The Effect of La3+ on the Methylene Blue Dye Removal Capacity of the La/ZnTiO3 Photocatalyst, a DFT Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
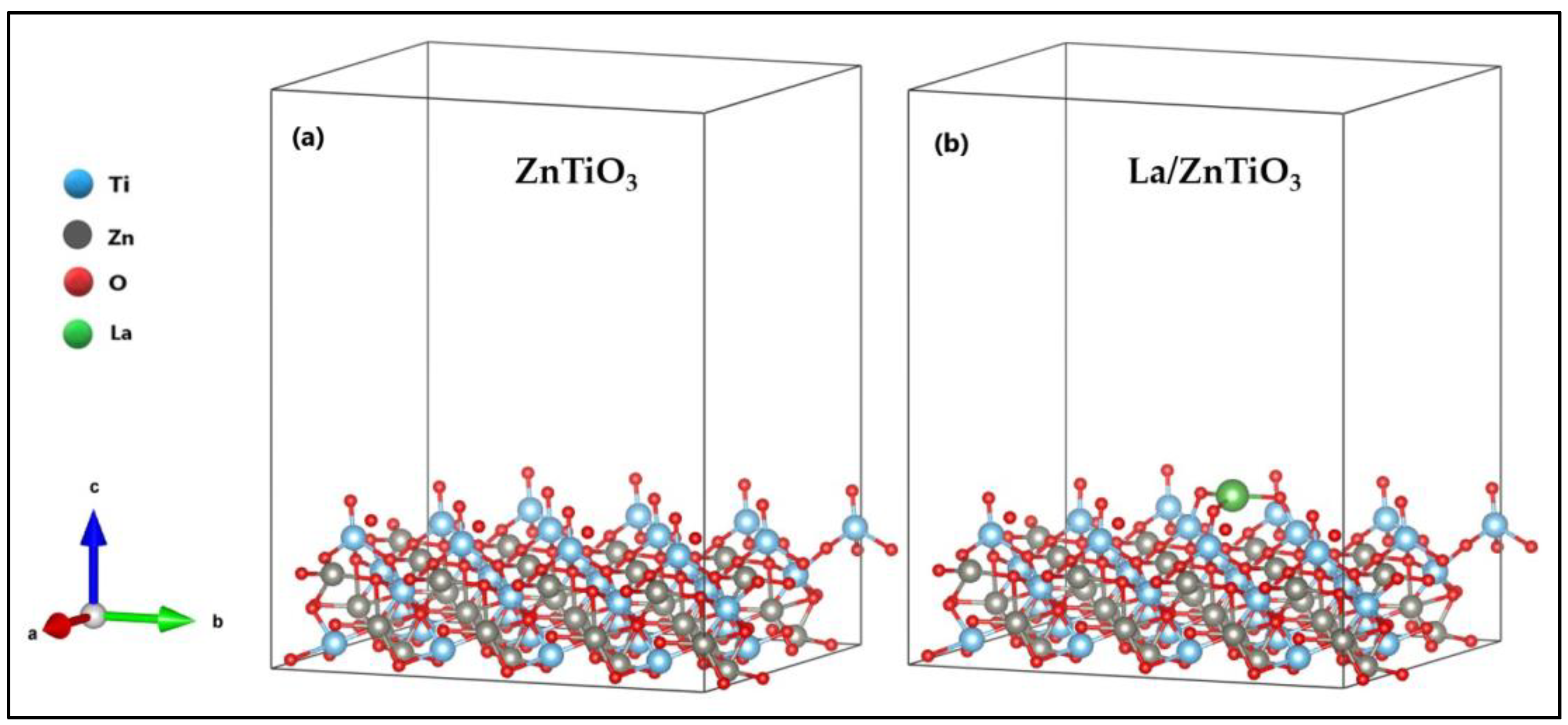
3.1. Optimization of La/ZnTiO3
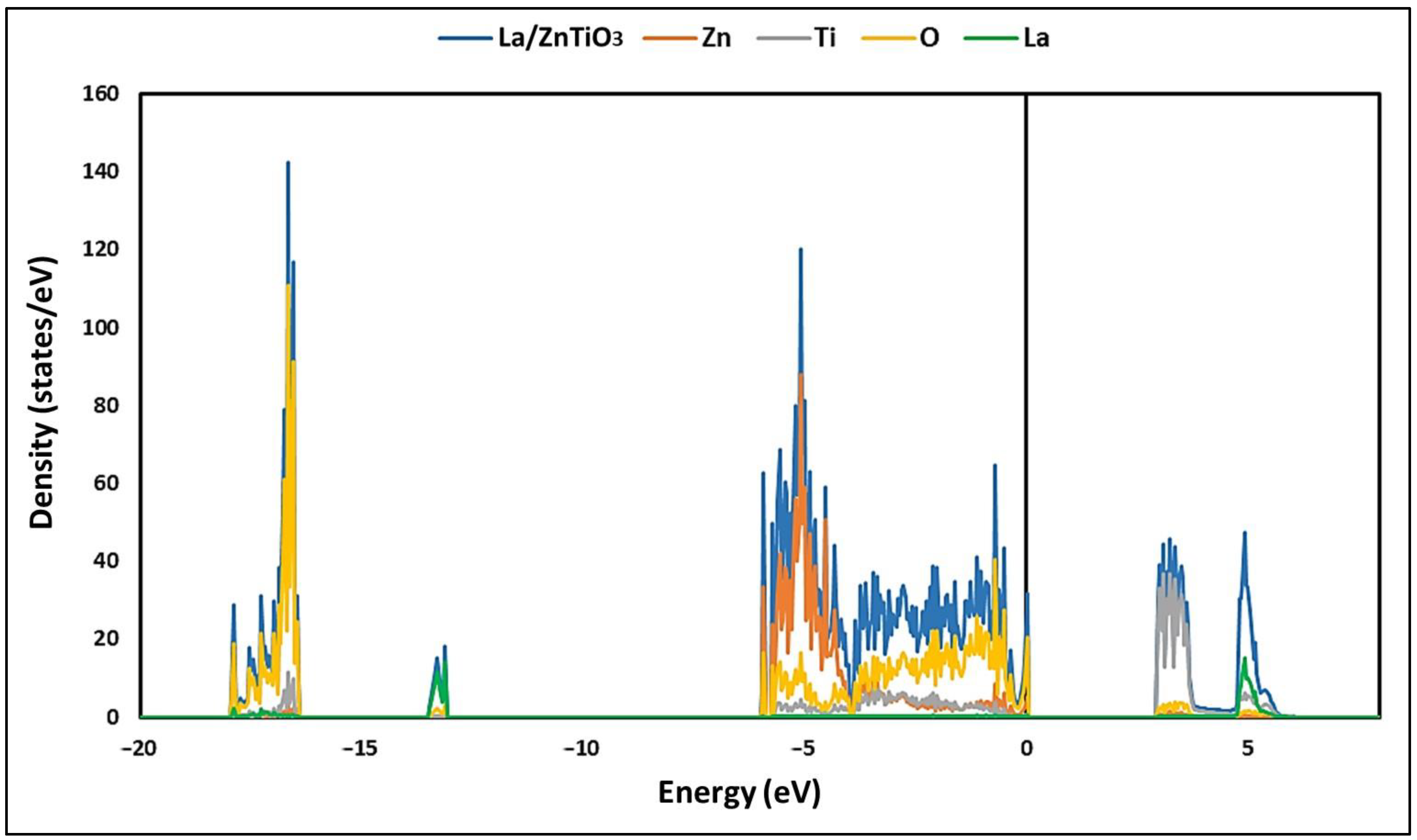
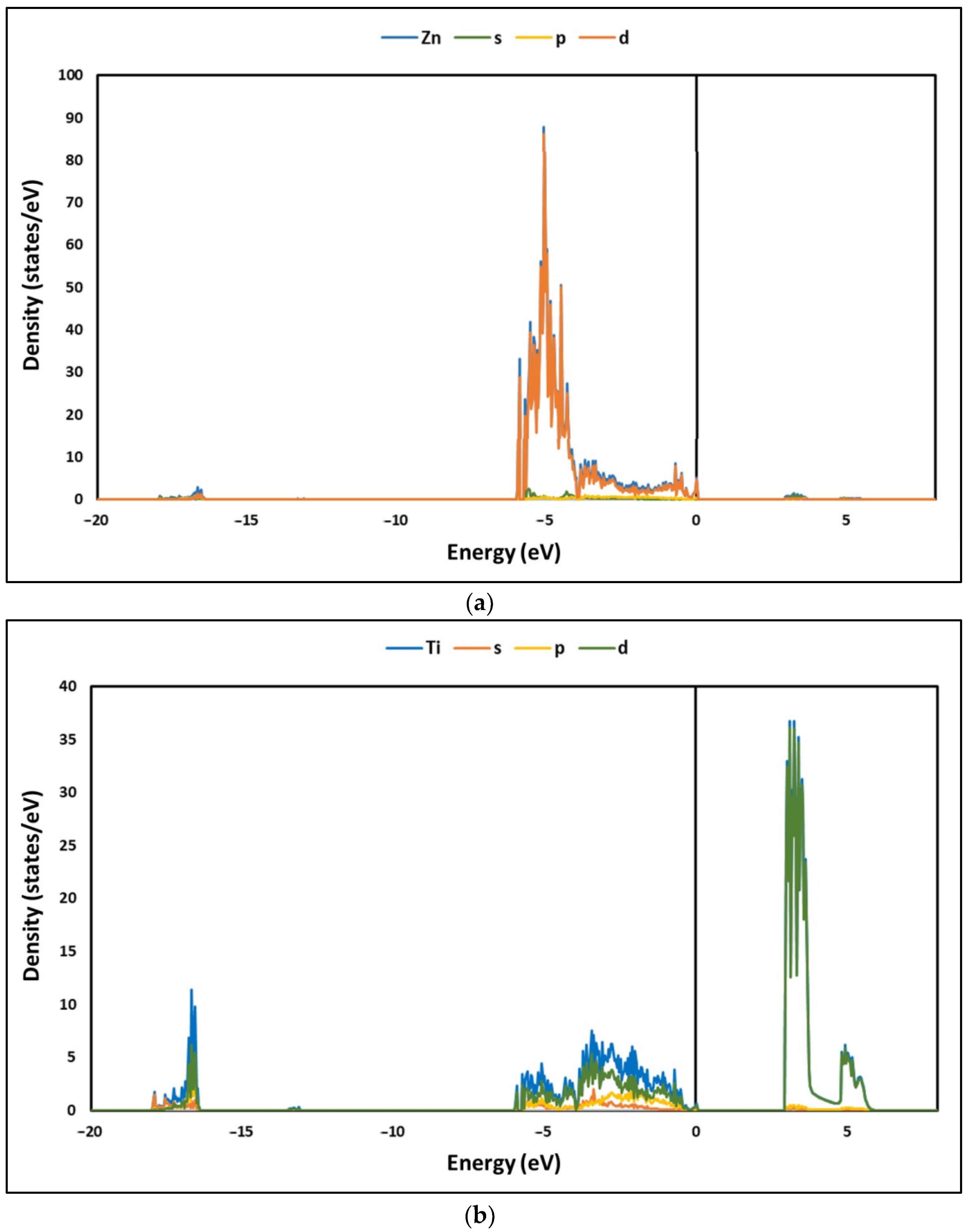
3.2. Electronic Structure of La/ZnTiO3
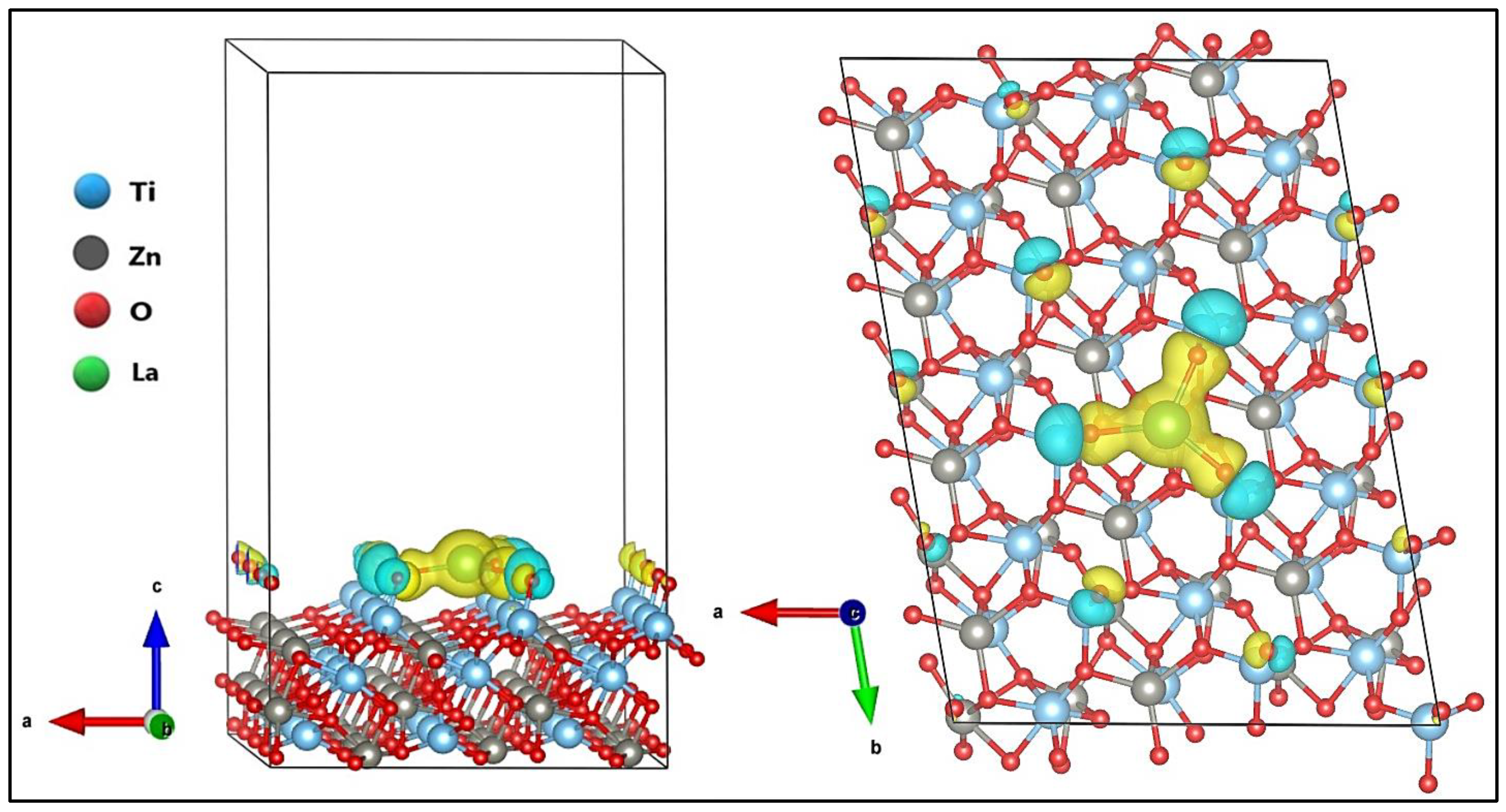
3.3. MB Adsorption on Surface (101) of ZnTiO3 and La/ZnTiO3
4. Discussion
4.1. Optimization of La/ZnTiO3
4.2. Electronic Structure of La/ZnTiO3
4.3. MB Adsorption on Surface (101) of ZnTiO3 and La/ZnTiO3
Proposed Photocatalytic Mechanism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; González, S.; Montesdeoca-Mendoza, F.; Medina, F. Structuring of ZnTiO3/TiO2 adsorbents for the removal of methylene blue, using zeolite precursor clays as natural additives. Nanomaterials 2021, 11, 898. [Google Scholar] [CrossRef]
- Wang, G.; Li, G.; Huan, Y.; Hao, C.; Chen, W. Acrylic acid functionalized graphene oxide: High-efficient removal of cationic dyes from wastewater and exploration on adsorption mechanism. Chemosphere 2020, 261, 127736. [Google Scholar] [CrossRef] [PubMed]
- Ambigadevi, J.; Kumar, P.S.; Vo, D.V.N.; Haran, S.H.; Raghavan, T.N.S. Recent developments in photocatalytic remediation of textile effluent using semiconductor based nanostructured catalyst: A review. J. Environ. Chem. Eng. 2021, 9, 104881. [Google Scholar] [CrossRef]
- Shi, X.; Wang, L.; Zuh, A.A.; Jia, Y.; Ding, F.; Cheng, H.; Wang, Q. Photo-Fenton reaction for the degradation of tetracycline hydrochloride using a FeWO4/BiOCl nanocomposite. J. Alloys Compd. 2022, 903, 163889. [Google Scholar] [CrossRef]
- Wang, L.; Ma, X.; Huang, G.; Lian, R.; Huang, J.; She, H.; Wang, Q. Construction of ternary CuO/CuFe2O4/g-C3N4 composite and its enhanced photocatalytic degradation of tetracycline hydrochloride with persulfate under simulated sunlight. J. Environ. Sci. 2022, 112, 59–70. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, D. Photocatalysis: Basic Principles, Diverse Forms of Implementations and Emerging Scientific Opportunities. Adv. Energy Mater. 2017, 7, 1700841. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Manuel, A.; Shankar, K. Hot Electrons in TiO2–Noble Metal Nano-Heterojunctions: Fundamental Science and Applications in Photocatalysis. Nanomaterials 2021, 11, 1249. [Google Scholar] [CrossRef]
- Upadhyay, G.K.; Rajput, J.K.; Pathak, T.K.; Kumar, V.; Purohit, L.P. Synthesis of ZnO:TiO2 nanocomposites for photocatalyst application in visible light. Vacuum 2019, 160, 154–163. [Google Scholar] [CrossRef]
- Lee, C.G.; Na, K.H.; Kim, W.T.; Park, D.C.; Yang, W.H.; Choi, W.Y. TiO2/ZnO nanofibers prepared by electrospinning and their photocatalytic degradation of methylene blue compared with TiO2 nanofibers. Appl. Sci. 2019, 9, 3404. [Google Scholar] [CrossRef]
