Investigation of a Novel Injectable Chitosan Oligosaccharide—Bovine Hydroxyapatite Hybrid Dental Biocomposite for the Purposes of Conservative Pulp Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of COS
2.2. Preparation of BHA
2.3. Synthesis of Experimental COS-BHA Pulp-Capping Material
2.4. Chemical Characterisation
Fourier Transform Infrared Spectroscopy (FTIR)
Inductively Coupled Mass Spectrometry (ICP-MS) Analysis
Scanning Electron Microscopy (SEM)/Energy-Dispersive X-ray Spectroscopy (EDX) Analysis
2.5. Physical Properties
Determination of pH and Solubility
Determination of Setting Time
Determination of Radiopacity
3. Results
3.1. Selection of the Appropriate Experimental Pulp-Capping Material
3.2. FTIR Analysis of COS, BHA, ZrO2, and the Biocomposite (Sample 4)
3.3. ICP-MS Biocomposite Analysis
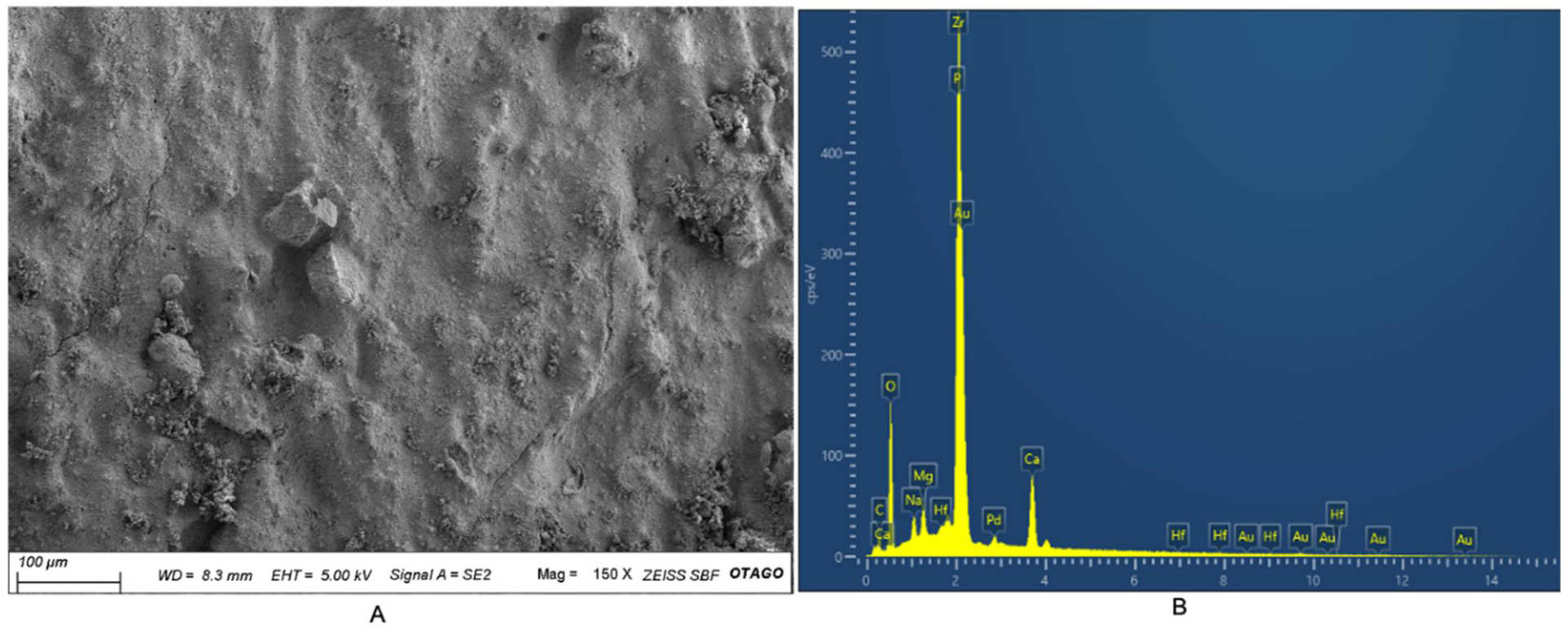
3.4. SEM-EDX Analysis
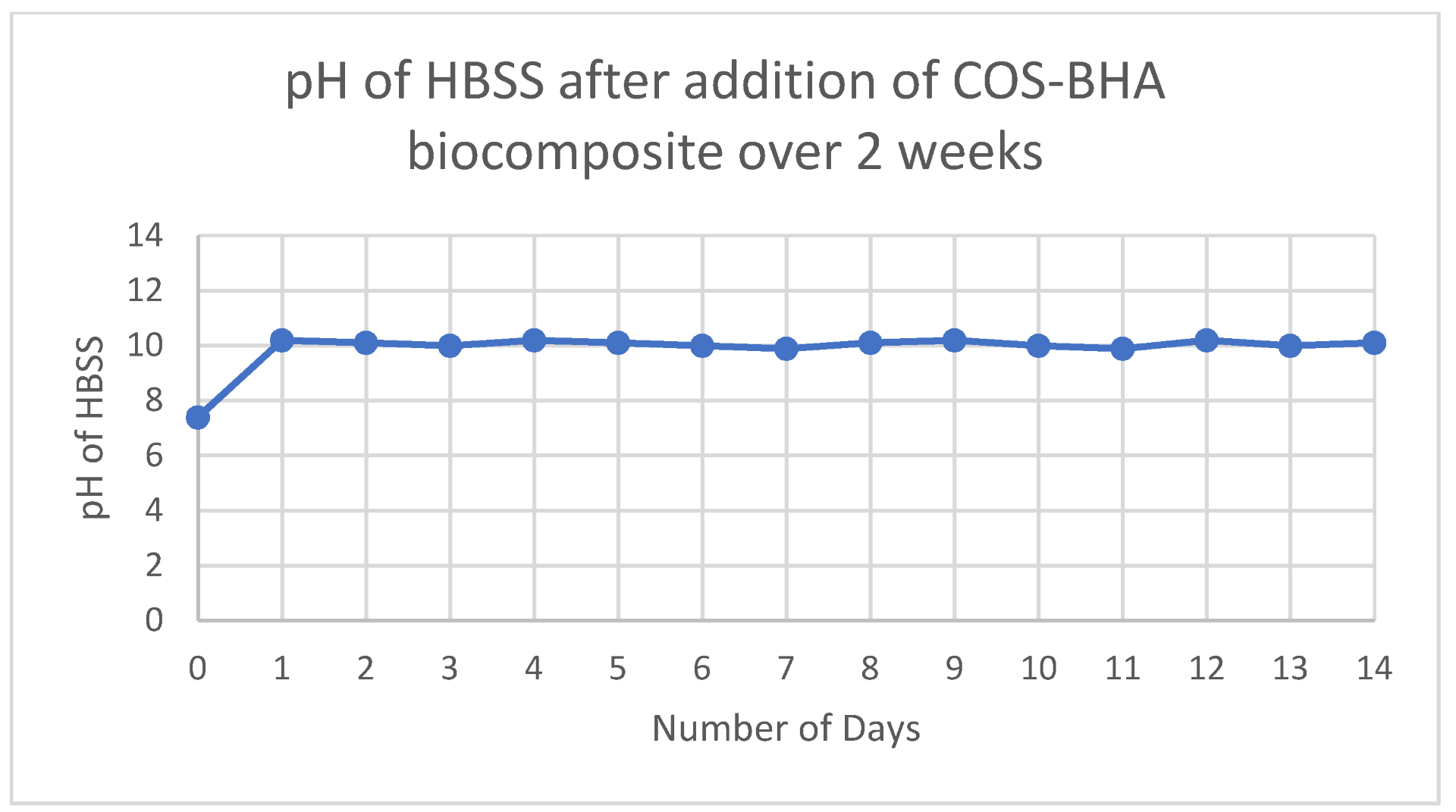
3.5. pH and Solubility of Sample 4
3.6. Biocomposite Setting Time
3.7. Biocomposite Radiopacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hilton, T.J. Keys to clinical success with pulp capping: A review of the literature. Oper. Dent. 2009, 34, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Yong, D.; Cathro, P. Conservative pulp therapy in the management of reversible and irreversible pulpitis. Aust. Dent. J. 2021, 66, S4–S14. [Google Scholar] [CrossRef] [PubMed]
- Komabayashi, T.; Zhu, Q.; Eberhart, R.; Imai, Y. Current status of direct pulp-capping materials for permanent teeth. Dent. Mater. J. 2016, 35, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Morotomi, T.; Washio, A.; Kitamura, C. Current and future options for dental pulp therapy. Jpn. Dent. Sci. Rev. 2019, 55, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Muanprasat, C.; Chatsudthipong, V. Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharmacol. Ther. 2017, 170, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Phil, L.; Sohail, M.; Hasnat, M.; Baig, M.M.F.A.; Ihsan, A.U.; Shumzaid, M.; Kakar, M.U.; Khan, T.M.; Akabar, M. Chitosan oligosaccharide (COS): An overview. Int. J. Biol. Macromol. 2019, 129, 827–843. [Google Scholar] [CrossRef] [PubMed]
- Odeh, A.; Osifo, P.; Noemagus, H. Chitosan: A low cost material for the production of membrane for use in PEMFC—A review. Energy Sources Part A Recovery Util. Environ. Eff. 2013, 35, 152–163. [Google Scholar] [CrossRef]
- Balhuc, S.; Campian, R.; Labunet, A.; Negucioiu, M.; Buduru, S.; Kui, A. Dental applications of systems based on hydroxyapatite nanoparticles—An evidence-based update. Crystals 2021, 11, 674. [Google Scholar] [CrossRef]
- Pu’ad, N.M.; Koshy, P.; Abdullah, H.; Idris, M.; Lee, T. Syntheses of hydroxyapatite from natural sources. Heliyon 2019, 5, e01588. [Google Scholar]
- Ratnayake, J.T.; Gould, M.L.; Shavandi, A.; Mucalo, M.; Dias, G.J. Development and characterization of a xenograft material from New Zealand sourced bovine cancellous bone. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, J.T.; Ross, E.D.; Dias, G.J.; Shanafelt, K.M.; Taylor, S.S.; Gould, M.L.; Guan, G.; Cathro, P.R. Preparation, characterisation and in-vitro biocompatibility study of a bone graft developed from waste bovine teeth for bone regeneration. Mater. Today Commun. 2020, 22, 100732. [Google Scholar] [CrossRef]
- Huang, J.; Ratnayake, J.; Ramesh, N.; Dias, G.J. Development and characterization of a biocomposite material from chitosan and New Zealand-sourced bovine-derived hydroxyapatite for bone regeneration. ACS Omega 2020, 5, 16537–16546. [Google Scholar] [CrossRef] [PubMed]
