Recent Progress in Morphology-Tuned Nanomaterials for the Electrochemical Detection of Heavy Metals
Abstract
:1. Introduction
2. Classification of Nanomaterial Morphology Based on Dimensions
2.1. Zero-Dimensional (0-D) Nanomaterials
2.2. One-Dimensional (1-D) Nanomaterials
2.3. Two-Dimensional (2-D) Nanomaterials
2.4. Three-Dimensional (3-D) Nanomaterials
3. Comparison of Sensitivities of Nanomaterials with Different Morphologies
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tchounwou, P.; Yedjou, C.; Patlolla, A.; Sutton, D. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology. Experientia Supplementum; Luch, A., Ed.; Springer: Basel, Switzerland; Berlin, Germany, 2012; Volume 101, pp. 133–164. [Google Scholar]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 6730305, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda. Available online: https://www.who.int/publications/i/item (accessed on 28 September 2022).
- Ali, M.M.; Hossain, D.; Al-Imran; Khan, M.S.; Begum, M.; Osman, M. Environmental Pollution with Heavy Metals: A Public Health Concern, IntechOpen. 2021. Available online: https://www.intechopen.com/chapters/76739 (accessed on 16 September 2022).
- Tchounwou, P.; Yedjou, C.; Patlolla, A.; Sutton, D. Heavy metals toxicity and the environment. Mol. Clin. Environ. Toxicol. 2012, 101, 133–164. [Google Scholar]
- L’opez, Y.; Ortega, G.; Reguera, E. Hazardous ions decontamination: From the element to the material. Chem. Eng. J. Adv. 2022, 11, 100297. [Google Scholar] [CrossRef]
- Corr, J.J.; Larsen, E.H. Arsenic speciation by liquid chromatography coupled with ionspray tandem mass spectrometry. J. Anal. Atomic Spectrom. 1996, 11, 1215–1224. [Google Scholar] [CrossRef]
- Gong, T.; Liu, J.; Liu, X.; Liu, J.; Xiang, J.; Wu, Y. A sensitive and selective platform based on CdTe QDs in the presence of L-cysteine for detection of silver, mercury, and copper ions in water and various drinks. Food Chem. 2016, 213, 306–312. [Google Scholar] [CrossRef]
- Bings, N.H.; Bogaerts, A.; Broekaert, J. Atomic spectroscopy: A review. Anal. Chem. 2006, 78, 3917–3946. [Google Scholar] [CrossRef]