- Sinha, D.; De, D.; Goswami, D.; Mondal, A.; Ayaz, A. ZnO and TiO2 Nanostructured Dye sensitized Solar Photovoltaic Cell. Mater. Today Proc. 2019, 11, 782–788. [Google Scholar] [CrossRef]
- Zalani, N.M.; Kaleji, B.K.; Mazinani, B. Synthesis and characterisation of the mesoporous ZnO-TiO2 nanocomposite; Taguchi optimisation and photocatalytic methylene blue degradation under visible light. Mater. Technol. 2020, 35, 281–289. [Google Scholar] [CrossRef]
- Siwińska-Stefańska, K.; Kubiak, A.; Piasecki, A.; Goscianska, J.; Nowaczyk, G.; Jurga, S.; Jesionowski, T. TiO2-ZnO Binary Oxide Systems: Comprehensive Characterization and Tests of Photocatalytic Activity. Materials 2018, 11, 841. [Google Scholar] [CrossRef]
- Tahay, P.; Khani, Y.; Jabari, M.; Bahadoran, F.; Safari, N.; Zamanian, A. Synthesis of cubic and hexagonal ZnTiO3 as catalyst support in steam reforming of methanol: Study of physical and chemical properties of copper catalysts on the H2 and CO selectivity and coke formation. Int. J. Hydrogen Energy 2020, 45, 9484–9495. [Google Scholar] [CrossRef]
- Pantoja-Espinoza, J.C.; Domínguez-Arvizu, J.L.; Jiménez-Miramontes, J.A.; Hernández-Majalca, B.C.; Meléndez-Zaragoza, M.J.; Salinas-Gutiérrez, J.M.; Herrera-Pérez, G.M.; Collins-Martínez, V.H.; López-Ortiz, A. Comparative study of Zn2Ti3O8 and ZnTiO3 photocatalytic properties for hydrogen production. Catalysts 2020, 10, 1372. [Google Scholar] [CrossRef]
- Baamran, K.S.; Tahir, M. Thermodynamic investigation and experimental analysis on phenol steam reforming towards enhanced H2 production over structured Ni/ZnTiO3 nanocatalyst. Energy Convers. Manag. 2019, 180, 796–810. [Google Scholar] [CrossRef]
- Ranjith, K.S.; Uyar, T. ZnO-TiO2 composites and ternary ZnTiO3 electrospun nanofibers: The influence of annealing on the photocatalytic response and reusable functionality. CrystEngComm 2018, 20, 5801–5813. [Google Scholar] [CrossRef]
- Sowmyashree, A.S.; Somya, A.; Kumar, C.B.P.; Rao, S. Novel nano corrosion inhibitor, integrated zinc titanate nano particles: Synthesis, characterization, thermodynamic and electrochemical studies. Surf. Interfaces 2021, 22, 100812. [Google Scholar] [CrossRef]
- Edalatfar, M.; Yazdani, F.; Salehi, M.B. Synthesis and identification of ZnTiO3 nanoparticles as a rheology modifier additive in water-based drilling mud. J. Pet. Sci. Eng. 2021, 201, 108415. [Google Scholar] [CrossRef]
- Zhu, B.; Yang, Q.; Zhang, W.; Cui, S.; Yang, B.; Wang, Q.; Li, S.; Zhang, D. A high sensitivity dual-mode optical thermometry based on charge compensation in ZnTiO3:M (M = Eu3+, Mn4+) hexagonal prisms. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 274, 121101. [Google Scholar] [CrossRef] [PubMed]
- Mofokeng, S.J.; Noto, L.L.; Dhlamini, M.S. Photoluminescence properties of ZnTiO3:Eu3+ phosphor with enhanced red emission by Al3+ charge compensation. J. Lumin. 2020, 228, 117569. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, B.; Wang, Y.S.; Qin, Z.; Ke, S.H. First-principles investigation of the ferroelectric, piezoelectric and nonlinear optical properties of LiNbO3-type ZnTiO3. Sci. Rep. 2019, 9, 17632. [Google Scholar] [CrossRef]
