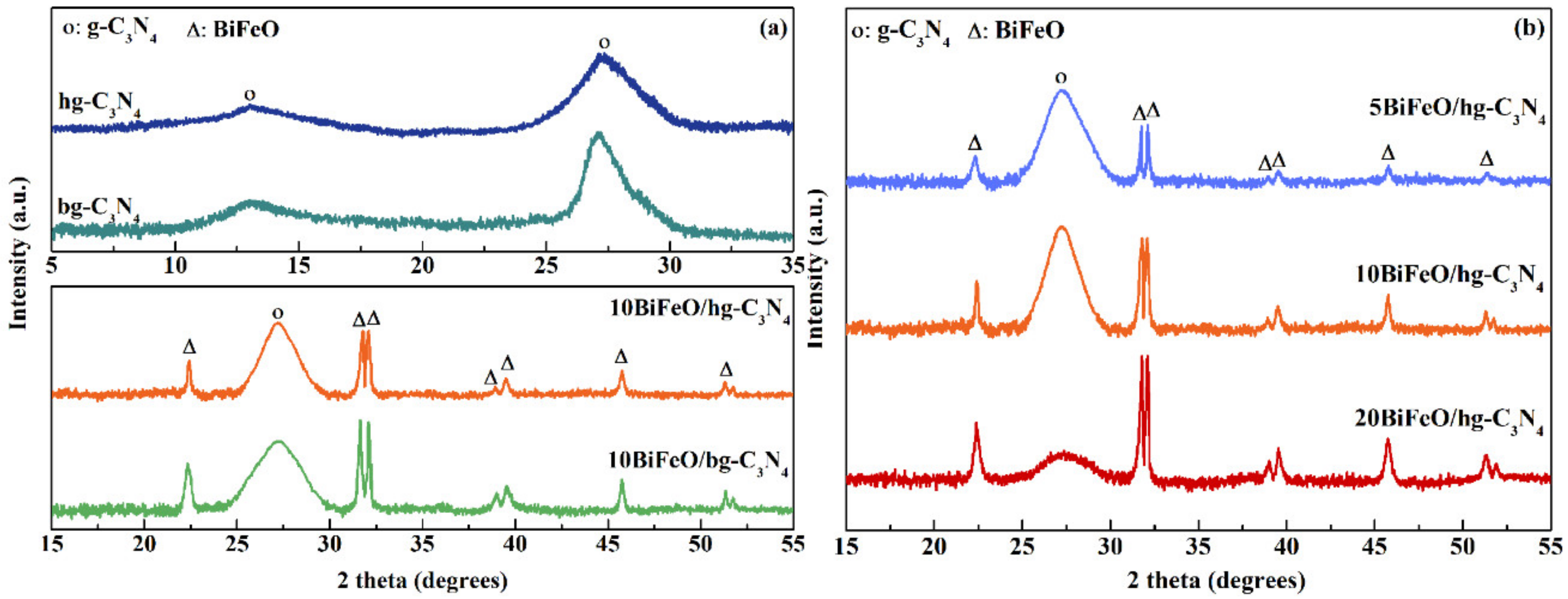




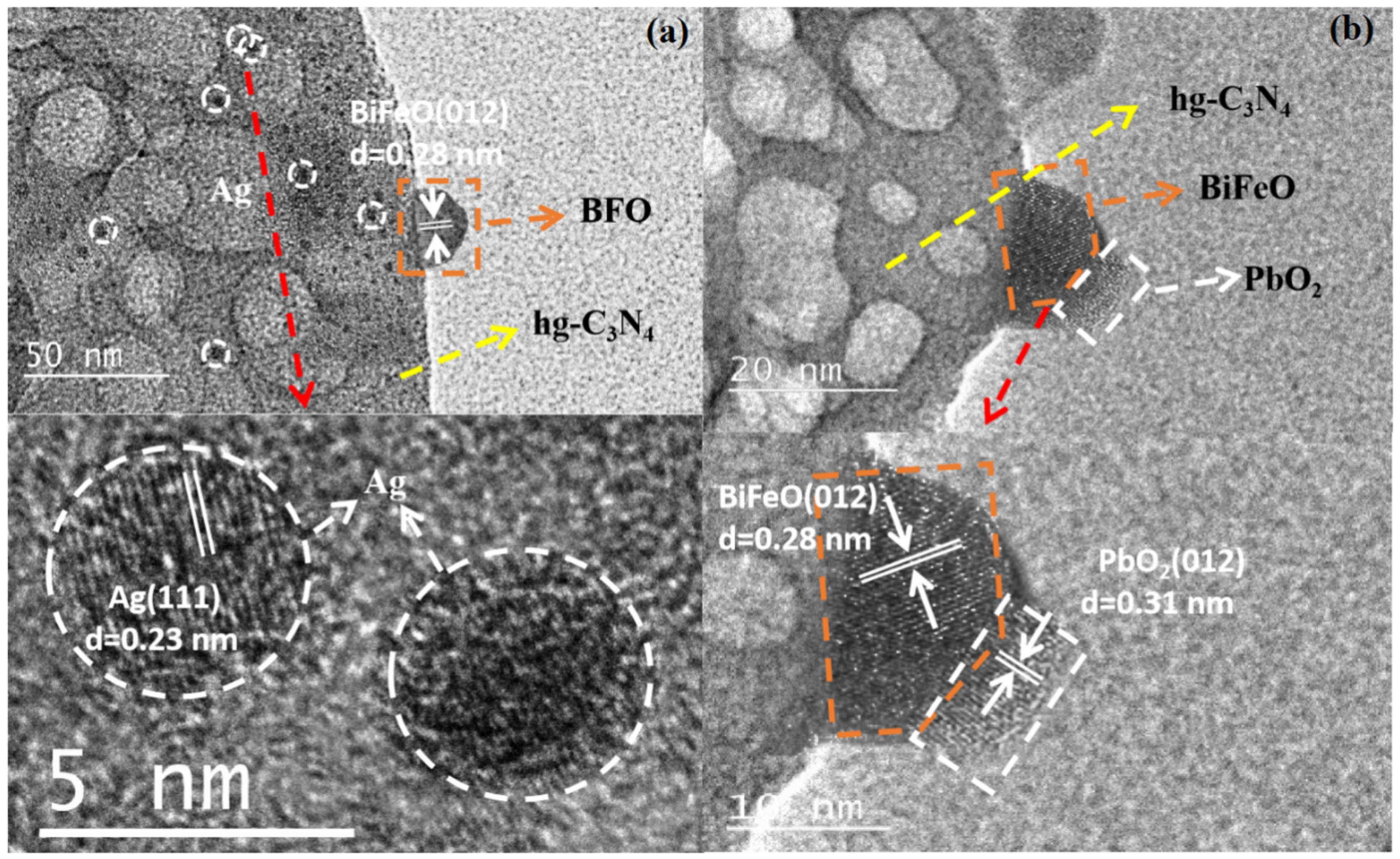
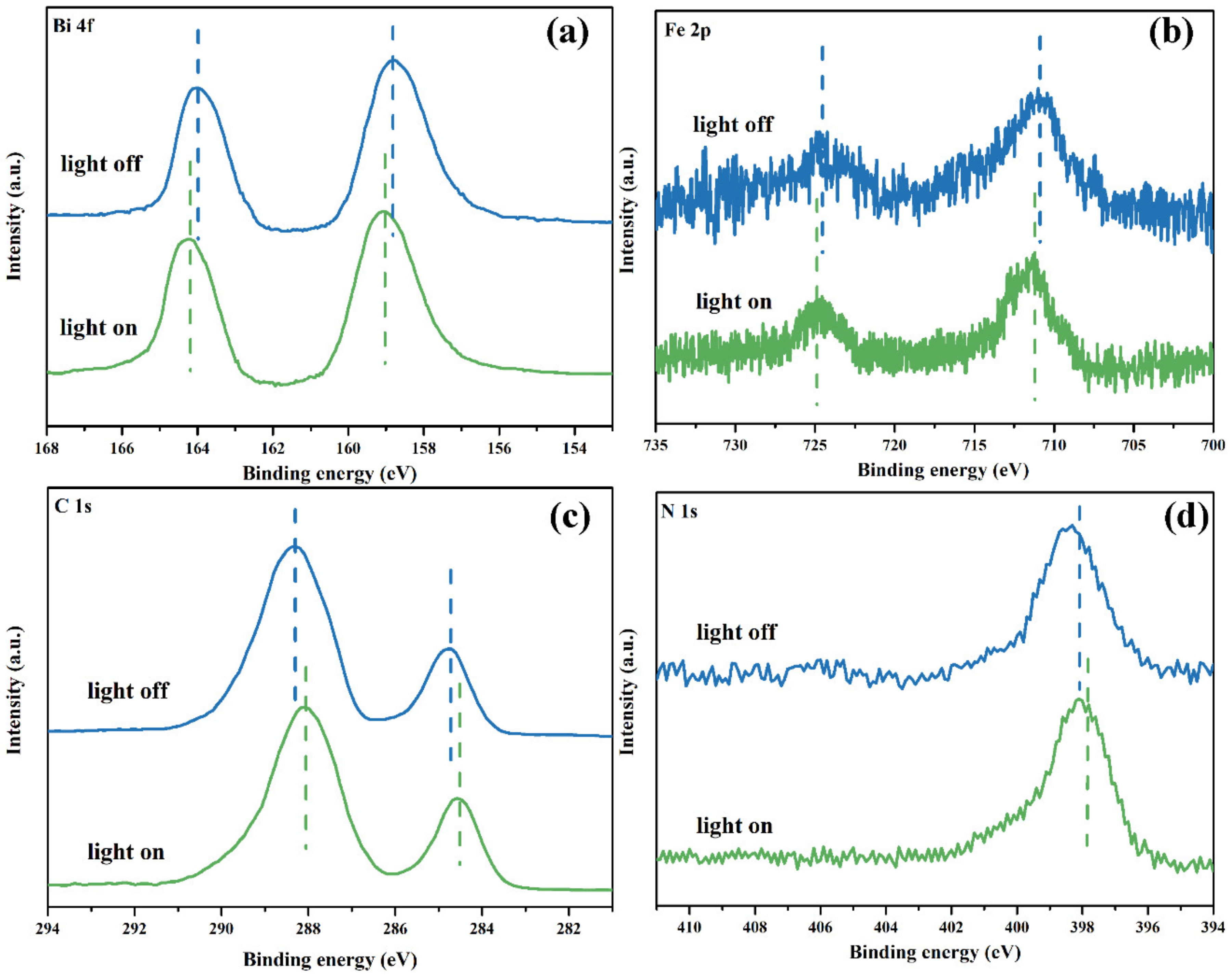
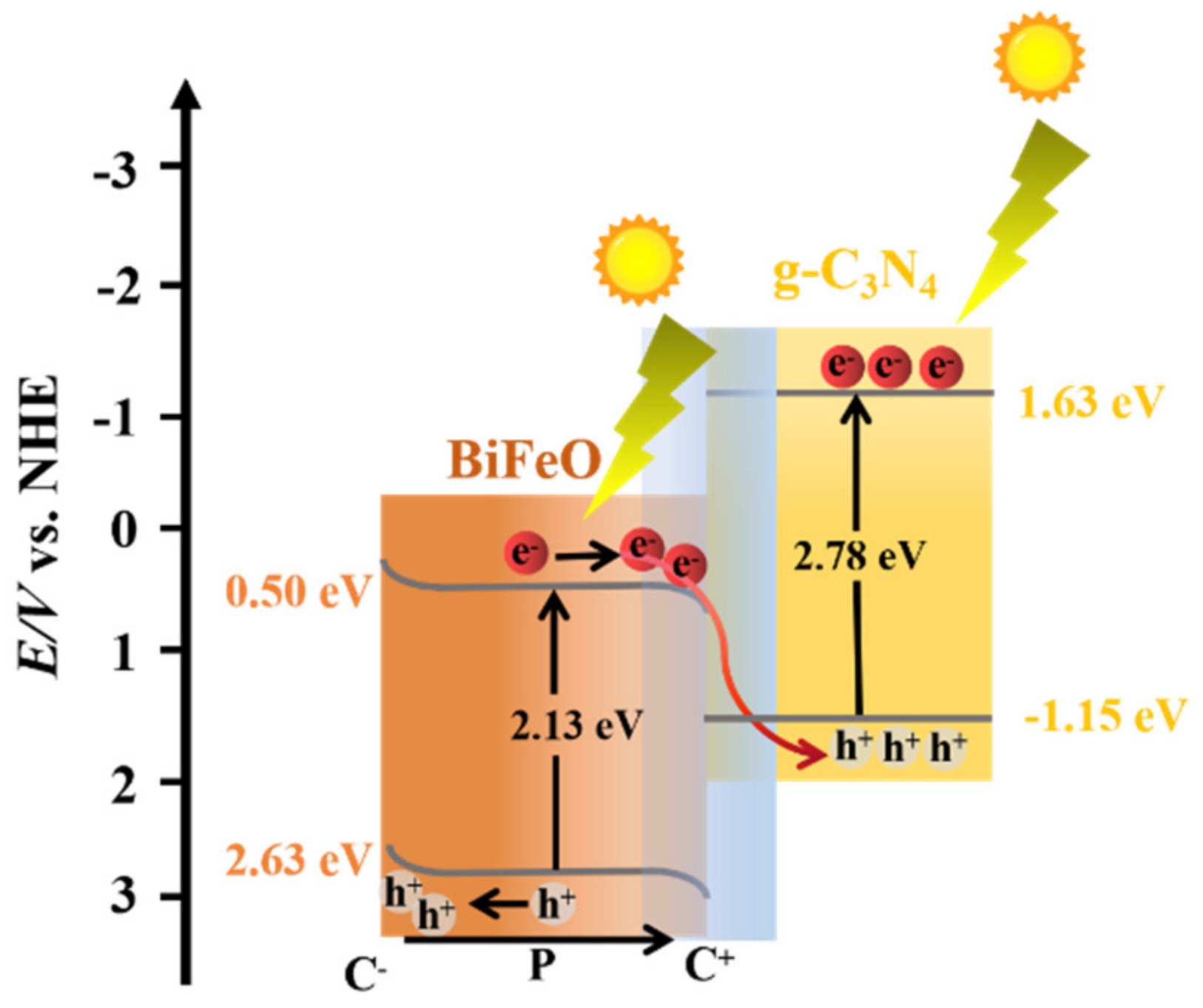
| Sample | BiFeO Content (wt. %) a | DBiFeO (nm) b | ABET (m2/g) c | VTotal (cm3/g) c |
|---|---|---|---|---|
| bg-C3N4 | - | - | 38.8 | 0.11 |
| hg-C3N4 | - | - | 61.4 | 0.19 |
| 10BiFeO/bg-C3N4 | 10.7 | 29.3 | 18.2 | 0.07 |
| 10BiFeO/hg-C3N4 | 9.2 | 18.5 | 41.8 | 0.12 |