- Sharma, V.; Saini, A.; Mobin, S. Multicolour fluorescent carbon nanoparticle probes for live cell imaging and dual palladium and mercury sensors. J. Mater. Chem. B 2016, 4, 2466–2476. [Google Scholar] [CrossRef]
- Losev, V.; Buyko, O.; Trofimchuk, A.; Zuy, O. Silica sequentially modified with polyhexamethylene guanidine and arsenazo I for preconcentration and ICPOES determination of metals in natural waters. Micro Chem. J. 2015, 123, 84–89. [Google Scholar] [CrossRef]
- Sawan, S.; Maalouf, R.; Errachid, A.; Renault, N. Metal and metal oxide nanoparticles in the voltammetric detection of heavy metals: A review. TrAC 2020, 131, 116014. [Google Scholar] [CrossRef]
- Tajik, S.; Dourandish, Z.; Jahani, P.; Sheikhshoaie, I.; Beitollahi, H.; Asl, M.; Jang, H.; Shokouhimehr, M. Recent developments in voltammetric and amperometric sensors for cysteine detection. RSC Adv. 2021, 11, 5411–5425. [Google Scholar] [CrossRef]
- Pletcher, D.; Greff, R.; Peat, R.; Peter, L.; Robinson, J. Instrumental Methods in Electrochemistry, 1st ed.; Woodhead Publishing: Cambridge, UK, 2001; pp. 18–228. [Google Scholar]
- Karimi-Maleh, H.; Karimi, F.; Alizadeh, M.; Sanati, A. Electrochemical sensors, a bright future in the fabrication of portable kits in analytical systems. Chem. Rec. 2020, 20, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Bansod, B.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Wang, Y.; Chen, Z.; Lou, T.; Qin, W. Nanomaterials/ionophore-based electrode for anodic stripping voltammetric determination of lead: An electrochemical sensing platform toward heavy metals. Anal. Chem. 2009, 81, 5088–5094. [Google Scholar] [CrossRef] [PubMed]
- Beaver, K.; Dantanarayana, A.; Minteer, S. Materials approaches for improving electrochemical sensor performance. J. Phys. Chem. B. 2021, 125, 11820–11834. [Google Scholar] [CrossRef]
- Venkatanarayanan, A.; Spain, E. Review of recent developments in sensing materials. In Comprehensive Materials Processing; Hashmi, S., Batalha, G., Tyne, C., Yilbas, B., Eds.; Elsevier: Dublin, Ireland, 2014; Volume 13, pp. 47–101. [Google Scholar]