- Djellabi, R.; Ordonez, M.F.; Conte, F.; Falletta, E.; Bianchi, C.L.; Rossetti, I. A Review of Advances in Multifunctional XTiO3 Perovskite-type Oxides as piezo-photocatalysts for Environmental Remediation and Energy Production. J. Hazard. Mater. 2021, 421, 126792. [Google Scholar] [CrossRef]
- Obodo, K.O.; Noto, L.L.; Mofokeng, S.J.; Ouma, C.N.M.; Braun, M.; Dhlamini, M.S. Influence of Tm, Ho and Er dopants on the properties of Yb activated ZnTiO3 perovskite: A density functional theory insight. Mater. Res. Express 2018, 5, 106202. [Google Scholar] [CrossRef]
- Sarkar, M.; Sarkar, S.; Biswas, A.; De, S.; Kumar, P.R.; Mothi, E.M.; Kathiravan, A. Zinc titanate nanomaterials—photocatalytic studies and sensitization of hydantoin derivatized porphyrin dye. Nano-Struct. Nano-Objects 2020, 21, 100412. [Google Scholar] [CrossRef]
- Kurajica, S.; Minga, I.; Blazic, R.; Muzina, K.; Tominac, P. Adsorption and Degradation Kinetics of Methylene Blue on As-prepared and Calcined Titanate Nanotubes. Athens J. Sci. 2018, 5, 7–22. [Google Scholar] [CrossRef]
- Sahu, A.; Chaurashiya, R.; Hiremath, K.; Dixit, A. Nanostructured zinc titanate wide band gap semiconductor as a photoelectrode material for quantum dot sensitized solar cells. Sol. Energy 2018, 163, 338–346. [Google Scholar] [CrossRef]
- Bhagwat, U.O.; Wu, J.J.; Asiri, A.M.; Anandan, S. Synthesis of ZnTiO3@TiO2 Heterostructure Nanomaterial as a Visible light Photocatalyst. Chem. Sel. 2019, 4, 6106–6112. [Google Scholar] [CrossRef]
- Rafieh, A.I.; Ekanayake, P.; Tan, A.L.; Lim, C.M. Effects of ionic radii of co-dopants (Mg, Ca, Al and La) in TiO2 on performance of dye-sensitized solar cells. Sol. Energy 2017, 141, 249–255. [Google Scholar] [CrossRef]
- Faisal, M.; Jalalah, M.; Harraz, F.A.; El-Toni, A.M.; Labis, J.P.; Al-Assiri, M.S. A novel Ag/PANI/ZnTiO3 ternary nanocomposite as a highly efficient visible-light-driven photocatalyst. Sep. Purif. Technol. 2021, 256, 117847. [Google Scholar] [CrossRef]
- Ozturk, B.; Soylu, G.S.P. Promoting role of transition metal oxide on ZnTiO3-TiO2 nanocomposites for the photocatalytic activity under solar light irradiation. Ceram. Int. 2016, 42, 11184–11192. [Google Scholar] [CrossRef]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Enhanced UV–Visible photocatalytic activity of Cu-doped ZnO/TiO2 nanoparticles. J. Mater. Sci. Mater. Electron. 2018, 29, 5480–5495. [Google Scholar] [CrossRef]
- Li, X.; Xiong, J.; Huang, J.; Feng, Z.; Luo, J. Novel g-C3N4/h′ZnTiO3-a′TiO2 direct Z-scheme heterojunction with significantly enhanced visible-light photocatalytic activity. J. Alloys Compd. 2019, 774, 768–778. [Google Scholar] [CrossRef]
- Abirami, R.; Kalaiselvi, C.R.; Kungumadevi, L.; Senthil, T.S.; Kang, M. Synthesis and characterization of ZnTiO3 and Ag doped ZnTiO3 perovskite nanoparticles and their enhanced photocatalytic and antibacterial activity. J. Solid State Chem. 2020, 281, 121019. [Google Scholar] [CrossRef]