| 5BiFeO/hg-C3N4 | 4.3 | 12.4 | 49.6 | 0.14 |
| 20BiFeO/hg-C3N4 | 21.5 | 36.8 | 22.7 | 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, H.; Wang, Z.; Cao, G.; Wu, Y.; Song, J.; Li, Y.; Zhang, L.; Mu, J.; Chou, X. Facilitated Photocatalytic Degradation of Rhodamine B over One-Step Synthesized Honeycomb-Like BiFeO3/g-C3N4 Catalyst. Nanomaterials 2022, 12, 3970. https://doi.org/10.3390/nano12223970
Cui H, Wang Z, Cao G, Wu Y, Song J, Li Y, Zhang L, Mu J, Chou X. Facilitated Photocatalytic Degradation of Rhodamine B over One-Step Synthesized Honeycomb-Like BiFeO3/g-C3N4 Catalyst. Nanomaterials. 2022; 12(22):3970. https://doi.org/10.3390/nano12223970
Chicago/Turabian StyleCui, Haoran, Zhipeng Wang, Guoqiang Cao, Yiwan Wu, Jian Song, Yu Li, Le Zhang, Jiliang Mu, and Xiujian Chou. 2022. "Facilitated Photocatalytic Degradation of Rhodamine B over One-Step Synthesized Honeycomb-Like BiFeO3/g-C3N4 Catalyst" Nanomaterials 12, no. 22: 3970. https://doi.org/10.3390/nano12223970
APA StyleCui, H., Wang, Z., Cao, G., Wu, Y., Song, J., Li, Y., Zhang, L., Mu, J., & Chou, X. (2022). Facilitated Photocatalytic Degradation of Rhodamine B over One-Step Synthesized Honeycomb-Like BiFeO3/g-C3N4 Catalyst. Nanomaterials, 12(22), 3970. https://doi.org/10.3390/nano12223970