- Torres-Rivero, K.; Florido, A.; Bastos-Arrieta, J. Recent trends in the improvement of the electrochemical response of screen-printed electrodes by their modification with shaped metal nanoparticles. Sensors 2021, 21, 2596. [Google Scholar] [CrossRef]
- Meier, J.; Schiøtz, J.; Liu, P.; Nørskov, J.; Stimming, U. Nano-scale effects in electrochemistry. Chem. Phys. Lett. 2004, 390, 440–444. [Google Scholar] [CrossRef]
- Li, W.; Yao, X.; Guo, Z.; Liu, J.; Huang, X. Fe3O4 with novel nanoplate-stacked structure: Surfactant-free hydrothermal synthesis and application in detection of heavy metal ions. J. Electroanal. Chem. 2015, 749, 75–82. [Google Scholar] [CrossRef]
- Liao, J.; Zhang, J.; Wang, C.; Lin, S. Electrochemical and density functional theory investigation on the differential behaviors of core-ring structured NiCo2O4 nanoplatelets toward heavy metal ions. Anal. Chim. Acta. 2018, 1022, 37–44. [Google Scholar] [CrossRef]
- Lei, P.; Zhou, Y.; Zhao, S.; Dong, C.; Shuang, S. Carbon-supported X-manganate (X-Ni, Zn, and Cu) nanocomposites for sensitive electrochemical detection of trace heavy metal ions. J. Hazard. Mater. 2022, 435, 129036. [Google Scholar] [CrossRef]
- Gleiter, H. Nanostructured materials: Basic concepts and microstructure. Acta Mater. 2000, 48, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Pokropivny, V.; Skorokhod, V. Classification of nanostructures by dimensionality and concept of surface forms engineering in nanomaterial science. Mater. Sci. Eng. C. 2007, 27, 990–993. [Google Scholar] [CrossRef]
- Bashir, S.; Liu, J. Advanced Nanomaterials and their Applications in Renewable Energy, 1st ed.; Elsevier: Texas, TX, USA, 2015; pp. 1–50. [Google Scholar]
- Wang, Z.; Hu, T.; Liang, R.; Wei, M. Application of zero dimensional nanomaterials in biosensing. Front. Chem. 2020, 8, 320. [Google Scholar] [CrossRef]
- Bhanjana, G.; Dilbaghi, N.; Kumar, R.; Umar, A.; Kumar, S. SnO2 quantum dots as novel platform for electrochemical sensing of cadmium. Electrochim. Acta 2015, 169, 97–102. [Google Scholar] [CrossRef]