- Ruzimuradov, O.; Hojamberdiev, M.; Fasel, C.; Riedel, R. Fabrication of lanthanum and nitrogen—Co-doped SrTiO3– TiO2 heterostructured macroporous monolithic materials for photocatalytic degradation of organic dyes under visible light. J. Alloys Compd. 2017, 699, 144–150. [Google Scholar] [CrossRef]
- Chaker, H.; Ameur, N.; Saidi-Bendahou, K.; Djennas, M.; Fourmentin, S. Modeling and Box-Behnken design optimization of photocatalytic parameters for efficient removal of dye by lanthanum-doped mesoporous TiO2. J. Environ. Chem. Eng. 2021, 9, 2213–3437. [Google Scholar] [CrossRef]
- Mazierski, P.; Lisowski, W.; Grzyb, T.; Winiarski, M.J.; Klimczuk, T.; Mikołajczyk, A.; Flisikowski, J.; Hirsch, A.; Kołakowska, A.; Puzyn, T.; et al. Enhanced photocatalytic properties of lanthanide-TiO2 nanotubes: An experimental and theoretical study. Appl. Catal. B Environ. 2017, 205, 376–385. [Google Scholar] [CrossRef]
- Prakash, J.; Kumar, A.; Dai, H.; Janegitz, B.C.; Krishnan, V.; Swart, H.C.; Sun, S. Novel rare earth metal–doped one-dimensional TiO2 nanostructures: Fundamentals and multifunctional applications. Mater. Today Sustain. 2021, 13, 100066. [Google Scholar] [CrossRef]
- Jian, S.; Tian, Z.; Hu, J.; Zhang, K.; Zhang, L.; Duan, G.; Yang, W.; Jiang, S. Enhanced visible light photocatalytic efficiency of La-doped ZnO nanofibers via electrospinning-calcination technology. Adv. Powder Mater. 2022, 1, 100004. [Google Scholar] [CrossRef]
- Shwetharani, R.; Sakar, M.; Chandan, H.R.; Balakrishna, R.G. Observation of simultaneous photocatalytic degradation and hydrogen evolution on the lanthanum modified TiO2 nanostructures. Mater. Lett. 2018, 218, 262–265. [Google Scholar] [CrossRef]
- Dal’Toé, A.T.O.; Colpani, G.L.; Padoin, N.; Fiori, M.A.; Soares, C. Lanthanum doped titania decorated with silver plasmonic nanoparticles with enhanced photocatalytic activity under UV-visible light. Appl. Surf. Sci. 2018, 441, 1057–1071. [Google Scholar] [CrossRef]
- Yan, Z.; Yang, X.; Gao, G.; Gao, R.; Zhang, T.; Tian, M.; Su, H.; Wang, S. Understanding of Photocatalytic Partial Oxidation of Methanol to Methyl Formate on Surface Doped La(Ce)-Tio2: Experiment and Dft Calculation. SSRN Electron. J. 2022, 411, 31–40. [Google Scholar] [CrossRef]
- Song, K.; Min, T.; Seo, J.; Ryu, S.; Lee, H.; Wang, Z.; Choi, S.Y.; Lee, J.; Eom, C.B.; Oh, S.H. Electronic and Structural Transitions of LaAlO3/SrTiO3 Heterostructure Driven by Polar Field-Assisted Oxygen Vacancy Formation at the Surface. Adv. Sci. 2021, 8, 2002073. [Google Scholar] [CrossRef]
- Coelho, L.L.; Hotza, D.; Estrella, A.S.; de Amorim, S.M.; Puma, G.L.; Moreira, R.d.P.M. Modulating the photocatalytic activity of TiO2 (P25) with lanthanum and graphene oxide. J. Photochem. Photobiol. A Chem. 2019, 372, 1–10. [Google Scholar] [CrossRef]