- Yong, D.; Choi, J.J.E.; Cathro, P.; Cooper, P.R.; Dias, G.; Huang, J.; Ratnayake, J. Development and Analysis of a Hydroxyapatite Supplemented Calcium Silicate Cement for Endodontic Treatment. Materials 2022, 15, 1176. [Google Scholar] [CrossRef] [PubMed]
- Radha, E.; Sudha, P. Synthesis and characterization of glutaraldehyde crosslinked chitosan oligosaccharide-graft-glycidylmethacrylate/poly propylene glycol blend. World J. Pharm. Res. 2017, 7, 1310–1324. [Google Scholar]
- Uysal, I.; Severcan, F.; Evis, Z. Characterization by Fourier transform infrared spectroscopy of hydroxyapatite co-doped with zinc and fluoride. Ceram. Int. 2013, 39, 7727–7733. [Google Scholar] [CrossRef]
- Das, R.S.; Warkhade, S.K.; Kumar, A.; Wankhade, A.V. Graphene oxide-based zirconium oxide nanocomposite for enhanced visible light-driven photocatalytic activity. Res. Chem. Intermed. 2019, 45, 1689–1705. [Google Scholar] [CrossRef]
- Coleman, N.J.; Li, Q. The impact of zirconium oxide radiopacifier on the early hydration behaviour of white Portland cement. Mater. Sci. Eng. C 2013, 33, 427–433. [Google Scholar] [CrossRef]
- Primus, C.M. Comments on testing for the presence of arsenic in MTA and portland cement. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 4, 479–480. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Zhou, X.-N.; Chen, W.-M. Self-etching adhesives: Possible new pulp capping agents to vital pulp therapy. Front. Med. 2011, 5, 77–79. [Google Scholar] [CrossRef] [PubMed]



| Sample | COS Sol (% by wt) | COS Sol (g) | BHA (% by wt) | BHA (g) | ZrO2 (% by wt) | ZrO2 (g) |
|---|---|---|---|---|---|---|
| 1 | 55 | 5 | 10 | 0.91 | 35 | 3.18 |
| 2 | 45 | 5 | 20 | 2.22 | 35 | 3.89 |