- Ting, S.; Ee, S.; Ananthanarayanan, A.; Leong, K.; Chen, P. Graphene quantum dots functionalized gold nanoparticles for sensitive electrochemical detection of heavy metal ions. Electrochim. Acta 2015, 172, 7–11. [Google Scholar] [CrossRef]
- Sui, X.; Downing, J.; Hersam, M.; Chen, J. Additive manufacturing and applications of nanomaterial-based sensors. Mater. Today Commun. 2021, 48, 35–154. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, J.; Zhu, H.; Yang, T.; Zou, M.; Zhang, M.; Du, M. Facile and green fabrication of size-controlled AuNPs/CNFs hybrids for the highly sensitive simultaneous detection of heavy metal ions. Electrochim. Acta 2016, 196, 422–430. [Google Scholar] [CrossRef]
- Bhanjana, G.; Dilbaghi, N.; Singhal, N.; Kim, K.; Kumar, S. Zinc oxide nanopillars as an electrocatalyst for direct redox sensing of cadmium. J. Ind. Eng. Chem. 2017, 53, 192–200. [Google Scholar] [CrossRef]
- Narouei, F.H.; Livernois, L.; Andreescu, D.; Andreescu, S. Highly sensitive mercury detection using electroactive gold-decorated polymer nanofibers. Sens. Actuators B Chem. 2021, 329, 129267. [Google Scholar] [CrossRef]
- Yıldız, C.; Bayraktepe, D.; Yazan, Z.; Önal, M. Bismuth nanoparticles decorated on Na-montmorillonite-multiwall carbon nanotube for simultaneous determination of heavy metal ions-electrochemical methods. J. Electroanal. Chem. 2022, 910, 116205. [Google Scholar] [CrossRef]
- Korotcenkov, G. Current trends in nanomaterials for metal oxide-based conductometric gas sensors: Advantages and limitations. part 1: 1D and 2D nanostructures. Nanomaterials 2020, 10, 1392. [Google Scholar] [CrossRef]
- Shan, Q.; Tian, J.; Ding, Q.; Wu, W. Electrochemical sensor based on metal-free materials composed of graphene and graphene oxide for sensitive detection of cadmium ions in water. Mater. Chem. Phys. 2022, 284, 126064. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, S.; Ye, H.; Yuan, J.; Song, P.; Hu, S. Graphene/CeO2 hybrid materials for the simultaneous electrochemical detection of cadmium(II), lead(II), copper(II), and mercury(II). J. Electroanal. Chem. 2015, 757, 235–242. [Google Scholar] [CrossRef]