- Priyanka, K.P.; Revathy, V.R.; Rosmin, P.; Thrivedu, B.; Elsa, K.M.; Nimmymol, J.; Balakrishna, K.M.; Varghese, T. Influence of La doping on structural and optical properties of TiO2 nanocrystals. Mater. Charact. 2016, 113, 144–151. [Google Scholar] [CrossRef]
- Wu, A.; Wang, D.; Wei, C.; Zhang, X.; Liu, Z.; Feng, P.; Ou, X.; Qiang, Y.; Garcia, H.; Niu, J. A comparative photocatalytic study of TiO2 loaded on three natural clays with different morphologies. Appl. Clay Sci. 2019, 183, 105352. [Google Scholar] [CrossRef]
- Eskandarloo, H.; Badiei, A.; Behnajady, M.A.; Tavakoli, A.; Ziarani, G.M. Ultrasonic-assisted synthesis of Ce doped cubic-hexagonal ZnTiO3 with highly efficient sonocatalytic activity. Ultrason. Sonochem. 2016, 29, 258–269. [Google Scholar] [CrossRef]
- Guo, W.; She, Z.; Yang, S.; Xue, H.; Zhang, X. Understanding the influence of Lu, La and Ga active elements on the bonding properties of Sn/SiO2 interfaces from first principle calculations. Ceram. Int. 2020, 46, 24737–24743. [Google Scholar] [CrossRef]
- Khan, S.; Cho, H.; Kim, D.; Han, S.S.; Lee, K.H.; Cho, S.H.; Song, T.; Choi, H. Defect engineering toward strong photocatalysis of Nb-doped anatase TiO2: Computational predictions and experimental verifications. Appl. Catal. B Environ. 2017, 206, 520–530. [Google Scholar] [CrossRef]
- Mazierski, P.; Mikolajczyk, A.; Bajorowicz, B.; Malankowska, A.; Zaleska-Medynska, A.; Nadolna, J. The role of lanthanides in TiO2-based photocatalysis: A review. Appl. Catal. B Environ. 2018, 233, 301–317. [Google Scholar] [CrossRef]
- Ako, R.T.; Ekanayake, P.; Tan, A.L.; Young, D.J. La modified TiO2 photoanode and its effect on DSSC performance: A comparative study of doping and surface treatment on deep and surface charge trapping. Mater. Chem. Phys. 2016, 172, 105–112. [Google Scholar] [CrossRef]
- Zhan, C.G. Development and application of first-principles electronic structure approach for molecules in solution based on fully polarizable continuum model. Wuli Huaxue Xuebao/Acta Physico. Chim. Sin. 2011, 27, 1–10. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter Mater. Phys. 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.C.; Tang, G.; Geng, W.T. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Sujith, C.P.; Joseph, S.; Mathew, T.; Mathew, V. First-principles investigation of structural, electronic and optical properties of quasi-one-dimensional barium cadmium chalcogenides Ba2CdX3 (X = S, Se, Te) using HSE06 and GGA-PBE functionals. J. Phys. Chem. Solids 2022, 161, 110488. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Quantum density oscillations in an inhomogeneous electron gas. Phys. Rev. 1965, 137, A1697. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; Hernández, K.; González, S. Cu(C3H3N3S3)3 Adsorption onto ZnTiO3/TiO2 for Coordination-Complex Sensitized Photochemical Applications. Materials 2022, 15, 3252. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- German, E.; Faccio, R.; Mombrú, A.W. Comparison of standard DFT and Hubbard-DFT methods in structural and electronic properties of TiO2 polymorphs and H-titanate ultrathin sheets for DSSC application. Appl. Surf. Sci. 2018, 428, 118–123. [Google Scholar] [CrossRef]