| 3 | 35 | 5 | 30 | 4.29 | 35 | 5.00 |
| 4 | 25 | 5 | 40 | 8.00 | 35 | 7.00 |
| 5 | 15 | 5 | 50 | 16.67 | 35 | 11.67 |
| Element (mg/kg) | Experimental Pulp-Capping Material |
|---|---|
| Zr | 68,100 |
| Ca | 55,300 |
| P | 14,600 |
| Na | 1320 |
| Mg | 914 |
| K | <900 |
| Al | <200 |
| B | <200 |
| Zn | <90 |
| Fe | <50 |
| Sr | 43 |
| Ba | 19 |
| Element | Weight (%) |
|---|---|
| C | 13.47 |
| O | 31.02 |
| Na | 1.17 |
| Mg | 1.28 |
| P | 2.66 |
| Ca | 6.01 |
| Zr | 43.72 |
| Hf | 0.66 |
| Total | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, M.; Ratnayake, J.; Cathro, P.; Gould, M.; Ali, A. Investigation of a Novel Injectable Chitosan Oligosaccharide—Bovine Hydroxyapatite Hybrid Dental Biocomposite for the Purposes of Conservative Pulp Therapy. Nanomaterials 2022, 12, 3925. https://doi.org/10.3390/nano12213925
Cai M, Ratnayake J, Cathro P, Gould M, Ali A. Investigation of a Novel Injectable Chitosan Oligosaccharide—Bovine Hydroxyapatite Hybrid Dental Biocomposite for the Purposes of Conservative Pulp Therapy. Nanomaterials. 2022; 12(21):3925. https://doi.org/10.3390/nano12213925
Chicago/Turabian StyleCai, Mingkai, Jithendra Ratnayake, Peter Cathro, Maree Gould, and Azam Ali. 2022. "Investigation of a Novel Injectable Chitosan Oligosaccharide—Bovine Hydroxyapatite Hybrid Dental Biocomposite for the Purposes of Conservative Pulp Therapy" Nanomaterials 12, no. 21: 3925. https://doi.org/10.3390/nano12213925
APA StyleCai, M., Ratnayake, J., Cathro, P., Gould, M., & Ali, A. (2022). Investigation of a Novel Injectable Chitosan Oligosaccharide—Bovine Hydroxyapatite Hybrid Dental Biocomposite for the Purposes of Conservative Pulp Therapy. Nanomaterials, 12(21), 3925. https://doi.org/10.3390/nano12213925