- Xu, C.; Wang, X.; Zhu, J. Graphene−metal particle nanocomposites. J. Phys. Chem. C 2008, 112, 19841–19845. [Google Scholar] [CrossRef]
- Li, S.; Xu, Q.; Xu, J.; Yan, G.; Zhang, Y.; Li, S.; Yin, L. Engineering Co2+/Co3+ redox activity of Ni-mediated porous Co3O4 nanosheets for superior Hg(II) electrochemical sensing: Insight into the effect of valence change cycle and oxygen vacancy on electroanalysis. Sens. Actuators B Chem. 2022, 354, 131095. [Google Scholar] [CrossRef]
- Peng, Q.; Guo, J.; Zhang, Q.; Xiang, J.; Liu, B.; Zhou, A.; Liu, R.; Tian, Y. Unique lead adsorption behavior of activated hydroxyl group in two-dimensional titanium carbide. J. Am. Chem. Soc. 2014, 136, 4113–4116. [Google Scholar] [CrossRef]
- Shahzad, F.; Zaidi, S.; Naqvi, R. 2D transition metal carbides (MXene) for electrochemical sensing: A review. Crit. Rev. Anal. Chem. 2022, 52, 848–864. [Google Scholar] [CrossRef]
- He, Y.; Ma, L.; Zhou, L.; Liu, G.; Jiang, Y.; Gao, J. Preparation and application of bismuth/MXene nano-composite as electrochemical sensor for heavy metal ions detection. Nanomaterials 2020, 10, 866. [Google Scholar] [CrossRef]
- Huang, H.; Chen, T.; Liu, X.; Ma, H. Ultrasensitive and simultaneous detection of heavy metal ions based on three-dimensional graphene-carbon nanotubes hybrid electrode materials. Anal. Chim. Acta 2014, 852, 45–54. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Li, Y.; Li, Y.; Li, Z.; Zhang, W.; Zou, X.; Shi, J.; Huang, X.; Liu, C.; et al. Rapid detection of cadmium ions in meat by a multi-walled carbon nanotubes enhanced metal-organic framework modified electrochemical sensor. Food Chem. 2021, 357, 129762. [Google Scholar] [CrossRef]
- Lu, Z.; Dai, W.; Lin, X.; Liu, B.; Zhang, J.; Ye, J.; Ye, J. Facile one-step fabrication of a novel 3D honeycomb-like bismuth nanoparticles decorated N-doped carbon nanosheet frameworks: Ultrasensitive electrochemical sensing of heavy metal ions. Electrochim. Acta 2018, 266, 94–102. [Google Scholar] [CrossRef]