- Cherifi, K.; Cheknane, A.; Hilal, H.S.; Benghia, A.; Rahmoun, K.; Benyoucef, B. Investigation of triphenylamine-based sensitizer characteristics and adsorption behavior onto ZnTiO3 perovskite (1 0 1) surfaces for dye-sensitized solar cells using first-principle calculation. Chem. Phys. 2020, 530, 110595. [Google Scholar] [CrossRef]
- Nor, N.U.M.; Mazalan, E.; Risko, C.; Crocker, M.; Amin, N.A.S. Unveiling the structural, electronic, and optical effects of carbon-doping on multi-layer anatase TiO2 (1 0 1) and the impact on photocatalysis. Appl. Surf. Sci. 2022, 586, 152641. [Google Scholar] [CrossRef]
- Samanta, P.K.; English, N.J. Opto-electronic properties of stable blue photosensitisers on a TiO2 anatase-101 surface for efficient dye-sensitised solar cells. Chem. Phys. Lett. 2019, 731, 136624. [Google Scholar] [CrossRef]
- Chang, X.; Li, X.; Xue, Q. Sensing mechanism of acetone adsorption on charged ZnO and ZnSe surfaces: Insights from DFT calculations. Mater. Today Commun. 2022, 31, 103238. [Google Scholar] [CrossRef]
- Lai, W.; Zhang, K.; Shao, P.; Yang, L.; Ding, L.; Pavlostathis, S.G.; Shi, H.; Zou, L.; Liang, D.; Luo, X. Optimization of adsorption configuration by DFT calculation for design of adsorbent: A case study of palladium ion-imprinted polymers. J. Hazard. Mater. 2019, 379, 120791. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; Capa, L.F.; Medina, F.; González, S. Dft study of methylene blue adsorption on ZnTiO3 and TiO2 surfaces (101). Molecules 2021, 26, 3780. [Google Scholar] [CrossRef]
- Paterson, A.L.; Hanson, M.A.; Werner-Zwanziger, U.; Zwanziger, J.W. Relating 139La Quadrupolar Coupling Constants to Polyhedral Distortion in Crystalline Structures. J. Phys. Chem. C 2015, 119, 25508–25517. [Google Scholar] [CrossRef]
- Maldonado, F.; Villamagua, L.; Rivera, R. DFT Analysis of the Adsorption of Phenol on the Nonpolar (1010) ZnO Surface. J. Phys. Chem. C 2019, 123, 12296–12304. [Google Scholar] [CrossRef]
- Hinuma, Y.; Pizzi, G.; Kumagai, Y.; Oba, F.; Tanaka, I. Band structure diagram paths based on crystallography. Comput. Mater. Sci. 2017, 128, 140–184. [Google Scholar] [CrossRef]
- Yu, M.; Trinkle, D.R. Accurate and efficient algorithm for Bader charge integration. J. Chem. Phys. 2011, 134, 064111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, W.; Wang, W.C.; Shi, X.Q. Ionicity of bonding in elemental solids. J. Phys. Commun. 2018, 2, 115009. [Google Scholar] [CrossRef]
- Kumar, P.S.V.; Raghavendra, V.; Subramanian, V. Bader’s Theory of Atoms in Molecules (AIM) and its Applications to Chemical Bonding. J. Chem. Sci. 2016, 128, 1527–1536. [Google Scholar] [CrossRef]