- Lu, Z.; Dai, W.; Liu, B.; Mo, G.; Zhang, J.; Ye, J.; Ye, J. One pot synthesis of dandelion-like polyaniline coated gold nanoparticles composites for electrochemical sensing applications. J. Colloid. Interface. Sci. 2018, 525, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Zhu, M.; Guo, H.; Ying, S.; Huang, X. Simple fabrication of a hexagonal prisms with hexagonal pyramid tips V2O5@MOF(V, Co) and its application as electrochemical sensor for Pb2+. Inorg. Chem. Commun. 2021, 133, 108966. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, K.; Sun, H.; Zhang, D.; Zhu, J.; Zhou, S.; Li, W.; Li, Y.; Wang, C.; Jia, X.; et al. Star-shaped porous nitrogen-doped metal-organic framework carbon as an electrochemical platform for sensitive determination of Cd(II) in environmental and tobacco samples. Anal. Chim. Acta 2022, 1228, 340309. [Google Scholar] [CrossRef] [PubMed]
- Naseri, M.; Mohammadniaei, M.; Ghosh, K.; Sarkar, S.; Sankar, R.; Mukherjee, S.; Pal, S.; Dezfouli, E.; Qiao, J.; Bhattacharyya, N.; et al. A robust electrochemical sensor based on butterfly-shaped silver nanostructure for concurrent quantification of heavy metals in water samples. Electroanalysis 2022, 34, 1–11. [Google Scholar] [CrossRef]
- Li, X.; Wen, H.; Fu, Q.; Peng, D.; Yu, J.; Zhang, Q.; Huang, X. Morphology-dependent NiO modified glassy carbon electrode surface for lead(II) and cadmium (II) detection. Appl. Surf. Sci. 2016, 363, 7–12. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Y.; Li, Y.; Zeng, T.; Wan, Q.; Yang, N. Morphology-controlled electrochemical sensing of environmental Cd2+ and Pb2+ ions on expanded graphite supported CeO2 nanomaterials. Anal. Chim. Acta 2020, 1126, 63–71. [Google Scholar] [CrossRef]
- Li, S.; Zhou, W.; Jiang, M.; Li, L.; Sun, Y.; Guo, Z.; Liu, J.; Huang, X. Insights into diverse performance for the electroanalysis of Pb(II) on Fe2O3 nanorods and hollow nanocubes: Toward analysis of adsorption sites. Electrochim. Acta 2018, 288, 42–51. [Google Scholar] [CrossRef]
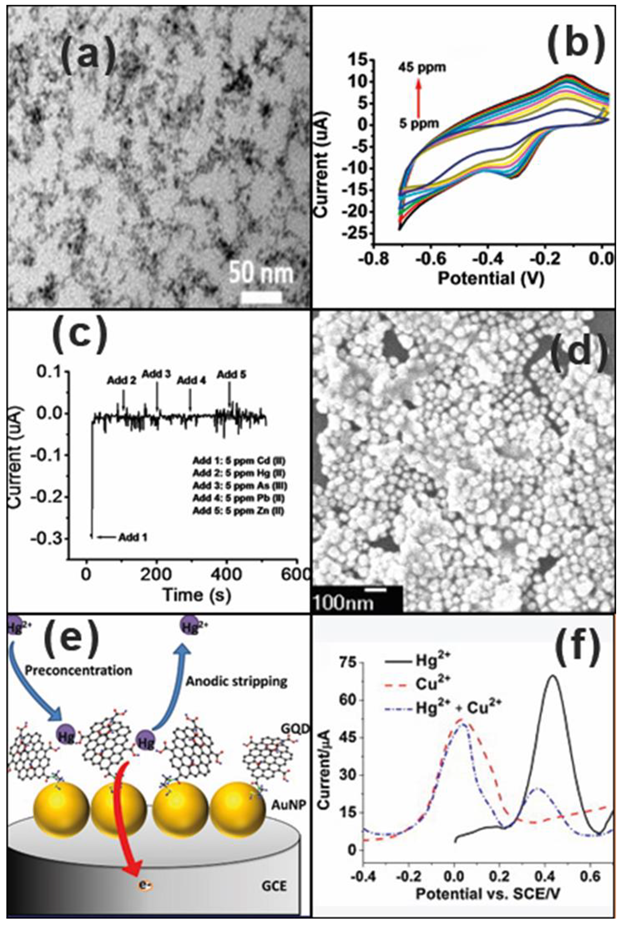
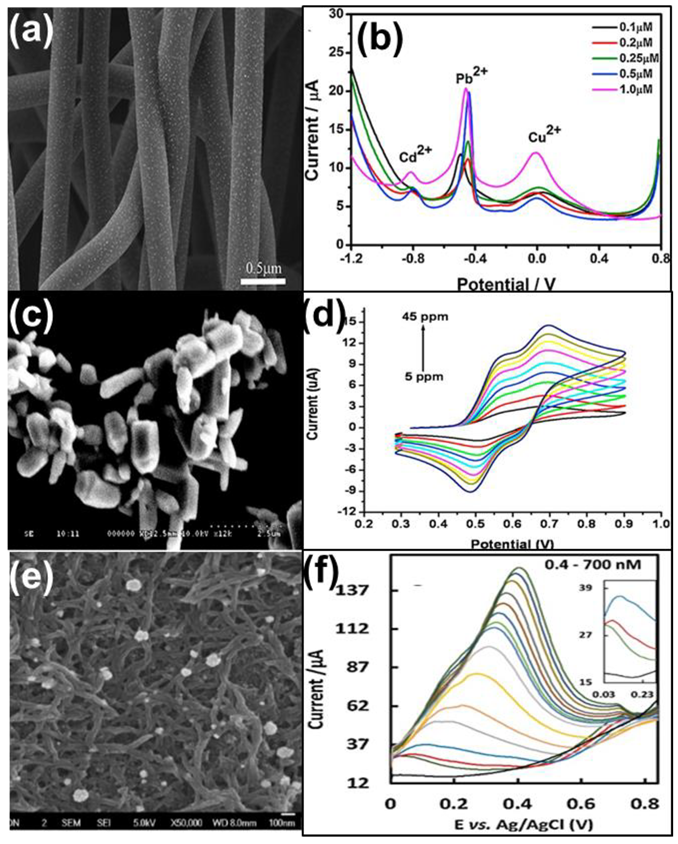
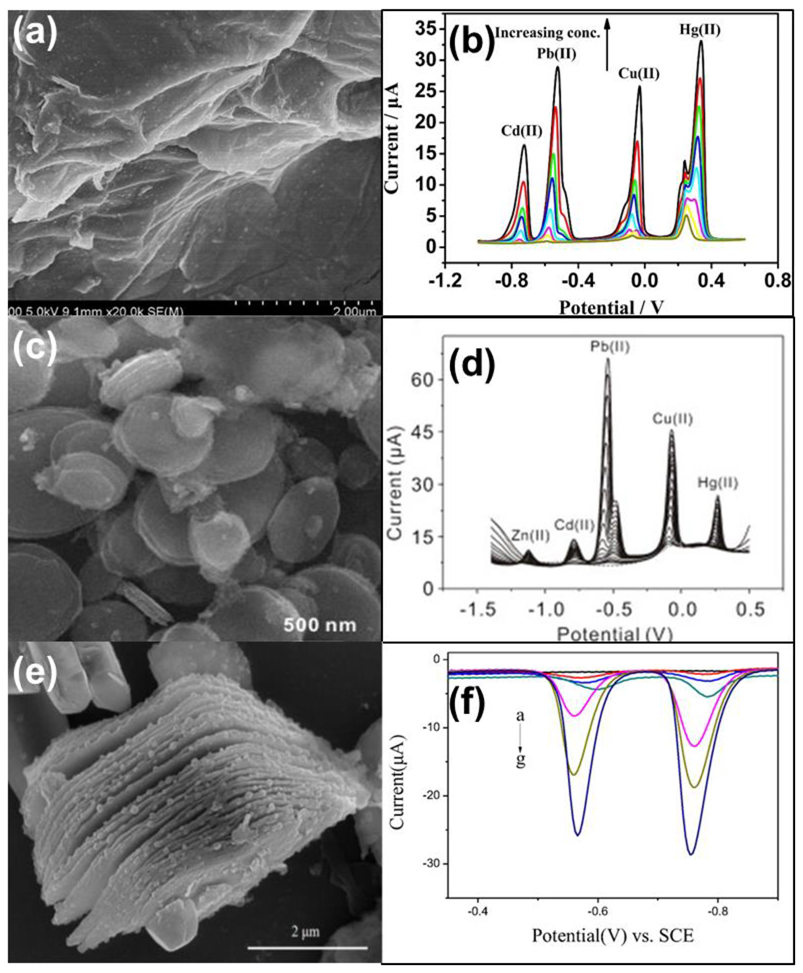
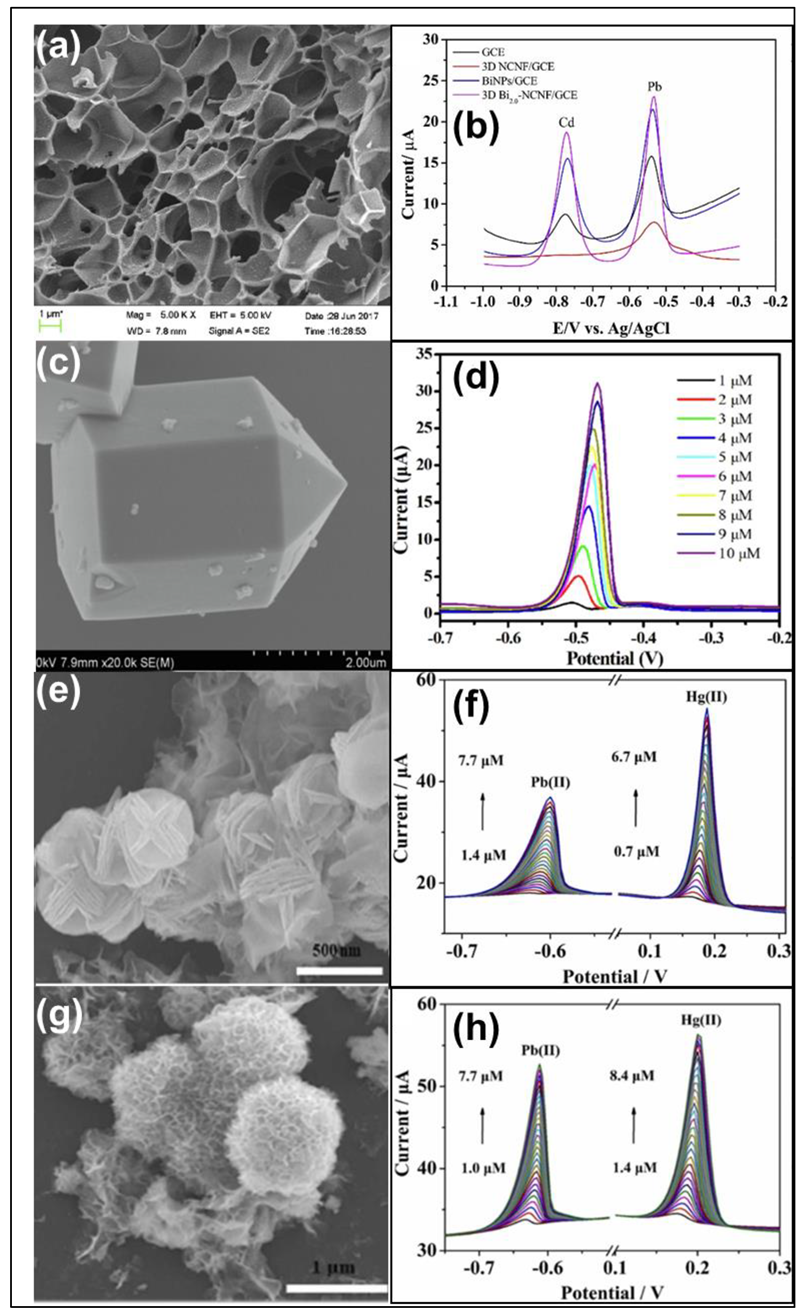