- Koch, D.; Golub, P.; Manzhos, S. Stability of charges in titanium compounds and charge transfer to oxygen in titanium dioxide. J. Phys. Conf. Ser. 2018, 1136, 12017. [Google Scholar] [CrossRef]
- Savin, A.; Nesper, R.; Wengert, S.; Fässler, T.F. ELF: The Electron Localization Function. Angew. Chemie Int. Ed. Engl. 1997, 36, 1808–1832. [Google Scholar] [CrossRef]
- Wen, C.; Zhu, Y.J.; Kanbara, T.; Zhu, H.Z.; Xiao, C.F. Effects of I and F codoped TiO2 on the photocatalytic degradation of methylene blue. Desalination 2009, 249, 621–625. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; González, S.; Medina, F. La-doped ZnTiO3 /TiO2 nanocomposite supported on ecuadorian diatomaceous earth as a highly efficient photocatalyst driven by solar light. Molecules 2021, 26, 6232. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, G.; Sun, Z.; Zheng, S.; Frost, R.L. A comparative study about the influence of metal ions (Ce, la and V) doping on the solar-light-induced photodegradation toward rhodamine B. J. Environ. Chem. Eng. 2015, 3, 1444–1451. [Google Scholar] [CrossRef]
- Armaković, S.J.; Grujić-Brojčin, M.; Šćepanović, M.; Armaković, S.; Golubović, A.; Babić, B.; Abramović, B.F. Efficiency of La-doped TiO2 calcined at different temperatures in photocatalytic degradation of β-blockers. Arab. J. Chem. 2019, 12, 5355–5369. [Google Scholar] [CrossRef]
- Surendar, T.; Kumar, S.; Shanker, V. Influence of La-doping on phase transformation and photocatalytic properties of ZnTiO3nanoparticles synthesized via modified sol-gel method. Phys. Chem. Chem. Phys. 2014, 16, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Elsellami, L.; Lachheb, H.; Houas, A. Synthesis, characterization and photocatalytic activity of Li-, Cd-, and La-doped TiO2. Mater. Sci. Semicond. Process. 2015, 36, 103–114. [Google Scholar] [CrossRef]
- Cherifi, K.; Cheknane, A.; Benghia, A.; Hilal, H.S.; Rahmoun, K.; Benyoucef, B.; Goumri-Said, S. Exploring N3 ruthenium dye adsorption onto ZnTiO3 (101) and (110) surfaces for dye sensitized solar cell applications: Full computational study. Mater. Today Energy 2019, 13, 109–118. [Google Scholar] [CrossRef]
- Shu, Q.; Liu, X.; Huo, Y.; Tan, Y.; Zhang, C.; Zou, L. Construction of a Brönsted-Lewis solid acid catalyst La-PW-SiO2/SWCNTs based on electron withdrawing effect of La(III) on π bond of SWCNTs for biodiesel synthesis from esterification of oleic acid and methanol. Chin. J. Chem. Eng. 2022, 44, 351–362. [Google Scholar] [CrossRef]
- Kumar, V.V.; Naresh, G.; Sudhakar, M.; Tardio, J.; Bhargava, S.K.; Venugopal, A. Role of Brønsted and Lewis acid sites on Ni/TiO2 catalyst for vapour phase hydrogenation of levulinic acid: Kinetic and mechanistic study. Appl. Catal. A Gen. 2015, 505, 217–223. [Google Scholar] [CrossRef]


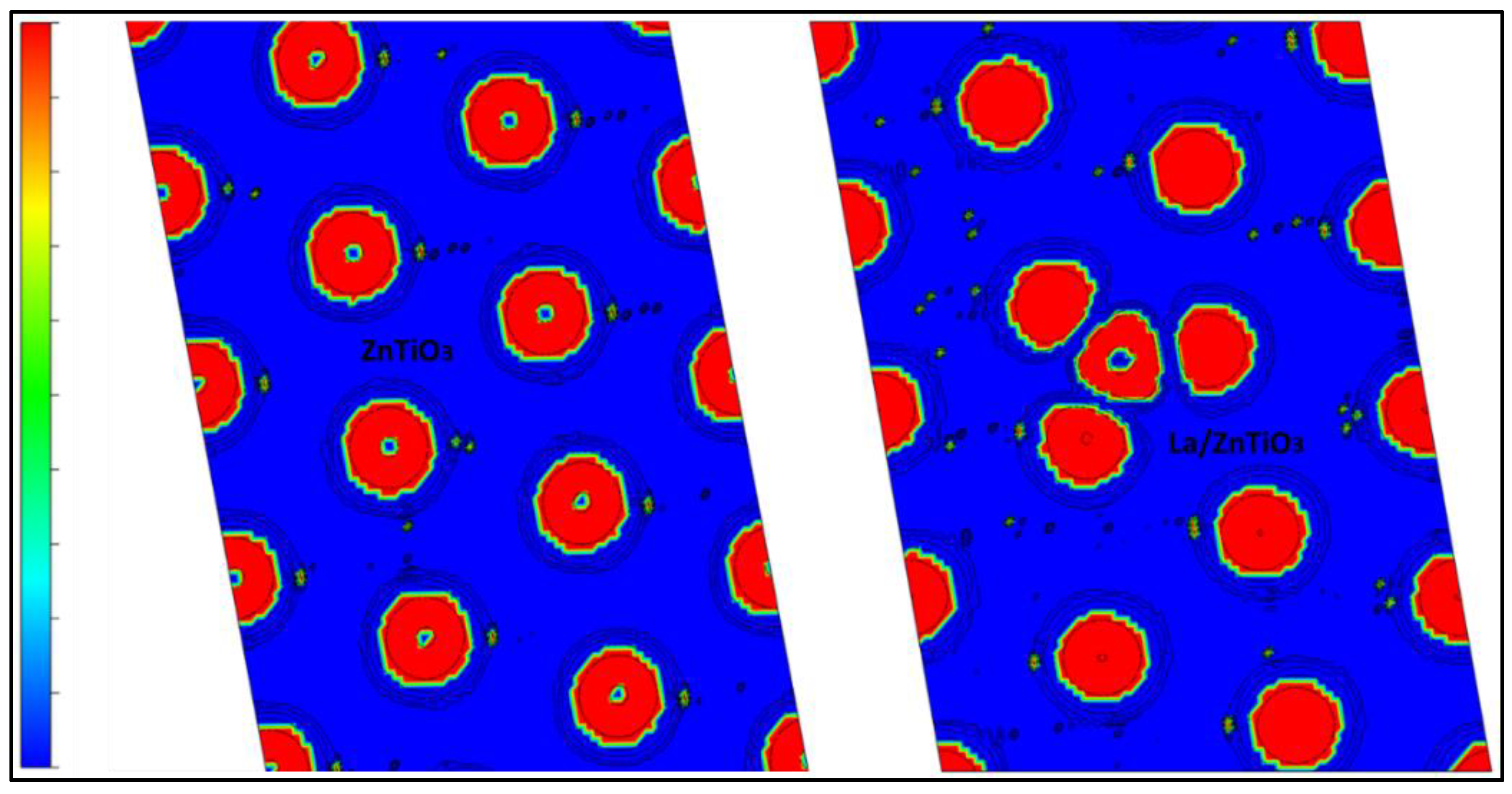
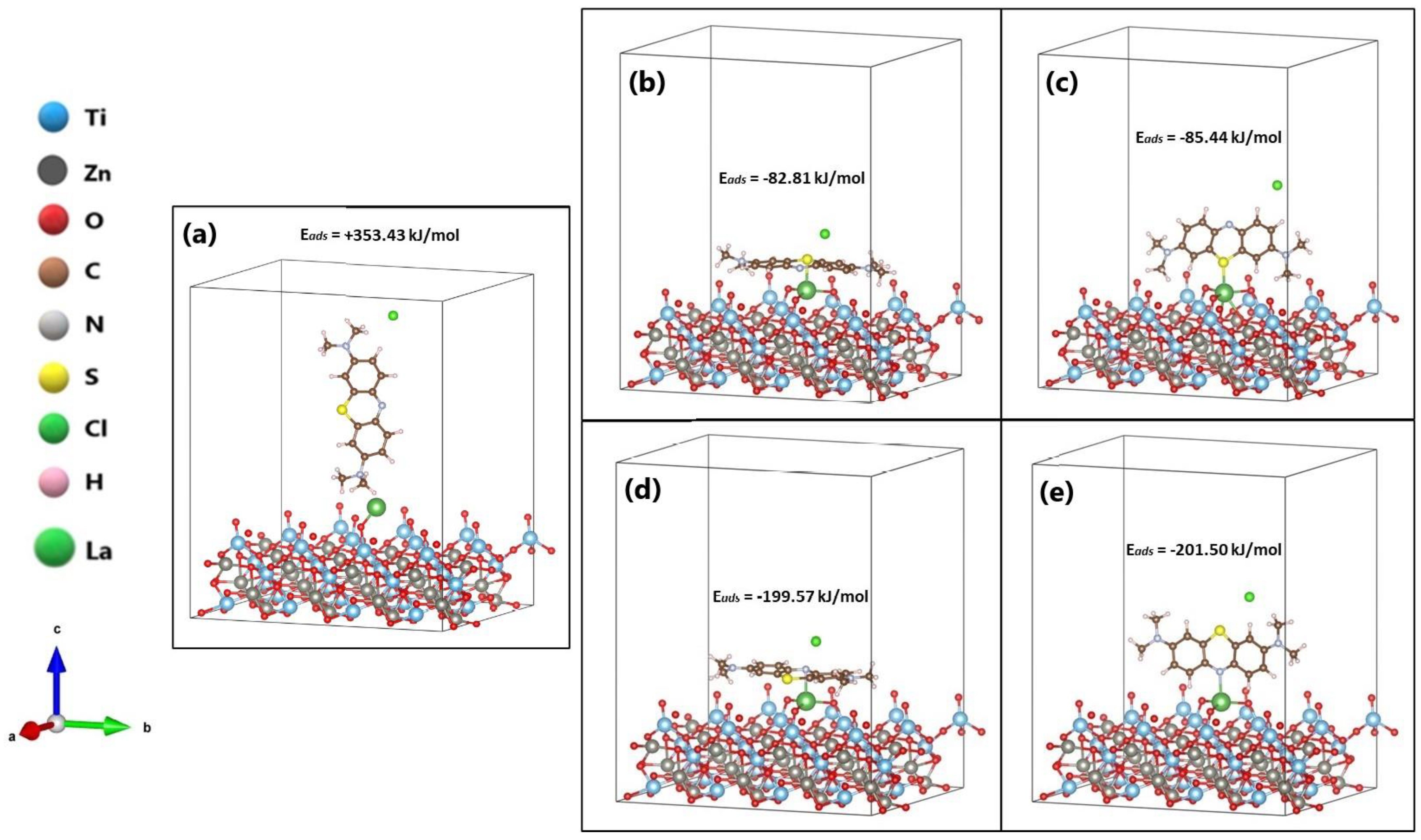
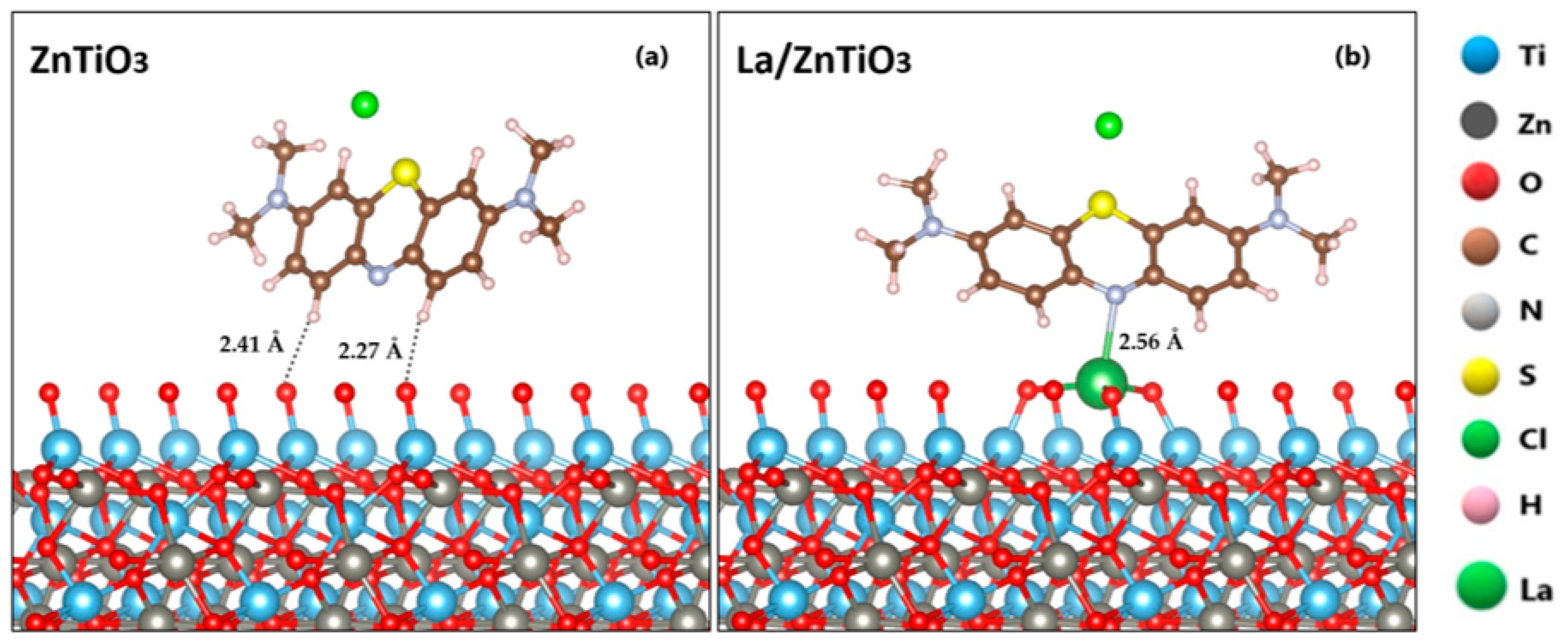
| Bond Length (Å) | Angle (°) | ||
|---|---|---|---|
| Atoms | La/ZnTiO3 | Atoms | La/ZnTiO3 |
| La-O4 | 2.32 | La-O4-Ti17 | 117.04 |