| Heavy Metal Ions (HMIs) | Guideline Value (μg/L) |
|---|---|
| As | 10 |
| Cd | 3 |
| Cr | 50 |
| Cu | 2000 |
| Pb | 10 |
| Hg | 6 |
| Ni | 70 |
| Zn | 3000 |
| Morphology | Electrode | Analyte | Electrochemical Technique | LOD | Linear Range | Sensitivity | Ref. |
|---|---|---|---|---|---|---|---|
| Quantum dots | SnO2 QD/Nafion/Au | Cd(II) | Amperometry | 0.5 ppm | 77.5 nA/ppm2 | [29] | |
| Quantum dots | GQD-Au/GCE | Hg(II) Cu(II) | ASV | 0.02 nM 0.05 nM | 2.47 μA/nM 3.69 μA/nM | [30] | |
| Nanopillars | ZnO/Nafion/Au | Cd(II) | CV | 4 ppb | 5–45 ppm | 10 μA/cm2/ppb | [33] |
| Nanofiber | AuNps/CNF/GCE | Cd(II) Pb(II) Cu(II) | SWASV | 0.1 μM 0.1 μM 0.1 μM | [32] | ||
| Nanotube Hollow nanocube | Fe2O3/GCE | Pb(II) | DPV | 0.0034 μM 0.083 μM | 109.67 μAμM−1 17.68 μAμM−1 | [53] | |
| Nanofiber | Au/PANOA/SPCE | Hg(II) | ASV | 0.23 nM | 0.8–12 nM | [34] | |
| Nanotube | BiNP/MWCNT-NNaM/PGE | Zn(II) Cd(II) Pb(II) Cu(II) | SWASV | 0.707 μM 0.097 μM, 0.008 μM, 0.157 μM | 2.36–40.0; 40.0–180.0 μM 0.32–2.0; 2.0–240.0 μM 0.03–5.0; 5.0–80.0 μM 0.52–10.0; 10.0–40.0 μM | [35] | |
| Nanosheet | GR/GO/GCE | Cd(II) | DPV | 0.087 μM | 0–10 μM | [37] | |
| Nanosheet | Graphene/CeO2/GCE | Cd(II) Pb(II) Cu(II) Hg(II) | DPASV | 0.1944 nM 0.1057 nM 0.1636 nM 0.2771 nM | 0.02–2.5 μM 0.01–2.5 μM 0.04–1 μM 0.002–0.12 μM | [38] | |
| Nanoplate | Fe3O4/GCE | Zn(II) Cd(II) Pb(II) Cu(II) Hg(II) | SWASV | 0.100 μM 0.213 μM 0.0595μM 0.221 μM 0.0587 μM | 0.4–1.8 μM 0.1–2 μM 0.04–20 μM 0.1–1.5 μM 1–8 μM | 3.38 μA μM−1 3.85 μA μM−1 33.3 μA μM−1 20.3 μA μM−1 11.4 μA μM−1 | [22] |
| Nanoplatelets | Core-ring NiCo2O4 nanoplatelets/GCE | Pb(II) Cd(II) Cu(II) Hg(II) | SWASV | 25.1 nM 104.1 nM 22.7 nM 10.8 nM | 0.1–1 μM | 45.97 μA/μM 6.41 μA/μM 16.18 μA/μM 58.5 μA/μM | [23] |
| Nanosheets | Ni/Co3O4 (NC5.0)/ GCE | Hg(II) | SWASV | 0.009 μM | 0–1.6 μM | 864.93 μA μM−1 cm−2 | [40] |
| Nanosheets | BiNPs@Ti3C2Tx/GCE | Pb(II) Cd(II) | ASV | 10.8 nM 12.4 nM | 0.06–0.6 μM 0.08–0.6 μM | [43] | |
| Three-dimensional hybrid structure | GR/MWCNTs/Bi | Cd(II) Pb(II) | DPASV | 0.1 μg/L 0.2 μg/L | 0.5–30 μg/L 0.5–30 μg/L | [44] | |