| La-O6 | 2.32 | La-O6-Ti26 | 116.25 |
| La-O8 | 2.38 | La-O8-Ti23 | 133.26 |
| Position | Bond | ∆Eads (kJ/mol) | ∆Gseg (kJ/mol) |
|---|---|---|---|
| ZnTiO3 | O-H | −64.06 | - |
| La/ZnTiO3: P0 | - | +353.43 | - |
| La/ZnTiO3: P1 | La-S | −82.81 | −57.00 |
| La/ZnTiO3: P2 | La-S | −85.44 | −57.00 |
| La/ZnTiO3: P3 | La-N | −199.57 | −58.19 |
| La/ZnTiO3: P4 | La-N | −201.50 | −58.19 |
| Absorption System | Atom | Total Electron (-e) before Adsorption | Total Electron (-e) after Adsorption | Transfer Charge (-e) |
|---|---|---|---|---|
| MB absorbed on ZnTiO3 | H1 | 0.89 | 0.88 | +0.02 |
| H2 | 0.88 | 0.86 | +0.01 | |
| O1 | 7.11 | 7.15 | −0.03 | |
| O2 | 7.11 | 7.14 | −0.03 | |
| MB absorbed on La/ZnTiO3 | N1 | 7.45 | 7.77 | −0.33 |
| La | 8.85 | 8.87 | +0.02 |
| Adsorbent | Method | Bandgap (eV) | Reference |
|---|---|---|---|
| ZnTiO3/TiO2 | Experimental | 3.07 | [78] |
| La/ZnTiO3/TiO2 | Experimental | 3.04 | [78] |
| ZnTiO3 | Experimental | 3.54 | [81] |
| La/ZnTiO3 (1%) | Experimental | 3.37 | [81] |
| La/ZnTiO3 (2%) | Experimental | 2.92 | [81] |
| La/ZnTiO3 (3%) | Experimental | 3.35 | [81] |
| La/ZnTiO3 (4%) | Experimental | 3.01 | [81] |
| La/ZnTiO3 (5%) | Experimental | 3.12 | [81] |
| ZnTiO3 | VASP (GGA/PBE+U) | 3.16 | [68] |
| La/ZnTiO3 | VASP (GGA/PBE+U) | 2.98 | This study |
| Adsorbent | Dye | Software Used | Adsorption (kJ/mol) | References |
|---|---|---|---|---|
| ZnTiO3 | s-Cu-TTC | VASP | −296.56 | [60] |
| ZnTiO3 | TPA-1 | CASTEP | −136.39 | [63] |
| ZnTiO3 | TPA-2 | CASTEP | −157.47 | [63] |
| ZnTiO3 | TPA-3 | CASTEP | −561.33 | [63] |
| ZnTiO3 | TPA-4 | CASTEP | −228.19 | [63] |
| ZnTiO3 (H) | MB | VASP | −126.76 | [68] |
| ZnTiO3 (SP) | MB | VASP | −282.05 | [68] |
| ZnTiO3 (P4) | MB | VASP | −64.06 | This study |
| La/ZnTiO3 (P1) | MB | VASP | −82.81 | This study |
| La/ZnTiO3 (P2) | MB | VASP | −85.44 | This study |
| La/ZnTiO3 (P3) | MB | VASP | −199.57 | This study |
| La/ZnTiO3 (P4) | MB | VASP | −201.50 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaramillo-Fierro, X.; Cuenca, G.; Ramón, J. The Effect of La3+ on the Methylene Blue Dye Removal Capacity of the La/ZnTiO3 Photocatalyst, a DFT Study. Nanomaterials 2022, 12, 3137. https://doi.org/10.3390/nano12183137
Jaramillo-Fierro X, Cuenca G, Ramón J. The Effect of La3+ on the Methylene Blue Dye Removal Capacity of the La/ZnTiO3 Photocatalyst, a DFT Study. Nanomaterials. 2022; 12(18):3137. https://doi.org/10.3390/nano12183137
Chicago/Turabian StyleJaramillo-Fierro, Ximena, Guisella Cuenca, and John Ramón. 2022. "The Effect of La3+ on the Methylene Blue Dye Removal Capacity of the La/ZnTiO3 Photocatalyst, a DFT Study" Nanomaterials 12, no. 18: 3137. https://doi.org/10.3390/nano12183137
APA StyleJaramillo-Fierro, X., Cuenca, G., & Ramón, J. (2022). The Effect of La3+ on the Methylene Blue Dye Removal Capacity of the La/ZnTiO3 Photocatalyst, a DFT Study. Nanomaterials, 12(18), 3137. https://doi.org/10.3390/nano12183137