| Three-dimensional honeycomb | Bi-NCNF/GCE | Cd(II) Pb(II) | SWASV | 0.02 μg/L 0.04 μg/L | [46] | ||
| Octahedron | UiO66-NH2@MWCNTs/GCE | Cd(II) | DPSV | 0.2 μg/L | 0.5–170 μg/L | [45] | |
| Dandelion | Au@PANI/GCE | Pb(II) Cu(II) | SWASV | 0.003 μM 0.008 μM | 0.02–0.72 μM 0.08–2.4 μM | [47] | |
| Hexagonal prisms | V2O5@MOF | Pb(II) | DPASV | 28.9 nM | 1–10 μM | [48] | |
| Star shape | Star ZIF-8-Nafion/ GCE | Cd(II) | SWASV | 0.5–30 μg/L 30–230 μg/L | 0.48 μg/L | [49] | |
| Butterfly shape | AgNS/SPCE | Cd(II) Pb(II) Cu(II) Hg(II) | DPSV | 0.4 ppb 2.5 ppb 7.3 ppb 0.7 ppb | 5–300 ppb 5–300 ppb 5–500 ppb 5–100 ppb | [50] | |
| Blooming flower carambola | ZMO-GR/GCE NMO-GR/GCE | Pb(II) Hg(II) | SWASV | 0.080 nM 0.040 nM 0.050 nM 0.027 nM | 1.0–7.7 μM 1.4–8.4 μM 1.4–7.7 μM 0.70–6.7 μM | 2.49 μA/μM 4.80 μA/μM 3 μA/μM 5.56 μA/μM | [24] |
| Nanorods Nanoflakes Nanoballs | NiO/GCE | Pb(II) Cd(II) | SWASV | 0.2 μM 0.084 μM 0.07 μM | 0.2–1.2 μM 0.1–1 μM 0.2–1 μM | 2.87 A/M 4.67 A/M 5.10 A/M | [51] |
| Nanorods | r-CeO2/EG/GCE | Cd(II) Pb(II) | DPV | 0.39 μg/L 0.21 μg/L | 5–100 μg/L 100–600 μg/L | [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gibi, C.; Liu, C.-H.; Barton, S.C.; Wu, J.J. Recent Progress in Morphology-Tuned Nanomaterials for the Electrochemical Detection of Heavy Metals. Nanomaterials 2022, 12, 3930. https://doi.org/10.3390/nano12223930
Gibi C, Liu C-H, Barton SC, Wu JJ. Recent Progress in Morphology-Tuned Nanomaterials for the Electrochemical Detection of Heavy Metals. Nanomaterials. 2022; 12(22):3930. https://doi.org/10.3390/nano12223930
Chicago/Turabian StyleGibi, Chinchu, Cheng-Hua Liu, Scott C. Barton, and Jerry J. Wu. 2022. "Recent Progress in Morphology-Tuned Nanomaterials for the Electrochemical Detection of Heavy Metals" Nanomaterials 12, no. 22: 3930. https://doi.org/10.3390/nano12223930
APA StyleGibi, C., Liu, C.-H., Barton, S. C., & Wu, J. J. (2022). Recent Progress in Morphology-Tuned Nanomaterials for the Electrochemical Detection of Heavy Metals. Nanomaterials, 12(22), 3930. https://doi.org/10.3390/nano12223930








